Abstract
Aims
Altered thyroid hormone metabolism characterized by a low triiodothyronine (T3), so‐called low‐T3 syndrome, is a common finding in patients with severe systemic diseases. Additionally, subclinical thyroid dysfunction, defined as abnormal thyroid stimulating hormone (TSH) and normal thyroxine (T4), causes left ventricular dysfunction. Our objective was to identify the prevalence and prognostic impact of low‐T3 syndrome and subclinical thyroid dysfunction in patients with acute decompensated heart failure (ADHF).
Methods and results
We examined 274 ADHF patients who were not receiving thyroid medication or amiodarone on admission (70 ± 15 years, 156 male), who underwent thyroid function tests. Euthyroidism was defined as TSH of 0.45 to 4.49 mIU/L; subclinical hypothyroidism as TSH of 4.5 to 19.9 mIU/L; and subclinical hyperthyroidism as TSH < 0.45 mIU/L, with normal free T4 level for the last two. Additionally, low‐T3 syndrome was defined as free T3 < 4.0 pmol/L among euthyroidism subjects. On admission, 188 patients (69%) showed euthyroidism, 58 (21%) subclinical hypothyroidism, 5 (2%) subclinical hyperthyroidism, and 95 (35%) low‐T3 syndrome. Cox proportional hazards models revealed that higher TSH, but not free T3 and free T4, was independently associated with composite cardiovascular events, including cardiac death and re‐hospitalization for heart failure. Indeed, subclinical hypothyroidism was an independent predictor (hazard ratio: 2.31; 95% confidence interval: 1.44 to 3.67; P < 0.001), whereas low‐T3 syndrome and subclinical hyperthyroidism were not.
Conclusions
Subclinical hypothyroidism on admission was an independent predictor of adverse cardiovascular outcomes in ADHF patients, suggesting a possible interaction between thyroid dysfunction and the pathophysiology of ADHF.
Keywords: Acute decompensated heart failure, Thyroid hormones, Subclinical thyroid dysfunction
Introduction
Heart failure is one of the most common causes of hospitalization and death, with an increasing trend in the number of patients living with heart failure.1 It has been acknowledged that several comorbid conditions and biomarkers are associated with disease progression and outcome in heart failure.2 Indeed, because the thyroid hormones increase heart rate and cardiac contractility and decrease systemic vascular resistance, both overt hyper‐ and hypothyroidism can be a cause of heart failure. Therefore, the American College of Cardiology/American Heart Association guidelines for the diagnosis and management of heart failure in adults recommend measurement of thyroid function.3
A low triiodothyronine (T3) concentration in nonthyroidal illness, so‐called low‐T3 syndrome, is considered to be an adaptive compensatory mechanism and a beneficial response to preserve energy consumption, and the magnitude of the change in T3 concentration varies according to the severity of the illness.4 It has been reported that low‐T3 syndrome was also observed in patients with heart failure, and that impairment of thyroxine (T4) to T3 conversion was found to be proportional to the clinical severity of heart failure.5, 6 Furthermore, it has been reported that the impaired concentration recovers to the baseline after adequate therapy for heart failure.7 Several studies indicated that low‐T3 syndrome was an independent prognostic factor in patients with heart failure.8, 9, 10
Meanwhile, subclinical thyroid dysfunction is defined biochemically as altered thyroid stimulating hormone (TSH) but normal thyroid hormones levels. Although thyroid hormones show normal levels, the cardiovascular alterations, such as left ventricular (LV) diastolic dysfunction and endothelial dysfunction, which have been reported in overt hypothyroidism have also been observed in patients with subclinical hypothyroidism.11 Additionally, the prognostic impact of subclinical hypothyroidism has been reported in patients with heart failure,10, 12 although the results were inconsistent.
As described above, it should be noted that lower thyroid hormones concentrations are observed in patients with more severe heart failure, and that lowered thyroid hormones concentrations return to the baseline when the patient recovers from a decompensated state of heart failure, and that the magnitude of the change is proportional to the severity of heart failure. Accordingly, its prognostic impact may also differ with the phase of heart failure. In previous studies of low‐T3 syndrome and subclinical thyroid dysfunction in patients with heart failure, thyroid hormones concentrations have been measured in the chronic or stable phase of heart failure. However, the prevalence and prognostic impact of abnormal thyroid function in an acute decompensated phase of heart failure have not been investigated.
We, therefore, examined the prevalence and prognostic impact of low‐T3 syndrome and subclinical thyroid dysfunction in patients with acute decompensated heart failure (ADHF).
Methods
Study population
From October 2007 to July 2012, 401 patients were hospitalized with ADHF in the National Cerebral and Cardiovascular Center. Diagnoses of heart failure were retrieved from the medical records and validated using the modified Framingham criteria.13 Among the 401 patients, 74 patients (18%) who did not receive thyroid function tests (TSH, free T4, and free T3 levels) on admission were excluded. Furthermore, 53 patients taking thyroid medication [antithyroid drug (n = 2) or thyroxine replacement (n = 31)] or amiodarone (n = 28) were excluded. Finally, 274 patients were enrolled. This study was approved by the ethics committee of the National Cerebral and Cardiovascular Center, and patients gave informed consent.
Thyroid hormone sampling and definition of subgroups of thyroid function
Thyroid hormones were measured immediately after sampling. Electrochemiluminescence Immunoassay (ECLIA) (Cobas® 8000 <e602>; Roche Diagnostics, Japan) was used for the analysis of TSH, free T3, and free T4 in our institution's laboratory. The normal reference intervals in our laboratory are: free T3, 4.0 to 7.1 pmol/L (2.6 to 4.6 pg/mL), and free T4, 14.2 to 23.2 pmol/L (1.1 to 1.8 ng/dL).
