Abstract
Aims
Loop diuretics are first‐line medications for congestive heart failure (CHF); however, they are associated with serious adverse effects, including decreased renal function, and sympathetic nervous and renin–angiotensin system activation. We tested whether tolvaptan, a vasopressin V2‐receptor antagonist, could reduce unfavourable furosemide‐induced effects during CHF treatment.
Methods and results
Sixty patients emergently hospitalized owing to CHF‐induced dyspnea were randomly assigned to receive either 40 mg intravenous furosemide daily or 7.5 mg oral tolvaptan for 5 days after admission. Both groups also received intravenous carperitide and canrenoate potassium. As results, baseline patient characteristics were similar between the furosemide (n = 30) and the tolvaptan (n = 30) groups, with no significant difference in 5 day urine volume or fluid balance. Brain natriuretic peptide and body weight improvements were similar between groups. However, serum creatinine (Cr) level did not increase, and the incidence of worsening renal function was significantly lower in the tolvaptan group. Consequently, the Cr increase to gain 1000 mL urine was 2.5‐fold lower in the tolvaptan group. Furthermore, the blood urea nitrogen (BUN)/Cr ratio significantly decreased in the tolvaptan group, suggesting that renal perfusion was preserved, and urea reuptake and passive water reabsorption were suppressed following tolvaptan treatment. Although catecholamine improvements after treatment were not significantly different, plasma renin activity was enhanced in the furosemide group.
Conclusions
As compared with furosemide, tolvaptan in patients with acute heart failure is associated with comparable decongestion, better preservation of renal function and less activation of renin–angiotensin system. (UMIN 000014134).
Keywords: Heart failure, Diuretic, Pharmacology, Kidney
Introduction
Congestive heart failure (CHF) is a major public health problem with high morbidity and mortality among aged populations.1, 2, 3 Despite therapeutic advances, the prognosis for acute decompensated CHF remains poor.4, 5, 6 Fluid retention and congestion are responsible for most HF hospitalizations,2, 4 and severe congestion is associated with poor outcomes.7 Therefore, appropriate acute CHF management may improve prognosis.
Loop diuretics are essential CHF medications that inhibit sodium reabsorption in Henle's loop, thereby passively increasing water excretion. Despite serious adverse effects, including decreased renal function and sympathetic nervous system (SNS) and renin–angiotensin–aldosterone system (RAAS) activation,8, 9, 10, 11 loop diuretics remain first line medications for acute CHF management. Additionally, loop diuretics directly affect the macula‐densa to activate renin, leading to afferent arteriole vasoconstriction and deteriorating renal function.12, 13, 14 In patients with decompensated CHF, acute kidney injury during the acute treatment phase leads to poor outcomes.15, 16, 17, 18 Furthermore, inappropriately high dose of intravenous furosemide within the first 72 h left room for improved long‐term prognoses.15, 19
Neurohumoral factors, such as SNS, RAAS, and arginine vasopressin (AVP), are biological defence mechanisms that preserve arterial volume and circulatory homeostasis during acute systemic volume depletion and low cardiac output. However, sustained neurohumoral factor activation causes progressive ventricular remodelling. Vasoconstriction and water retention induced by SNS and RAAS activation and AVP secretion can accelerate CHF progression. AVP regulates vascular tone and free‐water reabsorption through the vasopressin V1a and V2 receptors. Thus, these receptors represent potential neurohumoral targets for CHF treatment.20 Furthermore, preventing sustained SNS and RAAS activation using β blockers, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, and aldosterone antagonists is a fundamental CHF treatment strategy in patients with reduced ejection fractions.21, 22, 23, 24, 25, 26, 27, 28
Tolvaptan is a selective vasopressin V2 receptor antagonist with electrolyte‐free water diuretic properties, which acts on the distal nephron collecting.29 In the EVEREST trial, add‐on administration of tolvaptan to standard treatments significantly improved dyspnea and systemic edema, and reduced body weight in acute‐phase CHF.30 However, the role of V2 receptor antagonism on renal function and neurohumoral factors in CHF remains unclear. Therefore, we hypothesized that treatment with tolvaptan after hospitalization may ameliorate the adverse effects of furosemide treatment in CHF patients. We directly compared the effects of oral tolvaptan and intravenous furosemide on renal response and neurohumoral factor kinetics in patients with acute decompensated CHF.
Methods
Study population
This study included 60 patients (≥30 years) with dyspnea at rest [New York Heart Association (NYHA) classification IV] who were emergently hospitalized owing to worsening CHF in a single cardiovascular centre between January 2013 and February 2014. Patients were diagnosed with CHF using Framingham heart failure diagnostic criteria. The following patients were excluded: (i) patients with shock or preshock vital signs upon admission; (ii) patients already receiving daily tolvaptan; (iii) maintenance dialysis patients; (iv) patients with a malignant neoplasm whose life expectancy was less than 6 months; and (v) inadequate patients including patients who did not well understand the purpose of this study or patients with severe dementia, and patients who were uncooperative to medical practice. The study protocols conformed to the ethical guidelines of the 1975 Declaration of Helsinki. The ethics committee of our institution approved the study protocol. Written informed consent was obtained from all patients before study entry.
Treatment protocols
A randomized, controlled, open‐label trial was performed. Patients first received oxygen support, and vital signs were recorded upon admission. Blood samples were collected within 15 min of arrival. Patients were randomly assigned to receive 20 mg intravenous furosemide twice daily or 7.5 mg oral tolvaptan once daily. Additionally, both groups intravenously received continuous carperitide (0.025 µg/kg/min) and bolus injections of canrenoate potassium (100 mg twice daily). The prescribed treatment was administered for 5 days (‘intensive’ treatment period), unless systemic congestion was completely improved. We used carperitide as a vasodilator to relieve pulmonary artery congestion. Canrenoate potassium was used as a natriuretic to suppress endogenous aldosterone with low dehydrating effect. No limitations were set on standard CHF medication use except target diuretics.
Rationale for low‐dose furosemide and low‐dose tolvaptan
Appropriate furosemide dosing is required in CHF patients. We followed the American College of Cardiology/American Heart Association (ACC/AHA) guidelines, which stated that patients treated with loop diuretics should receive an initial intravenous dose equal to or greater than their chronic oral daily dose.28 In this study, patients received 37 ± 42 mg/day oral furosemide at admission. Therefore, a 40 mg/day intravenous bolus injection was administered. Additionally, 7.5 mg and 15 mg of tolvaptan exerted similar diuretic effects in patients with acute‐phase CHF.31 Based on these data, we compared the effect of low‐dose furosemide and tolvaptan.
Data collection
Vital signs, including physical findings, blood pressure, and urine volume, and lab data, including serum creatinine (Cr) and blood urea nitrogen (BUN) for renal function and plasma renin activity, plasma aldosterone concentration, brain natriuretic peptide (BNP), and catecholamines as neurohumoral factors, were evaluated daily during intensive treatment, and regularly afterwards.
