Abstract
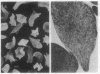
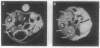
A line of transgenic mice with two cointegrated transgenes, the human beta S- and alpha 2-globin genes, linked to the beta-globin locus control region was produced and bred with mice carrying a deletion of the mouse beta major-globin gene. In transgenic mice homozygous for the beta major deletion (alpha H beta S[beta MDD]; where alpha H is human alpha-globin and MD is mouse deletion), 72.5 +/- 2.4% (mean +/- SD) of the beta-chains are beta S and the ratio of alpha H- to beta S-globin was 0.73. Introduction of a heterozygous mouse alpha-globin deletion into mice homozygous for the beta major deletion (alpha H beta S[alpha MD beta MDD]) resulted in 65.1 +/- 8.5% beta S and a human alpha/beta ratio of 0.89 +/- 0.2. Sickling occurs in 95% of erythrocytes from alpha H beta S[beta MDD] mice after slow deoxygenation. Transmission electron microscopy revealed polymer fiber formation but not fascicles of fiber. Increased organ weight was noted in lung, spleen, and kidney of transgenic mice vs. controls that may be due to hypertrophy or increased blood volume in the lungs and/or increased tissue water content. The hemoglobin content of lung, spleen, and kidney was also elevated in transgenic animals due to trapped hemoglobin and/or increased blood volume. When transgenic and control mice were examined by magnetic resonance imaging at 9.4 tesla, some transgenic animals had enlarged kidneys with prolonged relaxation time, consistent with increased organ weight and water content. The glomerular filtration rate was elevated in transgenic animals, which is characteristic of young sickle cell patients. Furthermore, exposure to hypoxia resulted in significantly decreased hematocrit, increased erythrocyte density, and induced a urine-concentrating defect. We conclude that the transgenic mouse line reported here has chronic organ damage and further hematological and organ dysfunction can be induced by hypoxia.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler D. D., Glazer G. M., Aisen A. M. MRI of the spleen: normal appearance and findings in sickle-cell anemia. AJR Am J Roentgenol. 1986 Oct;147(4):843–845. doi: 10.2214/ajr.147.4.843. [DOI] [PubMed] [Google Scholar]
- Bank N., Aynediian H. S. A micropuncture study of the effect of parathyroid hormone on renal bicarbonate reabsorption. J Clin Invest. 1976 Aug;58(2):336–344. doi: 10.1172/JCI108477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankir L., de Rouffignac C. Urinary concentrating ability: insights from comparative anatomy. Am J Physiol. 1985 Dec;249(6 Pt 2):R643–R666. doi: 10.1152/ajpregu.1985.249.6.R643. [DOI] [PubMed] [Google Scholar]
- Barabino G. A., McIntire L. V., Eskin S. G., Sears D. A., Udden M. Endothelial cell interactions with sickle cell, sickle trait, mechanically injured, and normal erythrocytes under controlled flow. Blood. 1987 Jul;70(1):152–157. [PubMed] [Google Scholar]
- Behringer R. R., Ryan T. M., Reilly M. P., Asakura T., Palmiter R. D., Brinster R. L., Townes T. M. Synthesis of functional human hemoglobin in transgenic mice. Science. 1989 Sep 1;245(4921):971–973. doi: 10.1126/science.2772649. [DOI] [PubMed] [Google Scholar]
- Bottomley P. A., Foster T. H., Argersinger R. E., Pfeifer L. M. A review of normal tissue hydrogen NMR relaxation times and relaxation mechanisms from 1-100 MHz: dependence on tissue type, NMR frequency, temperature, species, excision, and age. Med Phys. 1984 Jul-Aug;11(4):425–448. doi: 10.1118/1.595535. [DOI] [PubMed] [Google Scholar]
- ETTELDORF J. N., SMITH J. D., TUTTLE A. H., DIGGS L. W. Renal hemodynamic studies in adults with sickle cell anemia. Am J Med. 1955 Feb;18(2):243–248. doi: 10.1016/0002-9343(55)90239-4. [DOI] [PubMed] [Google Scholar]
- Fabry M. E., Fine E., Rajanayagam V., Factor S. M., Gore J., Sylla M., Nagel R. L. Demonstration of endothelial adhesion of sickle cells in vivo: a distinct role for deformable sickle cell discocytes. Blood. 1992 Mar 15;79(6):1602–1611. [PubMed] [Google Scholar]
