Abstract
Immune checkpoint inhibitors are designed to restore a patient's own antitumor immune response that has been suppressed during tumor development. The first monoclonal antibodies against the immune checkpoint programmed death 1 (PD‐1) receptor, nivolumab and pembrolizumab, are now approved for clinical use. Both agents are indicated for the treatment of advanced melanoma, as well as for the treatment of metastatic non–small cell lung cancer (NSCLC). Nivolumab is also approved for the treatment of advanced renal cell carcinoma. In patients with melanoma, these agents result in objective response rates of ~25–40%, with durable responses lasting more than 2 years in some cases. Results from phase III trials have shown improved survival with nivolumab versus standard‐of‐care chemotherapy in both patients with advanced melanoma and those with advanced NSCLC. In patients with advanced melanoma, both PD‐1 inhibitors (nivolumab and pembrolizumab) have shown improved survival versus ipilimumab. PD‐1 inhibitors are associated with adverse events that have immune etiologies, with grade greater than 3 adverse events typically reported in 16% or less of patients. However, most immune‐mediated adverse events (including grade 3–4 adverse events) can be managed by using published management algorithms without permanent discontinuation of the agent. As nivolumab and pembrolizumab enter the clinic, and with more PD‐1 pathway agents in development for a range of tumor types, this review aims to provide pharmacists with a basic understanding of the role of PD‐1 in modulating the immune system and their use in the cancer treatment. The most recent clinical efficacy and safety data are discussed, highlighting the response characteristics distinctive to immune checkpoint inhibitors, along with pharmacokinetic and pharmacodynamic data and cost considerations.
Keywords: adverse event, immune checkpoint blockade, programmed death‐1 pathway, PD‐1, immuno‐oncology, oncology, cancer
Immune checkpoint inhibitors are a new approach to cancer treatment. Whereas chemotherapies and most targeted agents interfere with key tumor signaling, cell growth, or cell division to reduce tumor cell proliferation or induce cell death, immune checkpoint inhibitors are designed to restore a patient's own antitumor immune response that was attenuated during the process of tumor development. Ipilimumab, a fully human, immunoglobulin (Ig) G1 monoclonal antibody directed against the cytotoxic T‐lymphocyte–associated antigen 4 (CTLA‐4),1 was the first immune checkpoint inhibitor approved by the U.S. Food and Drug Administration (FDA). Ipilimumab was approved in 2011 for the treatment of unresectable or metastatic melanoma on the basis of improved overall survival in two phase III trials.1
Nivolumab and pembrolizumab, both monoclonal antibodies against programmed death 1 (PD‐1), were granted accelerated FDA approval in 2014 for the treatment of patients with unresectable or metastatic melanoma and disease progression following treatment with ipilimumab and, if BRAF V600 mutation positive, a BRAF inhibitor.2, 3 In 2015, nivolumab and pembrolizumab received FDA approval for the treatment of metastatic squamous and nonsquamous non–small cell lung cancer (NSCLC), with progression on or after platinum‐based chemotherapy (patients with EGFR or ALK genomic tumor aberrations were required to have disease progression while receiving FDA‐approved therapy for these aberrations prior to receiving nivolumab).2, 3 Whereas pembrolizumab was only approved for patients whose tumors express programmed death‐ligand 1 (PD‐L1), nivolumab was approved for both PD‐L1 expressors and nonexpressors. Nivolumab is also approved as a single‐agent treatment for BRAF V600 wild‐type advanced melanoma and second‐line treatment of advanced renal cell carcinoma. These and other inhibitors of PD‐1 or its ligand, PD‐L1, are in advanced stages of development for the treatment of other cancer types, including head and neck cancer, bladder cancer, gastric cancer, glioblastoma, and other lung cancers (Table 1). As these agents become more commonly used in the clinic, pharmacists will need a basic understanding of the role of PD‐1 in modulating the immune system as well as in cancer overall, and they will need to know how inhibition of PD‐1 can lead to tumor reduction with associated immune‐mediated adverse events (AEs).
Table 1.
PD‐1 and PD‐L1 Immune Checkpoint Inhibitors: Approved Agents and Agents in Later‐Stage Clinical Development
| Approved agents | Description | Indications |
|---|---|---|
| Nivolumab | Fully human anti–PD‐1 IgG4 monoclonal antibody4 |
|
| Pembrolizumab | Humanized anti–PD‐1 IgG4‐κ isotype monoclonal antibody5 |
|
| Pipeline agents | Description | Trial phase | Tumor type |
|---|---|---|---|
| Durvalumab | Fully human, IgG1κ anti–PD‐L1 antibody6 |
III III II II |
NSCLC SCCHN Colorectal carcinoma Glioblastoma |
| Atezolizumab | Human anti–PD‐L1 monoclonal IgG1 antibody7 |
III III II |
Bladder cancer NSCLC Renal cell carcinoma |
| Nivolumab | Fully human anti–PD‐1 IgG4 monoclonal antibody4 |
III III III II II II II II II II |
Gastric cancer Glioblastoma SCCHN AML Anal canal cancer Cervical cancer Colon cancer HL, NHL Nasopharyngeal carcinoma Pancreatic cancer |
| Pembrolizumab | Humanized anti–PD‐1 IgG4‐κ isotype monoclonal antibody5 |
III III III III II II II II II |
Gastric/GEJ cancer NSCLC SCCHN Urothelial cancer Colorectal carcinoma Gastric/GEJ cancer Glioblastoma Merkel cell carcinoma HL, NHL |
| Pidilizumab (CT‐011) | Humanized anti–PD‐1 IgG1 monoclonal antibody8,a |
II II II II II II II II |
Multiple myeloma Pancreatic cancer Prostate cancer Renal cell carcinoma Sarcoma Thymic cancer NHL Renal cell carcinoma |
AML = acute myeloid leukemia; DLBCL = diffuse large B‐cell lymphoma; GEJ = gastroesophageal junction; HL = Hodgkin's lymphoma; NHL = non‐Hodgkin's lymphoma; NSCLC = non–small cell lung cancer; PD‐1 = programmed death 1; PD‐L1 = programmed death‐ligand 1; SCCHN = squamous cell carcinoma of the head and neck.
PD‐1 specificity not validated in any published material.9
Rationale for Immunotherapy
T‐cell activity and regulation are critical to tumor development because T cells have the ability to eliminate cancerous cells. Studies using several different tumor model systems identified CD8+ T cells as primarily responsible for eradicating tumor cells.10, 11, 12 Significantly, in these studies, elimination of regulatory T cells (Tregs) and exogenous support by CD4+ T cells, interleukin (IL)‐2, or other immune‐stimulating cytokines was needed to maximize the clearance of tumor cells by CD8+ T cells.10, 11, 12 Thus although CD8+ T cells have the ability to eradicate tumors, an immunosuppressive environment created by tumors can prevent an effective antitumor T‐cell response.
These earlier studies provide insight into the complexity of the interplay between the immune system and developing tumors. Some types of immunotherapy, including immune checkpoint inhibitors, aim to improve the antitumor function of the immune system and to reduce or destroy the immunosuppressive microenvironment of the tumor (Figure 1).
Figure 1.