The association between thyroid function on admission and cardiovascular events was examined. Thyroid function was analyzed as continuous variables using the data of free T3, free T4, and TSH, and as categorical variables. For categorical analysis, patients were assigned to the following groups using free T4 and TSH levels, using a common definition of subclinical thyroid dysfunction based on expert reviews and prior reports:14, 15 euthyroidism defined as TSH of 0.45 to 4.49 mIU/L; subclinical hypothyroidism defined as TSH of 4.5 to 19.9 mIU/L with normal free T4; subclinical hyperthyroidism defined as TSH < 0.45 mIU/L with normal free T4; overt hypothyroidism defined as TSH > 4.49 mIU/L with decreased free T4; overt hyperthyroidism defined as TSH < 0.45 mIU/L with elevated free T4. Those who did not fulfill these definitions were assigned to ‘undetermined’. Additionally, using free T3 level, low‐T3 syndrome was defined as TSH of 0.45 to 4.49 mIU/L with decreased free T3. For free T3 and free T4, we used our institution's cutoffs because free T3 and free T4 measurements show greater intermethod variation than do TSH assays.16, 17
Data collection and follow‐up
Patients underwent standardized evaluation including detailed medical history (comorbid conditions and medication), physical examination, blood chemistry, 12‐lead electrocardiogram, and chest X‐ray on admission. Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease equation: eGFR (mL/min/1.73m2) = 186 × [plasma creatinine]−1.154 × [age]−0.203 × [0.742 if female] × [1.212 if black].18 LV ejection fraction and LV dimensions were estimated by echocardiography in a stable state of heart failure after therapy for heart failure during hospitalization. Cardiac death and re‐hospitalization for heart failure were assessed as cardiac events after admission for ADHF. The primary endpoint of this study was composite cardiovascular events, defined as cardiac death and re‐hospitalization for heart failure. Cardiac death was defined as death attributable to ventricular arrhythmia or congestive heart failure. Follow‐up information was retrospectively obtained from medical records.
Statistical analysis
Statistical analyses were performed using JMP10 (SAS Institute, Cary, NC, USA). A P value less than 0.05 was considered statistically significant. The results were presented as mean ± SD, median values with their respective 25th and 75th percentiles, or number (%). Continuous variables were compared using unpaired Student's t‐test or ANOVA followed by Tukey's post‐test as appropriate. B‐type natriuretic peptide (BNP), free T3, free T4, and TSH levels were analyzed as continuous variables after natural logarithmic transformation to normalize their distribution. Categorical variables were compared among subgroups using chi‐squared test or Fisher's exact test as appropriate. Long‐term survival was estimated by Kaplan–Meier analysis, and differences in survival were assessed using the log‐rank test. Univariate and multivariate Cox proportional hazards regression models were constructed to investigate baseline data as predictors of composite cardiovascular events. Receiver‐operating characteristic (ROC) curves were used to examine the performance characteristics of the indices of thyroid function. Area under the curve (AUC) and 95% confidence of the ROC curves were calculated to provide a measure of the accuracy of the indices of thyroid function to predict composite cardiovascular events.
Results
Baseline characteristics of patients and prevalence of abnormal thyroid function in ADHF patients
Baseline characteristics of patients divided by free T4 and TSH levels are presented in Table 1. Among 274 patients, 188 patients (69%) showed euthyroidism and 58 (21%) showed subclinical hypothyroidism. Patients included in other criteria of thyroid function were not common (Figure 1).
Table 1.
Patient characteristics according to thyroid function status on admission
| Overall | Euthyroidism | Subclinical‐ | Subclinical‐ | Overt‐ | Overt‐ | |
|---|---|---|---|---|---|---|
| hypothyroidism | hyperthyroidism | hypothyroidism | hyperthyroidism | |||
| TSH, mIU/L | 0.45–4.49 | 4.5–19.9 | <0.45 | >4.49 | <0.45 | |
| Free T4, pmol/L | 14.2–23.2 | 14.2–23.2 | 14.2–23.2 | <14.2 | >23.2 | |
| (n = 274) | (n = 188) | (n = 58) | (n = 5) | (n = 17) | (n = 2) | |
| Age, years | 70 ± 15 | 70 ± 15 | 69 ± 12 | 54 ± 20 | 72 ± 16 | 74 ± 9 |
| Female sex | 118 (43) | 80 (43) | 21 (36) | 3 (60) | 9 (53) | 2 (100) |
| Body mass index | 23.4 ± 4.1 | 23.5 ± 3.9 | 22.6 ± 4.1 | 29.1 ± 4.7 | 23.2 ± 3.5 | 21.1 ± 7.2 |
| Etiology of heart failure | ||||||
| Ischemic heart disease | 31 (11) | 27 (14) | 2 (3)* | 0 (0) | 1 (6) | 0 (0) |
