Abstract
The pharmacokinetics of LY2605541 (basal insulin peglispro), a novel long‐acting basal insulin analogue, was evaluated in 5 groups of subjects with varying degrees of renal function based on creatinine clearance: normal renal function (>80 mL/min), mild renal impairment (51–80 mL/min), moderate renal impairment (30–50 mL/min), severe renal impairment (<30 mL/min), or end‐stage renal disease (ESRD) requiring hemodialysis. Serial blood samples for pharmacokinetic analyses were collected up to 12 days following a single 0.33 U/kg subcutaneous dose of LY2605541. The apparent clearance (CL/F) and half‐life across groups were not affected by renal function. Cmax values were lower in subjects with increasing severity of renal impairment; however, the small decrease in Cmax did not affect the overall exposure. Regression analysis showed that LY2605541 clearance is independent of renal function (slope = 0.000863; P = .885). The mean fraction of LY2605541 eliminated by a single hemodialysis session was 13% in subjects with ESRD. LY2605541 was generally well tolerated in healthy subjects and those with renal impairment following a single 0.33 U/kg subcutaneous dose. Given these data, no dose adjustment of LY2605541 based on pharmacokinetics is recommended in renal impairment or in patients undergoing hemodialysis.
Keywords: pharmacokinetics, long‐acting basal insulin analogue, renal impairment, insulin peglispro
The basal insulin analogue LY2605541 (basal insulin peglispro) is a novel long‐acting insulin analogue that consists of insulin lispro linked to a 20‐kDa polyethylene glycol (PEG) moiety. In a recent study, dynamic light scattering analysis indicated that LY2605541 has a hydrodynamic diameter of 7.9 ± 0.5 nm, a diameter 4 times larger than insulin lispro and similar to the size of a ∼75‐kDa globular protein.1 It has been shown that LY2605541 has hepato‐preferential action with reduced peripheral effects, a flat PK and PD profile, and a prolonged half‐life.1, 2, 3, 4
LY2605541 is being studied for use in patients with type 1 and type 2 diabetes mellitus, populations that are likely to have or to develop renal impairment. Recent estimates report that approximately 40% of patients with chronic kidney disease (CKD) are also diabetic, and, conversely, adults with diabetes are at an increased risk of developing CKD. Furthermore, inadequately controlled diabetes increases the progression of CKD to renal failure, requiring dialysis.5, 6
Typically, renal clearance contributes approximately 40% to 50% to the overall systemic clearance of insulin after the first‐pass metabolism of insulin produced in the pancreas.7, 8 The half‐life of insulin may be prolonged in diabetic patients with renal impairment because of lower levels of degradation. In addition, the clearance of insulin has been shown to be reduced as renal impairment increases.7, 8, 9 Consequently, diabetic patients have changing insulin requirements based on the state of their declining renal function and insulin treatment used.
PEG is a synthetic polymer that has been used successfully for many years in a wide range of therapeutic products, has an extensively evaluated safety profile, and may confer therapeutic advantages for the parenteral delivery of proteins.10 This study investigated the potential impact of pegylation of LY2605541 on renal clearance. The purpose of this study was to evaluate the pharmacokinetics (PK) and tolerability of a single 0.33 U/kg dose of LY2605541 in subjects with varying degrees of renal function.
Subjects and Methods
Subjects and Study Design
This phase 1 single‐dose, open‐label study was conducted at 4 centers in the United States. Subjects were enrolled into 1 of 5 renal function groups based on creatinine clearance (CrCl) by Cockcroft‐Gault estimation at screening: group 1 — normal renal function (>80 mL/min); group 2 — mild renal impairment (51–80 mL/min); group 3 — moderate renal impairment (30–50 mL/min); group 4 — severe renal impairment (< 30 mL/min) not requiring dialysis; or group 5 — end‐stage renal disease (ESRD) requiring hemodialysis.11, 12, 13 To ensure that sex and age distribution was similar, subjects with normal renal function (group 1) were matched with subjects in groups 2 through 5 with regard to sex and age (±15 years). Key inclusion criteria were male and female subjects aged 18 to 80 years (inclusive) with a body mass index (BMI) between 18.5 and 40.0 kg/m2 (inclusive). Subjects with type 2 diabetes mellitus were eligible to participate in this study; however, subjects with type 1 diabetes mellitus or subjects requiring insulin treatment for type 2 diabetes mellitus were excluded. Eligible subjects were permitted to continue ongoing medications needed to stabilize underlying medical conditions, for example, vitamin D compounds, antihypertensives, erythropoietin, antihyperuricemics, vasodilators, diuretics, and phosphate‐binding agents.
