Abstract
Aim
Although pedometer intervention is effective in increasing physical activity among adults with Type 2 diabetes, its impact on weight loss remains unclear. This meta‐analysis was aimed to assess whether pedometer intervention promotes weight loss.
Methods
Three different databases were searched for randomized controlled trials (RCTs) published in English up to April 2015. Studies were included if they investigated the effects of pedometer intervention on weight loss, as measured by BMI or weight. Effect sizes were aggregated using a random‐effects model. Subgroup and meta‐regression analyses were used to identify potential moderators. Eleven RCTs with 1258 participants were included. All enrolled participants were overweight or obese.
Results
Pedometer intervention led to significantly decreased BMI [weighted mean difference (WMD) −0.15 kg/m2, 95% confidence interval (CI) −0.29 to −0.02 kg/m2] and reduced weight (WMD −0.65 kg, 95% CI −1.12 to −0.17 kg). Dietary counselling seemed to be a key predictor of the observed changes. However, none of the following variables had a significant influence: step goal setting, baseline age, BMI, weight, sex distribution, disease duration, intervention duration, and baseline values or change scores for total or moderate‐to‐vigorous physical activity. After completion of the pedometer intervention, non‐significant declines in BMI and weight were observed during the follow‐up periods.
Conclusions
Pedometer intervention promotes modest weight loss, but its association with physical activity requires further clarification. Future studies are also required to document dietary and sedentary behaviour changes to facilitate the use of pedometers for weight loss in overweight and obese adults with Type 2 diabetes.
Introduction
Emerging evidence shows that there is a J‐shaped association between weight status as assessed by BMI and all‐cause mortality in adults with incident Type 2 diabetes 1. The evidence further points out that adults with Type 2 diabetes who were overweight or obese at diagnosis have higher mortality rates compared with their normal‐weight counterparts 1. Although intensive lifestyle intervention that promotes weight loss may not reduce the risk of cardiovascular morbidity or mortality 2, increasing evidence suggests that weight loss has clinically meaningful benefits in improving glycaemic control, lipid profiles, renal function, blood pressure and quality of life among adults with Type 2 diabetes 3, 4, 5, 6.
Pharmacological and surgical approaches are recognized as being highly effective in achieving substantial weight loss 7, 8, and dietary energy restriction is considered largely responsible for the initial weight loss in lifestyle‐intervention programmes 9, 10. However, regular physical activity, another important component of lifestyle intervention, remains a cornerstone in weight management 9, 11. In recent years, pedometer intervention designed for physical activity promotion and health improvement, including weight loss, has become increasingly popular among adults with Type 2 diabetes 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24. Although previous studies have clearly shown that pedometer intervention is remarkably effective in increasing physical activity among adults with Type 2 diabetes 24, 25, its effectiveness in losing weight remains poorly delineated. Moreover, although some studies pointed out that pedometer intervention is effective in decreasing BMI and reducing body weight, their analyses were conducted on highly heterogeneous populations 26, 27, making it questionable whether such conclusions could simply be transferred to adults with Type 2 diabetes. Furthermore, findings on weight loss through pedometer intervention are generally inconsistent across currently published randomized controlled trials (RCTs) 8, 12, 21, with most of showing no statistically significant reductions in weight 13, 15, 18, 19, 20, 23 and some having very limited statistical power due to the small sample sizes 13, 18.
In addition, preventing weight regain after successful weight loss interventions remains a great challenge 9, with poor adherence to increased or regular physical activity in the follow‐up periods one of the major contributing factors 28. Although there is evidence that the increased physical activity associated with pedometer intervention can be sustained in the follow‐up periods among adults with Type 2 diabetes 15, 20, it would be interesting to know whether a similar pattern for weight loss exists, given the important role of physical activity in weight management 9, 11.
Therefore, the primary objective of this meta‐analysis of RCTs was to investigate the impact of pedometer intervention on weight loss, as assessed by net BMI and weight changes in adults with Type 2 diabetes. The secondary objective was to evaluate whether pedometer intervention has a late effect on weight loss to prevent weight regain during the follow‐up periods.
Methods
This meta‐analysis was conducted and reported according to the outlines of the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement 29, and adhered to a prospectively registered protocol (PROSPERO CRD42015023178).
Data sources and search strategies
A structured and systemic literature search was conducted in the following databases up to 10 April 2015: PubMed, the Cochrane Central Register of Controlled Trials and Web of Science, using terms related to pedometer, Type 2 diabetes and RCTs (Table S1). In addition, reference lists of the retrieved relevant review article, systematic review and/or meta‐analysis were checked manually to search for more potentially suitable literature.
Study selection
Studies were included if: (1) all participants were adults (mean age ≥ 18 years) and had been diagnosed with Type 2 diabetes; (2) they received interventions using pedometers as motivational tools to increase unstructured activity (daily movement) with a minimum duration of 4 weeks, as suggested by Richardson et al. 27 and Hultquist et al. 30; (3) they were compared with control groups that did not receive any pedometer interventions or used pedometers only to record daily steps; (4) they reported outcomes assessing the effects of pedometer interventions on weight loss, as measured by BMI or weight 31 (primary outcome), or their sustained effects in the follow‐up periods after the completion of pedometer interventions (secondary outcome); and (5) they were RCTs and were published in English. Studies were excluded if the data of interest were not reported or could not be obtained after contacting the corresponding authors via emails. Studies were also excluded if they were not published in full‐texts (e.g. letters) 21, because of their limited information regarding the descriptions of the control and intervention details.