Prognosis and follow‐up
The in‐hospital prognoses were evaluated, including the duration of coronary care unit (CCU) stay, hospitalization duration, and in‐hospital mortality. After hospital discharge, outpatient office visits were scheduled monthly. Telephone contact was made if patients missed scheduled clinical visits. All‐cause deaths within 6 months and the survival rates were analysed by Kaplan–Meier method.
Statistical analysis
Values are expressed as the mean ± SD, median with interquartile range, and percentile. For 5 day total urine volume, 5 day total fluid balance, non‐invasive positive pressure ventilation (NPPV) duration, CCU stay, and total hospitalization, box and whisker plots were used. The bottom and top of the box are the first and third quartiles, and the band inside the box is the median. The ends of the whiskers represent the minimum and maximum of all data. The independent Student's t‐test or non‐parametric equivalent Mann–Whitney U‐test was used to compare continuous parameters between the furosemide and tolvaptan groups. The Kruskal–Wallis test was used to compare continuous parameters among ≥3 groups. To directly compare the difference between two groups after Kruskal–Wallis test, Tukey's multiple comparison test was applied. Wilcoxon's signed rank test was used to compare values between before and after the treatment. Pearson's chi‐squared test was used to evaluate categorical variables. Comparisons among ≥3 sets of consecutive samples from two groups were evaluated by two‐way ANOVA with a Sidak post hoc test, while a multiple t‐test with the Holm–Sidak method was used for two sets of samples from two groups. Two‐sided p‐values less than 0.05 were considered statistically significant. The survival rates after discharge were compared using Kaplan–Meier curves, and differences were assessed with the log‐rank test.
Results
Baseline characteristics
Baseline patient characteristics are shown in Table 1. Patients were typically elderly (>75 years old) with a normal body mass index and diverse comorbidities, including hypertension, diabetes, coronary artery disease, and chronic kidney disease. The two groups presented comparable clinical parameters at admission, although the prevalence of dyslipidemia was lower in the furosemide group (33 vs. 60%, P = 0.038). Both groups included 30–40% patients with reduced left ventricular ejection fraction (LVEF, 37 vs. 30%, P = 0.58). All patients were admitted because of dyspnea with hypoxemia (NYHA IV), and some of them needed respiratory support by NPPV (20 vs. 17 patients, P = 0.60). Patients from both groups showed similar baseline BNP levels (721 vs. 1196 pg/mL, P = 0.15). Laboratory analyses revealed poor lipid profiles, impaired vascular function, and low serum albumin levels. There was no significant difference in flow‐mediated dilatation, haemoglobin, low‐density lipoprotein (LDL)/high‐density lipoprotein (HDL) cholesterol ratio, or eicosapentaenoic acid/arachidonic acid (EPA/AA) ratio. Furthermore, both groups received similar pre‐hospital oral medications (Table 2). Transthoracic echocardiography exhibited similar cardiac parameters in both groups (Supplemental Table 1 ). The average target drug administration duration was 4.5 and 4.4 days for furosemide and tolvaptan, respectively. The inotropic drug use frequency during the intensive treatment period was low, and similar between groups (Supplemental Table 2 ).
Table 1.
Baseline patient characteristics
| Furosemide (n = 30) | Tolvaptan (n = 30) | P‐value | |
|---|---|---|---|
| Age, years old | 79 ± 11 | 79 ± 11 | 0.93 |
| >75 y/o | 21 (70%) | 20 (67%) | 0.78 |
| Male | 17 (57%) | 13 (43%) | 0.30 |
| BMI, kg/m2 | 24 ± 4.7 | 23 ± 4.8 | 0.64 |
| NYHA IV | 30 (100%) | 30 (100%) | – |
| Current smoking | 4 (13%) | 5 (17%) | 0.72 |
| Hypertension | 26 (87%) | 20 (67%) | 0.07 |
| Diabetes | 15 (50%) | 11 (37%) | 0.30 |
| Dyslipidemia | 18 (60%) | 10 (33%) | 0.038 |
| Hyperuricemia | 13 (43%) | 8 (27%) | 0.18 |
| Coronary artery disease | 15 (50%) | 13 (43%) | 0.60 |
| Prior revascularization | 7 (23%) | 3 (10%) | 0.17 |
| Prior myocardial infarction | 10 (33%) | 8 (27%) | 0.57 |
| Chronic kidney disease* | 19 (63%) | 14 (47%) | 0.19 |
| Atrial fibrillation | 13 (43%) | 11 (37%) | 0.60 |
| LV systolic dysfunction† | 11 (37%) | 9 (30%) | 0.58 |
| FMD, % | 3.4 ± 1.3 | 2.7 ± 1.8 | 0.052 |
| Lab data | |||
| BUN, mg/dL | 23.4 ± 9.6 | 26.0 ± 14.4 | 0.53 |
| Creatinine, mg/dL | 1.03 ± 0.49 | 1.01 ± 0.66 | 0.30 |
| eGFR, mL/min/1.73 m2 | 56.0 ± 27.5 | 63.9 ± 29.7 | 0.24 |
| Sodium, mEq/L | 138 ± 5.1 | 138 ± 6.0 | 0.67 |
| Potassium, mEq/L | 3.9 ± 0.8 | 4.0 ± 0.7 | 0.60 |
| BNP, ng/dL | 721 ± 497 | 1196 ± 901 | 0.15 |
| Albumin, g/dL | 3.2 ± 0.6 | 3.4 ± 0.6 | 0.29 |
| Haemoglobin, g/dL | 12 ± 2.4 | 11 ± 2.2 | 0.15 |
| LDL‐C/HDL‐C | 2.3 ± 1.0 | 2.0 ± 0.9 | 0.32 |
| EPA/AA | 0.38 ± 0.31 | 0.33 ± 0.21 | 1.00 |
BMI: body mass index; EPA/AA: eicosapentaenoic acid/arachidonic acid; FMD: flow mediated dilation; HDL‐C: high density lipoprotein cholesterol; LDL‐C: low density lipoprotein cholesterol; LV: left ventricular; NYHA: New York Heart Association.
Data presented as number (%), or mean ± SD.
eGFR < 60 mL/min./1.73 m2.
Ejection fraction < 40%.
Table 2.
Oral medications on admission
| Furosemide (n = 30) | Tolvaptan (n = 30) | P‐value | |
|---|---|---|---|
| Furosemide | 13 (43%) | 14 (47%) | 0.79 |
| Dose, mg/day | 20 (20–40) | 40 (20–40) | 0.61 |
| Aldosterone antagonist | 7 (23%) | 9 (30%) | 0.56 |
| Thiazide | 1 (3%) | 2 (7%) | 0.55 |
| Digoxin | 3 (10%) | 4 (13%) | 0.69 |
| β blocker | 12 (40%) | 12 (40%) | 1.00 |
| ACE inhibitor | 2 (7%) | 4 (13%) | 0.39 |
| ARB | 10 (33%) | 9 (30%) | 0.78 |
| Calcium channel blocker | 13 (43%) | 10 (33%) | 0.43 |
| ISDN | 4 (13%) | 4 (13%) | 1.00 |
| Statin | 10 (33%) | 5 (17%) | 0.14 |
ACE: angiotensin converting enzyme; ARB: angiotensin II receptor blocker; ISDN: isosorbide dinitrate.