Each study site received ethical approval to conduct the study from the Independent Investigational Review Board (Plantation, Florida). The study was conducted in accordance with the principles of the Declaration of Helsinki. Investigators obtained written informed consent from each subject prior to participation.
Study Procedures
Subjects were screened for eligibility up to 21 days before study treatment. Baseline evaluations were conducted on day ‐1 before study treatment. Subjects were admitted to the Clinical Research Unit (CRU) on day ‐1 and resided in the CRU until at least 48 hours after dosing. Subjects received a single 0.33 U/kg subcutaneous dose of LY2605541 on day 1. LY2605541 was administered in 1 of the 2 lower quadrants of the abdominal wall.
Blood samples for LY2605541 pharmacokinetic analysis were obtained at predose; 2, 4, 6, 8, and 12 hours on day 1; 24 and 36 hours on day 2; 48 hours on day 3; 72 hours on day 4; 120 hours on day 6; 168 hours on day 8; 216 hours on day 10; and 264 hours on day 12.
For subjects with ESRD, the LY2605541 dose was administered approximately 48 hours before the next hemodialysis session, and subjects underwent hemodialysis approximately 5 times over the 12‐day pharmacokinetic sampling period. Only high‐flux polysulfone hemodialysis membranes were used in the study.
To minimize the potential for hypoglycemic events, subjects were given 3 main meals during the day and 3 snacks after each main meal (midmorning, midafternoon, bedtime) every 24 hours for a minimum of 2 days after LY2605541 dose administration. In addition, to ensure subjects’ safety by monitoring blood glucose levels, blood samples were obtained every 1.5 to 3 hours or as clinically indicated from the time of LY2605541 administration to 48 hours postdose. All subjects were instructed to self‐monitor blood glucose on an outpatient basis from discharge until day 8.
Safety and tolerability were assessed by blood glucose measurements, vital signs measurements, physical examination, clinical laboratory tests, electrocardiograms, and monitoring of adverse events (AEs).
Bioanalytical Methods
Serum samples were analyzed for LY2605541 using a validated enzyme‐linked immunosorbent assay (ELISA) method at Charles River Laboratories Preclinical Services Montreal (Senneville, Quebec City, Canada). The method for human serum is similar to the method used for rat serum.14 Briefly, purified guinea pig anti‐human insulin antibody (data on file; Eli Lilly and Co.) is coated onto black Nunc MaxiSorp plates (Thermo Fisher, Pittsburgh, Pennsylvania), and I‐Block solution (Life Technologies, Grand Island, New York) is used to block free sites. Samples are loaded into the wells of the plate, and LY2605541 was allowed to bind to the immobilized insulin antibody. The bound complex is detected by the addition of rabbit monoclonal anti‐PEG‐biotin antibody (Abcam, Cambridge, Massachusetts). Following a wash step, peroxidase‐conjugated streptavidin is added to the plate, and signal is produced by adding QuantaBlu substrate (Thermo Scientific, Waltham, Massachusetts). The fluorescence signal was then measured at an excitation wavelength of 320 nm and an emission wavelength of 420 nm. The concentration of the samples was extrapolated from a standard curve fitted with a 5‐PL equation, with a weighting factor of 1/Y2. The concentrations of LY2605541 in the calibration standards, quality control samples, and study samples were directly proportional to the fluorescence measured in the wells of the plate.14
The validated ELISA method is selective for LY2605541, and endogenous substances in normal human serum do not cross‐react in the assay. Human insulin at concentrations up to 444 pmol/L does not interfere with the assay. The lower limit of quantification was 20.00 pmol/L, and the upper limit of quantification was 500.00 pmol/L. Samples above the limit of quantification were diluted and reanalyzed to yield results within the calibrated range. The interassay accuracy (% relative error) during validation ranged from 1.4% to 3.5%. The interassay precision (% relative standard deviation) during validation ranged from 5.2% to 8.5%.
Pharmacokinetic Analysis
The individual serum LY2605541 concentrations were analyzed by conventional noncompartmental pharmacokinetic analysis using WinNonlin Enterprise Edition software (version 5.3; Pharsight Corporation, St. Louis, Missouri). Concentrations below the quantification limit (BQL) at predose were treated as zero, whereas postdose BQL concentrations were treated as missing in noncompartmental analysis; BQL concentrations were treated as zero for generating the mean time–concentration profiles.