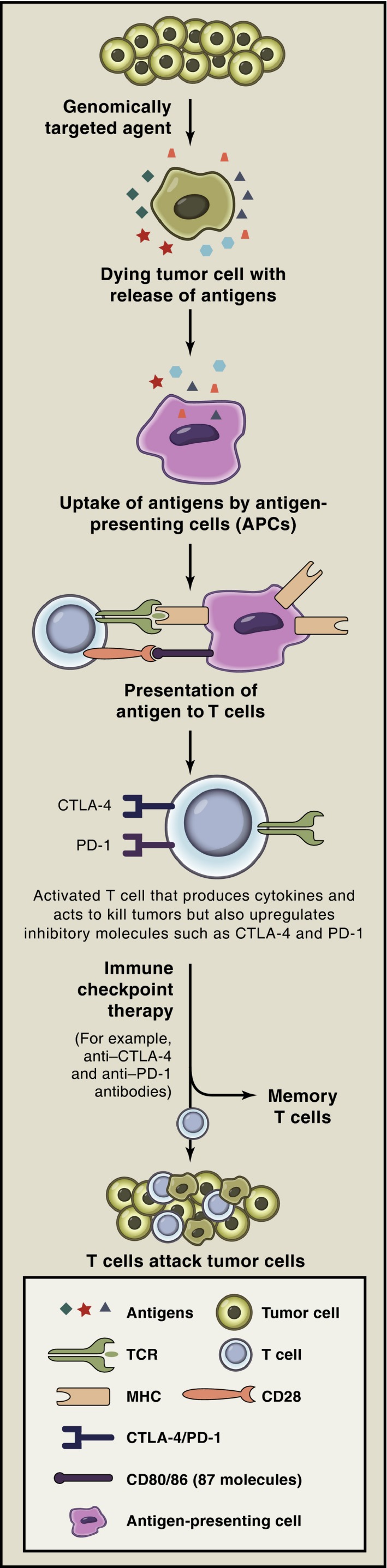
Combination strategies with immune checkpoint inhibitors may improve antitumor responses. Tumor cells die as a result of genomically targeted therapies with release of tumor antigens. Tumor antigens are taken up by antigen‐presenting cells (APCs) and are presented in the context of B7 costimulatory molecules to T cells. T cells recognize antigens on APCs to become activated; activated T cells also upregulate inhibitory checkpoints such as cytotoxic T‐lymphocyte‐associated antigen 4 (CTLA‐4) and programmed death 1 (PD‐1). Immune checkpoint therapy prevents attenuation of T‐cell responses, thereby allowing T cells to kill tumor cells, and T cells may differentiate into memory T cells that can reactivate in the presence of recurrent tumor. MHC = major histocompatibility complex; TCR = T‐cell receptor. (Reprinted with permission from reference 13.)
Immune Checkpoints and the Role of PD‐1 in Cancer
Immune checkpoints are cell surface receptors that, when bound to their cognate ligand, modulate immune responses. CTLA‐4 and PD‐1 are the best‐characterized immune checkpoints, but they are part of a large group of co‐inhibitory molecules that can be expressed by T cells.9, 14 In healthy, noncancerous conditions, immune checkpoint molecules negatively regulate the immune system to maintain peripheral self‐tolerance and prevent autoimmunity.9, 14
Tumors deploy multiple strategies to avoid elimination by the immune system, and exploiting PD‐1 is one key component. PD‐1 is expressed by T cells but also by other immune cells including B lymphocytes and natural killer cells.9, 14 The ligands for PD‐1, known as PD‐L1 and PD‐L2, are expressed by many different types of immune cells, but they can also be expressed by inflamed tissues including primary tumors and metastases.15, 16 Unlike CTLA‐4, which is thought to limit T‐cell activity early in the immune response, PD‐1 seems to reduce T‐cell activity later, during the course of the immune response, to prevent tissue damage at sites of chronic inflammation.9, 14 PD‐1 may also be important for the suppressive function of regulatory Tregs.17
Evidence for the importance of PD‐1 in evading immune detection comes from studies showing that expression of PD‐1 by tumor‐infiltrating lymphocytes is associated with reduced effector function with respect to impaired capacity to proliferate, reduced cytokine production, and diminished cytotoxic activity against tumor cells, as well as poorer outcomes.17 In some cases, the observed effects were reversed by blockade of PD‐L1.17, 18
Tumor PD‐L1 or PD‐L2 expression has been documented in numerous tumor types including melanoma, ovarian cancer, lung cancer, and renal cell carcinoma.16 Many studies across different tumor types have associated tumor PD‐L1 expression with larger tumor size or as a risk factor for poorer outcomes with respect to progression‐free survival (PFS) or overall survival (OS).16 To date, research suggests that tumor PD‐L2 expression has less clinical relevance than that of PD‐L1.19
PD‐1 and PD‐L1 Inhibitors: Mechanism of Action and Preclinical Evidence
Monoclonal antibodies directed against PD‐1 or PD‐L1 are designed to prevent PD‐1–mediated T‐cell inhibition so that antitumor immune responses can be maintained. Anti–PD‐1 antibodies should block PD‐1 binding to both of its ligands, PD‐L1 and PD‐L2, whereas anti‐PD‐L1 antibodies should be selective in preventing PD‐1 binding to PD‐L1, maintaining the ability for PD‐1 to interact and bind to PD‐L2.9
Preclinical studies in mice have shown that in vivo administration of either anti–PD–1 or anti–PD‐L1 antibodies inhibited the growth of myeloma cells and solid tumors, and prevented the metastatic spread of melanoma and colon cancer cells.17 Other studies have shown that blockade of the PD‐1 pathway in vitro or in vivo can rescue the effector function of T cells, promoting their proliferation and survival, and leading to increased inflammatory cytokine production and cytolytic activity against tumor cells.20, 21 Of interest, when both CTLA‐4 and PD‐1 blockade were used together, the antitumor effects were stronger than blockade of either pathway alone, suggesting that these two pathways have nonredundant effects in T‐cell downregulation.17
Clinical Activity
The attractiveness of immunotherapy, particularly activating T cells, can be seen from the study of high‐dose IL‐2 in patients with metastatic melanoma. As noted in an FDA review of IL‐2, the major advantage is the durability of response for the 16% of patients with metastatic melanoma who responded (10% partial response, 6% complete response) to treatment.22 In fact, 10 of the 17 complete responders were still in remission at the time of review (24+ to 106+ mo response duration). Unfortunately, the use of high‐dose IL‐2 is limited due to its toxicity. With the advanced understanding of the immune system, results from studies of new compounds that activate antitumor T cells, including the PD‐1 and PD‐L1 inhibitors, are very exciting. Based on the promising preclinical evidence, several PD‐1 and PD‐L1 inhibitors entered clinical development (Table 1).
Melanoma
Both pembrolizumab and nivolumab were given fast‐track FDA approval for the treatment of unresectable or metastatic melanoma based on initial data from phase I trials that showed relatively high response rates and durations of response lasting more than 2 years in some cases.5, 23 Phase III trials for PD‐1 immune checkpoint inhibitors are now starting to mature, and more robust efficacy data are available. In a phase III trial of patients who had previously received ipilimumab, the objective response rate (ORR) was ~32% in patients randomized to nivolumab 3 mg/kg every 2 weeks (Q2W) versus 11% in those receiving chemotherapy; 23% of patients receiving nivolumab and 34% of those receiving chemotherapy had stable disease (Table 2).24 This trial was open to patients with BRAF wild‐type or mutated tumors, and both subgroups benefited from nivolumab treatment compared with chemotherapy. OS data were not mature and are therefore not reported here.
Table 2.