Data presented as number (%), or median with interquartile range.
Fluid excretion
Furosemide increased urine volume on day 0, and gradually decreased over 5 days. In contrast, tolvaptan mildly increased daily urine volume on day 1 or 2, with a significant difference on day 0 (2646 vs. 1825 mL/day, P = 0.024, Figure 1 A). A similar fluid balance time‐course was observed between groups (Figure 1 B). Daily fluid balance on Day 0 also tended to be larger in the furosemide group than the tolvaptan group (−1988 vs. −1277 mL/day, P = 0.050). However, the 5 day total urine volume and fluid balance were similar (Figure 1 C, D). Furthermore, the two drugs similarly improved BNP (Figure 3 B) and central venous pressure (CVP) (Supplemental Figure 1A ). Body weight decreased over time in both groups, and changes from baseline were also similar between groups (Supplemental Figure 1B, C ). Reflecting to quicker increase of urine volume by furosemide, duration of NPPV use was 1 day shorter in the furosemide group; however, the difference did not reach statistical significance (2.3 vs. 3.2 days, P = 0.13, Supplemental Figure 1D ).
Figure 1.
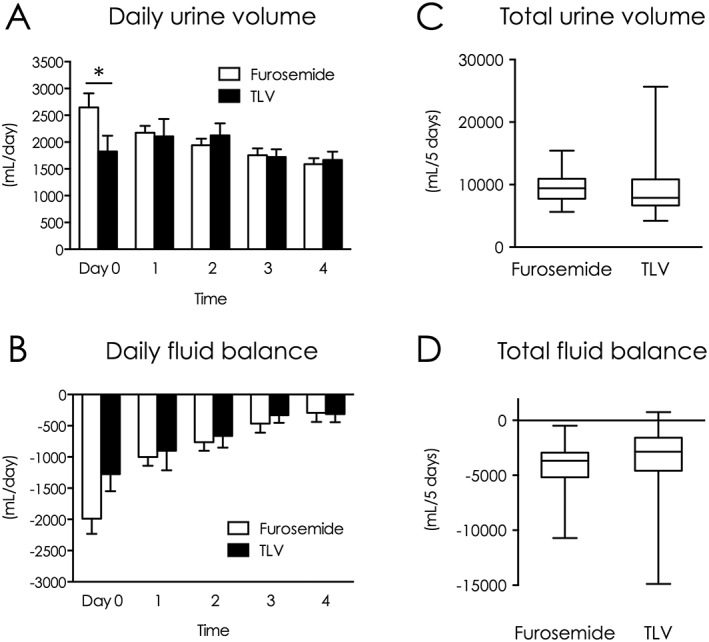
Comparison of urine volume and fluid balance during intensive treatment between groups. (A) Daily urine volume (days 0–4). (B) Daily fluid balance (days 0–4). (C) 5‐day total urine volume. (D) 5‐day total fluid balance. *P < 0.05 between groups. TLV indicates tolvaptan.
Figure 3.
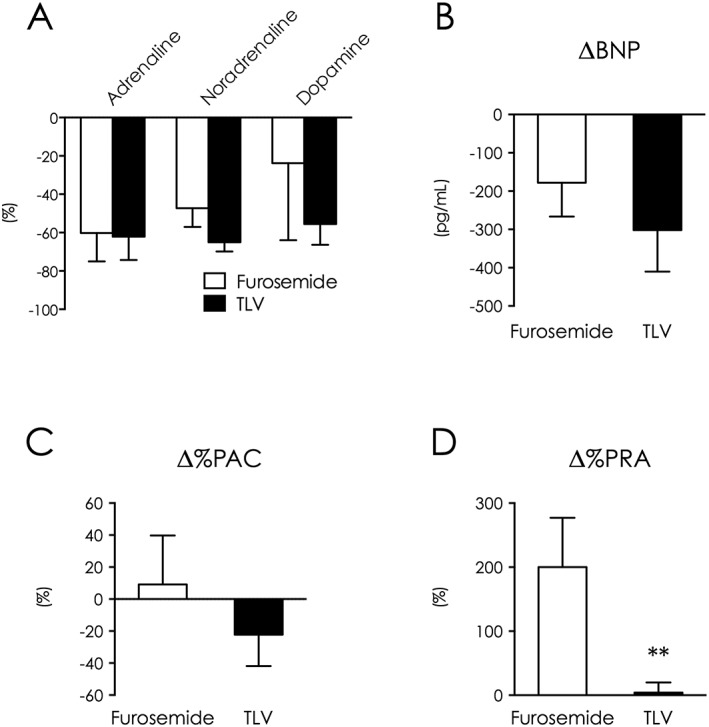
Tolvaptan decreased catecholamines and BNP without RAAS enhancement. Changes between baseline and intensive treatment end in catecholamines (A), BNP (B), plasma aldosterone concentration (C), and plasma renin activity (D). **P < 0.01 vs. furosemide. BNP indicates brain natriuretic peptide; PAC, plasma aldosterone concentration; PRA, plasma renin activity; TLV, tolvaptan.
Renal function
Furosemide worsened renal function, as indicated by a 20% increase in serum Cr (Figure 2 A) and a severe reduction in glomerular filtration rate (−5.9 mL/min/1.73 m2, Figure 2 B). In contrast, tolvaptan preserved renal function over 5 days of treatment (ΔCr: P = 0.033, ΔeGFR: P = 0.040, Figure 2 A,B, Supplemental Figure 2A ).
Figure 2.
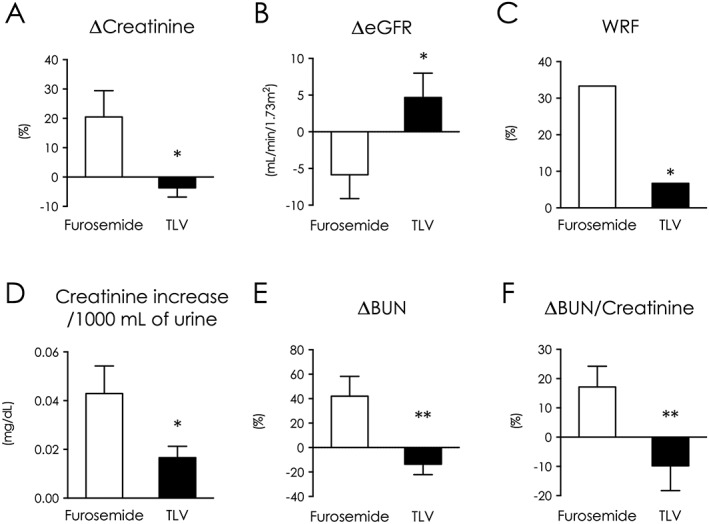
Tolvaptan preserved renal functions and decreased blood urea nitrogen/Cr ratio. Changes between baseline and intensive treatment end in serum Cr (A) and eGFR (B). (C) WRF incidence during intensive treatment. (D) Increase in serum Cr to gain 1000 mL of urine volume during intensive treatment. Changes between baseline and intensive treatment end in blood urea nitrogen (E) and blood urea nitrogen/Cr ratio (F). *P < 0.05, **P < 0.01 vs. furosemide. TLV indicates tolvaptan; WRF, worsening renal function.