Primary pharmacokinetic parameters were area under the concentration‐versus‐time curve from zero to infinity (AUC0–∞) and maximum concentration (Cmax). The values for AUC0–∞ and Cmax were log‐transformed and analyzed using an analysis of variance model with group as a factor to compare each level of the impaired renal function against the control group.
Pharmacokinetic parameters also included apparent total body clearance of drug calculated after extravascular administration (CL/F). To evaluate the relationships of CL/F, AUC0–∞ and Cmax with CrCl, linear regression analyses were performed.
The fraction of elimination by hemodialysis was calculated for subjects in the ESRD group as the difference between the observed and extrapolated predialysis LY2605541 serum concentrations relative to the observed predialysis LY2605541 serum concentration.15 The extrapolated predialysis concentration was derived from the linearly fit line on the semilogarithmic plot of the terminal phase.
Although patients with ESRD had more than 1 hemodialysis session during the pharmacokinetic sampling period, the fraction of dialysis elimination was calculated only during the first hemodialysis session of the pharmacokinetic sampling period.
Results
Subject Disposition and Demographics
Table 1 summarizes baseline characteristics by renal function group. Overall, 46 subjects participated in this study, with 8 to 12 subjects in each group. Eleven subjects with type 2 diabetes mellitus were enrolled across the renal impairment groups. There were 31 male subjects and 15 female subjects between the ages of 25 and 78 years of age (inclusive) in this study. The mean BMI was 26.7 kg/m2, and mean age in each group ranged from 44.4 to 66.9 years.
Table 1.
Demographic and Baseline Characteristics
| Estimated CrCl (mL/min) | ||||||
|---|---|---|---|---|---|---|
| Normal Function (>80), n = 12 | Mild Impairment (51–80), n = 8 | Moderate Impairment (30–50), n = 8 | Severe Impairment (<30), n = 9 | ESRD (Dialysis for >3 mo), n = 9 | Overall,a n = 46 | |
| Sex (male), n (%) | 9 (75.0) | 7 (87.5) | 5 (62.5) | 4 (44.4) | 6 (66.7) | 31 (67.4) |
| Age (y), mean (SD) | 45.5 (15.3) | 66.9 (8.9) | 61.0 (13.3) | 61.8 (11.5) | 44.4 (10.7) | 54.9 (15.1) |
| CrCla (mL/min), mean (SD) | 122.2 (26.5) | 63.4 (6.7) | 39.4 (6.7) | 21.9 (6.9) | 13.3 (5.0) | — |
| Weight (kg), mean (SD) | 81.3 (15.1) | 84.5 (13.0) | 73.7 (16.0) | 66.7 (10.1) | 79.9 (19.3) | 77.4 (15.7) |
| BMI (kg/m2), mean (SD) | 26.7 (3.1) | 28.8 (3.9) | 27.1 (5.7) | 24.7 (3.0) | 26.5 (6.3) | 26.7 (4.5) |
| Subjects with type 2 diabetes mellitus, n (%) | 0 (0.0) | 3 (37.5) | 2 (25.0) | 4 (44.4) | 2 (22.2) | 11 (23.9) |
| LY2605541 dose administered (U), mean (range) | 27.2 (18.7–37.8) | 28.1 (20.0–32.9) | 24.7 (16.4–32.4) | 22.3 (16.4– 28.4) | 26.7 (19.6– 38.2) | 25.9 (16.4–38.2) |
BMI, body mass index; CrCl, estimated creatinine clearance; ESRD, end‐stage renal disease; n, number of subjects; SAE, serious adverse event; SD, standard deviation.
Forty‐six subjects received LY2605541; 45 subjects completed the study, and 1 subject from the severe group discontinued the study because of an SAE (angina pectoris) judged to be unrelated to study treatment.
Of the 46 subjects who entered the study, 46 received the study drug, and 45 subjects completed the study. One subject did not complete the study because of a serious AE (SAE) of angina pectoris, which was determined by the study investigator to be unrelated to study treatment; this subject with severe renal impairment had a medical history of hypertension, coronary artery disease, and angina pectoris, and the SAE that occurred approximately 6 hours postdose and lasted approximately 1 day. Pharmacokinetic analyses were conducted using data from subjects receiving a dose of LY2605541 that have evaluable concentration data. PK was assessed in 43 of the 46 subjects enrolled in the study. Pharmacokinetic data were excluded for the subject who discontinued because of an SAE and 2 subjects because of the thawing of pharmacokinetic samples during transit to the bioanalytical laboratory.