Clinical Activity of PD‐1 Inhibitors from Select Key Clinical Trials in Patients with Advanced Tumors
| Agent | Trial phase | Cancer setting | No. of patients | Primary outcomes | Primary outcome significance | Secondary outcomes |
|---|---|---|---|---|---|---|
| Melanoma | ||||||
| Nivolumab vs dacarbazine | III25 | Unresectable, treatment naive | 418 |
Median OS: NR vs 10.8 mo 1‐yr OS: 72.9% vs 42.1% |
HR 0.42 (p<0.001) |
Median PFS: 5.1 vs 2.2 mo ORR: 40.0% vs 13.9% |
| Nivolumab vs chemotherapy | III24 | Unresectable; progression after ipilimumab or ipilimumab + BRAF inhibitor if BRAF V600 mutation‐positive | 405 |
ORR: 31.7% vs 10.6% OS: not mature |
No significance test reported | Median PFS: 4.7 vs 4.2 mo |
| Nivolumab + ipilimumab vs placebo + ipilimumab | II26 | Unresectable, treatment naive, known BRAF V600 mutation status | 142 (2:1 ratio) | ORR in patients with BRAF V600 WT: 61% vs 11% | OR 12.96 (p<0.001) |
PFS in patients with BRAF V600 WT: NR vs 4.4 mo ORR in BRAF V600+ patients: 52% vs 10% PFS in patients with BRAF V600+: 8.5 vs 2.7 mo |
| Pembrolizumab Q2W (A) vs pembrolizumab Q3W (B) vs ipilimumab (C) | III27 | Unresectable stage III/IV; ≤ 1 prior systemic therapy for advanced disease | 834 |
Median OS: NR (any group) 1‐yr OS: 74.1% vs 68.4% vs 58.2% Median PFS: 5.5 vs 4.1 vs 2.8 mo |
HR for OS: A vs C: 0.63 (p<0.0005) B vs C: 0.69 (p=0.0036) |
ORR: 33.7% vs 32.9% vs 11.9% |
| Pidilizumab | II28 | ≤ 3 prior lines of systemic therapy for metastatic melanoma | 103 | ORR: 6%a | NA |
Median PFS: 1.9 mo 1‐yr OS: 64.5% |
| NSCLC | ||||||
| Nivolumab | II29 | Squamous histology; refractory disease with progression after doublet platinum‐based chemotherapy and ≥ 1 other systemic therapy | 117 | ORR: 14.5% | NA |
Median PFS: 1.9 mo Median OS: 8.2 mo 1‐yr OS: 40.8% |
| Nivolumab vs docetaxel | III30 | Squamous histology; stage IIIB/IV recurrent disease after 1 platinum‐containing regimen | 272 |
Median OS: 9.2 vs 6 mo 1‐yr OS: 42% vs 24% |
HR 0.59 (p<0.001) |
Median PFS: 3.5 vs 2.8 mo ORR: 20% vs 9% |
| Nivolumab vs docetaxel | III31 | Nonsquamous histology; stage IIIB/IV recurrent disease after 1 platinum‐containing regimen | 582 |
Median OS: 12.2 vs 9.4 mo 1‐yr OS: 51% vs 39% 18‐mo OS: 39% vs 23% |
HR 0.73 (p=0.0015) |
Median PFS: 2.3 vs 4.2 mo ORR: 19% vs 12% |
| Pembrolizumab | PD‐L1 biomarker32 | Locally advanced or metastatic disease; treatment naive or previously treated | 495 |
ORR: 19.4% Median PFS: 3.7 mo Median OS: 12 mo (treatment naive, 16.2 mo; previously treated, 9.3 mo) |
NA | PD‐L1 ≥ 50% of tumor cells: ORR 45.2%; median PFS 6.3 mo |
| Pembrolizumab vs docetaxel | II/III33 | Previously treated, PD‐L1‐positive (TPS ≥ 1%) | 1034 |
Median OS: 10.4 (2 mg/kg) or 12.7 (10 mg/kg) vs 8.5 mo Median PFS: 3.9 (2 mg/kg) or 4.0 (10 mg/kg) vs 4.0 mo |
2 mg/kg: HR 0.71 (p=0.0008); 10 mg/kg: HR 0.61 (p<0.0001) 2 mg/kg: HR 0.88 (p=0.07); 10 mg/kg: HR 0.79 (p=0.004) |
ORR: 18% (2 mg/kg) or 18% (10 mg/kg) vs 9% |
| Renal cell carcinoma | ||||||
| Nivolumab (3 different doses) | II34, 35 | ≥ 1 prior antiangiogenic therapy in metastatic setting | 168 | Nivolumab 0.3 vs 2 vs 10 mg/kg: median PFS: 2.7 vs 4.0 vs 4.2 mo | For all dose comparisons: HR 1.0 (p=0.9) |
Nivolumab 0.3 vs 2 vs 10 mg/kg: ORR: 20% vs 22% vs 20% Median OS: 18.5 vs 25.5 vs 24.8 mo |
| Nivolumab vs everolimus | III36 | ≥ 1 prior antiangiogenic therapy in metastatic setting | 821 | Median OS: 25.0 vs 19.6 mo | HR 0.73 (p=0.002) |
ORR: 25% vs 5% Median PFS: 4.6 vs 4.4 mo |
HR = hazard ratio; NA = not available or reported; NR = not reached; NSCLC = non–small cell lung cancer; ORR = objective response rate; OR = odds ratio; OS = overall survival; PD‐1 = programmed death 1; PD‐L1 = programmed death‐ligand 1; PFS = progression‐free survival; Q2W = every 2 wks; Q3W = every 3 wks; TPS = tumor proportion score; WT = wild type.
Immune‐related response criteria.36
In a phase III trial of patients with previously untreated metastatic melanoma without BRAF mutation, 40% and 14% of those randomized to nivolumab 3 mg/kg Q2W or dacarbazine, respectively, had an objective response (Table 2), and an additional 17% of nivolumab‐treated patients and 22% of dacarbazine‐treated patients exhibited stable disease.25 Patients in the nivolumab group had a significant OS benefit compared with those in the dacarbazine group (hazard ratio [HR] for death 0.42 [99.8% confidence interval (CI) 0.25–0.73], p<0.001). At the time of analysis, median OS had not been reached in the nivolumab group and was 10.8 months (95% CI 9.3–12.1) in the dacarbazine group; 1‐year OS was 72.9% (95% CI 65.5–78.9) with nivolumab and 42.1% (95% CI 33.0–50.9) with dacarbazine. Based on interim results, the study was terminated early so that patients receiving dacarbazine could be switched to nivolumab. Although this trial was limited to patients with melanoma with wild‐type BRAF, other studies (including the phase III trial described earlier) have reported that nivolumab exhibited similar clinical activity, regardless of BRAF mutation status.24
Pembrolizumab was compared with the investigator's choice chemotherapy in patients with ipilimumab‐refractory melanoma in a phase II trial.37 The response rate with pembrolizumab at the approved dose (2 mg/kg every 3 wks [Q3W]) was 21% versus 4% with chemotherapy (p<0.0001); 6‐month PFS was 34% and 16%, respectively; HR 0.57 (95% CI 0.45–0.73, p<0.0001). A phase III trial compared the efficacy of pembrolizumab with ipilimumab (Table 2).27 Pembrolizumab 10 mg/kg was given intravenously either Q2W or (in a separate arm of the trial) Q3W. Pembrolizumab significantly improved OS in patients with advanced melanoma (~66% treatment naive and 34% previously treated) versus ipilimumab, regardless of which schedule was used. HR for pembrolizumab Q2W versus ipilimumab was 0.63 (95% CI 0.47–0.83, p<0.0005) and for pembrolizumab Q3W was 0.69 (95% CI 0.52–0.90, p=0.0036). Median OS was not reached in any group, but 1‐year OS rates were 74.1%, 68.4%, and 58.2% in the pembrolizumab Q2W, pembrolizumab Q3W, and ipilimumab groups, respectively. Pembrolizumab also significantly improved PFS versus ipilimumab (HR 0.58 [95% CI 0.46–0.72, p<0.001] for the Q2W regimen and 0.58 [95% CI 0.47–0.72, p<0.001] for the Q3W regimen), as well as ORR (p<0.001). This promising trial suggests that PD‐1 inhibitors may represent an advance in care compared with ipilimumab. Combination therapy with both types of immune checkpoint inhibitors in melanoma is also under investigation (discussed later).