Worsening renal function (WRF) is defined as serum Cr > 0.3 mg/dL during intensive treatment periods. WRF occurred more frequently in the furosemide group (33%) than the tolvaptan group (6.7%; P < 0.01, Figure 2 C). Consequently, increases in serum Cr per 1000 mL urine were 2.5‐fold higher in the furosemide group (0.043 vs. 0.017 mg/dL/1000 mL, P = 0.028) (Figure 2 D). Among patients with WRF, a persistent increase in Cr was observed in three patients treated with furosemide (33 vs. 0%). BUN increased in the furosemide group and decreased in the tolvaptan group (42 vs. −14%, P < 0.01, Figure 2 E). Patient with BUN >21.0 mg/dL was similarly prevalent in both groups at baseline (53% vs. 53%); however, it was significantly higher in the furosemide group at the end of intensive treatment (87% vs. 27%, P < 0.01). Changes in the BUN/Cr ratio were similar to BUN kinetics, with a lower change in ratio in the tolvaptan group (17 vs. −9.8%, P < 0.01, Figure 2 F, Supplemental Figure 2A ).
Neurohumoral factors
Tolvaptan decreased catecholamine and BNP levels similar to standard furosemide therapy (adrenaline: −60 vs. −62%, P = 0.38, noradrenaline: −47 vs. −65%, P = 0.36, dopamine: −24 vs. −56%, P = 0.68, Figure 3 A; BNP: −178 vs. −302 pg/mL, P = 0.27, Figure 3 B, Supplemental Figure 2B ). Although plasma aldosterone concentration changes were not statistically different between groups under the use of 200 mg daily dose of anti‐aldosterone drug (9.1 vs. −22%, P = 0.48, Figure 3 C), plasma renin was highly enhanced in the furosemide group (200 vs. 4%, P = 0.014, Figure 3 D, Supplemental Figure 2B ).
Systemic blood pressure
Average blood pressure (BP) on admission was similar in both groups (systolic: 132 vs. 132 mmHg, P = 0.76; diastolic: 75 vs. 78 mmHg, P = 0.82). Systolic BP was maintained in the tolvaptan group, but was significantly decreased through day 1 to 5 in the furosemide group with carperitide, because of its hypotensive effect (132 to 117 mmHg, P < 0.01, Figure 4 A). Although diastolic BP in both groups decreased over time and the difference between groups did not reach a statistical significance, it made a significant decrease through day 1 to day 5 in the furosemide group (75 to 61 mmHg, P < 0.01, Figure 4 B).
Figure 4.
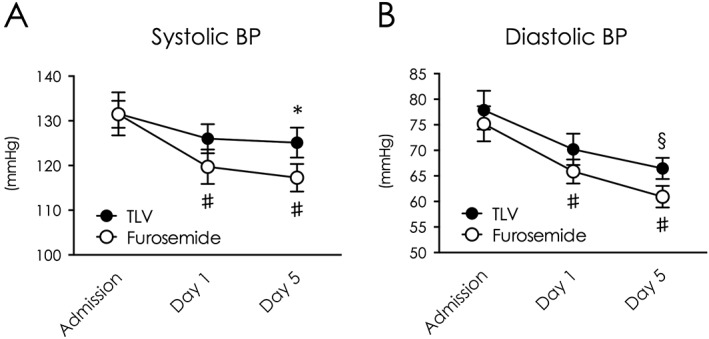
Tolvaptan preserved systemic BP during intensive treatment. Changes of systolic BP (A) and diastolic BP (B) from baseline to intensive treatment end. *P < 0.05 vs. furosemide. #P < 0.05 vs. baseline in the furosemide group. §P < 0.05 vs. admission. TLV indicates tolvaptan.
Effect of left ventricular systolic function on tolvaptan
To determine if LV systolic function influences the effect of tolvaptan, we classified the study population by LVEF (cut‐off value: 40%) into patients with preserved LVEF (HFpEF) and reduced LVEF (HFrEF). Patient baseline clinical profiles were significantly different among four groups, because the HFpEF population more frequently included females and older patients with lower haemoglobin (Supplemental Table 3 ). Total 5 day urine volume was similar; however, total fluid balance was significantly different among groups (P = 0.18, P = 0.035, respectively, Figure 5 A, B). Serum creatinine was significantly increased only in furosemide‐treated HFpEF patients. Furthermore, the WRF rate was greater than 40% in furosemide‐treated HFpEF patients (Figure 5 C, D). BUN levels were consistent with serum creatinine changes (Figure 5 E). Systolic BP in furosemide‐treated HFpEF patients decreased compared with other groups, although the difference was not significant (Figure 5 F, G). Reductions in CVP, BNP, and PAC were similar among groups (Figure 5 H–J). In contrast, furosemide‐treated HFpEF patients exhibited increased PRA (Figure 5 K).
Figure 5.