Pharmacokinetic Evaluations
Mean serum LY2605541 concentration–time profiles after subcutaneous administration of LY2605541 0.33 U/kg were similar between renal function groups (Figure 1). Table 2 summarizes pharmacokinetic parameter estimates by renal function group. Overall, the Cmax of serum LY2605541 tended to be lower in subjects with increasing severity of renal impairment; however, this small decrease in Cmax did not affect AUC. In addition, the half‐life tended to be higher in the renal impairment groups compared with the control group. The AUC was lower in the ESRD group compared with the normal renal function group, likely because of LY2605541 elimination in dialysis. Statistical analysis showed no statistically significant difference in AUC0–∞ or Cmax between each renal impairment group and the control group (Table 2). In addition, review of the individual pharmacokinetic parameters did not suggest that age was a factor for any differences in the pharmacokinetic parameters.
Figure 1.
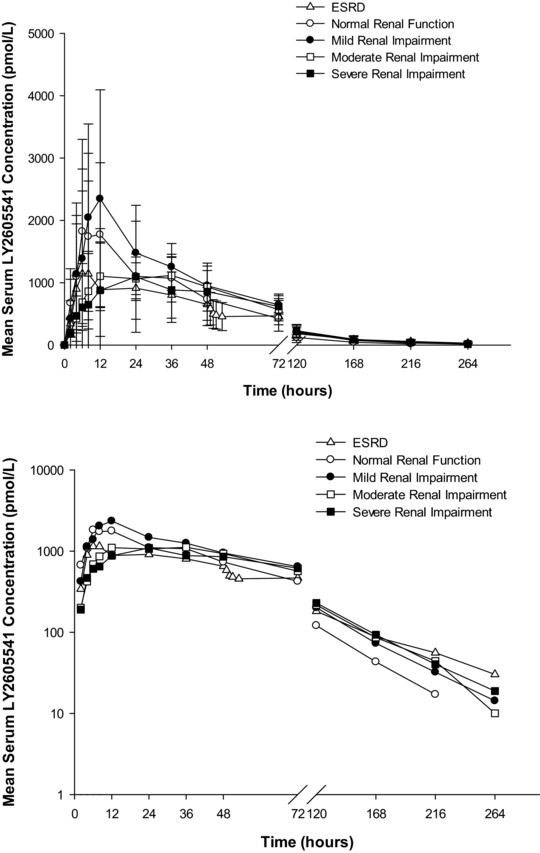
Mean ± SD LY2605541 serum concentration‐versus‐time profiles in subjects with varying degrees of renal function: linear scale (top), semilogarithmic scale (bottom). SD, standard deviation; ESRD, end‐stage renal disease.
Table 2.
Summary and Statistical Comparison of LY2605541 Pharmacokinetic Parameters in Subjects With Varying Degrees of Renal Function
| Estimated CrCl (mL/min) | |||||
|---|---|---|---|---|---|
| Normal Function (>80), n = 10 | Mild Impairment (51–80), n = 8 | Moderate Impairment (30–50), n = 8 | Severe Impairment (<30), n = 8 | ESRD (Dialysis for >3 mo), n = 9 | |
| Summary of PK parameters, geometric mean (CV%) | |||||
| Cmax, pmol/L | 1700 (77) | 2090 (88) | 1310 (29) | 1030 (77) | 1180 (124) |
| 2090 (1390)c | 2630 (1730)c | 1360 (421)c | 1290 (965)c | 1730 (1610)c | |
| AUC0–∞, pmol·h/L | 84 100 (32) | 110 000 (37) | 90 400 (28) | 82 400 (44) | 73 400 (48) |
| 88 000 (28 100)c | 116 000 (37 800)c | 93 500 (25 600)c | 89 400 (40 500)c | 79 600 (30 900)c | |
| CL/F, L/h | 2.84 (36) | 2.21 (31) | 2.33 (38) | 2.36 (39) | 3.10 (61) |
| 3.0 (1.0)c | 2.3 (0.7)c | 2.5 (1.1)c | 2.5 (0.9)c | 3.7 (2.9)c | |
| t1/2, h | 34.9 (50) | 37.2 (31) | 43.7 (48) | 42.4 (16) | 45.7 (24) |
| 39.7 (27.1)c | 38.7 (11.6)c | 48.8 (30.3)c | 42.9 (7.2)c | 46.8 (11.2)c | |
| Fraction eliminated by dialysis | — | — | — | — | 0.128a (92) |
| 0.076a (0.182)c | |||||
| Statistical comparison of PK parameters, ratio of LS geometric means (90%CI)b | |||||
| Cmax, pmol/L | — | 1.23 (0.69–2.18) | 0.77 (0.43–1.37) | 0.61 (0.34–1.08) | 0.69 (0.40–1.21) |
| AUC0–∞, pmol·h/L | — | 1.30 (0.97–1.75) | 1.08 (0.80–1.45) | 0.98 (0.73–1.32) | 0.87 (0.65–1.16) |
AUC0–∞, area under the concentration‐versus‐time curve from time zero to infinity; CI, confidence interval; CL/F, apparent total body clearance of drug calculated after extravascular administration; Cmax, maximum observed drug concentration; CrCl, estimated creatinine clearance; CV%, coefficient of variation; ESRD, end‐stage renal disease; n, number of subjects; PK, pharmacokinetic; t1/2, half‐life associated with the terminal rate constant in noncompartmental analysis.
