Summary
Aim
The aim of this study was to assess the effects of ursodeoxycholic acid composite (URSA‐S) on fatigue in patients with elevated liver function tests and/or fatty liver disease.
Methods
In this multi‐centre randomised double‐blinded placebo‐controlled trial, 168 adults who were diagnosed with fatigue based on our criteria and had elevated liver function tests (but not > 5 times the normal level) and/or fatty liver on ultrasonography, were randomised to either the placebo or URSA‐S administration group. The rate of improvement of checklist individual strength (CIS) using a cut‐off of 76 points at the end of the study (8 weeks), the change in fatigue scale [CIS score and visual analogue scale (VAS)] were evaluated. The adverse effects of URSA‐S were also recorded.
Results
The rate of CIS improvement at the end‐point was 79.76% and 45.68% in the therapy and placebo groups, respectively (p < 0.05). The fatigue recovery rate of the CIS score and VAS were higher in the therapy (−25.44 ± 18.57, −27.84 ± 2.70) than in the placebo group (−16.59 ± 17.29, −19.46 ± 2.81) (p < 0.05). The difference in fatigue recovery rate between the therapy and placebo groups was significant after 8 weeks. When analysed separately in patients with abnormal liver function tests and fatty liver disease, the fatigue recovery rate of the CIS score and VAS at 8 weeks was higher in the therapy than in the placebo group (p < 0.05). The frequency of adverse events in the therapy group was not significantly higher than that in the placebo group.
Conclusion
URSA‐S is effective for alleviating fatigue in patients with liver dysfunction and/or fatty liver. The adverse effects of URSA‐S are not significant. This study is registered at https://clinicaltrials.gov/ct2/show/ NCT02415777.
Review criteria
We gathered the information for the review via research database (such as PubMed, Ovid, Medline) and search engine (such as Google©). Systemic review such as meta‐analysis, RCT articles were considered more important than the others.
Message for the clinic
Urosodeoxycholic acid composite can be prescribed to alleviate fatigue in patients with abnormal liver function tests (but less than five times of upper limits) and/or fatty liver disease. It should be taken for 8 weeks to be effective.
Introduction
Fatigue is defined as an overwhelming sense of tiredness, lack of energy and feeling of exhaustion 1. Fatigue is highly prevalent in various disorders and can have a negative impact on the quality‐of‐life and physical performance. Fatigue is considered as the bodily experience of exhaustion following strenuous physical effort. Mental or central fatigue is the subjective self‐reported feeling of fatigue 2, 3. Several studies have demonstrated that energy metabolism is involved in the pathophysiology of fatigue. Various supplements and nutraceuticals have been used to relieve fatigue. However, only a few studies have evaluated the efficacy and safety of drugs for alleviating fatigue in patients with abnormal liver function and/or fatty liver diseases.
URSA‐S comprises 50 mg of ursodeoxycholic acid (UDCA), 10 mg of thiamine nitrate, and 5 mg of riboflavin. It is used for the treatment of cholestatic liver diseases, gallstone and fatty liver, as well as among patients with hepatitis virus infection to ameliorate the elevated alanine aminotransferase levels in East Asia 4, 5, 6. URSA‐S is also beneficial for liver regeneration in cases of non‐alcoholic fatty liver disease (NAFLD) 7. The suggested mechanisms of URSA‐S include the improvement of bile acid transport and/or detoxification, cytoprotection and anti‐apoptotic effects 8, 9, 10. URSA‐S activates AMP‐activated protein kinase (AMPK) in the liver, which suggests that URSA‐S may serve as an AMPK agonist 11. It is also known to play a major role in energy homoeostasis.
These findings suggest that URSA‐S has a beneficial effect on the regulation of energy production. However, to our knowledge, no clinical trials have been conducted on the effects of URSA‐S on fatigue in patients with hepatopathy. In the present multi‐centre, randomised, and double‐blinded placebo‐controlled study, we aimed to evaluate the effect of URSA‐S on fatigue in cases with abnormal liver function and/or fatty liver. The fatigue level was evaluated by using the checklist individual strength (CIS) and visual analogue scale (VAS) scores.