Non–Small Cell Lung Cancer
Although NSCLC has not typically been considered an immunoresponsive tumor, squamous NSCLC in particular is associated with high mutation frequency and multiple novel tumor antigens that may make it more sensitive to immune recognition.38, 39 The importance of the immune system in regulating growth of these tumors is suggested by the identification of inactivating mutations in the human leukocyte antigen A class I major histocompatibility gene in some squamous cell NSCLCs, possibly providing one route of avoiding destruction by the immune system.39
Several anti–PD‐1 and anti–PD‐L1 antibodies have shown signs of antitumor activity in NSCLC. In a phase I expansion cohort of patients with heavily pretreated NSCLC who received nivolumab, ORR was 17% and median OS was 9.9 months, with 1‐, 2‐, and 3‐year survival rates of 42%, 24%, and 18%, respectively; efficacy was similar in patients with squamous and nonsquamous histology.40 Phase II and III data with nivolumab confirmed these promising results (Table 2). In a phase III trial comparing nivolumab with docetaxel in patients with previously treated advanced squamous NSCLC, median OS was 9.2 months (95% CI 7.3–13.3) with nivolumab versus 6.0 months (95% CI 5.1–7.3) with docetaxel, and the HR for death was 0.59 (95% CI 0.44–0.79, p<0.001).30 In a second phase III trial comparing nivolumab with docetaxel in patients with previously treated nonsquamous NSCLC, median OS was 12.2 months with nivolumab versus 9.4 months with docetaxel, and the HR for death was 0.73 (95% CI 0.59–0.89, p=0.0015).31 Nivolumab was approved for the treatment of patients with squamous and nonsquamous metastatic NSCLC after failure of platinum‐based chemotherapy based on these trials.3
In a multicohort phase I study of 280 patients with metastatic NSCLC whose disease had progressed with prior platinum‐based or EGFR‐ALK therapy, treatment with pembrolizumab resulted in an ORR of 41.0% in a prospectively defined subgroup of patients (n=61) with a PD‐L1 tumor proportion score (TPS) of 50% or higher (50% or more of tumor cells expressed PD‐L1).32 On the basis of these data, pembrolizumab was approved for previously treated patients with metastatic NSCLC whose PD‐L1 TPS was 50% or higher.2
A phase II/III randomized study of 1034 patients with previously treated PD‐L1–positive NSCLC evaluated the efficacy of pembrolizumab 2 mg/kg (Q3W) or pembrolizumab 10 mg/kg (Q3W) versus docetaxel. Median OS was significantly longer with pembrolizumab 2 mg/kg (14.9 mo; HR: 0.54, 95% CI 0.38–0.77; p=0.0002) or pembrolizumab 10 mg/kg (17.3 mo; 0.50, 0.36–0.70; p<0.0001) versus docetaxel (8.2 mo) in patients whose PD‐L1 TPS was 50% or higher.33
Atezolizumab (MPDL3280A) and durvalumab (MEDI4736) have both been granted FDA breakthrough therapy or fast‐track designation for treatment in patients with NSCLC on the basis of early clinical trial data showing evidence of efficacy.41
Other Tumor Types
Exploratory phase I studies of anti–PD‐1 and anti–PD‐L1 monoclonal antibodies that enrolled patients with multiple tumor types reported tumor responses in patients with renal cell carcinoma, pancreatic cancer, gastric, and head and neck cancer in addition to melanoma and NSCLC.41 Responses to these agents have also been reported in patients with bladder cancer, ovarian cancer, and hematologic malignancies, highlighting the potential applicability of this therapeutic approach to a broad range of malignancies.41
Apart from melanoma and NSCLC, the most data on PD‐1 pathway inhibitors are from patients with renal cell carcinoma receiving nivolumab. A phase II trial reported durable tumor responses with nivolumab, with some responses lasting more than 2 years, and many in heavily pretreated patients; OS data compared favorably with historical data.34, 35 Recent phase III trial data versus everolimus in previously treated patients with advanced renal cell carcinoma reported longer median OS with nivolumab (25.0 vs 19.6 mo, p=0.002).36
Promising activity with the anti–PD‐L1 inhibitor atezolizumab in bladder cancer led to its FDA‐granted breakthrough therapy designation in 2014; in a phase I expansion study, ORR was 43% and 11% in patients with previously treated bladder cancer with and without PD‐L1–expressing immune cells, respectively.7 A phase III study comparing atezolizumab with chemotherapy in advanced urothelial bladder cancer after a platinum‐containing regimen is ongoing (ClinicalTrials.gov identifier NCT02302807). Responses in head and neck cancer have been sufficiently encouraging at phase I41 to lead to phase III studies with pembrolizumab, durvalumab, and nivolumab.
Combination Therapy
Results from studies combining ipilimumab with nivolumab in patients with advanced melanoma suggest that the combination is more efficacious than ipilimumab alone, and possibly also more active than nivolumab alone. In a randomized placebo‐controlled phase II study, the addition of nivolumab to ipilimumab significantly improved ORR compared with ipilimumab alone (61% vs 11%) in patients with BRAF wild‐type melanoma (OR 12.96 [95% CI 3.91–54.49, p<0.001]), and improved PFS (median not reached with combination therapy vs 4.4 mo with ipilimumab [HR 0.40, 95% CI 0.23–0.68, p<0.001]).26 Similar improvements in both parameters were also seen in patients with BRAF mutation–positive tumors. A double‐blind phase III study comparing nivolumab monotherapy or nivolumab plus ipilimumab with ipilimumab monotherapy in previously untreated patients with metastatic melanoma reported improved PFS with the combination treatment. Median PFS was 11.5 months (95% CI 8.9–16.7) for nivolumab plus ipilimumab versus 2.9 months (95% CI 2.8–3.4) for ipilimumab alone (HR 0.42, 99.5% CI 0.31–0.57, p<0.001), and 6.9 months (95% CI 4.3–9.5) for nivolumab alone (HR vs ipilimumab alone 0.57, 99.5% CI 0.43–0.76, p<0.001).42 On the basis of the phase II study, nivolumab in combination with ipilimumab was approved by the FDA for the treatment of BRAF wild‐type advanced melanoma.1, 3
Combinations of CTLA‐4 and PD‐1 inhibitors are also being investigated in patients with numerous other tumor types including advanced NSCLC and renal cell carcinoma. In patients with metastatic renal cell carcinoma, preliminary data show a higher ORR with combination blockade at tolerated doses (38–40%) than was seen with PD‐1 inhibition alone (20–22%).34, 43 Similarly, in a phase I/II trial of patients with extensive‐disease small cell lung cancer, median OS was higher with ipilimumab plus nivolumab versus nivolumab alone: 7.8 months versus 3.6 months.44 However, early data from a NSCLC trial have not shown increased efficacy with combination blockade (ORR 13–39% across all dosing and histology groups tested in patients with chemotherapy‐naive NSCLC) versus PD‐1 blockade alone (ORR 23% across all histologies).45, 46 These combination data should be treated with some caution because the total number of patients treated is still relatively small.
Response Kinetics
As with conventional agents, most responses to PD‐1 or PD‐L1 inhibitors in clinical trials were detected 3 months or less after treatment initiation.5, 34, 47 However, reports of first responses (including complete responses) occurring after 5 months or more after starting treatment have been reported.29, 34, 40, 47 Most responses seen in clinical trials (typically more than 90%) meet traditional Response Evaluation Criteria in Solid Tumors (RECIST) criteria.24, 25, 40, 48, 49 However, “unconventional” or “immune‐related” responses (classified using immune‐related response criteria49) have occurred in some cases after apparent initial disease progression.24, 25, 40, 48, 49 This may be true tumor progression followed by a delayed antitumor immune response, or it may be tumor enlargement as a result of immune cell infiltration in the presence of a rapidly mobilized immune response, known as “pseudo progression,” followed by visible regression.50 Because of the occurrence of unconventional responses, protocols of some PD‐1 inhibitors have allowed continued treatment in patients with possible tumor progression in the context of other favorable clinical parameters.5, 24, 25, 29, 34, 40, 48 The clinical importance of unconventional tumor responses (which would be classified as progressive disease per RECIST v1.1 criteria) was highlighted in a recent analysis showing that they were associated with better OS than responses classified as progressive disease by both sets of response criteria.49
Another consistent and intriguing observation is the long duration of response seen with PD‐1 inhibitors, persisting even in patients who have stopped treatment for reasons other than disease progression. In the phase I trial of nivolumab, the median response duration was 2 years (range 4–27+ mo) in patients with advanced melanoma, 17.0 months (range 1.4+ to 36.8+ mo) in patients with NSCLC, and 12.9 months (range 8.4–29.1+ mo) in patients with renal cell carcinoma.23, 40, 48 Later‐phase nivolumab trials, as well as trials with other agents (pembrolizumab, atezolizumab), have also shown durable responses, but follow‐up times have been shorter (Table 2).
Safety Profile of PD‐1 Pathway Inhibitors
Across the broad clinical experience of nivolumab and pembrolizumab trials, the safety profile has remained consistent without identification of new safety signals. PD‐1 immune checkpoint inhibitors are associated with AEs that have possible immune etiologies (immune‐mediated AEs).5, 23, 25 Similar AEs were reported with ipilimumab,51 although the safety profile with PD‐1 or PD‐L1 inhibition seems different. Results from trials with both ipilimumab and pembrolizumab or nivolumab arms suggest that PD‐1 inhibitors have fewer grade 3 or higher treatment‐related AEs than ipilimumab. In patients with advanced melanoma, grade 3 or higher treatment‐related AEs occurred in 10–13% of patients receiving pembrolizumab versus 20% with ipilimumab.27 In patients with previously untreated metastatic melanoma, these rates were 17% with nivolumab and 28% with ipilimumab.42 PD‐1 inhibitors appear to have fewer gastrointestinal immune‐mediated AEs of any grade versus ipilimumab: diarrhea 1–17% versus 23–27%; colitis 1–4% versus 8% (Table 3).27, 42
Table 3.