1 unit LY2605541 = 9000 pmol.
n = 6; 3 subjects had negative fraction of dialysis elimination values estimated and were excluded in the summary statistics, as it is not meaningful to have a negative fraction of dialysis elimination.
Renally impaired groups (mild, moderate, severe, ESRD) versus control (normal renal function).
Arithmetic mean (standard deviation).
Figure 2 illustrates the concentration profile of LY2605541 during the hemodialysis session. A single hemodialysis session, initiated approximately 48 hours postdose, resulted in a mean 13% (range, 3%–25%) elimination of LY2605541 (Table 2).
Figure 2.
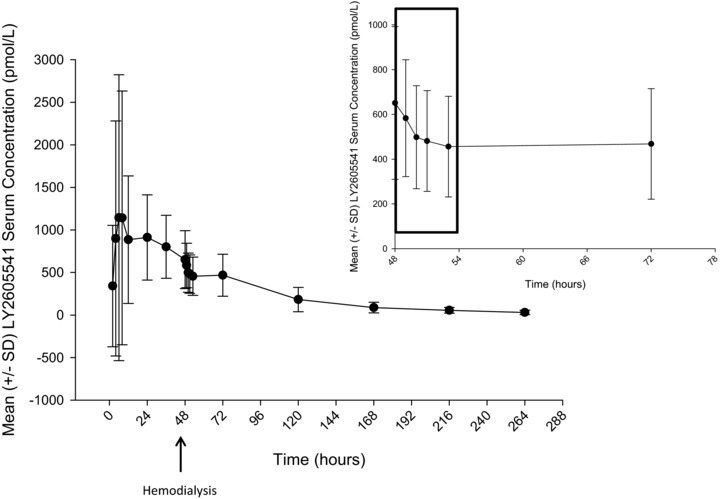
Mean ± SD LY2605541 serum concentration‐versus‐time profile in ESRD subjects showing the effect of hemodialysis. SD, standard deviation.
Figure 3 depicts the relationship between LY2605541 apparent clearance and CrCl. There was no direct relationship between the apparent clearance of LY2605541 and CrCl (R 2 = 0.000513, slope = 0.000863, P = .885). Similarly, there was no direct relationship between Cmax and AUC0–∞ with CrCl (Figure S1).
Figure 3.
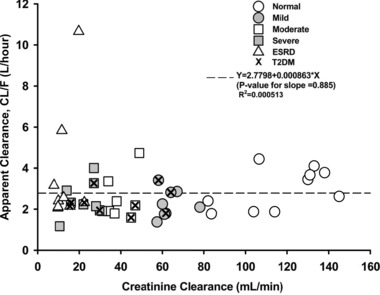
Relationship between apparent LY2605541 clearance and creatinine clearance. CL/F, apparent total body clearance of drug calculated after extravascular administration; T2DM, type 2 diabetes mellitus; ESRD, end‐stage renal disease.
A population pharmacokinetic model was used to simulate the median LY2605541 serum concentration profiles at steady state for once‐daily 0.33 U/kg LY2605541 dosing. Simulated profiles showed similar exposures during the 24‐hour dosing period for the control and all renal impairment groups (Figure 4).
Figure 4.
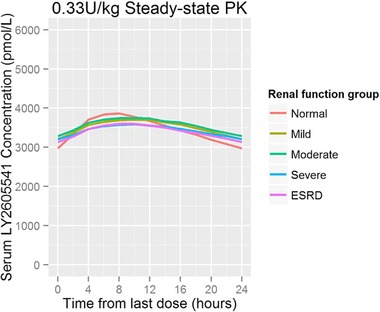
Simulated median steady‐state LY2605541 concentration profiles (0.33 U/kg daily). ESRD, end‐stage renal disease; PK, pharmacokinetics.
