In 1982 the group of Harald zur Hausen discovered genomes of human papillomaviruses in cervical cancer biopsies and cell lines derived from them (reviewed in1). It quickly became clear that human papillomavirus genomes can be found in the vast majority of cervical cancers but also in a substantial fraction of vaginal, vulvar, penile, anal, and oropharyngeal cancers.2 These findings stimulated a tremendous amount of activity to search for oncogenic HPV sequences in other non‐anogenital cancers as well, including breast cancer,3 bladder cancer,4 colon cancer,5 and esophageal squamous cell carcinoma (ESCC) (reviewed in Halec et al., this issue and6) . In particular, the prevalence of potentially oncogenic HPV genotypes in ESCC has been investigated in more than 100 original reports (reviewed in7 and8). The reported prevalence of HPV genotypes in these studies ranged from 0 to 100 %, leaving the scientific community in the dark about the true role of oncogenic HPV types in the pathogenesis of ESCC.
In the vast majority of these previously published reports, only the prevalence of HPV genome fragments was investigated while functional aspects of what causative role HPV genomes may play were largely neglected. Thus, it was difficult to discern whether these HPV genome fragments were just silent passengers or indeed encompassed active viral oncogenes to trigger the development of ESCC.
The pathognomonic role of oncogenic HPV types in cervical and oropharyngeal cancers has been well established by a plethora of epidemiological studies linked to extensive functional analysis, clearly documenting that in these cancers the neoplastic phenotype is unequivocally linked to the genetic activity of the papillomavirus genes E6 and E7.9 The criteria now accepted to document the causative role of HPV genotypes in human cancers include the following three aspects:
At least one HPV genome equivalent should be present in each neoplastic cell of the suspected cancer.
The HPV oncogenes E6 and E7 should be transcribed in the cancer cells and their transcripts should be translated into the respective viral oncoproteins.
Suppression of their activity by either inhibiting their expression or abrogating their function should result in loss of the neoplastic phenotype of the respective cancer cell. This aspect has been referred to as an “oncogene addiction” of HPV‐triggered cancers from the continuous expression of the viral E6 and E7 oncogenes.
As the third criterion requires complex experimental studies, it cannot be applied in epidemiological studies. Therefore, surrogate parameters needed to be defined that strongly suggest not only the presence but also the functional activity of HPV oncogenes in human cancers. The oncogenic activity of HPVs (i.e., the active expression of the viral E6 and E7 oncogenes in each affected cancer cell) cannot be documented by conventional histopathological criteria. Therefore, it was essential to define additional criteria to document the functional activity of oncogenic HPV genomes by surrogate parameters. One of them is the detection of active mRNAs transcribed from the HPV E6 and E7 genes. The presence of a spliced version of the E6 transcript, the E6*I mRNA proved to be a good expression marker.10 A more indirect, but technically simpler approach is to detect surrogate markers such as the cyclindependent kinase inhibitor p16INK4a (reviewed in11) by simple immunohistochemistry techniques. p16INK4a is substantially upregulated in all HPV‐transformed cells in reaction to the oncogenic stress triggered by the HPV E7 protein and its associated modification of the chromatin and histone scaffold of the p16INK4a gene locus.12 However, sometimes cancer cells do overexpress p16INK4a, also despite the absence of oncogenic HPV infections.13 Taken together, these observations suggest that it is highly likely that a cancer is caused by oncogenic HPV infections if the following three criteria are fulfilled (figure 1):
Figure 1.