- Fabry M. E., Kaul D. K., Raventos-Suarez C., Chang H., Nagel R. L. SC erythrocytes have an abnormally high intracellular hemoglobin concentration. Pathophysiological consequences. J Clin Invest. 1982 Dec;70(6):1315–1319. doi: 10.1172/JCI110732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabry M. E., Nagel R. L., Pachnis A., Suzuka S. M., Costantini F. High expression of human beta S- and alpha-globins in transgenic mice: hemoglobin composition and hematological consequences. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12150–12154. doi: 10.1073/pnas.89.24.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabry M. E., Romero J. R., Buchanan I. D., Suzuka S. M., Stamatoyannopoulos G., Nagel R. L., Canessa M. Rapid increase in red blood cell density driven by K:Cl cotransport in a subset of sickle cell anemia reticulocytes and discocytes. Blood. 1991 Jul 1;78(1):217–225. [PubMed] [Google Scholar]
- Greaves D. R., Fraser P., Vidal M. A., Hedges M. J., Ropers D., Luzzatto L., Grosveld F. A transgenic mouse model of sickle cell disorder. Nature. 1990 Jan 11;343(6254):183–185. doi: 10.1038/343183a0. [DOI] [PubMed] [Google Scholar]
- Hebbel R. P., Yamada O., Moldow C. F., Jacob H. S., White J. G., Eaton J. W. Abnormal adherence of sickle erythrocytes to cultured vascular endothelium: possible mechanism for microvascular occlusion in sickle cell disease. J Clin Invest. 1980 Jan;65(1):154–160. doi: 10.1172/JCI109646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herfkens R. J., Sievers R., Kaufman L., Sheldon P. E., Ortendahl D. A., Lipton M. J., Crooks L. E., Higgins C. B. Nuclear magnetic resonance imaging of the infarcted muscle: a rat model. Radiology. 1983 Jun;147(3):761–764. doi: 10.1148/radiology.147.3.6844611. [DOI] [PubMed] [Google Scholar]
- Kaul D. K., Fabry M. E., Nagel R. L. Microvascular sites and characteristics of sickle cell adhesion to vascular endothelium in shear flow conditions: pathophysiological implications. Proc Natl Acad Sci U S A. 1989 May;86(9):3356–3360. doi: 10.1073/pnas.86.9.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C., Fabry M. E., Nagel R. L. The unique red cell heterogeneity of SC disease: crystal formation, dense reticulocytes, and unusual morphology. Blood. 1991 Oct 15;78(8):2104–2112. [PubMed] [Google Scholar]
- Mozzarelli A., Hofrichter J., Eaton W. A. Delay time of hemoglobin S polymerization prevents most cells from sickling in vivo. Science. 1987 Jul 31;237(4814):500–506. doi: 10.1126/science.3603036. [DOI] [PubMed] [Google Scholar]
- Ratner A. V., Okada R. D., Newell J. B., Pohost G. M. The relationship between proton nuclear magnetic resonance relaxation parameters and myocardial perfusion with acute coronary arterial occlusion and reperfusion. Circulation. 1985 Apr;71(4):823–828. doi: 10.1161/01.cir.71.4.823. [DOI] [PubMed] [Google Scholar]
- Rhoda M. D., Domenget C., Vidaud M., Bardakdjian-Michau J., Rouyer-Fessard P., Rosa J., Beuzard Y. Mouse alpha chains inhibit polymerization of hemoglobin induced by human beta S or beta S Antilles chains. Biochim Biophys Acta. 1988 Jan 29;952(2):208–212. doi: 10.1016/0167-4838(88)90117-3. [DOI] [PubMed] [Google Scholar]
- Rubin E. M., Lu R. H., Cooper S., Mohandas N., Kan Y. W. Introduction and expression of the human Bs-globin gene in transgenic mice. Am J Hum Genet. 1988 Apr;42(4):585–591. [PMC free article] [PubMed] [Google Scholar]
- Rubin E. M., Witkowska H. E., Spangler E., Curtin P., Lubin B. H., Mohandas N., Clift S. M. Hypoxia-induced in vivo sickling of transgenic mouse red cells. J Clin Invest. 1991 Feb;87(2):639–647. doi: 10.1172/JCI115041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan T. M., Townes T. M., Reilly M. P., Asakura T., Palmiter R. D., Brinster R. L., Behringer R. R. Human sickle hemoglobin in transgenic mice. Science. 1990 Feb 2;247(4942):566–568. doi: 10.1126/science.2154033. [DOI] [PubMed] [Google Scholar]
- Solanki D. L., Kletter G. G., Castro O. Acute splenic sequestration crises in adults with sickle cell disease. Am J Med. 1986 May;80(5):985–990. doi: 10.1016/0002-9343(86)90649-2. [DOI] [PubMed] [Google Scholar]
- Trudel M., Saadane N., Garel M. C., Bardakdjian-Michau J., Blouquit Y., Guerquin-Kern J. L., Rouyer-Fessard P., Vidaud D., Pachnis A., Roméo P. H. Towards a transgenic mouse model of sickle cell disease: hemoglobin SAD. EMBO J. 1991 Nov;10(11):3157–3165. doi: 10.1002/j.1460-2075.1991.tb04877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick T. M., Moake J. L., Udden M. M., Eskin S. G., Sears D. A., McIntire L. V. Unusually large von Willebrand factor multimers increase adhesion of sickle erythrocytes to human endothelial cells under controlled flow. J Clin Invest. 1987 Sep;80(3):905–910. doi: 10.1172/JCI113151. [DOI] [PMC free article] [PubMed] [Google Scholar]