Comparison of Key Immune‐Mediated Adverse Events by Organ Category with Ipilimumab and PD‐1/PD‐L1 Immune Checkpoint Inhibitorsa
| Organ category | Immune‐mediated adverse events (%)b | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ipilimumabc | Nivolumabd | Pembrolizumabe | Pidilizumabf | Durvalumabg | Atezolizumabh | |||||||
| Any grade | Grade 3–4 | Any grade | Grade 3–4 | Any grade | Grade 3–4 | Any grade | Grade 3–4 | Any grade | Grade 3–4 | Any grade | Grade 3–4 | |
| Endocrine | 8 | 4 | 4–8 | ≤ 1 | NR | NR | NR | NR | NR | NR | NR | NR |
| Hypothyroidism | 2 | 0 | 4–6 | 0 | 2–10 | ≤ 1 | NR | NR | 4–5 | 0 | NR | NR |
| Hyperthyroidism | 0–2 | < 1 | 2–3 | 0 | 2–7 | 0 | NR | NR | 4 | < 1 | NR | NR |
| Gastrointestinal | 29 | 8 | 8–17 | 1–2 | NR | NR | NR | NR | NR | NR | NR | NR |
| Diarrhea | 23–27 | 3–5 | 8–16 | ≤ 1 | 1–17 | ≤ 3 | 16 | 0 | 7–8 | < 1 | 10 | 0 |
| Colitis | 8 | 5–7 | 1 | ≤ 1 | 1–4 | 1–3 | NR | NR | NR | NR | NR | NR |
| Hepatic | 4 | 0 | 2–4 | ≤ 1 | NR | NR | NR | NR | NR | NR | NR | NR |
| ALT level increased | 2 | 0 | 1–3 | ≤ 1 | ≤ 2 | ≤ 1 | NR | NR | 3 | < 1 | 2 | 1 |
| AST level increased | < 1 | 0 | 1–4 | ≤ 1 | 1–3 | ≤ 1 | NR | NR | 2–3 | ≤ 1 | 1 | 1 |
| Pulmonary | 0 | 0 | 1–5 | ≤ 1 | NR | NR | NR | NR | NR | NR | NR | NR |
| Pneumonitis | < 1 | < 1 | 1–5 | ≤ 1 | ≤ 5 | ≤ 2 | NR | 1 | 1–3 | 0 | NR | 0 |
| Renal | 0 | 0 | 1–3 | ≤ 1 | NR | NR | NR | NR | NR | NR | NR | NR |
| Increased serum creatinine | ≤ 3 | 0 | NR | NR | NR | NR | NR | NR | NR | NR | ||
| Renal failure | ≤ 1 | ≤ 1 | 1 | < 1 | NR | NR | NR | NR | NR | NR | ||
| Skin | 44 | 2 | 9–37 | ≤ 2 | NR | NR | NR | NR | NR | NR | NR | NR |
| Pruritus | 24–25 | < 1 | 2–17 | ≤ 1 | 2–14 | 0 | NR | NR | 4–7 | 0 | 8 | 0 |
| Rash | 14–19 | ≤ 1 | 4–15 | ≤ 1 | 2–15 | < 1 | NR | NR | 7–8 | 0 | 10 | 0 |
| Hypersensitivity/infusion reaction | 0 | 0 | ≤ 2 | ≤ 1 | 3 | < 1 | NR | NR | < 1 | 0 | NR | NR |
PD‐1 = programmed death 1; PD‐L1 = programmed death‐ligand 1; ALT = alanine aminotransferase; AST = aspartate aminotransferase; NR = not reported.
Any adverse event with the correct term was included, whether or not it was defined as immune mediated. Data are not from head‐to‐head trials; thus comparisons across agents should be made with caution.
Key events are only listed for each organ category, so the sum of individual events may be less than the total for a given category. In some cases, because patients may have experienced more than one event, the total of individual events may exceed the sum for the organ category.
N=102.28
N=277.47
In clinical trials, most immune‐mediated AEs occurred within the first 6 months of treatment initiation, and increased exposure did not increase the risk of such events.2, 3, 25, 30 In a phase III trial of patients with advanced NSCLC who received nivolumab, gastrointestinal AEs tended to occur first (median time to onset 3 wks), followed by endocrine and skin AEs (median time to onset ~7 wks), renal AEs (median time to onset 10.5 wks), and pulmonary and hepatic AEs (median time to onset 15–18 wks).30 However, wide variation exists in the reported times of onset of immune‐mediated AEs with PD‐1 inhibitors.2, 3, 30
An analysis of pembrolizumab data showed that the safety profile was similar in patients with and without prior ipilimumab treatment.52 In a phase III nivolumab trial in patients who had previously received ipilimumab (6 wks or more prior), none of the grade 3–4 immune‐mediated AEs reported with ipilimumab were reproduced with nivolumab,24 suggesting that AEs with ipilimumab do not predispose patients to an increased risk of AEs with PD‐1 inhibitors.
Based on the mechanism of action, it follows that PD‐1 inhibition may facilitate activation of potentially autoreactive T cells, leading to inflammatory AEs across a range of tissues. For this reason, patients with a history of autoimmune diseases or systemic immune suppression were excluded from clinical trials of PD‐1 pathway inhibitors.5, 7, 25, 26, 27, 29, 34, 37, 42 Management strategies for immune‐mediated AEs have been published for the PD‐1 immune checkpoint inhibitor nivolumab.24, 25, 26 These include treatment interruption or discontinuation, and, in keeping with their inflammatory etiology, involve use of corticosteroids with a long taper over a period of at least 1 month (Table 4). The guidelines recommend escalation to other immunosuppressive agents if certain AEs prove refractory to corticosteroids; these agents include infliximab for diarrhea, colitis, or pneumonitis, and mycophenolate mofetil for pneumonitis or hepatic inflammation.24, 25, 26 Unlike most immune‐mediated AEs with PD‐1 inhibitors, endocrinopathies associated with treatment may be irreversible but can be managed with hormone replacement therapy.24, 25, 26
Table 4.
Management Strategies for Immune‐Mediated Adverse Events Associated with PD‐1 Inhibitors25
| Adverse event gradea | Management | Follow‐up |
|---|---|---|
| Gastrointestinal (diarrhea/colitis) | ||
| Grade 2 |
|
If persists > 5–7 days or recurs:
If worsens or persists > 3–5 days with oral steroids:
|
| Grade 3–4 |
|
If persists > 3–5 days or recurs:
|
| Hepatic (liver enzyme level elevation) | ||
| Grade 2 |
|
If persists > 5–7 days or worsens:
|
| Grade 3–4 |
|
If does not improve in 3–5 days, worsens, or rebounds:
|
| Skin (rash) | ||
| Grade 1–2 |
|
If persists > 1–2 wks or recurs:
If worsens:
|
| Grade 3–4 |
|
Can resume I‐O if resolution to grade 1 |
| Endocrine | ||
| Symptomatic |
Normal laboratory test results/pituitary scan:
Abnormal laboratory test results /pituitary scan:
|
If improves (with or without hormone replacement):
|
| Suspected adrenal crisis |
|
|
| Renal (increased serum creatinine) | ||
| Grade 2–3 |
|
If serum creatinine concentration elevations persist > 7 days or worsen:
|
| Grade 4 |
|
|
| Pulmonary (pneumonitis) | ||
| Grade 2 |
|
Re‐image every 1–3 days If not improving after 14 days or worsening:
|
| Grade 3–4 |
|
If not improving after 48 hrs or worsening:
|
ID = infectious disease; I‐O = immuno‐oncology agent; i.v. = intravenous; IVIG = intravenous immunoglobulin; MP = methylprednisolone; MRI = magnetic resonance imaging; PD‐1 = programmed death 1.
National Cancer Institute Common Terminology Criteria for Adverse Events, v.4.0.
If improves to grade 1, taper steroids > 1 mo, and consider prophylactic antibiotics for opportunistic infections; I‐O therapy can be resumed for mild to moderate immune‐mediated adverse events that have returned to baseline.