Safety
Single doses of LY2605541 were generally well tolerated in all renal impairment groups. Of the 46 subjects who received LY2605541, 26 subjects reported treatment‐emergent AEs (TEAEs). Thirteen subjects reported 19 TEAEs that were related to LY2605541 as judged by the investigator. The most common study drug‐related TEAEs were hypoglycemia (9 events reported by 7 subjects) and headache (5 events reported by 5 subjects); see Table 3.
Table 3.
Frequency of LY2605541 Treatment‐Emergent Adverse Events
| Number of Adverse Events (Number of Subjects With Adverse Event) | ||||||
|---|---|---|---|---|---|---|
| MedDRA Preferred Term | Normal Function (>80), n = 12 | Mild Impairment (51–80), n = 8 | Moderate Impairment (30–50), n = 8 | Severe Impairment (<30), n = 9 | ESRD (Dialysis for >3 mo), n = 9 | Overall, n = 46 |
| Hypoglycemia | 2 (2) | 0 (0) | 1 (1) | 0 (0) | 6 (4) | 9 (7) |
| Headache | 1 (1) | 1 (1) | 1 (1) | 2 (2) | 0 (0) | 5 (5) |
| Dizziness | 2 (2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (2) |
| Diarrhea | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 1 (1) |
| Urine abnormality | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) |
| Oropharyngeal swelling | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 1 (1) |
| Overall total | 6 (4) | 1 (1) | 3 (2) | 2 (2) | 7 (4) | 19 (13) |
Only the maximum severity of each adverse event is reported. MedDRA, Medical Dictionary for Regulatory Activities; n, number of subjects.
Of the 9 hypoglycemic AEs reported, 3 were symptomatic (blood glucose range, 43–66 mg/dL), and 6 were characterized as asymptomatic (blood glucose range, 52–67 mg/dL). In 1 of the 9 cases, the hypoglycemia resolved spontaneously, and in the remaining cases it was treated with a snack or oral glucose. The hypoglycemic events occurred in 4 subjects without and in 3 subjects with type 2 diabetes mellitus. The majority of hypoglycemic events were experienced by subjects in the ESRD group (6 of 9 events). The onset of the 9 hypoglycemic events ranged from 2 hours to 2 days postdose, with an average onset of 23 hours postdose.
Four subjects experienced SAEs during the study, all of which were judged to be unrelated to LY2605541 and were attributed to other medical conditions. The SAEs reported were acute asthma attack, graft thrombosis (clotted dialysis graft), viral infection, and angina pectoris. All SAEs resolved before the end of the study.
Apart from the hypoglycemic events, there were no clinically relevant changes in clinical laboratory parameters and no safety concerns following a single dose of LY2605541.
Discussion
The primary objective of this single‐dose study was to assess the effect of renal impairment on the PK of LY2605541, obtain pharmacokinetic and tolerability data, as well as provide guidance on dose adjustments. This study was conducted as a single‐dose study in which subjects were administered a 0.33 U/kg dose of LY2605541. Previous data had shown only a minimal glycemic response after a similar single dose, and it was therefore not deemed necessary to use a euglycemic clamp methodology in this study or to continuously supplement with glucose.
Study results indicated that renal function impairment had no significant effect on the pharmacokinetic profile of LY2605541 after single doses in subjects with varying degrees of renal function. Overall, the mean CL/F of LY2605541 appeared to be slightly lower in the mild, moderate, and severe groups, although a regression analysis of CL/F of LY2605541 against CrCl confirmed that LY2605541 clearance was independent of renal function. Statistical analysis of the AUC0–∞ and Cmax showed no statistically significant difference between each renal impairment group and the control group.11 Similar results were obtained when the same analysis was conducted using the renal impairment classification based on the 2010 Food and Drug Administration (FDA) guidance.16 According to Meibohm and Zhou, protein molecules with a molecular weight less than 60 kDa may be expected to be impacted by renal impairment, as this is the cutoff for glomerular filtration.17 LY2605541 has a molecular weight of 26 kDa; however, molecular weight is not sufficient to explain the lack of relationship between clearance and renal function.
Although the half‐life tended to be higher in renal impairment groups, it was not substantially altered. In addition, although the geometric mean showed an approximate 25% higher half‐life for ESRD patients (compared with healthy subjects with normal renal function), this increase was within the expected variability in LY2605541 exposure between patients, and, as such, it was not expected to necessitate dose adjustment for patients with ESRD. Using the pharmacokinetic data after a single dose of LY2605541, simulated median steady‐state pharmacokinetic profiles showed similar LY2605541 exposure in patients with renal impairment compared with patients with normal renal function (Figure 4).