| Idiopathic dilated cardiomyopathy | 47 (17) | 32 (17) | 10 (17) | 3 (60)* | 2 (12) | 0 (0) |
| Hypertensive heart disease | 40 (15) | 28 (15) | 7 (12) | 1 (20) | 2 (12) | 1 (50) |
| Valvular heart disease | 95 (35) | 64 (34) | 23 (40) | 1 (20) | 5 (29) | 1 (50) |
| Others | 61 (22) | 37 (20) | 16 (28) | 0 (0) | 7 (41)* | 0 (0) |
| Comorbidity | ||||||
| Atrial fibrillation | 140 (51) | 89 (47) | 36 (62)* | 3 (60) | 10 (59) | 1 (50) |
| Hypertension | 162 (59) | 119 (63) | 23 (40)* | 4 (80) | 10 (59) | 2 (100) |
| Diabetes mellitus | 71 (26) | 54 (29) | 10 (17) | 2 (40) | 3 (18) | 1 (50) |
| Dyslipidemia | 86 (31) | 67 (36) | 13 (22) | 1 (20) | 5 (29) | 0 (0) |
| Medication on admission | ||||||
| ACEI and/or ARB | 125 (46) | 88 (47) | 26 (45) | 1 (20) | 7 (41) | 2 (100) |
| Beta blocker | 108 (39) | 76 (40) | 21 (36) | 2 (40) | 7 (41) | 1 (50) |
| Spironolactone or eplerenone | 83 (30) | 57 (30) | 18 (31) | 1 (20) | 8 (47) | 1 (50) |
| Diuretic (loop or thiazide) | 164 (60) | 112 (60) | 43 (74)* | 0 (0)* | 5 (29) | 0 (0) |
| Statin | 46 (17) | 38 (20) | 6 (10) | 0 (0) | 0 (0)* | 1 (50) |
| Systolic blood pressure on admission, mmHg | 131 ± 29 | 132 ± 30 | 123 ± 24 | 133 ± 32 | 134 ± 29 | 143 ± 60 |
| Diastolic blood pressure on admission, mmHg | 75 ± 20 | 77 ± 21 | 68 ± 14 | 79 ± 12 | 75 ± 21 | 74 ± 13 |
| Heart rate on admission, bpm | 88 ± 26 | 90 ± 27 | 77 ± 19* | 122 ± 32 | 85 ± 25 | 92 ± 23 |
| LV ejection fraction, % | 40 ± 17 | 40 ± 17 | 40 ± 17 | 36 ± 21 | 44 ± 20 | 47 ± 29 |
| LV diastolic dimension, mm | 57 ± 12 | 57 ± 12 | 59 ± 12 | 60 ± 6 | 53 ± 10 | 58 ± 6 |
| LV systolic dimension, mm | 44 ± 14 | 44 ± 14 | 44 ± 14 | 48 ± 9 | 40 ± 14 | 42 ± 16 |
| Laboratory parameters on admission | ||||||
| TSH, mIU/L | 2.70 | 2.00 | 6.27 | 0.30 | 8.56 | 0.01 |
| (1.36–4.80) | (1.22–2.99) | (5.12–8.74) | (0.03–0.38) | (6.21–24.05)* | (0.01–0.01) | |
| Free T3, pmol/L | 3.84 | 3.84 | 3.84 | 3.23 | 3.23 | 14.98 |
| (3.23–4.30) | (3.23–4.45) | (3.38–4.30) | (2.53–4.84) | (2.38–3.69) | (7.83–22.12)* | |
| Free T4, pmol/L | 16.73 | 18.02 | 16.73 | 16.73 | 11.58 | 69.50 |
| (15.44–19.31) | (15.44–19.31) | (15.44–18.02) | (14.80–19.31) | (10.94–12.87)* | (39.90–99.10)* | |
| Serum blood urea nitrogen, mg/dL | 23 ± 12 | 22 ± 11 | 24 ± 12 | 18 ± 7 | 25 ± 14 | 19 ± 6 |
| Serum creatinine, mg/dL | 1.05 ± 0.47 | 1.04 ± 0.46 | 1.11 ± 0.50 | 0.72 ± 0.17 | 1.19 ± 0.46 | 0.68 ± 0.09 |
| eGFR, mL/min/1.73m2 | 79.7 ± 32.6 | 80.2 ± 32.2 | 76.0 ± 32.0 | 122.5 ± 30.9* | 68.7 ± 24.6 | 92.2 ± 16.8 |
| Serum sodium, mmol/L | 139 ± 4 | 140 ± 4 | 139 ± 4 | 142 ± 4 | 139 ± 5 | 137 ± 3 |
| Hemoglobin, g/dL | 12.2 ± 2.2 | 12.4 ± 2.2 | 12.2 ± 2.2 | 12.2 ± 2.5 | 11.0 ± 2.2 | 12.6 ± 2.1 |
| Serum total bilirubin, mg/dL | 1.1 ± 0.8 | 1.0 ± 0.7 | 1.4 ± 0.9* | 0.9 ± 1.0 | 0.8 ± 0.8 | 1.2 ± 1.0 |
| Serum uric acid, mg/dL | 7.2 ± 2.5 | 7.1 ± 2.5 | 7.7 ± 2.3 | 6.4 ± 3.0 | 7.3 ± 2.2 | 5.1 ± 0.3 |
| Serum C‐reactive protein, mg/dL | 0.32 | 0.31 | 0.28 | 0.50 | 0.45 | 1.67 |
| (0.10–0.89) | (0.11–0.89) | (0.09–0.64) | (0.27–6.01) | (0.09–0.96) | (0.01–3.33) | |
| Serum total cholesterol, mg/dL | 162 ± 42 | 164 ± 43 | 153 ± 40 | 156 ± 37 | 174 ± 45 | 128 ± 16 |
| Serum triglyceride, mg/dL | 93 ± 54 | 96 ± 55 | 82 ± 53 | 78 ± 21 | 100 ± 55 | 72 ± 52 |
| Serum HDL cholesterol, mg/dL | 46 ± 18 | 46 ± 18 | 47 ± 16 | 44 ± 17 | 52 ± 25 | 36 ± 2 |
| Serum LDL cholesterol, mg/dL | 99 ± 35 | 101 ± 35 | 90 ± 36 | 99 ± 23 | 104 ± 42 | 74 ± 7 |
| BNP, pg/mL | 628 | 623 | 736 | 434 | 814 | 290 |
| (250–1287) | (230–1228) | (288–1346) | (226–679) | (217–1454) | (61–518) |
Binary data are presented as number (%); continuous variables are presented as mean (SD) or median (interquartile range). ACEI, angiotensin converting enzyme inhibitor; BNP, B‐type natriuretic peptide; ARB, angiotensin II receptor blocker; eGFR, estimated glomerular filtration rate; HDL, high density lipoprotein; LDL, low density lipoprotein; LV, left ventricular; TSH, thyroid stimulating hormone; T3, triiodothyronine; T4, thyroxine. *P value < 0.05 vs. Euthyroidism.
Figure 1.
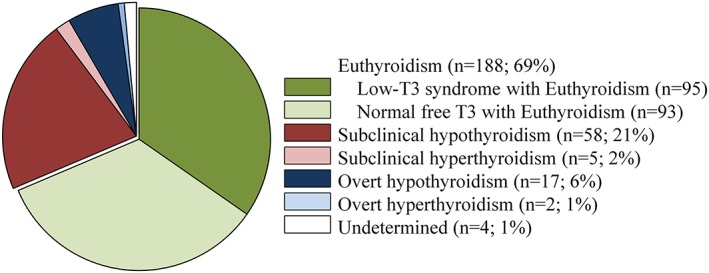
Prevalence of the abnormality of thyroid function and low‐T3 syndrome among euthyroidism subjects.
No differences were found regarding age, sex, systolic blood pressure, LV ejection fraction, and plasma BNP among the groups. Compared with euthyroidism patients, subclinical hypothyroidism patients had a lower prevalence of ischemic heart disease and hypertension, higher prevalence of atrial fibrillation and diuretic (loop and/or thiazide) use, lower heart rate, and higher total bilirubin level. Thyroid hormones concentrations, free T3 and free T4 levels, were similar between patients with subclinical hypothyroidism and euthyroidism.