Clinical trial experience has demonstrated the importance of close patient monitoring and prompt intervention for successful management of immune‐mediated AEs.24, 25, 26 Suspected events should be thoroughly investigated and, if deemed to be immune related (and not an infection, for example), appropriate treatment should be started. Limited data suggest no clear relationship between steroid use and continued efficacy of PD‐1 inhibitors53; however, further investigation is needed, including any effects of prolonged and/or high‐dose steroids. In nivolumab trials, involvement of an appropriate specialist (dependent on the toxicity observed) was recommended for most cases of grade 3 or higher AEs.24, 25, 26 Using the provided management algorithms, most of all grade 3–4 immune‐mediated AEs reported in the two published phase III nivolumab melanoma trials (10/12 [83%]) resolved.24, 25
Pharmacokinetics and Pharmacodynamics
Among the PD‐1 immune checkpoint inhibitors currently approved or in development, pembrolizumab and nivolumab have the most pharmacokinetic data available. Both of these fully human (nivolumab) or humanized (pembrolizumab) IgG4 monoclonal antibodies have an elimination half‐life of ~26–27 days,2, 3 whereas pidilizumab (a humanized IgG1 monoclonal antibody) has a half‐life of 9–17 days.8 Clearance of nivolumab and pembrolizumab increases with increasing body weight, supporting body weight‐based dosing.2, 3 None of the other parameters evaluated (age, sex, glomerular filtration rate, race/ethnicity, Eastern Cooperative Oncology Group performance status, lactate dehydrogenase level, hepatic function, baseline tumor size, tumor type, immunogenicity, and PD‐L1 expression) had a clinically relevant effect on clearance.2, 3
Effects on Cytochrome P450 Enzymes
Therapeutic monoclonal antibodies are not thought to interact directly with cytochrome P450 (CYP) enzymes.54 However, cytokines produced by activated T cells can affect the regulation of many drug transporters and CYP enzyme levels54; therefore, immunomodulatory antibodies may indirectly affect exposure to small molecule drugs.
A study evaluating the effect of PD‐1 inhibition on peripheral blood levels of cytokines known to modulate CYP enzymes found that most inflammatory cytokines (IL‐2 soluble receptor α, IL‐1A, IL‐1B, IFN‐γ, tumor necrosis factor‐α, IL‐12P, and IL‐23M) were below the level of detection for the assay both before and after nivolumab dosing.55 Levels of IL‐2, IL‐6, and IL‐10 were essentially unaffected during nivolumab treatment, with no systematic changes observed. The authors concluded that nivolumab has no or low potential for modulating CYP enzymes and thus a low risk of a therapeutic protein–drug interaction.
Antidrug Antibodies
To date, no evidence suggests that formation of antidrug antibodies should impact clinical decision making with any anti–PD‐1 or PD‐L1 antibody that is either approved or in development. Further analyses and a larger clinical experience will show whether this remains the case in the future.
A relatively low risk of antidrug antibodies is expected for fully human antibodies such as nivolumab, durvalumab, and atezolizumab. Among 281 patients treated with nivolumab 3 mg/kg Q2W, 24 (8.5%) were positive for antidrug antibodies, with neutralizing antibodies detected in two patients (less than 1%).3 In an analysis of pooled data from three nivolumab studies, the incidence of persistent anti‐nivolumab antibodies was less than 1% in more than 500 evaluable patients, and the incidence of neutralizing antibodies was less than 1% in over 250 evaluable patients.56 However, population pharmacokinetic and exposure‐response analyses show that the development of antidrug antibodies has minimal or no impact on nivolumab clearance, and there is no evidence of an altered nivolumab safety profile.3, 56 Similarly, there is no evidence to date of durvalumab immunogenicity affecting the pharmacokinetic or pharmacodynamic activity of this antibody at the dose used in phase III trials.
Antidrug antibodies may be more likely for the humanized but not fully human antibodies pembrolizumab and pidilizumab. In this type of antibody, the mouse constant regions and variable framework regions are replaced with human sequences to minimize immunogenicity, so that only the complementarity‐determining parts of the variable regions are of murine origin. Limited immunogenicity studies for pembrolizumab found no treatment‐emergent antidrug antibodies in 97 patients treated with 2 mg/kg Q3W.2 To date, no antibody data have been published for pidilizumab.
Pharmacodynamics/Biomarkers
Limited pharmacodynamics information is available for PD‐1 and PD‐L1 inhibitors, although ongoing trials are evaluating their pharmacodynamics and potential biomarkers. Tumor or immune cell expression of PD‐L1 has been investigated as a potential marker of response to PD‐1 or PD‐L1 inhibitors. Across studies using varying methodologies, higher response rates were associated with PD‐L1–positive tumors at baseline in patients with a range of different tumor types; however, responses were in almost every case also seen in patients with tumors with low or negative PD‐L1 expression.7, 24, 25, 29, 32, 47, 49
As trials mature, survival data by PD‐L1 expression are becoming available. In a trial of pembrolizumab in patients with NSCLC (all histologies), high baseline tumor expression of PD‐L1 (TPS of 50% or higher) was associated with improved survival: median OS had not been reached in those with a proportion score of 50% or higher and appeared longer than for patients with a proportion score of 49% or lower, with clear separation of the curves by Kaplan‐Meier analysis.32 In the phase II/III NSCLC trial, median OS was 10.4 months for patients with a TPS of 1% or higher and 14.9 months for those with a TPS of 50% or higher receiving the approved 2 mg/kg pembrolizumab dose.33 In a phase III trial of nivolumab in patients with nonsquamous NSCLC, median OS was 19.4 months versus 9.9 months in patients with 10% or more and less than 10% baseline PD‐L1 tumor expression, respectively.31 However, in patients with squamous NSCLC receiving nivolumab, a less pronounced association was found between tumor PD‐L1 status and survival: median OS was 11.0 months (10% or more expression) and 8.2 months (less than 10% expression).30 Overall, these data suggest that the level of PD‐L1 expression may be predictive of the degree of benefit achieved with PD‐1 inhibitors in patients but do not show that those with low or negative PD‐L1 expression will not benefit from treatment. Interestingly, in patients with PD‐L1–negative melanoma, PFS was higher with combination CTLA‐4 and PD‐1 blockade than with either nivolumab or ipilimumab alone. In contrast, PFS was similar with combination blockade or nivolumab in patients with PD‐L1–positive melanoma.42 It should be noted that PD‐L1 expression testing has not been standardized; hence results should not be compared across studies.
Some research suggests that PD‐L1 expression on tumor‐infiltrating immune cells may be more predictive of response to PD‐1 pathway inhibitors than tumor cell PD‐L1 expression. A study of atezolizumab across multiple tumor types found a significant association between treatment response and tumor‐infiltrating immune cell PD‐L1 expression (p=0.007) but a nonsignificant trend between response and tumor cell PD‐L1 expression (p=0.079).47 There was also a trend for increased PFS with increasing PD‐L1 expression by tumor‐infiltrating immune cells; however, further studies are needed in this area.
An interesting study showed that patients with tumors deficient in mismatch‐repair enzymes had a greatly enhanced antitumor response with pembrolizumab than those without the deficiency.57 It is thought that mismatch‐repair deficiency creates a higher number of mutations and novel tumor antigens, making the tumor more visible to the immune system. Because many different tumor types can have mismatch‐repair deficiency, it is possible that screening for mismatch‐repair status could potentially identify those patients who are likely to benefit from treatment with PD‐1 pathway inhibitors.
Dosing and Administration
All PD‐1 pathway inhibitors currently approved or in development have been tested across a range of doses. For pembrolizumab, nivolumab, and pidilizumab, no maximum tolerated dose was defined in phase I trials.8, 23, 58 Table 5 provides recommendations for dosing, treatment withdrawal, and permanent discontinuation for nivolumab and pembrolizumab. No premedication is recommended for infusion of these approved agents, and infusion‐related reactions are rare.2, 3
Table 5.
Dosing Recommendations for FDA‐Approved PD‐1 Inhibitors
| Dose | Nivolumab3 | Pembrolizumab2 |
|---|---|---|
| As recommended | 3 mg/kg administered as an i.v. infusion over 60 min every 2 wks | 2 mg/kg administered as an i.v. infusion over 30 min every 3 wks |
| Withhold |
|
|
| Permanently discontinue |
|
|
ALT = alanine aminotransferase; AST = aspartate aminotransferase; FDA = U.S. Food and Drug Administration; ULN = upper limit of normal.