Data extraction and quality assessment
Following the literature search and the removal of duplicates, the titles, abstracts or full‐texts of the retrieved publications were reviewed to select potentially eligible studies based on the inclusion and exclusion criteria. Data were extracted using a structured form, which included general information on the included studies (authors and year of publication), characteristics of enrolled participants [sample sizes, sex distribution (proportion of women), disease duration and baseline mean age, weight, BMI and physical activity], details of pedometer interventions (intervention duration, follow‐up period after the completion of pedometer interventions, step goal setting and dietary counselling) and their respective controls, outcome variables of interest (net changes in BMI or weight from baseline) and some other data (dropout rates and countries of origin).
The methodological quality of each included RCT was evaluated using the Cochrane Collaboration's ‘Risk of Bias’ tool 32. This tool includes six items in general, which are random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias) and selective reporting (reporting bias). Based on the criteria for risk of bias judgement described in the Cochrane Handbook 32, each item was judged to be of low, unclear or high risk of bias.
Two researchers (S.Q. and X.C.) performed the literature selection, data extraction and quality assessment. Disagreements between researchers were resolved through discussion with a third researcher (U.S.) when they occurred.
Data synthesis and statistical analysis
Net mean change from baseline in BMI or weight (in kg) and its sd for each group from each study were calculated if they were not reported, using the formulas provided by Cochrane Handbook 32. Specifically, the sd of the mean change was imputed using a correlation coefficient as suggested 32, which was calculated to be 0.98 for both intervention and control groups based on the reported data 22. When a study reported data of BMI and/or weight for more than one time‐point, data from the last available time‐point were chosen. Finally, if one study compared more than one pedometer intervention with the same control group, these intervention groups were combined into one to overcome the unit‐of‐analysis error resulted from double counting of the participants in the ‘shared’ group 32.
All available data related to the primary or secondary outcome were pooled using a random‐effects model to assess the summary effect size estimates [i.e. weighted mean differences (WMDs)] with the corresponding 95% confidence intervals (95% CI) 32. Heterogeneity was examined using the Cochrane Q and I 2 statistics, with either the P‐value for the Cochrane Q statistic < 0.10 or I 2 ≥ 50% indicative of substantial heterogeneity. Subgroup analyses were conducted to examine the associations of pedometer intervention with weight loss on the basis of step goal setting (with vs. without), dietary counselling (with vs. without) and data analysis (intention‐to‐treat vs. per‐protocol analyses). Univariate weighted random‐effects meta‐regression analyses were also performed to identify the potential modifiers (source of heterogeneity) of BMI or weight changes based on the characteristics of participants and pedometer interventions. These modifiers included baseline mean age, weight, BMI, sex distribution and disease duration, pedometer intervention duration, as well as baseline and change scores for total physical and moderate‐to‐vigorous physical activity. Specifically, total physical activity was assessed using pedometers or accelerometers with the unit of steps/day, and moderate‐to‐vigorous physical activity was measured using accelerometers or questionnaires with the unit of minutes/day. Sensitivity analyses were carried out to assess the robustness of the findings by removing each individual study sequentially. Publication bias was assessed quantitatively using the Begg's rank correlation test and the Egger's asymmetry test, with either of the P < 0.10 considered to be significant.
The above analyses were conducted mainly on the primary outcome (that is, the immediate post‐intervention effect of pedometer intervention on weight loss), because it was later found that only two studies 15, 23 reported the secondary outcome (i.e. the late effect of pedometer intervention on weight loss in the follow‐up periods). All analyses were performed using STATA software (v. 12.0, College Station, Texas, USA) and Review Manager (v. 5.2, the Nordic Cochrane Centre, Copenhagen, Denmark). A two‐tailed P < 0.05 was considered statistically significant, unless otherwise stated.
Results
Of the 261 unique studies identified, a total of 11 RCTs 12, 13, 14, 15, 16, 17, 18, 19, 20, 22, 23 met the inclusion criteria and were included in the current meta‐analysis upon the removal of duplicate and the review of title, abstract and full‐text (Fig. 1). All the included RCTs (Table 1) were published as full paper articles between 2004 and 2013. The sample sizes of individual RCTs varied from 30 to 494 participants, and the mean ages of enrolled participants ranged from 49.0 to 68.3 years. All participants were overweight or obese, and had baseline mean BMI > 25.0 or 30.0 kg/m2. The durations of pedometer interventions lasted from 6 to 48 weeks. Of the included RCTs, seven clearly stated the use of step goals 13, 14, 15, 16, 18, 22, 23, while the other four failed to specify 12, 17, 19, 20. Besides, two RCTs provided dietary counselling in addition to pedometer intervention 12, 17, and two reported follow‐ups after the completion of pedometer intervention, assessing the sustained effect of pedometer intervention on weight loss 15, 23. The majority of the RCTs had been conducted in North American or European countries. Four RCTs had dropout rates > 20% 14, 17, 20, 22. The methodological quality of included RCTs was low to moderate in general. Among them, six did not describe the methods of randomization 13, 14, 17, 18, 22, 23, and five used per‐protocol analyses rather than intention‐to‐treat analyses 14, 17, 18, 20, 22, leading to the potentially high risk of attrition bias of these studies (Table S2).
Figure 1.