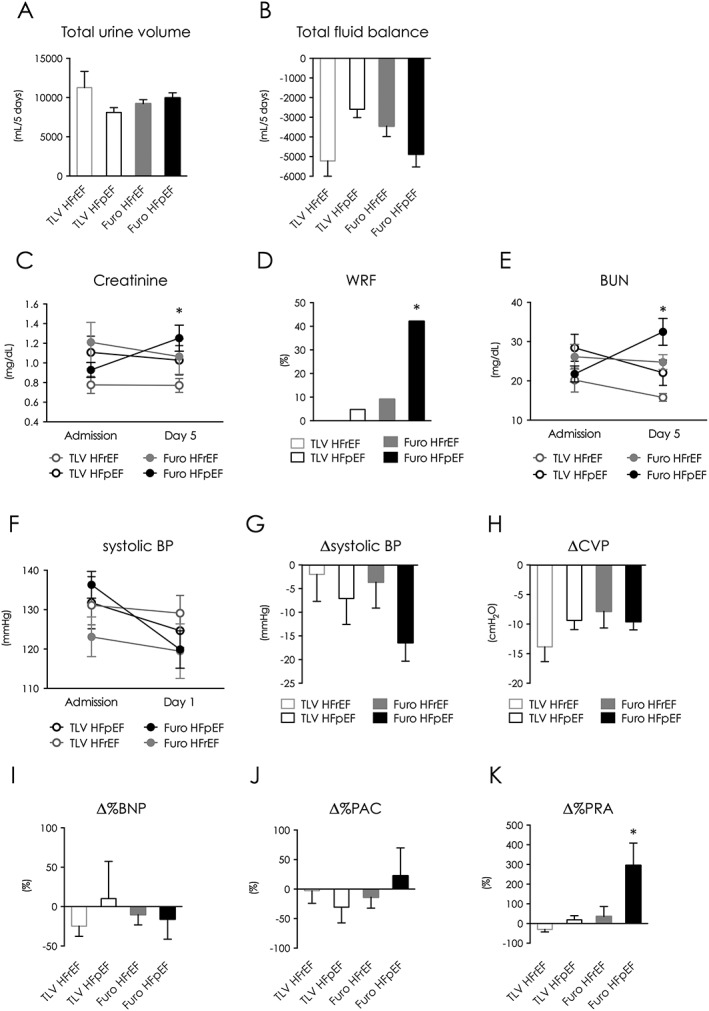
Patient characteristics and clinical outcomes in patients classified by left ventricular ejection fraction. (A) 5‐day total urine volume. (B) 5‐day total fluid balance. (C) Serum creatinine values from baseline to intensive treatment end. (D) WRF incidence during intensive treatment. (E) blood urea nitrogen values from baseline to intensive treatment end. (F) Systolic BP at baseline and day 1. (G) Change in systolic BP between baseline and day 1. (H–K) Change between baseline and intensive treatment end in CVP (H), BNP (I), PAC (J), and PRA (K). *P < 0.05. BNP indicates brain natriuretic peptide; BP, blood pressure; BUN, blood urea nitrogen; CVP, central venous pressure; HFp(r)EF, heart failure patient with preserved (reduced) ejection fraction; TLV, tolvaptan; WRF, worsening renal function.
Electrolyte kinetics
Serum sodium concentration on admission was not significantly different between groups (138 vs. 138 mEq/L, P = 0.67), and was not altered upon discharge in the furosemide group. In contrast, tolvaptan significantly increased sodium concentrations on day 1, and these values remained high until treatment end (Admission vs. Day 5, P < 0.01, Supplemental Figure 3A ). Although hyponatremia patients (less than 135 mEq/L) were similarly prevalent in both groups on admission (17 vs. 20%, P = 0.74), there were significantly fewer hyponatremia patients following intensive treatment in the tolvaptan group (30 vs. 3.3%, P < 0.01). However, interestingly in the tolvaptan group, 14 patients who received tolvaptan only for the first 5 days of treatment (temporary tolvaptan group) showed similar serum sodium changes to patients who received tolvaptan throughout their hospital stay (continual tolvaptan group) (Supplemental Figure 3B ). Potassium changes were similar between groups during the intensive treatment period with intravenous canrenoate potassium (Supplemental Figure 3C ).
In‐hospital and mid‐term prognosis
CCU stay and total hospitalization duration were not statistically different between groups (CCU: 6.4 vs. 5.2 days, P = 0.76; total hospitalization: 27 vs. 21 days, P = 0.88, Supplemental Figure 4A, B ). In‐hospital mortality rates were low and similar between groups (3.3 vs. 10%, P = 0.30), and all were cardiovascular‐related deaths. After discharge, tolvaptan was continually administered to 48% and 10% of the tolvaptan and furosemide groups, respectively (P < 0.01). Continuation of oral furosemide administration was significantly higher in the furosemide group (97%) than the tolvaptan group (78%; P = 0.034). Furthermore, the furosemide group required higher doses of furosemide (33 mg/day) than the tolvaptan group (23 mg/day, P = 0.058). Administration of other diuretics and cardiovascular medications was similar between groups (Supplemental Table 4 ). Finally, the survival rate up to 180 days was similar between groups (P = 0.17, Supplemental Figure 4C ).
Discussion
High dose of loop diuretic in CHF patients is correlated with renal function deterioration and long‐term adverse clinical outcomes, and even after adjustment for covariates, it emerged as an independent risk factor for mortality.32, 33, 34 However, furosemide still is the standard treatment for both of acute and chronic CHF patients.
Tolvaptan is a unique diuretic, in terms of providing aquaresis, or excretion of water without electrolyte loss, in contrast to furosemide‐induced natriuresis via sodium reabsorption blocking. A numbers of trials and observations using tolvaptan in an ‘add‐on’ manner failed to improve clinical outcomes for CHF patients. There has been no evidence about the impact of tolvaptan alone in acute heart failure patients. A recent article from Udelson and colleagues provided important information to help answer the question. They demonstrated that short‐term tolvaptan, with or without concomitant furosemide, resulted in a higher urine volume than that observed with furosemide monotherapy in chronic HFrEF patients.35 We directly compared the effects of tolvaptan and furosemide in patients with acute‐phase decompensated CHF. Oral tolvaptan exerted superior effects on renal function and plasma renin activation compared with those of intravenous furosemide as well as comparable decongestion efficacy, suggesting that tolvaptan is a potential alternative to furosemide during intensive treatment of CHF.
Tolvaptan prevents the adverse effects of furosemide
Maintenance of serum sodium concentration by tolvaptan retains plasma osmolality, preserves systemic hemodynamics, and possibly avoids renal hypoperfusion. This mechanism was supported by decreased BUN/Cr ratio, and by the lack of change in plasma renin activity after tolvaptan treatment.
The SNS, RAAS, and AVP maintain arterial blood volume integrity.14 Inappropriately high furosemide doses lead to decreased catecholamines and BNP, and RAAS activation.36 During the acute phase of CHF, the primary factor for furosemide to activate RAAS is the change in sodium concentration at the macula densa.12, 13, 14 In the following period, sustained RAAS activation associated with insufficient intravascular volume replenishment from the extravascular space (plasma refill rate) may contribute to it.37 In the chronic phase of CHF, renin is activated by overdiuresis with furosemide leading to volume depletion and the subsequent engagement of renal baroreceptor‐mediated additional activation of the RAAS and SNS.38, 39 In contrast, tolvaptan significantly preserved RAAS activity and reduced SNS activation. Renin activation was 50‐fold lower with tolvaptan treatment than with furosemide. This ‘non‐activation’ effect could be important in improve CHF prognosis.
Tolvaptan preserved BP better than furosemide during intensive treatment, which may prevent a decrease in renal blood flow, resulting in preservation of renal function and avoidance of RAAS activation. This phenomenon was seen prominently in CHF patients with preserved LVEF. When classifying the study population by LV systolic function, furosemide‐treated HFpEF patients showed deteriorated renal function with increased renin activity. They achieved a similar improvement in fluid retention as that shown by the other groups. In contrast, the decrease in systolic BP was greater. These results were consistent with those of the ROSE trial.40 Thus, close hemodynamic observation is necessary, especially in furosemide‐treated HFpEF patients.