Among 188 euthyroidism patients, 95 patients (35% of the whole population) showed decreased free T3 (<4.0 pmol/L) and 93 patients showed normal free T3 (4.0–7.1 pmol/L) (Figure 1). Baseline characteristics of patients with low‐T3 syndrome on admission are shown in Table 2. Compared with patients with normal free T3, low‐T3 syndrome patients were older and had lower levels of eGFR, hemoglobin, and triglycerides, but a higher level of C‐reactive protein.
Table 2.
Baseline characteristics in subjects with normal free T3 and low‐T3 syndrome
| Normal free T3 | Low‐T3 syndrome | ||
|---|---|---|---|
| TSH, mIU/L | 0.45–4.49 | 0.45–4.49 | |
| Free T3, pmol/L | 4.0–7.1 | <4.0 | |
| (n = 93) | (n = 95) | P value | |
| Age, years | 66 ± 15 | 74 ± 14 | <0.001 |
| Female sex | 34 (38) | 45 (47) | 0.177 |
| Body mass index | 24.0 ± 3.7 | 23.1 ± 4.1 | 0.138 |
| Etiology of heart failure | |||
| Ischemic heart disease | 10 (11) | 17 (18) | 0.238 |
| Idiopathic dilated cardiomyopathy | 15 (16) | 17 (18) | 0.720 |
| Hypertensive heart disease | 11 (12) | 17 (18) | 0.252 |
| Valvular heart disease | 32 (34) | 32 (34) | 0.866 |
| Others | 25 (27) | 12 (13) | 0.007 |
| Comorbidity | |||
| Atrial fibrillation | 42 (45) | 47 (49) | 0.554 |
| Hypertension | 52 (56) | 67 (71) | 0.038 |
| Diabetes mellitus | 25 (27) | 29 (31) | 0.581 |
| Dyslipidemia | 35 (38) | 32 (34) | 0.572 |
| Medication on admission | |||
| ACEI and/or ARB | 46 (49) | 42 (44) | 0.471 |
| Beta blocker | 40 (43) | 36 (38) | 0.475 |
| Spironolactone or eplerenone | 33 (35) | 24 (25) | 0.127 |
| Diuretic (loop or thiazide) | 54 (58) | 58 (61) | 0.676 |
| Statin | 17 (18) | 21 (22) | 0.514 |
| Systolic blood pressure on admission, mmHg | 129 ± 29 | 135 ± 32 | 0.177 |
| Diastolic blood pressure on admission, mmHg | 75 ± 22 | 80 ± 20 | 0.118 |
| Heart rate on admission, bpm | 84 ± 26 | 96 ± 27 | 0.002 |
| LV ejection fraction, % | 42 ± 17 | 39 ± 17 | 0.253 |
| LV diastolic dimension, mm | 57 ± 12 | 57 ± 11 | 0.805 |
| LV systolic dimension, mm | 44 ± 15 | 44 ± 14 | 0.846 |
| Laboratory parameters on admission | |||
| TSH, mIU/L | 2.11 (1.27–3.07) | 1.92 (1.03–2.94) | 0.346 |
| Free T3, pmol/L | 4.45 (4.15–4.76) | 3.23 (2.76–3.53) | <0.001 |
| Free T4, pmol/L | 18.02 (16.73–19.31) | 16.73 (15.44–19.31) | 0.006 |
| Serum blood urea nitrogen, mg/dL | 21 ± 13 | 23 ± 10 | 0.453 |
| Serum creatinine, mg/dL | 1.00 ± 0.46 | 1.07 ± 0.47 | 0.336 |
| eGFR, mL/min/1.73m2 | 84.9 ± 31.6 | 75.6 ± 32.3 | 0.047 |
| Serum sodium, mmol/L | 140 ± 4 | 139 ± 4 | 0.059 |
| Hemoglobin, g/dL | 12.8 ± 2.3 | 12.0 ± 2.0 | 0.007 |
| Serum total bilirubin, mg/dL | 1.0 ± 0.4 | 1.1 ± 0.9 | 0.300 |
| Serum uric acid, mg/dL | 7.2 ± 2.3 | 7.0 ± 2.6 | 0.535 |
| Serum C‐reactive protein, mg/dL | 0.21 (0.08–0.41) | 0.63 (0.19–1.89) | <0.001 |
| Serum total cholesterol, mg/dL | 166 ± 43 | 162 ± 42 | 0.497 |
| Serum triglyceride, mg/dL | 109 ± 60 | 83 ± 47 | 0.003 |
| Serum HDL cholesterol, mg/dL | 45 ± 14 | 47 ± 22 | 0.508 |
| Serum LDL cholesterol, mg/dL | 105 ± 35 | 96 ± 34 | 0.131 |
| BNP, pg/mL | 605 (221–1180) | 627 (253–1381) | 0.076 |
Binary data are presented as number (%); continuous variables are presented as mean (SD) or median (interquartile range). Abbreviations are as in Table 1.
Impact of thyroid function indices on predicting cardiovascular events
The mean duration of follow‐up was 31.8 ± 21.7 months. During the follow‐up period, 27 (10%) patients died of heart disorders, including 6 in‐hospital deaths and 21 deaths after discharge. Among the patients, 20 patients died of heart failure and 7 patients died of ventricular arrhythmia. Additionally, 86 (32% of discharged patients) patients were re‐hospitalized for heart failure.
In univariate Cox regression analysis, a high TSH level was significantly associated with an elevated risk of composite cardiovascular events (hazard ratio: 1.34 per 1SD increment in log‐transformed TSH value; 95% confidence interval: 1.09 to 1.64; P = 0.006), whereas free T3 and free T4 levels were not. In multiple Cox regression analysis adjusting for age, heart rate, eGFR, and hemoglobin, the prognostic impact of TSH level persisted (Table 3). In the present patient population, ROC curve analysis indicated that TSH on admission had modest ability to predict composite cardiovascular events (AUC = 0.651; 95% confidence interval: 0.579 to 0.718). A cutoff level of TSH of ≥4.39 mIU/L best predicted composite cardiovascular events, with sensitivity of 47% and specificity of 81%. The test's positive and negative predictive values were 56% and 74%, respectively.
Table 3.