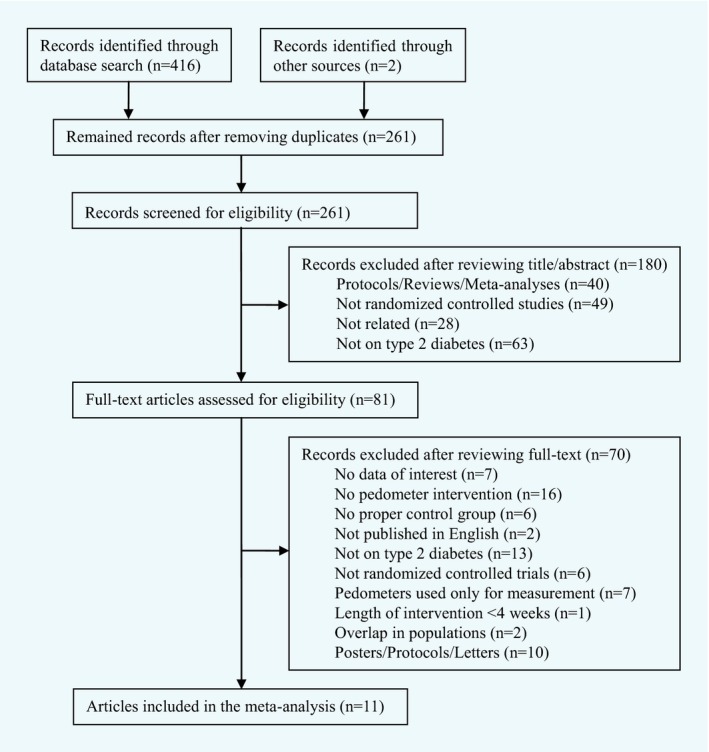
Flowchart of included studies.
Table 1.
Characteristics of each included RCT
| Source | Characteristics of participantsa | Intervention and control groups | Intervention details | |||||
|---|---|---|---|---|---|---|---|---|
| Sample sizeb | Age, year | BMI, kg/m2 | Weight, kg | Duration, weeks | Dietary advice | Step goal | ||
| Andrews et al., 2011 12 | 246 | 60.0 | 31.6 | 91.1 | Intervention: received pedometer‐based walking interventions and dietary consultation with nurse support. | 48 | With | NS |
| 248 | 60.1 | 31.5 | 90.2 | Control: received dietary consultation with nurse support. | ||||
| Araiza et al., 2006 13 | 15 | 49 | 30.0 | NA | Intervention: received pedometer‐based walking interventions. | 6 | Without | Yes |
| 15 | 51 | 33.5 | NA | Control: were asked to maintain normal activity habits. | ||||
| Bjorgaas et al., 2008 14 | 23 | 56.4 | 31.2 | 94.8 | Intervention: received pedometers and were encouraged to increase daily time on walking. | 24 | Without | Yes |
| 25 | 61.2 | 31.5 | 95.2 | Control: were encouraged to increase daily time on walking. | ||||
| De Greef et al., 2010c 15 | 20 | 61.3 | 29 | 83.5 | Intervention: received cognitive–behavioural pedometer‐based interventions. | 12 | Without | Yes |
| 21 | 61.3 | 31.5 | 92.6 | Control: received usual care. | ||||
| De Greef et al., 2011d 16 | 43 | 68.3 | 29.7 | NA | Intervention: received pedometer‐based physical activity programmes with individual or group counselling. | 12 | Without | Yes |
| 24 | 66.0 | 31.5 | NA | Control: received general care. | ||||
| Diedrich et al., 2010 17 | 16 | 56.7 | NA | 94.8 | Intervention: received pedometer‐based programmes, books (Manpo‐kei) and usual diabetes education. | 12 | With | NS |
| 17 | 54.9 | NA | 107.1 | Control: received usual diabetes education. | ||||
| Engel and Linder 2006 18 | 22 | 60.5 | 32.7 | 91.9 | Intervention: received pedometers and health‐related coaching. | 24 | Without | Yes |
| 28 | 64 | 31.2 | 84.9 | Control: only received health‐related coaching. | ||||
| Kirk et al., 2009d 19 | 99 | 62.1 | 32.8 | NA | Intervention: received pedometers, physical activity consultation and telephone call. | 48 | Without | NS |
| 35 | 59.2 | 34.9 | NA | Control: received standard care and telephone call. | ||||
| Plotnikoff et al., 2013d 20 | 139 | 61.8 | 30.2 | NA | Intervention: received pedometers, theory‐based behavioural interventions and physical activity education. | 48 | Without | NS |
| 83 | 61.0 | 30.2 | NA | Control: received standard physical activity education. | ||||
| Tudor‐Locke et al., 2004 22 | 24 | 52.8 | 34.1 | 96.8 | Intervention: received pedometers with instructions for goal‐setting and motivational postcards. | 16 | Without | Yes |
| 23 | 52.5 | 32.5 | 92.3 | Control: only received motivational postcards for thanks. | ||||
| Van Dyck et al., 2013c 23 | 60 | 62 | 30.2 | 89.2 | Intervention: received pedometer‐based physical activity interventions with telephone support. | 24 | Without | Yes |
| 32 | 29.7 | 84.5 | Control: received usual care. | |||||
Data for age, BMI, weight were imputed using baseline mean values.
Number of participants included in the per‐protocol or intention‐to‐treat analyses.
Reported follow‐up data of BMI and weight after the completion of pedometer interventions.
Included two intervention groups and both were combined into one group.
NS, not specified; NA, not applicable.