Cost Considerations
To date, no analyses of the cost‐effectiveness of treating melanoma with PD‐1 or PD‐L1 inhibitors have been published. However, a recent analysis of ipilimumab compared with best supportive care in patients with previously treated melanoma, based on a U.S. payer perspective, estimated that the incremental cost‐effectiveness ratio was $78,218 per life‐year gained and $128,656 per quality‐adjusted life‐year (QALY).59 This study also estimated that ipilimumab had a 95% chance of being cost‐effective at a willingness to pay of $146,000 per QALY. Some have argued that the U.S. Panel on Cost‐Effectiveness in Health and Medicine, while providing guidance on the conduct of cost‐effectiveness studies, has failed to recommend an acceptable level of cost‐effectiveness.60 Based on a review of relevant literature, the same authors suggested that an acceptable cost‐effectiveness threshold in the United States approached $200,000.60 The estimated cost‐effectiveness of ipilimumab falls well within this limit. The cost‐effectiveness of ipilimumab has been the subject of some debate in both the United States and the United Kingdom.61
In addition to drug cost, critical factors in modeling cost‐effectiveness include survival benefit compared with other treatments and the cost of disease management and toxicity management. The American Society of Clinical Oncology Task Force on the Cost of Cancer Care has proposed a conceptual framework for assessing the value of advanced cancer treatment options.62 In the advanced disease framework, points are awarded (or subtracted) in the categories of clinical benefit (OS, PFS, or ORR) and toxicity. Bonus points are added if a regimen shows statistically significant improvement in palliation of symptoms and/or treatment‐free interval compared with the control treatment in a clinical trial. Clinical benefit, toxicity, and bonus points are combined to generate a net health benefit score, which is then juxtaposed against the direct cost of the treatment to provide an overall summary assessment.
The improved OS, PFS and/or ORR and reduction in grade 3 or higher treatment‐related AEs reported with nivolumab and pembrolizumab versus standard‐of‐care treatments, including ipilimumab,25, 27, 30, 31, 37 suggests that PD‐1 inhibitors would have a high net health benefit score using the advanced cancer framework. Direct costs would have to be incorporated into the framework to assess the value of these treatments.
The encouraging results with combination immunotherapy have also raised questions and concerns about the price of combination regimens, including drug costs and the cost of managing the higher occurrence of AEs versus single‐agent therapy. This is likely to become an increasingly important topic of discussion and research if combination therapies are approved by the FDA.
Conclusion
Immunotherapy with PD‐1 and PD‐L1 immune checkpoint inhibitors is a rapidly growing area and appears to have broad applications across a number of tumor types. PD‐1 expression on T cells is a hallmark of T‐cell exhaustion, consistent with the finding that tumor‐infiltrating lymphocytes expressing PD‐1 possess curtailed effector function. These observations point to a central role of PD‐1 and its ligands in enabling tumors to evade elimination by the immune system and provide a rationale for therapy with PD‐1 or PD‐L1 inhibitors. Early clinical data validated this strategy, and more mature phase I and emerging phase III data have so far confirmed the clinical benefits offered by these approaches for patients with advanced melanoma and advanced NSCLC. Toxicities associated with nivolumab and pembrolizumab appear similar but are generally less severe than those reported with the CTLA‐4 inhibitor ipilimumab, and they are manageable using published algorithms. Many questions remain about the optimum way to use and combine inhibitors of PD‐1 and PD‐L1, but based on currently available information, we anticipate that these agents will ultimately play a key role in the management of patients with a range of tumor types.
Acknowledgments
Professional medical writing assistance was provided by Britt Anderson and Jean Scott, and professional editing assistance was provided by Lisa Sullivan at StemScientific, an Ashfield Company, funded by Bristol‐Myers Squibb. Bristol‐Myers Squibb generated the concept for this review article; however, the authors developed the content. Bristol‐Myers Squibb reviewed a draft for medical accuracy only. Neither Bristol‐Myers Squibb nor StemScientific influenced the content of the manuscript, nor did the authors receive financial compensation for authoring the manuscript.
Source of support: Professional medical writing and medical editing assistance was provided by StemScientific, an Ashfield Company, funded by Bristol‐Myers Squibb.
References
- 1. Yervoy (ipilimumab) prescribing information. Princeton, NJ: Bristol‐Myers Squibb; 2015. [Google Scholar]
- 2. Keytruda (pembrolizumab) prescribing information. Whitehouse Station, NJ: Merck & Co; 2015. [Google Scholar]
- 3. Opdivo (nivolumab) prescribing information. Princeton, NJ: Bristol‐Myers Squibb; 2015. [Google Scholar]
- 4. Wang C, Thudium KB, Han M, et al. In vitro characterization of the anti–PD‐1 antibody nivolumab, BMS‐936558, and in vivo toxicology in non‐human primates. Cancer Immunol Res 2014;2:846–56. [DOI] [PubMed] [Google Scholar]
- 5. Robert C, Ribas A, Wolchok JD, et al. Anti‐programmed‐death‐receptor‐1 treatment with pembrolizumab in ipilimumab‐refractory advanced melanoma: a randomised dose‐comparison cohort of a phase 1 trial. Lancet 2014;384:1109–17. [DOI] [PubMed] [Google Scholar]
- 6. Rizvi NA, Brahmer JR, Ou S‐HI, et al. Safety and clinical activity of MEDI4736, an anti‐programmed cell death‐ligand 1 (PD‐L1) antibody, in patients with non‐small cell lung cancer (NSCLC) [abstract 8032]. J Clin Oncol 2015;33(Suppl). Available from http://meetinglibrary.asco.org/content/108561?media=sl. Accessed July 14, 2015. [Google Scholar]
- 7. Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti–PD‐L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014;515:558–62. [DOI] [PubMed] [Google Scholar]
- 8. Berger R, Rotem‐Yehudar R, Slama G, et al. Phase I safety and pharmacokinetic study of CT‐011, a humanized antibody interacting with PD‐1, in patients with advanced hematologic malignancies. Clin Cancer Res 2008;14:3044–51. [DOI] [PubMed] [Google Scholar]
- 9. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hanson HL, Donermeyer DL, Ikeda H, et al. Eradication of established tumors by CD8+ T cell adoptive immunotherapy. Immunity 2000;13:265–76. [DOI] [PubMed] [Google Scholar]
- 11. Klebanoff CA, Gattinoni L, Torabi‐Parizi P, et al. Central memory self/tumor‐reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA 2005;102:9571–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+ CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol 1999;163:5211–8. [PubMed] [Google Scholar]
- 13. Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 2015;161:205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fife BT, Bluestone JA. Control of peripheral T‐cell tolerance and autoimmunity via the CTLA‐4 and PD‐1 pathways. Immunol Rev 2008;224:166–82. [DOI] [PubMed] [Google Scholar]
- 15. Taube JM, Klein AP, Brahmer JR, et al. Association of PD‐1, PD‐1 ligands, and other features of the tumor immune microenvironment with response to anti–PD‐1 therapy. Clin Cancer Res 2014;20:5064–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zou W, Chen L. Inhibitory B7‐family molecules in the tumour microenvironment. Nat Rev Immunol 2008;8:467–77. [DOI] [PubMed] [Google Scholar]
- 17. Baksh K, Weber J. Immune checkpoint protein inhibition for cancer: preclinical justification for CTLA‐4 and PD‐1 blockade and new combinations. Semin Oncol 2015;42:363–77. [DOI] [PubMed] [Google Scholar]
- 18. Zhang Y, Huang S, Gong D, Qin Y, Shen Q. Programmed death‐1 upregulation is correlated with dysfunction of tumor‐infiltrating CD8+ T lymphocytes in human non‐small cell lung cancer. Cell Mol Immunol 2010;7:389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rozali EN, Hato SV, Robinson BW, Lake RA, Lesterhuis WJ. Programmed death ligand 2 in cancer‐induced immune suppression. Clin Dev Immunol 2012;2012:656340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peng W, Liu C, Xu C, et al. PD‐1 blockade enhances T‐cell migration to tumors by elevating IFN‐gamma inducible chemokines. Cancer Res 2012;72:5209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou Q, Xiao H, Liu Y, et al. Blockade of programmed death‐1 pathway rescues the effector function of tumor‐infiltrating T cells and enhances the antitumor efficacy of lentivector immunization. J Immunol 2010;185:5082–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. U.S. Food and Drug Administration . Medical Reviewers Report Product: Proleukin (aldesleukin), Proleukin, interleukin‐2 (IL‐2). Available from http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/ucm094495.pdf. Accessed August 6, 2015.