Materials and methods
Recruitment
Among the male and female individuals aged ≥ 19 years who visited one of the five general hospitals in Korea, we enrolled patients who presented with persistent fatigue for > 1 month and at least one abnormal liver function parameter within the last 4 weeks or fatty liver, from October 2014 to March 2015. The presence of fatty liver within the last 3 months was proved by using the medical records. Patients were considered to be eligible for study enrolment if they met all the following inclusion criteria: (i) > 19 years of age; (ii) persistent or chronic fatigue for ≥ 1 month upon screening; (iii) total CIS score of ≥ 76 points upon screening and at baseline; (iv) Hospital Anxiety and Depression Scale (HADS) score of ≤ 10 points upon screening; (v) abnormal serum ALT levels within the last 4 weeks or presence of fatty liver on ultrasonography within the last 3 months and (vi) voluntary agreement to participate in the clinical trials and provision of informed consent.
Patients were excluded from the study if they met one of the following conditions: (i) liver cirrhosis, liver cancer, severe hepatic dysfunction (five times more than the normal upper limit of serum ALT level), renal dysfunction (two times more than the normal upper limit of serum creatinine) and chronic fatigue syndrome; (ii) known underlying cause of fatigue (such as malignant tumour, active pulmonary tuberculosis, asthma, glaucoma, multiple sclerosis, hypothyroidism, HIV positive status, anaemia, chronic inflammatory diseases such as rheumatoid arthritis etc.); (iii) psychiatric diseases (major depressive disorder, bipolar disorder, schizophrenia, delusional disorder, dementia, etc.); (iv) uncontrolled hypertension (≥ 170/110 mmHg), uncontrolled diabetes (HbA1c ≥ 8.0%) and obesity (BMI ≥ 30); (v) taking medicines or supplements related to fatigue for more than 4 days in 2 weeks (such as beta blockers, glucocorticoids, immune modulators, antidepressants, anxiolytics, sedatives, antipsychotics, vitamins, ginseng, non‐steroidal anti‐inflammatory drug (NSAID), hormones, traditional oriental medicinal management and materials that can alter liver function as determined by the physicians participating in this study); (vi) pregnant women, breast feeding women, women with an expected delivery date during of within 2 months after the study; (vii) history of alcohol or drug abuse; (viii) individuals who participated in other clinical trials within 1 month of this study; and (ix) other conditions that may make participation in this study inappropriate.
The protocol was approved by the ethical committee of each institution participating in the study. IRB number of the main hospital is IB‐1409‐040. Patients were informed about the details of the clinical study and voluntarily agreed to participate in the study. We conducted this clinical study in accordance with the Declaration of Helsinki and good clinical practice guidelines. This study was registered at ClinicalTrials.gov (NCT02415777). A central study coordinator monitored all the procedures and entered the data into a central database.
Study design
This clinical trial was planned as a multi‐centre, randomised, double‐blinded, placebo‐controlled study to evaluate the effect of URSA‐S on fatigue in patients with abnormal liver function and/or fatty liver disease. The eligible participants were assigned to one of two groups with equal probability according to a randomisation code. The randomisation code was prepared by a block randomisation method stratified (according to the clinical research centres) by a statistician in a clinical trial centre (C&R Research, Seoul, South Korea).
After the patients provided informed consent, their medical history and records, laboratory test results, and CIS, VAS and HADS scores were reviewed. The subjects were randomised to the treatment or placebo group, and took either URSA‐S or placebo three times daily for 8 weeks. During the study, the participants were excluded if any protocol violation was noted, such as dropping out of the study, lack of fulfilment of the inclusion and exclusion criteria, taking of materials related to fatigue, and low compliance (< 80%) (Figure 1).
Figure 1.
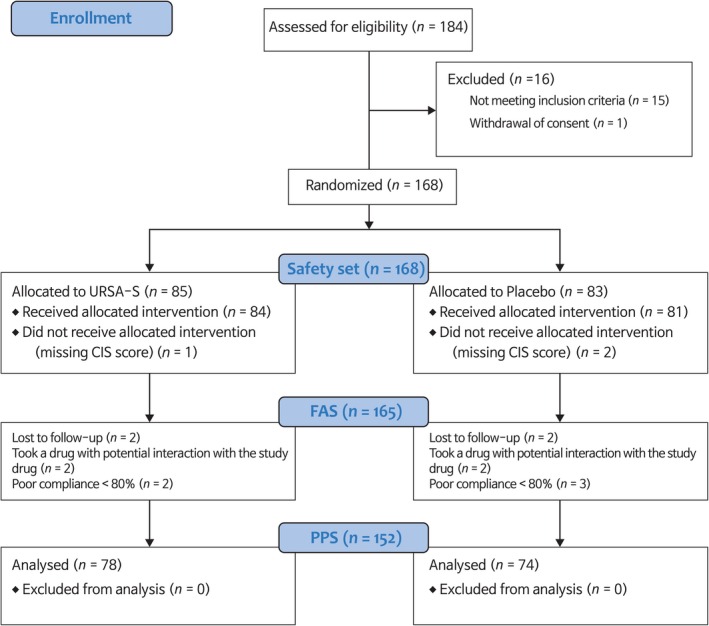
Study participation flow chart. FAS, full analysis set; PPS, per‐protocol set
Checklist individual strength scores; VAS scores; vital signs including blood pressure and pulse pressure; and laboratory examination data were assessed at baseline and 4 and 8 weeks after the start of the study. Adverse events, along with their severity and perceived relation to the study medication, were recorded throughout the study. Serious adverse events (for example, those requiring admission to hospital or that resulted in a persistent or significant disability or incapacity) were also recorded.