Immediate post‐intervention effect of pedometer intervention on weight loss
Eight RCTs with 1130 participants reported immediate post‐intervention outcomes on BMI related to pedometer intervention 12, 13, 15, 16, 18, 19, 20, 23, and seven RCTs enrolling 805 participants reported such outcomes on weight 13, 14, 15, 17, 18, 22, 23. The mean net BMI (BMI post‐intervention minus pre‐intervention) was −0.14 kg/m2 (sd 0.37 kg/m2) for control groups and −0.3 kg/m2 (sd 0.46 kg/m2) for pedometer interventions. The mean net weight (weight post‐intervention minus pre‐intervention) was −1.12 kg (sd 1.34 kg) for control groups and −1.34 kg (sd 1.38 kg) for pedometer interventions. The meta‐analyses showed that pedometer intervention was associated with a significant decrease in BMI (WMD −0.15 kg/m2, 95% CI −0.29 to −0.02 kg/m2; I 2 = 18.7%, P for heterogeneity = 0.28; Fig. 2), and a significant reduction in weight (WMD −0.65 kg, 95% CI −1.12 to −0.17 kg; I 2 <1%, P for heterogeneity = 0.80; Fig. 3) when compared with controls.
Figure 2.
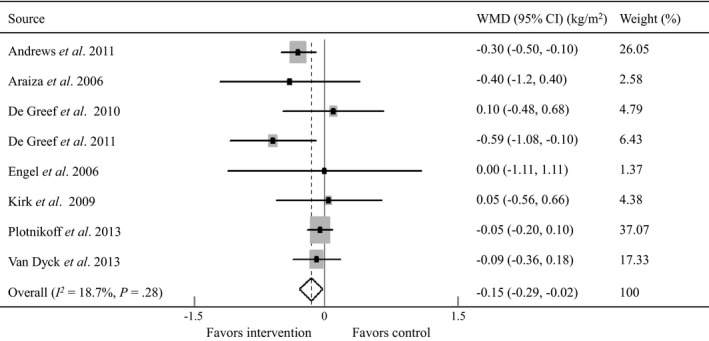
Forest plot for net changes in BMI using a random‐effects model. WMD, weighted mean difference; CI, confidence interval. Andrews et al. 12, Araiza et al.13, De Greef et al. 15, 16, Engel and Linder 18, Kirk et al. 19, Plotnikoff et al. 20, Van Dyck et al. 23.
Figure 3.
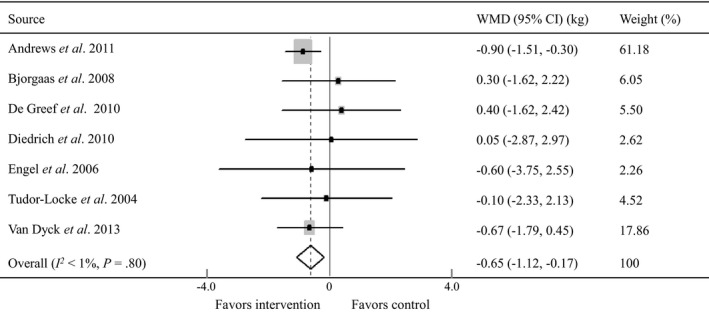
Forest plot for net changes in weight using a random‐effects model. WMD, weighted mean difference; CI, confidence interval. Andrews et al. 12, Bjorgaas et al. 14, De Greef et al. 15, Diedrich et al. 17 Engel and Linder 18, Tudor‐Locke et al. 22, Van Dyck et al. 23.
Subgroup analyses showed that pedometer intervention with dietary counselling resulted in significant declines in BMI (WMD −0.30 kg/m2, 95% CI −0.50 to −0.10 kg/m2) and weight (WMD −0.86 kg, 95% CI −1.45 to −0.27 kg) compared with controls who received dietary counselling. However, pedometer intervention alone showed only some trends towards decreased BMI and reduced weight compared with controls lacking dietary counselling (Table 2). Step goal setting did not predict any significant changes in BMI or weight (Table 2). Interestingly, RCTs employing intention‐to‐treat analyses showed that pedometer intervention significantly decreased BMI (WMD −0.22 kg/m2, 95% CI −0.39 to −0.05 kg/m2) and reduced weight (WMD −0.77 kg, 95% CI −1.28 to −0.25 kg). Meta‐regression analyses revealed that none of the variables described in the Methods section significantly influenced the BMI or weight outcome related to pedometer intervention (Table 3). Sensitivity analyses by excluding one RCT at a time showed that both BMI and weight outcomes were not substantially affected by any particular study in general. No evidence of publication bias was detected for BMI outcome (Begg's test, P = 0.71; Egger's test, P = 0.70), while some minor bias was observed for weight outcome using Egger's test (P = 0.02) but not using Begg's test (P = 0.76).
Table 2.
Subgroup analyses for BMI and weight
| Subgroups | BMI | Weight | ||||
|---|---|---|---|---|---|---|
| No. studies (subjects) | Effect size (kg/m2) | No. studies (subjects) | Effect size (kg) | |||
| WMD | 95% CI | WMD | 95% CI | |||
| Step goal use | ||||||
| With | 5 (280) | −0.18 | −0.41 to 0.05 | 5 (278) | −0.27 | −1.06 to 0.52 |
| Without | 3 (850) | −0.14 | −0.34 to 0.06 | 2 (527) | −0.86 | −1.45 to −0.27 |
| Dietary counselling | ||||||
| With | 1 (494) | −0.30 | −0.50 to −0.10 | 2 (527) | −0.86 | −1.45 to −0.27 |
| Without | 7 (636) | −0.09 | −0.20 to 0.03 | 5 (278) | −0.27 | −1.06 to 0.52 |
| Data analysing | ||||||
| ITT analysis | 6 (858) | −0.22 | −0.39 to −0.05 | 3 (627) | −0.77 | −1.28 to −0.25 |
| Per‐protocol analysis | 2 (272) | −0.05 | −0.20 to 0.10 | 4 (178) | 0.01 | −1.12 to 1.21 |
Table 3.