In addition, a single dose of LY2605541 at 0.33 U/kg was generally well tolerated in all renal function groups. There were 9 postdose events of hypoglycemia; however, reduction in plasma glucose was anticipated because of the insulin action of LY2605541. The hypoglycemic events were experienced mainly in the group with ESRD. Compared with the other patients who did not experience hypoglycemia, LY2605541 exposures (both Cmax and AUC) were not higher in the patients with ESRD, who may be more susceptible to hypoglycemia because of altered glucose homeostasis related to decreased kidney function. Hemodialysis may also predispose patients to hypoglycemia18 or in some cases lead to severe hypoglycemia in patients with impaired renal function.19
Although the need for dosage adjustment may arise when a significant fraction of drug or active metabolite is removed by the hemodialysis process, results from this study demonstrated that the hemodialysis procedure was unlikely to result in significant elimination of LY2605541. In this study, only a small fraction of LY2605541 was eliminated during a single hemodialysis session of LY2605541. Therefore, the impact of a single hemodialysis session on overall exposure of LY2605541 with daily dosing was expected to be small. The high‐flux polysulfone dialysis membranes were used in this study, but it is possible that other types of dialysis membranes with different protein permeability may have an effect on the LY2605541 fraction that is eliminated. Under the conditions of this study, the data suggest that no additional dose adjustment would be needed to maintain therapeutic drug concentrations in ESRD patients undergoing dialysis.
In this study creatinine clearance was estimated using the C‐G method. Although this method is commonly used and consistent with regulatory guidence,11 it has some limitations. For example, it relies on a stable serum creatinine level and may be influenced by variations in muscle mass or dietary intake; these factors are not taken into account in the C‐G equation.
Overall, renal impairment and hemodialysis in ESRD subjects did not appreciably alter the pharmacokinetic profile, safety, or tolerability of single doses of LY2605541; however, assessing the effect of renal impairment and hemodialysis on safety and efficacy after multiple dosing with LY2605541 is warranted in long‐term clinical studies. The use of basal insulins in diabetic patients who have renal impairment is challenging. This is, in part, because of glycemic variability, but pharmacokinetic variability can also contribute to the overall variability in insulin effects. Typically, if renal clearance is a significant contributor to the overall clearance, then as renal function worsens, one would expect an increase in drug exposure. With LY2605541, the pharmacokinetic profile is not altered with renal impairment; thus, no dose adjustments are recommended in subjects with various degrees of renal impairment.
Declaration of Conflicting Interests
V.P.S. is a former employee of Eli Lilly and Company and has a patent pending related to LY2605541 (Eli Lilly and Company is the assignee); E.C.Q.L. and S.L.C. are current employees of Eli Lilly and Company; H.L., K.F.M., and T.S.H. are current employees of, and hold stock in, Eli Lilly and Company. V.P.S. was an employee at Eli Lilly at the time the research was conducted, and the views expressed in this article do not necessarily reflect those of the FDA.
Funding
The study was supported by Eli Lilly and Company.
Supporting information
Additional supporting information may be found in the online version of this article at the publisher's web‐site.
Supplementary Material
Acknowledgments
The authors acknowledge the clinical trial investigators and site staff who contributed to this study: Thomas C. Marbury, MD (Orlando Clinical Research Center, Orlando, Florida); Kenneth C. Lasseter, MD (Clinical Pharmacology of Miami, Inc., Miami, Florida); Harry Alcorn, PharmD (DaVita Clinical Research, Minneapolis, Minnesota); Kambiz Farbakhsh, MD (DaVita Clinical Research, Minneapolis, Minnesota); Heather Muster, MD (DaVita Clinical Research, Minneapolis, Minnesota); Mark A. Matson MD (Prism Research, St. Paul, Minnesota). The ethics committee for all 4 clinical sites was the Independent Investigational Review Board, Plantation, Florida. The authors also thank the subjects who participated in this study. The authors acknowledge Parag Garhyan (Eli Lilly and Company, Indianapolis, Indiana) for his expertise in reviewing this article, as well as Aik Hoe Seah (Lilly‐NUS Clinical Pharmacology Pte Ltd, Singapore) for statistical computing and analysis support. Writing and editorial assistance were provided by Stephanie Brillhart, MSCI, CCRP, Cindi Wood, MS, and Gina Moore, MS from inVentiv Health Clinical.