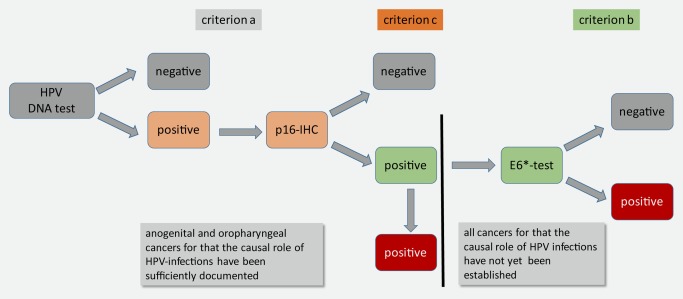
Minimal criteria to prove a causal role of HPV infections in carcinogenesis are schematically outlined by this diagnostic algorithm. Upon detection of HPV‐DNA fragments (at least one genome equivalent per cell, criterion a), samples should be stained for p16INK4a using immunohistochemistry (criterion c). For practical reasons p16‐immunohistochemisty (p16‐IHC) may be used as “search test” for HPV‐associated cancers and followed by HPV‐DNA detection.14 For samples of the oropharyngeal or anogenital tract, the causal role of oncogenic HPV types is sufficiently documented to assume that if they are positive for HPV‐DNA and p16 IHC, that the oncogenic HPV infection is most likely the causative agent. For all other cancer samples for which the causal role of oncogenic HPV infections has not yet been established, further proof by detecting the expression of the viral oncogenes using the E6*‐PCR (criterion b) is highly recommended as described in the text.
presence of at least one genome equivalent of oncogenic HPV types in each cancer cell
active expression of HPV oncogene transcript evidenced by the detection of the E6*I transcript and
overexpression of the cyclin‐dependent kinase inhibitor p16INK4a in each cell as surrogate parameter for the activity of the E7 oncoprotein.
For anogenital and oropharyngeal cancers it is meanwhile broadly accepted that criteria a.) and c.) are sufficient to document the causal involvement of HPV as causative carcinogens.14 However, in cases in which the carcinogenic role of HPV is less established, it is indeed mandatory to require that all three criteria sufficiently document the pathogenic role of HPV infections (figure 1).
In the paper of Gordana Halec and her colleagues, this question has been systematically addressed to test whether ESCC are linked to oncogenic HPV infections. In their study they analyzed a cohort of 133 patients that came from high incidence areas for ESCC in South Africa, China, and Iran. All 133 patients displayed evidence for HPV infections as they were seropositive for antibodies against HPV early proteins. The respective tumor samples were tested for the presence of oncogenic HPV types by broad‐spectrum HPV genotyping methods. In addition they quantified the viral loads by quantitative real‐time PCR, documented the expression of the viral oncogenes by type‐specific mRNA analysis using E6*1 RT‐PCR,10 and checked the expression of p16INK4a.11
They detected DNA of oncogenic HPV types in 10 (8%) of the samples, including a few samples in which multiple HPV types were found in the same sample (multiple infection). However, these HPV‐positive samples displayed only low copy numbers of HPV genotypes, suggesting that the first of the aforementioned criteria requiring that at least one genome copy equivalent was present in each cancer cell was not fulfilled. One of the samples showed p16INK4a upregulation; however, HPV E6*I mRNA was not detected, suggesting that in this case overexpression of p16INK4a was triggered by HPV‐independent mechanisms, a phenomenon frequently found in other cancer entities, too.13 Here, criteria 1 and 3 were fulfilled, but not criterion 2, suggesting again that the HPV infection was not causative for the development of this ESCC. All in all, none of the 133 investigated cases form three high incidence areas of ESCC fulfilled the three required criteria to document a causal relationship between HPV infection and carcinogenesis. As so many reports have been published over the past decades (reviewed in15, 16) reporting highly divergent results on the prevalence and the potentially pathognomonic role of HPV types in human cancers, the three criteria listed above should now be accepted as minimal standard criteria by all editors of scientific journals to document a causative role of HPV infections in human cancer. This would substantially clear up the true role of oncogenic HPV infections in human carcinogenesis and avoid further ongoing scientific confusion as to which cancers might and which might not be caused by oncogenic HPV infections.