Univariate weighted meta‐regression analyses for BMI and weight
| Variables | BMI | Weight | ||||
|---|---|---|---|---|---|---|
| No. of studies | Coefficient | P | No. of studies | Coefficient | P | |
| Baseline mean agea | 8 | −0.24 | 0.89 | 7 | −4.15 | 0.57 |
| Sex distributionb | 7 | 0.01 | 0.21 | 7 | 0.02 | 0.64 |
| Baseline BMI/weight | 8 | −0.11 | 0.19 | 7 | 0.05 | 0.60 |
| Disease duration | 4 | −0.005 | 0.96 | 4 | −0.03 | 0.40 |
| Baseline total PAc | 6 | 0.00007 | 0.54 | 3 | 0.008 | 0.53 |
| Changes in total PAc | 6 | −0.0001 | 0.36 | 3 | 0.001 | 0.54 |
| Baseline MVPAd | 4 | −0.02 | 0.33 | NA | NA | NA |
| Changes in MVPAd | 4 | −0.04 | 0.13 | NA | NA | NA |
| Intervention duration | 8 | 0.003 | 0.62 | 7 | −0.03 | 0.21 |
Age data were log‐transformed.
It represented the proportion of women.
Physical activity was assessed using pedometers or accelerometers with the unit of steps per day.
MVPA was assessed using accelerometers (objective methods) in two studies 12, 15 and questionnaires (subjective methods) in the other two studies 16, 20.
PA, physical activity; MVPA, moderate‐to‐vigorous physical activity; NA, not applicable.
Late effect of pedometer intervention on weight loss in follow‐up periods
Two RCTs involving 133 participants reported follow‐up outcomes on both BMI and weight after the completion of pedometer intervention 15, 23, with a mean follow‐up period of 33.5 weeks (Table 1). During this period, one RCT utilized a booster session aiming to help participants in the pedometer intervention group to increase self‐efficacy and set up long‐term action plans 15, and the other had no further intervention 23. The meta‐analyses showed that participants who received pedometer intervention exhibited some, albeit non‐significant declines in BMI (WMD −0.21 kg/m2, 95% CI −1.06 to 0.65 kg/m2) and weight (WMD −0.05 kg, 95% CI −1.06 to 0.95 kg) compared with controls who did not receive such intervention during the follow‐up periods.
Discussion
Summary
To our knowledge, this is the first meta‐analysis of RCTs addressing the impact of pedometer intervention on BMI and weight among adults with Type 2 diabetes; in particular, among those who are overweight or obese. This study provides evidence that pedometer intervention led to modestly but significantly decreased BMI and reduced weight compared with controls, and dietary counselling seemed to be a key predictor of these changes. However, we did not find clear evidence that these changes were likely to be moderated by baseline age, BMI or weight, sex distribution, disease duration, intervention duration, baseline values or change scores of total or moderate‐to‐vigorous physical activity, as well as the presence of step goal setting. Moreover, the evidence remains insufficient regarding the late or the sustained effects of pedometer intervention in losing weight or preventing weight regain in the follow‐up periods due to the limited number of studies.
Interpretations
The main findings of our study are generally consistent with those observed in the previous systematic review and meta‐analysis, which reported comparable modest declines in BMI (WMD −0.38 kg/m2, 95% CI −0.72 to −0.05 kg/m2) and weight (WMD −1.27 kg, 95% CI −1.85 to −0.70 kg) associated with pedometer intervention 26, 27. However, one should keep in mind that both studies failed to specify these effects among a particular population such as patients with Type 2 diabetes, who are more likely to be overweight or obese 1 and might show greater difficulties in losing weight compared with the population without diabetes 33, 34. Moreover, they did not restrict studies to RCTs, lowering their degree of generalizability and subsequently the level of evidence. In addition, Bravata et al. found that having a step goal and older age were key predictors of reduced BMI among pedometer users 26, and Richardson et al. observed that longer duration of pedometer intervention was associated with more weight reduction 27. However, in contrast to their findings, our subgroup and meta‐regression analyses did not identify these moderators. It seems likely that the different target populations and the different study selection criteria might largely contribute to these discrepancies. There is some evidence suggesting that compared with pedometer intervention alone, participants would achieve more weight loss by adding an additional dietary component, such as dietary counselling, which could reinforce the implementation of individual dietary management 27. Partly in support of this, our indirect comparison showed that pedometer intervention in conjunction with dietary counselling seemed to yield more reductions in BMI and weight than pedometer intervention alone, although these results were found to be statistically non‐significant. Yet it is important to acknowledge that such reductions might be underestimated, because the control group used in the subgroup analysis of the combined effects of pedometer intervention with dietary counselling on weight loss received dietary counselling, while the one in another subgroup analysis did not. Therefore, more RCTs with head‐to‐head study designs are required in the future to address the additional effects of dietary counselling in pedometer users. Our meta‐regression analyses also showed that the modest weight loss related to pedometer intervention seemed to be non‐significantly associated with the baseline values or the change scores of total physical activity (presented as steps/day), which is in agreement with the results reported in the previous study 26. Given the accumulating evidence that moderate‐to‐vigorous physical activity leads to reduced weight regardless of its amount 35, 36, one possible explanation for our findings is that the intensity of physical activity seems to be essential in the weight management. However, meta‐regression analyses from our meta‐analysis did not show adequate evidence that moderate‐to‐vigorous physical activity was related to the weight loss associated with pedometer use. This is likely due to the limited power of meta‐regression analysis to detect the significant moderator, since our meta‐regression analyses were conducted using the averages of patient characteristics for each RCT rather than the individual patient data. Moreover, the potential heterogeneity of the methods used to assess the moderate‐to‐vigorous physical activity (objective vs. subjective methods 37, Table 3) might also lead to the inconsistency.