Advantages of tolvaptan in patients with refractory congestive heart failure
In chronic CHF patients, BUN levels above 21.0 mg/dL increase the risk of death with loop diuretics.41 Although patients with baseline BUN levels > 21.0 mg/dL were similarly distributed between groups in this study, only tolvaptan‐treated patients showed a significant BUN decrease. The tubular reabsorption of urea is highly dependent both on direct and indirect neurohumoral activation.42, 43, 44 As a direct effect, AVP increases urea reabsorption in the collecting duct,45 and therefore, vasopressin receptor antagonism augments urea excretion. Indirect effects such as decreased renal blood flow and increased proximal tubular reabsorption of water and solute could be improved by tolvaptan. Furosemide‐induced neurohumoral activation mediates renally; therefore, clearance of BUN would parallel the adverse effects of furosemide.
Hyponatremia is common in hospitalized heart failure patients and associated with longer hospital stays and higher mortality.46 Persistent hyponatremia is also a predictor of long‐term mortality in CHF patients with preserved systolic left ventricular function.47 Tolvaptan significantly increased serum sodium level over the intensive treatment period. However, the levels decreased at discharge to similar levels as those in furosemide‐treated patients, despite continual tolvaptan administration in 48% of patients. Furthermore, when comparing sodium concentrations between continual and temporary tolvaptan administration, no significant difference was observed. Thus, natriuretics may primarily regulate electrolytes after the compensation of acute worsening of CHF.
Impact of worsening renal function
WRF during acute‐phase CHF has diverse causes, including overdiuresis leading to volume depletion and reduced renal blood flow, GFR, and cardiac output. Transient reductions in intravascular volume may also induce WRF, owing to insufficient plasma refill rate.37 Even if creatinine levels and GFR return to baseline, a significant reduction in renal functional reserve (RFR) occurs, increasing the susceptibility to AKI after future exposure.48 Repeated WRF steadily reduces RFR. The WRF incidence during intensive treatment was higher in patients treated with intravenous furosemide, and frequently persisted. This may contribute to mid‐term prognosis. Acute decompensation repeats in CHF patients, which could increase WRF risk. Therefore, WRF prevention is essential for long‐term clinical outcomes in CHF patients.
Future prospects
The results of the present study suggest that a larger trial focused on long‐term prognosis is warranted. A randomized trial should be conducted wherein two groups would be treated with tolvaptan, one group in which tolvaptan alone is continued after discharge and another in which standard therapy is initiated at discharge. We could evaluate the impact of the anti‐AVP effect of continual tolvaptan after the discharge on clinical prognosis in chronic‐phase CHF patients. Additionally, future trials could also examine the relative roles of adjunctive therapies for decongestion, particularly in view of the recent study suggesting adverse effects of carperitide in acute CHF patients.49
Study limitations
Our sample size was small, especially among the non‐randomized population in the analysis of LVEF. Although Kaplan–Meier statistics overlapped owing to a small population size, differences in survival rate were observed after 3 months. It is difficult to conclude that tolvaptan improves prognosis. Longer, large‐scale follow‐up is necessary. Second, we used low‐dose furosemide for the control group, by simply referring to the prehospital oral furosemide dose. Therefore, dose‐dependent effects of both diuretics were unclear. Third, we used carperitide as a vasodilator in both groups. This recombinant human atrial natriuretic peptide used in 58.2% of hospitalized CHF patients in Japan50 has some neurohumoral inhibitory effects. Thus, it may affect clinical outcomes, and the findings in this study are generalized only for patients receiving this vasodilator. Fourth, we did not set optimal criteria for tolvaptan continuation at discharge. Thus, we could not determine the effect of tolvaptan continuation on mid‐term prognosis. Finally, no urine data were collected; therefore, we could not determine urine sodium excretion.
Conclusions
Initiation of tolvaptan therapy in acute CHF patients exerted potent dehydration effects and preserved renal function and the RAAS. This novel therapy may prevent adverse furosemide‐induced effects in CHF patients.
Funding
This study was not financially supported by any company, grant, or fund.
Disclosures
Not applicable
Conflict of interest
None declared.
Supporting information
Table S1. Echocardiographic findings.
Table S2. Intravenous inotropic reagent use.
Table S3. Baseline patient characteristics classified by LVEF.
Table S4. Oral medications at discharge.
Figure S1. Water excretion was similar during intensive treatment between the furosemide and tolvaptan groups.
Figure S2. Tolvaptan preserved renal functions and neurohumoral parameters.
Figure S3. Tolvaptan increased serum sodium concentration during intensive treatment period.
Figure S3. In‐hospital and mid‐term prognoses were not significantly different between the furosemide and tolvaptan group.
Supporting info item
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Jujo, K. , Saito, K. , Ishida, I. , Furuki, Y. , Kim, A. , Suzuki, Y. , Sekiguchi, H. , Yamaguchi, J. , Ogawa, H. , and Hagiwara, N. (2016) Randomized pilot trial comparing tolvaptan with furosemide on renal and neurohumoral effects in acute heart failure. ESC Heart Failure, 3: 177–188. doi: 10.1002/ehf2.12088.
The work presented in this manuscript was performed at Nishiarai Heart Center Hospital, 1‐12‐8 Nishiarai‐honcho, Adachi‐ku, Tokyo, Japan, 123‐0845.