Univariate and multivariate Cox proportional hazards models for composite cardiovascular events
| Univariate Analysis | Multivariate Analysis (Model 1) | Multivariate Analysis (Model 2) | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Age, yearsa | 1.37 (1.16 to 1.63) | <0.001 | 1.17 (0.98 to 1.42) | 0.085 | 1.20 (0.99 to 1.47) | 0.059 |
| Female | 1.29 (0.86 to 1.94) | 0.217 | ||||
| Systolic blood pressure, mmHgb | 0.96 (0.89 to 1.03) | 0.296 | ||||
| Heart rate, bpmc | 0.95 (0.91 to 0.99) | 0.012 | 0.99 (0.94 to 1.03) | 0.647 | 1.00 (0.96 to 1.05) | 0.853 |
| eGFR, mL/min/1.73m2 d | 0.91 (0.85 to 0.97) | 0.005 | 0.95 (0.89 to 1.02) | 0.181 | 0.96 (0.90 to 1.03) | 0.246 |
| Serum sodium, mmol/Le | 0.99 (0.78 to 1.29) | 0.916 | ||||
| Hemoglobin, g/dLf | 0.80 (0.73 to 0.88) | <0.001 | 0.86 (0.77 to 0.96) | 0.007 | 0.85 (0.76 to 0.95) | 0.005 |
| Serum C‐reactive protein, mg/dLg | 0.95 (0.85 to 1.03) | 0.285 | ||||
| LV ejection fraction, %h | 1.01 (0.95 to 1.07) | 0.858 | ||||
| BNP, pg/mLi | 1.08 (0.88 to 1.34) | 0.463 | ||||
| Free T3, pmol/Lj | 1.03 (0.85 to 1.25) | 0.749 | ||||
| Free T4, pmol/Lk | 0.94 (0.78 to 1.15) | 0.554 | ||||
| TSH, mIU/Ll | 1.34 (1.09 to 1.64) | 0.006 | 1.25 (1.01 to 1.53) | 0.041 | ||
| Subclinical hypothyroidism | 2.37 (1.52 to 3.65) | <0.001 | 2.31 (1.44 to 3.67) | <0.001 | ||
| (vs. Euthyroidism) | ||||||
| Low‐T3 syndrome | 1.05 (0.60 to 1.84) | 0.858 | ||||
| (vs. Normal free T3) | ||||||
Age reflects risk with increase of 10 years.
With increase of 10 mmHg.
With increase of 5 bpm.
With increase of 10 mL/min/1.73m2.
With increase of 5 mmol/L.
With increase of 1 g/dL.
With increase of 1 mg/dL.
With increase of 5%.
With increase of 1SD in log BNP.
With increase of 1SD in log free T3.
With increase of 1SD in log free T4.
With increase of 1SD in log TSH.
BNP, B‐type natriuretic peptide; CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; LV, left ventricular; T3, triiodothyronine: T4, thyroxine: TSH, thyroid stimulating hormone.
Fifty (27%) euthyroidism patients, 34 (59%) subclinical hypothyroidism, 8 (47%) overt hypothyroidism, and 1 (50%) overt hyperthyroidism had adverse cardiovascular events. No adverse cardiovascular events were observed in patients with subclinical hyperthyroidism. In the present study, we examined the prognostic impact of subclinical hypothyroidism, compared with euthyroidism. Figure 2 displays the Kaplan–Meier plots of groups with euthyroidism and subclinical hypothyroidism. The survival rate of patients with subclinical hypothyroidism was lower than that of patients with euthyroidism (log‐rank: χ2 = 15.99; P < 0.001). In multivariate Cox proportional hazards regression among variables including age, heart rate, eGFR, and hemoglobin, patients with subclinical hypothyroidism showed a 2.31‐fold higher risk of composite cardiovascular events when compared with euthyroidism patients (Table 3, hazard ratio: 2.31; 95% confidence interval:1.44 to 3.67; P < 0.001).
Figure 2.
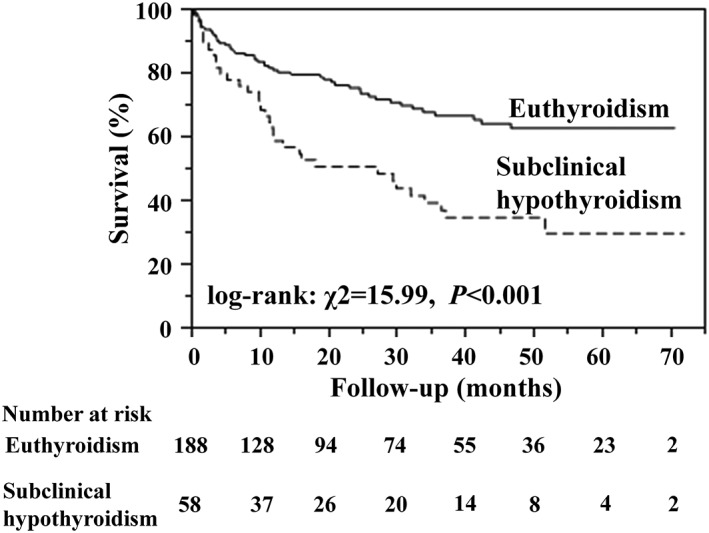
Kaplan–Meier survival curves for composite cardiovascular events for patients with euthyroidism and subclinical hypothyroidism.
We also examined the prognostic significance of low‐T3 syndrome. As demonstrated in the Kaplan–Meier plots of groups with normal free T3 and low‐T3 syndrome, the survival rate was comparable between patients with normal free T3 and low‐T3 syndrome (Figure 3).
Figure 3.