There are some other explanations for the observed declines in BMI and weight resulting from pedometer intervention among overweight and obese adults with Type 2 diabetes. It is well documented that participants with Type 2 diabetes who received pedometer intervention would become more active than those who did not receive such intervention 24, 38. Given the fact that physically active participants are more likely to have better healthy eating index scores 39, lower fat intake, and more dietary restraint compared with those who are physically inactive 40, it is plausible that pedometer intervention would lead to subsequent weight loss due changes in dietary behaviour. However, none of the included studies assessed such change. In addition to that, sedentary behaviour change might be another possible explanation for our findings, because pedometer use results in significantly reduced sedentary time 12, 41, which is shown to be associated with decreased BMI 42, 43. However, only two of the included studies recorded sedentary behaviour change using accelerometers, and both failed to investigate this relationship 12, 15.
It should be also mentioned that these declines in BMI and body weight were modest, which might be due in part to the fact that none of the included studies was initially or specifically designed to assess the effects of pedometer interventions on weight loss. In addition, participants with Type 2 diabetes showed somehow poor compliance with the pedometer invention programmes, with adherence rates of only around 80% 15, 20, 22 together with high dropout rates of > 20% 14, 17, 20, 22. Moreover, it is well‐recognized that diabetes medication, such as metformin, sulfonylureas, insulin and glucagon‐like peptide–1 receptor agonists, potentially affect body weight; however, almost all included studies failed to assess such confounding effects following pedometer interventions. Furthermore, as indicated by our subgroup analyses (intention‐to‐treat vs. per‐protocol analyses), the approaches for handling missing data could also influence the final outcomes. Therefore, future research is required to address these concerns.
In addition to weight loss, preventing weight regain after successful weight loss is another key goal for a weight management programme 9. Although our study did not find that pedometer intervention had a sustained effect in maintaining weight loss in the follow‐up periods after the completion of the intervention, there is some evidence that pedometer intervention may have a late effect in preventing weight regain for patients with Type 2 diabetes. However, because of the limited number of studies included (only two), this finding was likely to be underestimated and deserves further attention. Consequently, more research is required on this topic. Moreover, in order to achieve further weight loss or maintain weight loss in the follow‐up periods after pedometer intervention, studies that provide ongoing support, such as encouraging participants to use pedometers continuously or take part in telephone‐ 44 or web‐based lifestyle interventions 45, should be given priority.
Strengths and limitations
Our study has some strengths, which include the use of a pre‐specified protocol and the inclusion of only RCTs. In addition, because only one of the eight RCTs assessing changes in BMI reported statistically significant results 16, this highlights the superiority of a meta‐analysis in identifying the important summary estimate with increased and improved statistical power (e.g. large sample size) and the necessity to conduct a meta‐analysis in order to obtain stringent evidence.
Our study also has several limitations. First, despite undetected publication bias by the Begg's test or the Egger's test, there remains some possibility of this bias because of the unsearched ‘grey literature’ (e.g. dissertation) and the language restriction to English. Second, although this study did not find any significant moderators using meta‐regression analyses, one should be aware of the possibility that meta‐regression analyses may have limited power to detect these moderators. Third, the robustness of our findings from the meta‐analysis might be weakened because of the selection bias that resulted from the enrolled participants who were all overweight or obese rather than a clinically representative population with Type 2 diabetes. Moreover, the robustness might be further weakened since there was some evidence of publication bias for the meta‐analysis of weight. Fourth, the methodological quality of some included RCTs was judged to be low due to the attrition bias resulting from their per‐protocol analyses, and this will lower the level of the current evidence obtained. Fifth, all included RCTs were conducted in high‐income countries. It remains unknown whether these findings could be used as guides for participants from low‐ or middle‐income nations to use pedometers. Sixth, although BMI and weight are well‐recognized markers for assessing weight loss, it might be also useful to choose some other markers such as waist circumference and waist‐to‐hip ratio for analysis. However, our meta‐analysis failed to do that. Finally, despite some observed weight loss related to pedometer intervention, it is of great interest to investigate whether there are any changes in body composition, such as body fat percentages or lean body mass. However, very few studies evaluated these changes 12, 17, limiting the further exploration by using meta‐analytical approaches consequently. Therefore, future studies are worth being conducted on this topic.
Conclusion
In conclusion, pedometer intervention is a promising approach for promoting weight loss in overweight and obese adults with Type 2 diabetes that modestly reduces BMI and weight. In order to better understand the association between pedometer intervention and weight loss, future studies are required to document the changes in physical activity, dietary behaviour and sedentary time, as well as to investigate changes in body composition. Furthermore, future studies are also required to provide ongoing support after pedometer intervention to maintain weight loss, and to analyse the cost‐effectiveness of pedometer intervention for its better promotion, given the fact that pedometer is inexpensive, but its use only leads to a modest weight loss.
Funding sources
None.
Competing interests
None declared.