Magnus von Knebel Doeberitz Associate Editor, MD Department of Applied Tumor Biology Institute of Pathology University of Heidelberg Im Neuenheimer Feld 224 D‐69120 Heidelberg Email: magnus.knebel-doeberitz@med.uni-heidelberg.de DOI:10.1002/ijc.30059
Reference
- 1. zur Hausen H. Papillomaviruses in the causation of human cancers ‐ a brief historical account. Virology 2009;384:260–5. [DOI] [PubMed] [Google Scholar]
- 2. de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 2012;13:607–15. [DOI] [PubMed] [Google Scholar]
- 3. Joshi D, Buehring GC. Are viruses associated with human breast cancer? Scrutinizing the molecular evidence. Breast Cancer Res Treat 2012;135:1–15. [DOI] [PubMed] [Google Scholar]
- 4. Shigehara K, Sasagawa T, Namiki M. Human papillomavirus infection and pathogenesis in urothelial cells: a mini‐review. J Infect Chemother 2014;20:741–7. [DOI] [PubMed] [Google Scholar]
- 5. Damin DC, Ziegelmann PK, Damin AP. Human papillomavirus infection and colorectal cancer risk: a meta‐analysis. Colorectal Dis 2013;15:e420–8. [DOI] [PubMed] [Google Scholar]
- 6. Zhang SK, Guo LW, Chen Q, Zhang M, Liu SZ, Quan PL, Lu JB, Sun XB. The association between human papillomavirus 16 and esophageal cancer in Chinese population: a meta‐analysis. BMC Cancer 2015;15:1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Halec G, Schmitt M, Egger S, Abnet CC, Babb C, Dawsey SM, Flechtenmacher C, Gheit T, Hale M, Holzinger D, Malekzadeh R, Taylor PR, et al. Mucosal Alpha‐Papillomaviruses are not associated with Esophageal Squamous Cell Carcinomas: Lack of Mechanistic Evidence from South Africa, China and Iran and from a World‐Wide Meta‐Analysis. Int J Cancer 2016;139:85–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ludmir EB, Stephens SJ, Palta M, Willett CG, Czito BG. Human papillomavirus tumor infection in esophageal squamous cell carcinoma. J Gastrointest Oncol 2015;6:287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mighty KK, Laimins LA. The role of human papillomaviruses in oncogenesis. Recent Results Cancer Res 2014;193:135–48. [DOI] [PubMed] [Google Scholar]
- 10. Halec G, Schmitt M, Dondog B, Sharkhuu E, Wentzensen N, Gheit T, Tommasino M, Kommoss F, Bosch FX, Franceschi S, Clifford G, Gissmann L, et al. Biological activity of probable/possible high‐risk human papillomavirus types in cervical cancer. Int J Cancer 2013;132:63–71. [DOI] [PubMed] [Google Scholar]
- 11. Bergeron C, Ronco G, Reuschenbach M, Wentzensen N, Arbyn M, Stoler M, von Knebel Doeberitz M. The clinical impact of using p16(INK4a) immunochemistry in cervical histopathology and cytology: an update of recent developments. Int J Cancer 2015;136:2741–51. [DOI] [PubMed] [Google Scholar]
- 12. McLaughlin‐Drubin ME, Crum CP, Munger K. Human papillomavirus E7 oncoprotein induces KDM6A and KDM6B histone demethylase expression and causes epigenetic reprogramming. Proc Natl Acad Sci U S A 2011;108:2130‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Romagosa C, Simonetti S, Lopez‐Vicente L, Mazo A, Lleonart ME, Castellvi J, Ramon y Cajal S. p16(Ink4a) overexpression in cancer: a tumor suppressor gene associated with senescence and high‐grade tumors. Oncogene 2011;30:2087–97. [DOI] [PubMed] [Google Scholar]
- 14. Smeets SJ, Hesselink AT, Speel EJ, Haesevoets A, Snijders PJ, Pawlita M, Meijer CJ, Braakhuis BJ, Leemans CR, Brakenhoff RH. A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer 2007;121:2465–72. [DOI] [PubMed] [Google Scholar]
- 15. Syrjanen K, Syrjanen S. Detection of human papillomavirus in esophageal papillomas: systematic review and meta‐analysis. APMIS 2013;121:363–74. [DOI] [PubMed] [Google Scholar]
- 16. Xu W, Liu Z, Bao Q, Qian Z. Viruses, Other Pathogenic Microorganisms and Esophageal Cancer. Gastrointest Tumors 2015;2:2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]