References
- 1. Beals JM, Cutler GB Jr., Vick A, et al. LY2605541: leveraging hydrodynamic size to develop a novel basal insulin [abstract]. Diabetologia. 2012;55(Suppl 1):S23. [Google Scholar]
- 2. Sinha VP, Howey DC, Choi SL, Mace KF, Heise T. Steady‐state pharmacokinetics and glucodynamics of the novel, long‐acting basal insulin LY2605541 dosed once‐daily in patients with type 2 diabetes mellitus. Diabet Obes Metab. 2014;16:344–350. [DOI] [PubMed] [Google Scholar]
- 3. Moore MC, Smith MS, Sinha VP. Novel PEGylated insulin LY2605541 has a preferential hepatic effect on glucose metabolism. Diabetes. 2014;63:494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Henry RR, Mudaliar S, Choi SL, et al. Basal insulin peglispro demonstrates preferential hepatic vs. peripheral action relative to insulin glargine in healthy subjects [abstract]. Diabetes. 2014;63(Suppl 1):A226. [DOI] [PubMed] [Google Scholar]
- 5. U.S. Renal Data System . USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End‐Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. 2014. http://www.USRDS.ORG/2012/VIEW/DEFAULT.ASPX?ZOOM_HIGHLIGHT=ATLAS. Accessed November 17, 2014.
- 6. National Kidney Foundation . KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Diabetes and Chronic Kidney Disease. Am J Kid Dis. 2007;49:S1–S179. [DOI] [PubMed] [Google Scholar]
- 7. Rabkin R, Ryan MP, Duckworth WC. The renal metabolism of insulin. Diabetologia. 1984;27:351–357. [DOI] [PubMed] [Google Scholar]
- 8. Rave K, Heise T, Pfutzner A, Heinemann L, Sawicki PT. Impact of diabetic nephropathy on pharmacodynamic and pharmacokinetic properties of insulin in type 1 diabetic patients. Diabetes Care. 2001;24:886–890. [DOI] [PubMed] [Google Scholar]
- 9. Cavanaugh KL. Diabetes management issues for patients with chronic kidney disease. Clin Diabetes. 2007;25:90–97. [Google Scholar]
- 10. Hinderer W. Biosimilar Drugs. In: Kaysser O, Warzecha H, eds. Pharmaceutical Biotechnology: Drug Discovery and Clinical Applications. 2nd ed Weinheim: Wiley‐Blackwell; 2012;313–316. [Google Scholar]
- 11. U.S. Food and Drug Administration . Guidance for industry: pharmacokinetics in patients with impaired renal function‐study design, data analysis, and impact on dosing and labeling. 1998. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072127.pdf. Accessed November 17, 2014.
- 12. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. [DOI] [PubMed] [Google Scholar]
- 13. European Medicines Agency . Note for guidance on the evaluation of the pharmacokinetics of medicinal products with impaired renal function. 2004. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003123.pdf. Accessed November 17, 2014.
- 14. Knadler MP, Ellis BB, Brown‐Augsburger PL, Murphy AT, Martin JA, Wroblewski VJ. Disposition of basal insulin peglispro compared with 20‐kDa polyethylene glycol in rats following a single intravenous or subcutaneous dose. Drug Metab Dispos. 2015;43:1477–1483. [DOI] [PubMed] [Google Scholar]
- 15. Amdisen A, Skjoldborg H. Haemodialysis for lithium poisoning. Lancet. 1969;2:213. [DOI] [PubMed] [Google Scholar]
- 16. U.S. Food and Drug Administration . Guidance for industry: pharmacokinetics in patients with impaired renal function—study design, data analysis, and impact on dosing and labeling. 2010. http://www.fda.gov/downloads/Drugs/Guidances/UCM204959.pdf
- 17. Meibohm B, Zhou H. Characterizing the impact of renal impairment on the clinical pharmacology of biologics. J Clin Pharmacol. 2012;52(Suppl 1):54S–62S. [DOI] [PubMed] [Google Scholar]
- 18. Jackson MA, Holland MR, Nicholas J, Lodwick R, Forster D, Macdonald IA. Hemodialysis‐induced hypoglycemica in diabetic patients. Clin Nephrol. 2000;54:30–34. [PubMed] [Google Scholar]
- 19. Greenblatt DJ. Fatal hypoglycaemia occurring after peritoneal dialysis. Br Med J. 1972;2:270–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article at the publisher's web‐site.
Supplementary Material