Supporting information
Table S1. Search strategies.
Table S2. Bias assessment of each randomized controlled trials.
Diabet. Med. 33, 1035–1044 (2016)
References
- 1. Tobias DK, Pan A, Jackson CL, O'Reilly EJ, Ding EL, Willett WC et al Body‐mass index and mortality among adults with incident type 2 diabetes. N Engl J Med 2014; 370: 233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Look ARG, Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM et al Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013; 369: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Espeland MA, Rejeski WJ, West DS, Bray GA, Clark JM, Peters AL et al Intensive weight loss intervention in older individuals: results from the Action for Health in Diabetes Type 2 diabetes mellitus trial. J Am Geriatr Soc 2013; 61: 912–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jesudason DR, Pedersen E, Clifton PM. Weight‐loss diets in people with type 2 diabetes and renal disease: a randomized controlled trial of the effect of different dietary protein amounts. Am J Clin Nutr 2013; 98: 494–501. [DOI] [PubMed] [Google Scholar]
- 5. Stevens VJ, Obarzanek E, Cook NR, Lee IM, Appel LJ, Smith West D et al Long‐term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med 2001; 134: 1–11. [DOI] [PubMed] [Google Scholar]
- 6. Williamson DA, Rejeski J, Lang W, Van Dorsten B, Fabricatore AN, Toledo K et al Impact of a weight management program on health‐related quality of life in overweight adults with type 2 diabetes. Arch Intern Med 2009; 169: 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rucker D, Padwal R, Li SK, Curioni C, Lau DC. Long term pharmacotherapy for obesity and overweight: updated meta‐analysis. BMJ 2007; 335: 1194–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dixon JB, O'Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S et al Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008; 299: 316–323. [DOI] [PubMed] [Google Scholar]
- 9. Klein S, Sheard NF, Pi‐Sunyer X, Daly A, Wylie‐Rosett J, Kulkarni K et al Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care 2004; 27: 2067–2073. [DOI] [PubMed] [Google Scholar]
- 10. Norris SL, Zhang X, Avenell A, Gregg E, Brown TJ, Schmid CH et al Long‐term non‐pharmacologic weight loss interventions for adults with type 2 diabetes. Cochrane Database Syst Rev 2005; CD004095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qiu S, Cai X, Schumann U, Velders M, Sun Z, Steinacker JM. Impact of walking on glycemic control and other cardiovascular risk factors in type 2 diabetes: a meta‐analysis. PLoS One 2014; 9: e109767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Andrews RC, Cooper AR, Montgomery AA, Norcross AJ, Peters TJ, Sharp DJ et al Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the Early ACTID randomised controlled trial. Lancet 2011; 378: 129–139. [DOI] [PubMed] [Google Scholar]
- 13. Araiza P, Hewes H, Gashetewa C, Vella CA, Burge MR. Efficacy of a pedometer‐based physical activity program on parameters of diabetes control in type 2 diabetes mellitus. Metabolism 2006; 55: 1382–1387. [DOI] [PubMed] [Google Scholar]
- 14. Bjorgaas MR, Vik JT, Stolen T, Lydersen S, Grill V. Regular use of pedometer does not enhance beneficial outcomes in a physical activity intervention study in type 2 diabetes mellitus. Metabolism 2008; 57: 605–611. [DOI] [PubMed] [Google Scholar]
- 15. De Greef K, Deforche B, Tudor‐Locke C, De Bourdeaudhuij I. A cognitive–behavioural pedometer‐based group intervention on physical activity and sedentary behaviour in individuals with type 2 diabetes. Health Educ Res 2010; 25: 724–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Greef K, Deforche B, Tudor‐Locke C, De Bourdeaudhuij I. Increasing physical activity in Belgian type 2 diabetes patients: a three‐arm randomized controlled trial. Int J Behav Med 2011; 18: 188–198. [DOI] [PubMed] [Google Scholar]
- 17. Diedrich A, Munroe DJ, Romano M. Promoting physical activity for persons with diabetes. Diabetes Educ 2010; 36: 132–140. [DOI] [PubMed] [Google Scholar]
- 18. Engel L, Lindner H. Impact of using a pedometer on time spent walking in older adults with type 2 diabetes. Diabetes Educ 2006; 32: 98–107. [DOI] [PubMed] [Google Scholar]
- 19. Kirk A, Barnett J, Leese G, Mutrie N. A randomized trial investigating the 12‐month changes in physical activity and health outcomes following a physical activity consultation delivered by a person or in written form in Type 2 diabetes: Time2Act. Diabet Med 2009; 26: 293–301. [DOI] [PubMed] [Google Scholar]
- 20. Plotnikoff RC, Karunamuni N, Courneya KS, Sigal RJ, Johnson JA, Johnson ST. The Alberta Diabetes and Physical Activity Trial (ADAPT): a randomized trial evaluating theory‐based interventions to increase physical activity in adults with type 2 diabetes. Ann Behav Med 2013; 45: 45–56. [DOI] [PubMed] [Google Scholar]
- 21. Tubili C, Di Flaviani A, Morviducci L, Nardone R, Altieri N. Pedometer use is beneficial for type 2 diabetes mellitus patients if included in educational programs. Metabolism 2010; 59: E1–2; author reply E3–4. [DOI] [PubMed] [Google Scholar]
- 22. Tudor‐Locke C, Bell RC, Myers AM, Harris SB, Ecclestone NA, Lauzon N et al Controlled outcome evaluation of the First Step Program: a daily physical activity intervention for individuals with type II diabetes. Int J Obes Relat Metab Disord 2004; 28: 113–119. [DOI] [PubMed] [Google Scholar]