The study was registered at the University Hospital Medical Information Network Clinical Trials Registry (UMIN‐CTR) with the identifier UMIN000014134. http://www.umin.ac.jp/ctr/index.htm
References
- 1. Jessup M, Brozena S. Heart failure. N Engl J Med 2003; 348: 2007–2018. [DOI] [PubMed] [Google Scholar]
- 2. Lloyd‐Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel‐Smoller S, Wong N, Wylie‐Rosett J, Hong Y, American Heart Association Statistics C, Stroke Statistics S . Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009; 119: 480–486. [DOI] [PubMed] [Google Scholar]
- 3. McMurray JJ, Pfeffer MA. Heart failure. Lancet 2005; 365: 1877–1889. [DOI] [PubMed] [Google Scholar]
- 4. Adams KF Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP, Committee ASA, Investigators . Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 2005; 149: 209–216. [DOI] [PubMed] [Google Scholar]
- 5. O'Connor CM, Hasselblad V, Mehta RH, Tasissa G, Califf RM, Fiuzat M, Rogers JG, Leier CV, Stevenson LW. Triage after hospitalization with advanced heart failure: the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) risk model and discharge score. J Am Coll Cardiol 2010; 55: 872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ross JS, Chen J, Lin Z, Bueno H, Curtis JP, Keenan PS, Normand SL, Schreiner G, Spertus JA, Vidan MT, Wang Y, Wang Y, Krumholz HM. Recent national trends in readmission rates after heart failure hospitalization. Circ Heart Fail 2010; 3: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gheorghiade M, Follath F, Ponikowski P, Barsuk JH, Blair JE, Cleland JG, Dickstein K, Drazner MH, Fonarow GC, Jaarsma T, Jondeau G, Sendon JL, Mebazaa A, Metra M, Nieminen M, Pang PS, Seferovic P, Stevenson LW, van Veldhuisen DJ, Zannad F, Anker SD, Rhodes A, McMurray JJ, Filippatos G, European Society of C , European Society of Intensive Care M . Assessing and grading congestion in acute heart failure: a scientific statement from the acute heart failure committee of the heart failure association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail 2010; 12: 423–433. [DOI] [PubMed] [Google Scholar]
- 8. Sarraf M, Masoumi A, Schrier RW. Cardiorenal syndrome in acute decompensated heart failure. Clin J Am Soc Nephrol 2009; 4: 2013–2026. [DOI] [PubMed] [Google Scholar]
- 9. Chen HH, Redfield MM, Nordstrom LJ, Cataliotti A, Burnett JC, Angiotensin II Jr. AT1 receptor antagonism prevents detrimental renal actions of acute diuretic therapy in human heart failure. Am J Physiol Renal Physiol 2003; 284: F1115–1119. [DOI] [PubMed] [Google Scholar]
- 10. Gottlieb SS, Brater DC, Thomas I, Havranek E, Bourge R, Goldman S, Dyer F, Gomez M, Bennett D, Ticho B, Beckman E, Abraham WT. BG9719 (CVT‐124), an A1 adenosine receptor antagonist, protects against the decline in renal function observed with diuretic therapy. Circulation 2002; 105: 1348–1353. [DOI] [PubMed] [Google Scholar]
- 11. Vallon V. Tubuloglomerular feedback and the control of glomerular filtration rate. News Physiol Sci 2003; 18: 169–174. [DOI] [PubMed] [Google Scholar]
- 12. Kubo SH, Clark M, Laragh JH, Borer JS, Cody RJ. Identification of normal neurohormonal activity in mild congestive heart failure and stimulating effect of upright posture and diuretics. Am J Cardiol 1987; 60: 1322–1328. [DOI] [PubMed] [Google Scholar]
- 13. Francis GS, Siegel RM, Goldsmith SR, Olivari MT, Levine TB, Cohn JN. Acute vasoconstrictor response to intravenous furosemide in patients with chronic congestive heart failure. Activation of the neurohumoral axis. Ann Intern Med 1985; 103: 1–6. [DOI] [PubMed] [Google Scholar]
- 14. Sarraf M, Schrier RW. Cardiorenal syndrome in acute heart failure syndromes. Int J Nephrol 2011; Mar 2; 2011: 293938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Forman DE, Butler J, Wang Y, Abraham WT, O'Connor CM, Gottlieb SS, Loh E, Massie BM, Rich MW, Stevenson LW, Young JB, Krumholz HM. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol 2004; 43: 61–67. [DOI] [PubMed] [Google Scholar]
- 16. Hata N, Yokoyama S, Shinada T, Kobayashi N, Shirakabe A, Tomita K, Kitamura M, Kurihara O, Takahashi Y. Acute kidney injury and outcomes in acute decompensated heart failure: evaluation of the RIFLE criteria in an acutely ill heart failure population. Eur J Heart Fail 2010; 12: 32–37. [DOI] [PubMed] [Google Scholar]
- 17. Shirakabe A, Hata N, Kobayashi N, Shinada T, Tomita K, Tsurumi M, Matsushita M, Okazaki H, Yamamoto Y, Yokoyama S, Asai K, Mizuno K. Long‐term prognostic impact after acute kidney injury in patients with acute heart failure. Int Heart J 2012; 53: 313–319. [DOI] [PubMed] [Google Scholar]
- 18. Shirakabe A, Hata N, Kobayashi N, Shinada T, Tomita K, Tsurumi M, Matsushita M, Okazaki H, Yamamoto Y, Yokoyama S, Asai K, Mizuno K. Prognostic impact of acute kidney injury in patients with acute decompensated heart failure. Circ J 2013; 77: 687–696. [DOI] [PubMed] [Google Scholar]
- 19. Shah RV, McNulty S, O'Connor CM, Felker GM, Braunwald E, Givertz MM. Effect of admission oral diuretic dose on response to continuous versus bolus intravenous diuretics in acute heart failure: an analysis from diuretic optimization strategies in acute heart failure. Am Heart J 2012; 164: 862–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chatterjee K. Neurohormonal activation in congestive heart failure and the role of vasopressin. Am J Cardiol 2005; 95: 8B–13B. [DOI] [PubMed] [Google Scholar]
- 21. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N Engl J Med 1987; 316: 1429–1435. [DOI] [PubMed] [Google Scholar]
- 22. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med 1991; 325: 293–302. [DOI] [PubMed] [Google Scholar]
- 23. The Cardiac Insufficiency Bisoprolol Study II (CIBIS‐II): a randomised trial. Lancet 1999; 353: 9–13. [PubMed] [Google Scholar]
- 24. Cohn JN, Tognoni G, Valsartan Heart Failure Trial I . A randomized trial of the angiotensin‐receptor blocker valsartan in chronic heart failure. N Engl J Med 2001; 345: 1667–1675. [DOI] [PubMed] [Google Scholar]
- 25. McMurray JJ, Ostergren J, Swedberg K, Granger CB, Held P, Michelson EL, Olofsson B, Yusuf S, Pfeffer MA, Investigators C, Committees . Effects of candesartan in patients with chronic heart failure and reduced left‐ventricular systolic function taking angiotensin‐converting‐enzyme inhibitors: the CHARM‐Added trial. Lancet 2003; 362: 767–771. [DOI] [PubMed] [Google Scholar]
- 26. Packer M, Fowler MB, Roecker EB, Coats AJ, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Staiger C, Holcslaw TL, Amann‐Zalan I, DeMets DL, Carvedilol Prospective Randomized Cumulative Survival Study G . Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation 2002; 106: 2194–2199. [DOI] [PubMed] [Google Scholar]