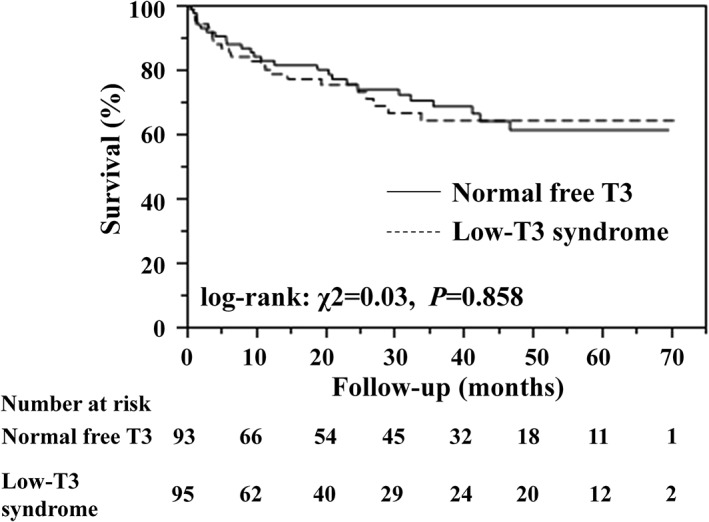
Kaplan–Meier survival curves for composite cardiovascular events for patients with normal free T3 and low‐T3 syndrome.
Discussion
We demonstrated that the presence of subclinical hypothyroidism on admission for ADHF was an independent predictor of long‐term adverse cardiovascular events including cardiac death and re‐hospitalization for heart failure, although the presence of low T3 concentration on admission was not. These findings suggest that measurements of thyroid hormones concentrations could be useful for early risk stratification of patients with ADHF.
Subclinical hypothyroidism is characterized by a high serum TSH concentration with normal levels of serum T4 (and T3).15 Thyroid hormones are regulated via a feedback loop of TSH and thyroid releasing hormone. As long as the regulation system is preserved, an increase in TSH level indicates deficiency of circulating thyroid hormones. Therefore, TSH is a sensitive indicator of thyroid status. The prevalence of subclinical hypothyroidism has been reported to be between 4 and 20% of community‐based populations,19, 20 and subclinical hypothyroidism is an independent risk factor for coronary heart disease,21 heart failure,15, 22 and cardiac death23 in community‐based populations, whereas a large population study of Danish National Patient Registry has demonstrated that risk of major adverse cardiovascular events and developing heart failure was not elevated in patients with subclinical hypothyroidism.24
A few previous studies examining the prognostic impact of subclinical hypothyroidism in patients with heart failure have been reported. Although, they have apparently shown inconsistent results, it is noteworthy that the study endpoints differed among the studies. For example, Iacoviello et al. demonstrated that a higher TSH level was independently associated with a higher incidence of composite outcomes including death, urgent heart transplantation, or hospitalization because of worsening heart failure.12 On the other hand, Frey et al. reported that subclinical hypothyroidism was not associated with all‐cause death, 42% of which comprised non‐cardiac deaths.10 In the present study, we demonstrated that a higher TSH level and the presence of subclinical hypothyroidism were independent predictors of composite cardiovascular events including cardiac death and hospitalization for worsening heart failure. Considering these results, higher TSH level or the presence of subclinical hypothyroidism might be associated with higher incidence of cardiac events, rather than non‐cardiac death, suggesting that a close relationship between the thyroid and cardiovascular system could exist in patients with heart failure.
During severe systemic illness, the alteration of thyroid hormones levels is also affected by the factors other than the hypothalamic‐pituitary‐thyroid axis. More than 80% of T3, which is considered to be the biologically active form of thyroid hormones, derives from peripheral conversion of the prohormone T4 via a reaction catalyzed by 5′‐monodeiodinases in organs such as the liver, kidney, and skeletal muscle.25, 26 It has been reported that a decrease in 5′‐monodeiodination leads to low serum T3 concentration in patients with any severe systemic illness, including acute myocardial infarction,27 and chronic heart failure.8 Besides the deiodination of T4, the alterations in thyroid hormones binding to serum proteins and T4 transport to T3‐producing tissues are involved in such transient changes in plasma concentrations of free thyroid hormones.28, 29, 30 Proinflammatory cytokines such as interleukin‐6 (IL‐6) and tumor necrosis factor‐α (TNF‐α), inducing the release of IL‐6, are considered to contribute to the transient change in the thyroid hormones levels. Administration of IL‐6 and TNF‐α into healthy subjects reproduces alterations in both peripheral and central thyroid hormones similar to low‐T3 syndrome.31, 32 Actually, the increase of plasma IL‐6 and TNF‐α is observed in patients with heart failure and associated with poor prognosis.33, 34 Therefore, the transient increase in proinflammatory cytokines in ADHF patients gives rise to temporary changes in peripheral, and central thyroid hormones not only via the hypothalamic‐pituitary‐thyroid axis but possibly also because of a direct negative effect of the cytokines on both hypothalamic and/or pituitary levels.35
T3 level alters with the severity and stage of the illness via the mechanisms described above; T3 becomes lower as the illness becomes more severe, probably in order to suppress systemic energy consumption, and returns to the baseline as the illness resolves.7, 36 Actually, in a previous report, subclinical thyroid dysfunction at discharge had normalized spontaneously six months after discharge in four fifths of patients hospitalized for heart failure,10 suggesting the presence of the transient subclinical thyroid dysfunction in the course of heart failure. In the extremely severe stage of heart failure, peripheral T4 to T3 conversion would be more strongly suppressed, and subsequently plasma TSH level might increase via a feedback loop of the hypothalamic‐pituitary‐thyroid axis. Therefore, elevated TSH level on admission of ADHF patients might indicate that the patients were in the very severe or life‐threatening systemic state in the ADHF phase.
Frey et al. reported that low‐T3 syndrome is an independent prognostic factor in patients with ADHF.10 However, we demonstrated that low‐T3 syndrome was not associated with composite cardiovascular events in ADHF patients. The difference in the timing of evaluating thyroid function may account for the inconsistent results. As described above, the temporal alteration of thyroid hormones levels exists in the clinical course of heart failure.10 In the stable phase of heart failure, lowered T3 level may indicate the intrinsic severity of heart failure, so that low‐T3 syndrome might have a poor prognosis. On the other hand, lowered T3 level in the acute decompensated phase may reflect temporal hemodynamic deterioration that could be restored by adequate therapy. Therefore, the discrepancy in the prognostic impact might be demonstrated between the studies.