- 23. Van Dyck D, De Greef K, Deforche B, Ruige J, Bouckaert J, Tudor‐Locke CE et al The relationship between changes in steps/day and health outcomes after a pedometer‐based physical activity intervention with telephone support in type 2 diabetes patients. Health Educ Res 2013; 28: 539–545. [DOI] [PubMed] [Google Scholar]
- 24. Qiu S, Cai X, Chen X, Yang B, Sun Z. Step counter use in type 2 diabetes: a meta‐analysis of randomized controlled trials. BMC Med 2014; 12: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Funk M, Taylor EL. Pedometer‐based walking interventions for free‐living adults with type 2 diabetes: a systematic review. Curr Diabetes Rev 2013; 9: 462–471. [DOI] [PubMed] [Google Scholar]
- 26. Bravata DM, Smith‐Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R et al Using pedometers to increase physical activity and improve health: a systematic review. JAMA 2007; 298: 2296–2304. [DOI] [PubMed] [Google Scholar]
- 27. Richardson CR, Newton TL, Abraham JJ, Sen A, Jimbo M, Swartz AM. A meta‐analysis of pedometer‐based walking interventions and weight loss. Ann Fam Med 2008; 6: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Elfhag K, Rossner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev 2005; 6: 67–85. [DOI] [PubMed] [Google Scholar]
- 29. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P, Altman D et al Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. J Clin Epidemiol 2009; 62: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 30. Hultquist CN, Albright C, Thompson DL. Comparison of walking recommendations in previously inactive women. Med Sci Sports Exerc 2005; 37: 676–683. [DOI] [PubMed] [Google Scholar]
- 31. Drager LF, Brunoni AR, Jenner R, Lorenzi‐Filho G, Bensenor IM, Lotufo PA. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta‐analysis of randomised trials. Thorax 2015; 70: 258–264. [DOI] [PubMed] [Google Scholar]
- 32. The Cochrane Collaboration . Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Available at: http://www.cochrane-handbook.org Last accessed 1 January 2016.
- 33. Wing RR, Koeske R, Epstein LH, Nowalk MP, Gooding W, Becker D. Long‐term effects of modest weight loss in type II diabetic patients. Arch Intern Med 1987; 147: 1749–1753. [PubMed] [Google Scholar]
- 34. Lau DC, Teoh H. Benefits of modest weight loss on the management of type 2 diabetes mellitus. Can J Diabetes 2013; 37: 128–134. [DOI] [PubMed] [Google Scholar]
- 35. Slentz CA, Duscha BD, Johnson JL, Ketchum K, Aiken LB, Samsa GP et al Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE – a randomized controlled study. Arch Intern Med 2004; 164: 31–39. [DOI] [PubMed] [Google Scholar]
- 36. Palakodeti S, Uratsu CS, Schmittdiel JA, Grant RW. Changes in physical activity among adults with diabetes: a longitudinal cohort study of inactive patients with Type 2 diabetes who become physically active. Diabet Med 2015; 32: 1051–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dyrstad SM, Hansen BH, Holme IM, Anderssen SA. Comparison of self‐reported versus accelerometer‐measured physical activity. Med Sci Sports Exerc 2014; 46: 99–106. [DOI] [PubMed] [Google Scholar]
- 38. Yamanouchi K, Shinozaki T, Chikada K, Nishikawa T, Ito K, Shimizu S et al Daily walking combined with diet therapy is a useful means for obese NIDDM patients not only to reduce body weight but also to improve insulin sensitivity. Diabetes Care 1995; 18: 775–778. [DOI] [PubMed] [Google Scholar]
- 39. Monfort‐Pires M, Salvador EP, Folchetti LD, Siqueira‐Catania A, Barros CR, Ferreira SRG. Diet quality is associated with leisure‐time physical activity in individuals at cardiometabolic risk. J Am Coll Nutr 2014; 33: 297–305. [DOI] [PubMed] [Google Scholar]
- 40. Catenacci VA, Odgen L, Phelan S, Thomas JG, Hill J, Wing RR et al Dietary habits and weight maintenance success in high versus low exercisers in the National Weight Control Registry. J Phys Act Health 2014; 11: 1540–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Qiu S, Cai X, Ju C, Sun Z, Yin H, Zugel M et al Step counter use and sedentary time in adults: a meta‐analysis. Medicine 2015; 94: e1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Helmink JH, Kremers SP, van Brussel‐Visser FN, de Vries NK. Sitting time and Body Mass Index in diabetics and pre‐diabetics willing to participate in a lifestyle intervention. Int J Environ Res Public Health 2011; 8: 3747–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stamatakis E, Hamer M, Tilling K, Lawlor DA. Sedentary time in relation to cardio‐metabolic risk factors: differential associations for self‐report vs accelerometry in working age adults. Int J Epidemiol 2012; 41: 1328–1337. [DOI] [PubMed] [Google Scholar]
- 44. Liu F, Kong X, Cao J, Chen S, Li C, Huang J et al Mobile phone intervention and weight loss among overweight and obese adults: a meta‐analysis of randomized controlled trials. Am J Epidemiol 2015; 181: 337–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kodama S, Saito K, Tanaka S, Horikawa C, Fujiwara K, Hirasawa R et alEffect of Web‐based lifestyle modification on weight control: a meta‐analysis. Int J Obes (Lond) 2012; 36: 675–685. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Search strategies.
Table S2. Bias assessment of each randomized controlled trials.