- 23. Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long‐term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014;32:1020–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weber JS, D'Angelo SP, Minor D, et al. Nivolumab compared with chemotherapy in patients with advanced melanoma who progressed after anti‐CTLA‐4 therapy. Lancet Oncol 2015;16:375–84. [DOI] [PubMed] [Google Scholar]
- 25. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–30. [DOI] [PubMed] [Google Scholar]
- 26. Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:30–9. [DOI] [PubMed] [Google Scholar]
- 28. Atkins MB, Kudchadkar RR, Sznol M, et al. Pidilizumab in metastatic melanoma; results from a multicenter phase II, open label randomized trial [abstract 9001]. J Clin Oncol 2014;32(5s). [Google Scholar]
- 29. Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti–PD‐1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non‐small‐cell lung cancer (CheckMate 063): a phase 2, single‐arm trial. Lancet Oncol 2015;16:257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015;373:123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Borghaei H, Paz‐Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced non‐squamous non‐small‐cell lung cancer. N Engl J Med 2015;373:1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med 2015;21(372):2018–28. [DOI] [PubMed] [Google Scholar]
- 33. Herbst RS, Baas P., Kim D‐W et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): a randomised controlled trial. N Engl J Med 2015. >. Epub ahead of print 19 December. http://dx.doi.org/10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 34. Motzer R, Rini B, McDermott D, et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol 2015;33:1430–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Plimack ER, Hammers HJ, Rini BI, et al. Updated survival results from a randomized, dose‐ranging phase II study of nivolumab (NIVO) in metastatic renal cell carcinoma (mRCC) [abstract 4533]. J Clin Oncol 2015;33(Suppl). [Google Scholar]
- 36. Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal‐cell carcinoma. N Engl J Med 2015;373:1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator‐choice chemotherapy for ipilimumab‐refractory melanoma (KEYNOTE‐002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015;16:908–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rizvi NA, Hellmann MD, Snyder A, et al. Mutational landscape determines sensitivity to PD‐1 blockade in non‐small cell lung cancer. Science 2015;348:124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cancer Genome Atlas Research Network . Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012;489:519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long‐term safety of nivolumab (anti–PD‐1 antibody, BMS‐936558, ONO‐4538) in patients with previously treated advanced non‐small‐cell lung cancer. J Clin Oncol 2015;33:2004–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sunshine J, Taube JM. PD‐1/PD‐L1 inhibitors. Curr Opin Pharmacol 2015;23:32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Larkin J, Chiarion‐Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hammers HJ, Plimack ER, Infante JR, et al. Expanded cohort results from CheckMate 016: A phase I study of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma (mRCC) [abstract 4516]. J Clin Oncol 2015;33(Suppl). Available from http://meetinglibrary.asco.org/content/109741?media=sl. Accessed July 14, 2015. [Google Scholar]
- 44. Calvo E, López‐Martin JA, Bendell J, et al. Nivolumab (NIVO) monotherapy or in combination with ipilimumab (IPI) for treatment of recurrent small cell lung cancer (SCLC), European Cancer Congress. Vienna, Austria, 2015. Available from http://poster-submission.com/ecc2015. Accessed November 10, 2015.
- 45. Hellmann MD, Rizvi NA, Gettinger SN, et al. Safety and efficacy of first‐line nivolumab and ipilimumab in non‐small cell lung cancer (NSCLC), European Cancer Congress. Vienna, Austria, 2015. Available from http://poster-submission.com/ecc2015. Accessed November 10, 2015.
- 46. Gettinger SN, Hellmann MD, Shepherd FA, et al. First‐line monotherapy with nivolumab in advanced non‐small cell lung cancer (NSCLC): safety, efficacy, and biomarker analyses, European Cancer Congress. Vienna, Austria, 2015. Available from http://poster-submission.com/ecc2015. Accessed November 10, 2015.
- 47. Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti–PD‐L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McDermott DF, Drake CG, Sznol M, et al. Survival, durable response, and long‐term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J Clin Oncol 2015;33:2013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hodi FS, Ribas A, Daud A, et al. Evaluation of immune‐related response criteria (irRC) in patients (pts) with advanced melanoma (MEL) treated with the anti–PD‐1 monoclonal antibody MK‐3475 [abstract 3006]. J Clin Oncol 2014;32(5s). Available from http://meetinglibrary.asco.org/content/94752?media=vm. Accessed July 14, 2015. [Google Scholar]
- 50. Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune‐related response criteria. Clin Cancer Res 2009;15:7412–20. [DOI] [PubMed] [Google Scholar]
- 51. Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ribas A, Hodi FS, Kefford R, et al. Efficacy and safety of the anti–PD‐1 monoclonal antibody MK‐3475 in 411 patients (pts) with melanoma (MEL) [abstract LBA9000]. J Clin Oncol 2014;32(5s): Available from http://meetinglibrary.asco.org/content/93504?media=sl. Accessed July 14, 2015. [Google Scholar]
- 53. Leighl N, Gandhi L, Hellmann MD, et al. Pembrolizumab for NSCLC: immune‐mediated adverse events and corticosteroid use, 16th World Conference on Lung Cancer. Denver, CO, USA, 2015. Available from http://library.iaslc.org/virtual-library. Accessed November 10, 2015.
- 54. Christensen H, Hermann M. Immunological response as a source to variability in drug metabolism and transport. Front Pharmacol 2012;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Passey C, Simon J, Hong Q, Roy A, Agrawal S. Assessment of drug interaction potential by nivolumab using cytokine modulation data. Clin Pharm Ther 2015;97(Suppl 1):S96. [Google Scholar]
- 56. Agrawal S, Roy A, Feng Y, et al. Immunogenicity of nivolumab and its impact on pharmacokinetics and safety in subjects with metastatic solid tumors. Clin Pharm Ther 2015;97(Suppl 1):S95. [Google Scholar]
- 57. Le DT, Uram JN, Wang H, et al. PD‐1 blockade in tumors with mismatch‐repair deficiency. N Engl J Med 2015;372:2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Patnaik A, Kang SP, Rasco D, et al. Phase I study of pembrolizumab (MK‐3475; anti–PD‐1 monoclonal antibody) in patients with advanced solid tumors. J Clin Oncol 2015;21:4286–93. [DOI] [PubMed] [Google Scholar]
- 59. Barzey V, Atkins MB, Garrison LP, Asukai Y, Kotapati S, Penrod JR. Ipilimumab in 2nd line treatment of patients with advanced melanoma: a cost‐effectiveness analysis. J Med Econ 2013;16:202–12. [DOI] [PubMed] [Google Scholar]
- 60. Ubel PA, Hirth RA, Chernew ME, Fendrick AM. What is the price of life and why doesn't it increase at the rate of inflation? Arch Intern Med 2003;163:1637–41. [DOI] [PubMed] [Google Scholar]
- 61. Fellner C. Ipilimumab (Yervoy) prolongs survival in advanced melanoma: serious side effects and a hefty price tag may limit its use. P T 2012;37:503–30. [PMC free article] [PubMed] [Google Scholar]
- 62. Schnipper LE, Davidson NE, Wollins DS, et al. American Society of Clinical Oncology statement: a conceptual framework to assess the value of cancer treatment options. J Clin Oncol 2015;33:2563–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Segal NH, Ou S‐HI, Balmanoukian AS, et al. Safety and efficacy of MEDI4736, an anti–PD‐L1 antibody, in patients from a squamous cell carcinoma of the head and neck (SCCHN) expansion cohort [abstract 3011]. J Clin Oncol 2015;33(Suppl). Available from http://meetinglibrary.asco.org/content/110673?media=sl. Accessed July 14, 2015. [Google Scholar]