We demonstrated that TSH level on admission was an independent prognostic marker in ADHF patients, and had modest ability to predict adverse outcomes. Although some indicators, including sodium and BNP levels, are considered to be useful to predict cardiac events, it has been reported that few indicators evaluated at the decompensated stage are independent prognostic markers. For example, a high BNP level at discharge is generally considered to be a strong independent marker of death or re‐admission for heart failure.37, 38 However, the prognostic impact of BNP level on admission on ADHF appears to be weak and is less than that of BNP level at discharge after appropriate treatment for ADHF. Indeed, BNP level on admission was not a prognostic marker of cardiovascular events in our study. In contrast, the present study indicated that higher TSH level on admission for ADHF was independently associated with adverse cardiovascular events after discharge. Thus, TSH level could be a practical indicator to stratify the risk of future adverse cardiovascular events, even in the early stage of treatment for ADHF.
Our study has several limitations. First, this was an observational study with a retrospective design. Second, in view of the nature of the study design, we cannot infer cause‐effect relationship between thyroid hormones and heart failure, suggesting that this study cannot be used to support the clinical benefit of replacement therapy with thyroid hormones. Third, although we demonstrated that the presence of subclinical hypothyroidism at admission was a predictor of worse prognosis in the present study, we cannot determine whether a raise of TSH level on admission for ADHF had been preexisting before developing ADHF, or arose in this ADHF phase. In other words, we cannot mention the main pathophysiology of worse prognosis: preexisting subclinical thyroid dysfunction and/or the acute exacerbation of heart failure which causes severe general condition and instability of the hypothalamic‐pituitary‐thyroid axis. TSH level before hospitalization or after discharge, as well as anti‐thyroid peroxidase antibodies, may be useful to refer to the point, although we do not have sufficient information on them. Prospective follow‐up studies are needed to further elucidate this issue.
Conclusions
We demonstrated that the presence of subclinical hypothyroidism on admission for ADHF was an independent predictor of adverse cardiovascular events. Elevated TSH level could serve as a promising marker to predict cardiovascular events in ADHF patients.
Conflict of interest
None declared.
Hayashi, T. , Hasegawa, T. , Kanzaki, H. , Funada, A. , Amaki, M. , Takahama, H. , Ohara, T. , Sugano, Y. , Yasuda, S. , Ogawa, H. , and Anzai, T. (2016) Subclinical hypothyroidism is an independent predictor of adverse cardiovascular outcomes in patients with acute decompensated heart failure. ESC Heart Failure, 3: 168–176. doi: 10.1002/ehf2.12084.
This study was performed in the Department of Cardiovascular Medicine, National Cerebral and Cardiovascular Center, Suita, Japan.
References
- 1. Roger VL, Weston SA, Redfield MM, Hellermann‐Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community‐based population. JAMA 2004; 292: 344–350. [DOI] [PubMed] [Google Scholar]
- 2. Nagarajan V, Tang WH. Biomarkers in advanced heart failure: diagnostic and therapeutic insights. Congest Heart Fail 2011; 17: 169–174. [DOI] [PubMed] [Google Scholar]
- 3. Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, Konstam MA, Mancini DM, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 Focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 2009; 119: 1977–2016. [DOI] [PubMed] [Google Scholar]
- 4. Utiger RD. Altered thyroid function in nonthyroidal illness and surgery. To treat or not to treat? N Engl J Med 1995; 333: 1562–1563. [DOI] [PubMed] [Google Scholar]
- 5. Iervasi G, Pingitore A, Landi P, Raciti M, Ripoli A, Scarlattini M, L'Abbate A, Donato L. Low‐T3 syndrome: a strong prognostic predictor of death in patients with heart disease. Circulation 2003; 107: 708–713. [DOI] [PubMed] [Google Scholar]
- 6. Schmidt‐Ott UM, Ascheim DD. Thyroid hormone and heart failure. Curr Heart Fail Rep 2006; 3: 114–119. [DOI] [PubMed] [Google Scholar]
- 7. Baloch Z, Carayon P, Conte‐Devolx B, Demers LM, Feldt‐Rasmussen U, Henry JF, LiVosli VA, Niccoli‐Sire P, John R, Ruf J, Smyth PP, Spencer CA. Stockigt JR; Guidelines Committee, National Academy of Clinical Biochemistry. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid 2003; 13: 3–126. [DOI] [PubMed] [Google Scholar]
- 8. Hamilton MA, Stevenson LW, Luu M, Walden JA. Altered thyroid hormone metabolism in advanced heart failure. J Am Coll Cardiol 1990; 16: 91–95. [DOI] [PubMed] [Google Scholar]
- 9. Opasich C, Pacini F, Ambrosino N, Riccardi PG, Febo O, Ferrari R, Cobelli F, Tavazzi L. Sick euthyroid syndrome in patients with moderate‐to‐severe chronic heart failure. Eur Heart J 1996; 17: 1860–1866. [DOI] [PubMed] [Google Scholar]
- 10. Frey A, Kroiss M, Berliner D, Seifert M, Allolio B, Güder G, Ertl G, Angermann CE, Störk S, Fassnacht M. Prognostic impact of subclinical thyroid dysfunction in heart failure. Int J Cardiol 2013; 168: 300–305. [DOI] [PubMed] [Google Scholar]
- 11. Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev 2008; 29: 76–131. [DOI] [PubMed] [Google Scholar]
- 12. Iacoviello M, Guida P, Guastamacchia E, Triggiani V, Forleo C, Catanzaro R, Cicala M, Basile M, Sorrentino S, Favale S. Prognostic role of sub‐clinical hypothyroidism in chronic heart failure outpatients. Curr Pharm Des 2008; 14: 2686–2692. [DOI] [PubMed] [Google Scholar]
- 13. Senni M, Tribouilloy CM, Rodeheffer RJ, Jacobsen SJ, Evans JM, Bailey KR, Redfield MM. Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation 1998; 98: 2282–2289. [DOI] [PubMed] [Google Scholar]