- 27. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341: 709–717. [DOI] [PubMed] [Google Scholar]
- 28. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013; 128: 1810–1852. [DOI] [PubMed] [Google Scholar]
- 29. Yamamura Y, Nakamura S, Itoh S, Hirano T, Onogawa T, Yamashita T, Yamada Y, Tsujimae K, Aoyama M, Kotosai K, Ogawa H, Yamashita H, Kondo K, Tominaga M, Tsujimoto G, Mori T. OPC‐41061, a highly potent human vasopressin V2‐receptor antagonist: pharmacological profile and aquaretic effect by single and multiple oral dosing in rats. J Pharmacol Exp Ther 1998; 287: 860–867. [PubMed] [Google Scholar]
- 30. Gheorghiade M, Niazi I, Ouyang J, Czerwiec F, Kambayashi J, Zampino M, Orlandi C, Tolvaptan I. Vasopressin V2‐receptor blockade with tolvaptan in patients with chronic heart failure: results from a double‐blind, randomized trial. Circulation 2003; 107: 2690–2696. [DOI] [PubMed] [Google Scholar]
- 31. Inomata T, Izumi T, Matsuzaki M, Hori M, Hirayama A, Tolvaptan I. Phase III clinical pharmacology study of tolvaptan. Cardiovasc Drugs Ther 2011; 25(Suppl 1): S57–65. [DOI] [PubMed] [Google Scholar]
- 32. Domanski M, Norman J, Pitt B, Haigney M, Hanlon S, Peyster E, Studies of Left Ventricular D . Diuretic use, progressive heart failure, and death in patients in the Studies Of Left Ventricular Dysfunction (SOLVD). J Am Coll Cardiol 2003; 42: 705–708. [DOI] [PubMed] [Google Scholar]
- 33. Ahmed A, Husain A, Love TE, Gambassi G, Dell'Italia LJ, Francis GS, Gheorghiade M, Allman RM, Meleth S, Bourge RC. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J 2006; 27: 1431–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kapelios CJ, Kaldara E, Ntalianis A, Sousonis V, Repasos E, Sfakianaki T, Vakrou S, Pantsios C, Nanas JN, Terrovitis JV. High furosemide dose has detrimental effects on survival of patients with stable heart failure. Hellenic J Cardiol 2015; 56: 154–159. [PubMed] [Google Scholar]
- 35. Udelson JE, Bilsker M, Hauptman PJ, Sequeira R, Thomas I, O'Brien T, Zimmer C, Orlandi C, Konstam MA. A multicenter, randomized, double‐blind, placebo‐controlled study of tolvaptan monotherapy compared to furosemide and the combination of tolvaptan and furosemide in patients with heart failure and systolic dysfunction. J Card Fail 2011; 17: 973–981. [DOI] [PubMed] [Google Scholar]
- 36. Bayliss J, Norell M, Canepa‐Anson R, Sutton G, Poole‐Wilson P. Untreated heart failure: clinical and neuroendocrine effects of introducing diuretics. Br Heart J 1987; 57: 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goldsmith SR, Bart BA, Burnett J. Decongestive therapy and renal function in acute heart failure: time for a new approach? Circ Heart Fail 2014; 7: 531–535. [DOI] [PubMed] [Google Scholar]
- 38. Scholz H, Vogel U, Kurtz A. Interrelation between baroreceptor and macula densa mechanisms in the control of renin secretion. J Physiol 1993; 469: 511–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Harris RC, Zhang MZ, Cheng HF. Cyclooxygenase‐2 and the renal renin–angiotensin system. Acta Physiol Scand 2004; 181: 543–547. [DOI] [PubMed] [Google Scholar]
- 40. Chen HH, Anstrom KJ, Givertz MM, Stevenson LW, Semigran MJ, Goldsmith SR, Bart BA, Bull DA, Stehlik J, LeWinter MM, Konstam MA, Huggins GS, Rouleau JL, O'Meara E, Tang WH, Starling RC, Butler J, Deswal A, Felker GM, O'Connor CM, Bonita RE, Margulies KB, Cappola TP, Ofili EO, Mann DL, Davila‐Roman VG, McNulty SE, Borlaug BA, Velazquez EJ, Lee KL, Shah MR, Hernandez AF, Braunwald E, Redfield MM, Network NHFCR . Low‐dose dopamine or low‐dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA 2013; 310: 2533–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Testani JM, Cappola TP, Brensinger CM, Shannon RP, Kimmel SE. Interaction between loop diuretic‐associated mortality and blood urea nitrogen concentration in chronic heart failure. J Am Coll Cardiol 2011; 58: 375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fenton RA. Essential role of vasopressin‐regulated urea transport processes in the mammalian kidney. Pflugers Arch 2009; 458: 169–177. [DOI] [PubMed] [Google Scholar]
- 43. Schrier RW. Blood urea nitrogen and serum creatinine: not married in heart failure. Circ Heart Fail 2008; 1: 2–5. [DOI] [PubMed] [Google Scholar]
- 44. Kazory A. Emergence of blood urea nitrogen as a biomarker of neurohormonal activation in heart failure. Am J Cardiol 2010; 106: 694–700. [DOI] [PubMed] [Google Scholar]
- 45. Bankir L, Bichet DG, Bouby N. Vasopressin V2 receptors, ENaC, and sodium reabsorption: a risk factor for hypertension? Am J Physiol Renal Physiol 2010; 299: F917–928. [DOI] [PubMed] [Google Scholar]
- 46. Gheorghiade M, Abraham WT, Albert NM, Gattis Stough W, Greenberg BH, O'Connor CM, She L, Yancy CW, Young J, Fonarow GC, Investigators O‐H, Coordinators . Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE‐HF registry. Eur Heart J 2007; 28: 980–988. [DOI] [PubMed] [Google Scholar]
- 47. Rusinaru D, Buiciuc O, Leborgne L, Slama M, Massy Z, Tribouilloy C. Relation of serum sodium level to long‐term outcome after a first hospitalization for heart failure with preserved ejection fraction. Am J Cardiol 2009; 103: 405–410. [DOI] [PubMed] [Google Scholar]
- 48. Sharma A, Mucino MJ, Ronco C. Renal functional reserve and renal recovery after acute kidney injury. Nephron Clin Pract 2014; 127: 94–100. [DOI] [PubMed] [Google Scholar]
- 49. Matsue Y, Kagiyama N, Yoshida K, Kume T, Okura H, Suzuki M, Matsumura A, Yoshida K, Hashimoto Y. Carperitide is associated with increased in‐hospital mortality in acute heart failure: a propensity score‐matched analysis. J Card Fail 2015; 21: 859–864. [DOI] [PubMed] [Google Scholar]
- 50. Sato N, Kajimoto K, Keida T, Mizuno M, Minami Y, Yumino D, Asai K, Murai K, Muanakata R, Aokage T, Sakata Y, Mizuno K, Takano T, Investigators T . Clinical features and outcome in hospitalized heart failure in Japan (from the ATTEND Registry). Circ J 2013; 77: 944–951. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Echocardiographic findings.
Table S2. Intravenous inotropic reagent use.
Table S3. Baseline patient characteristics classified by LVEF.
Table S4. Oral medications at discharge.
Figure S1. Water excretion was similar during intensive treatment between the furosemide and tolvaptan groups.
Figure S2. Tolvaptan preserved renal functions and neurohumoral parameters.
Figure S3. Tolvaptan increased serum sodium concentration during intensive treatment period.
Figure S3. In‐hospital and mid‐term prognoses were not significantly different between the furosemide and tolvaptan group.
Supporting info item