- 14. Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH, Franklyn JA, Hershman JM, Burman KD, Denke MA, Gorman C, Cooper RS, Weissman NJ. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA 2004; 291: 228–238. [DOI] [PubMed] [Google Scholar]
- 15. Gencer B, Collet TH, Virgini V, Bauer DC, Gussekloo J, Cappola AR, Nanchen D, den Elzen WP, Balmer P, Luben RN, Iacoviello M, Triggiani V, Cornuz J, Newman AB, Khaw KT, Jukema JW, Westendorp RG, Vittinghoff E, Aujesky D, Rodondi N; Thyroid Studies Collaboration . Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation 2012; 126: 1040–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stockigt JR. Free thyroid hormone measurement. A critical appraisal. Endocrinol Metab Clin North Am 2001; 30: 265–289. [DOI] [PubMed] [Google Scholar]
- 17. Giovannini S, Zucchelli GC, Iervasi G, Iervasi A, Chiesa MR, Mercuri A, Renieri A, Prontera C, Conte R, Clerico A. Multicentre comparison of free thyroid hormones immunoassays: the Immunocheck study. Clin Chem Lab Med 2011; 49: 1669–1676. [DOI] [PubMed] [Google Scholar]
- 18. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461–470. [DOI] [PubMed] [Google Scholar]
- 19. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med 2000; 160: 526–534. [DOI] [PubMed] [Google Scholar]
- 20. Razvi S, Weaver JU, Pearce SH. Subclinical thyroid disorders: significance and clinical impact. J Clin Pathol 2010; 63: 379–386. [DOI] [PubMed] [Google Scholar]
- 21. Walsh JP, Bremner AP, Bulsara MK, O'Leary P, Leedman PJ, Feddema P, Michelangeli V. Subclinical thyroid dysfunction as a risk factor for cardiovascular disease. Arch Intern Med 2005; 165: 2467–2472. [DOI] [PubMed] [Google Scholar]
- 22. Rodondi N, Newman AB, Vittinghoff E, de Rekeneire N, Satterfield S, Harris TB, Bauer DC. Subclinical hypothyroidism and the risk for heart failure, other cardiovascular events, and death. Arch Intern Med 2005; 165: 2460–2466. [DOI] [PubMed] [Google Scholar]
- 23. Tseng FY, Lin WY, Lin CC, Lee LT, Li TC, Sung PK, Huang KC. Subclinical hypothyroidism is associated with increased risk for all‐cause and cardiovascular mortality in adults. J Am Coll Cardiol 2012; 60: 730–737. [DOI] [PubMed] [Google Scholar]
- 24. Selmer C, Olesen JB, Hansen ML, von Kappelgaard LM, Madsen JC, Hansen PR, Pedersen OD, Faber J, Torp‐Pedersen C, Gislason GH. Subclinical and overt thyroid dysfunction and risk of all‐cause mortality and cardiovascular events: a large population study. J Clin Endocrinol Metab 2014; 99: 2372–2382. [DOI] [PubMed] [Google Scholar]
- 25. Maia AL, Kim BW, Huang SA, Harney JW, Larsen PR. Type 2 iodothyronine deiodinase is the major source of plasma T3 in euthyroid humans. J Clin Invest 2005; 115: 2524–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 2002; 23: 38–89. [DOI] [PubMed] [Google Scholar]
- 27. Friberg L, Werner S, Eggertsen G, Ahnve S. Rapid down‐regulation of thyroid hormones in acute myocardial infarction: is it cardioprotective in patients with angina? Arch Intern Med 2002; 162: 1388–1394. [DOI] [PubMed] [Google Scholar]
- 28. Chopra IJ, Teco GN, Nguyen AH, Solomon DH. In search of an inhibitor of thyroid hormone binding to serum proteins in nonthyroidal illnesses. J Clin Endocrinol Metab 1979; 49: 63–69. [DOI] [PubMed] [Google Scholar]
- 29. Lim CF, Docter R, Visser TJ, Krenning EP, Bernard B, van Toor H, de Jong M, Hennemann G. Inhibition of thyroxine transport into cultured rat hepatocytes by serum of nonuremic critically ill patients: effects of bilirubin and nonesterified fatty acids. J Clin Endocrinol Metab 1993; 76: 1165–1172. [DOI] [PubMed] [Google Scholar]
- 30. Wartofsky L, Burman KD. Alterations in thyroid function in patients with systemic illness: the “euthyroid sick syndrome”. Endocr Rev 1982; 3: 164–217. [DOI] [PubMed] [Google Scholar]
- 31. Torpy DJ, Tsigos C, Lotsikas AJ, Defensor R, Chrousos GP, Papanicolaou DA. Acute and delayed effects of a single‐dose injection of interleukin‐6 on thyroid function in healthy humans. Metabolism 1998; 47: 1289–1293. [DOI] [PubMed] [Google Scholar]
- 32. van der Poll T, Romijn JA, Wiersinga WM, Sauerwein HP. Tumor necrosis factor: a putative mediator of the sick euthyroid syndrome in man. J Clin Endocrinol Metab 1990; 71: 1567–1572. [DOI] [PubMed] [Google Scholar]
- 33. Tsutamoto T, Hisanaga T, Wada A, Maeda K, Ohnishi M, Fukai D, Mabuchi N, Sawaki M, Kinoshita M. Interleukin‐6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin‐6 is an important prognostic predictor in patients with congestive heart failure. J Am Coll Cardiol 1998; 31: 391–398. [DOI] [PubMed] [Google Scholar]
- 34. Ferrari R, Bachetti T, Confortini R, Opasich C, Febo O, Corti A, Cassani G, Visioli O. Tumor necrosis factor soluble receptors in patients with various degrees of congestive heart failure. Circulation 1995; 92: 1479–1486. [DOI] [PubMed] [Google Scholar]
- 35. Lyson K, McCann SM. The effect of interleukin‐6 on pituitary hormone release in vivo and in vitro. Neuroendocrinology 1991; 54: 262–266. [DOI] [PubMed] [Google Scholar]
- 36. Zaloga GP, Chernow B, Smallridge RC, Zajtchuk R, Hall‐Boyer K, Hargraves R, Lake CR, Burman KD. A longitudinal evaluation of thyroid function in critically ill surgical patients. Ann Surg 1985; 201: 456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Logeart D, Thabut G, Jourdain P, Chavelas C, Beyne P, Beauvais F, Bouvier E, Solal AC. Predischarge B‐type natriuretic peptide assay for identifying patients at high risk of re‐admission after decompensated heart failure. J Am Coll Cardiol 2004; 43: 635–641. [DOI] [PubMed] [Google Scholar]
- 38. Cournot M, Mourre F, Castel F, Ferrières J, Destrac S. Optimization of the use of B‐type natriuretic peptide levels for risk stratification at discharge in elderly patients with decompensated heart failure. Am Heart J 2008; 155: 986–991. [DOI] [PubMed] [Google Scholar]