The primary efficacy variable of this study was the rate of improvement in CIS scores at the end‐point (8 weeks). Improvement was considered a change in the CIS score to ≤ 76. The secondary efficacy variables included changes in the fatigue scale scores (CIS score and VAS).
Measurement
During screening, the demographic information (age, gender, smoking, drinking and other information), medical history, and medication history of the subjects were investigated and recorded. When clinically significant test results were observed, the investigator decided whether to enrol the subject in the experiment.
The laboratory tests were conducted at baseline, week 4, and week 8. The assessments included complete blood count, liver function test, renal function test, lipid profile and urinalysis conducted at each institute. Haemoglobin A1c level assessment and human immunodeficiency virus test were only performed at the initial visit.
Fatigue alleviation was the primary outcome and was evaluated using self‐administered measures of fatigue, including the CIS and VAS scores. The CIS questionnaire consists of four subscales: fatigue severity (CIS‐fatigue, eight items), concentration (five items), motivation (four items) and activity level (three items), each of which is scored on a 7‐point Likert‐scale. Higher total scores represent a higher degree of fatigue. The CIS covers several aspects of fatigue, such as severity, motivation, concentration, and physical activity level, which adhere to the concept of prolonged fatigue. The total CIS cut‐off point of 76 was determined, with a specificity of 90% and a sensitivity of 73% 12. The CIS total cut‐off point is defined as a score indicating a fatigue level that places the individual ‘at risk’ for sick leave or work disability, and appears to be appropriate for use in studies on fatigue in the working population. The VAS is used to assess the impact of fatigue on daily life, and the answers range from ‘no influence at all’ to ‘a lot of influence’ along a line of 100 mm (range 0–100).
Statistical analysis
The primary objective of this trial was to evaluate the rate of improvement in fatigue symptoms or CIS scores during the study as a result of drug administration, to determine its statistical superiority as compared to the placebo. The assumed rate of CIS scores that would improve to below 76 points is 72% in the therapy group and 49% in the placebo group, based on previous research on fatigue 13. When this study was tested under the conditions of a two‐sided significance level of 0.05, a statistical power of 80%, and 1:1 assignment, the minimum required number of test subjects was calculated as 67 people per group. Considering the expected drop out rate of 20%, we decided to register 84 people per group (total, 168 patients).
All efficacy analyses were based on the full analysis set (FAS). Missing data were imputed by the last observation carried forward approach (LOCF). The primary outcome variable, the fatigue recovery rate of the CIS scores, was analysed by using a Cochran–Mantel–Haenszel test, while adjusting for alcohol consumption within 2 years. An analysis of covariance (ANCOVA) model, with alcohol consumption within 2 years as a covariate, was used to analyse the change in CIS and VAS scores during the study. The change in blood chemistry data, including the levels of liver enzymes during the study, was compared with ANCOVA by using rank transformation with alcohol consumption within 2 years as a covariate. Pearson's Chi‐squared test or Fisher's exact test was conducted to compare the adverse events between the groups.
All analyses were conducted using the SAS™ system (SAS Institute, Inc., Cary, NC) version 9.3 for Windows software. P‐values < 0.05 were considered statistically significant.
Results
Subject participation
During the clinical trial, a total of 184 subjects were screened, and 168 were appropriately identified as having fatigue. Subjects were randomised into the therapy (n = 85) or placebo (n = 83) groups. Hence, the safety set included a total of 168 subjects. As 7 (8.2%, 7/85) subjects dropped out of the therapy group during the observation period (8 weeks), 78 (91.8%, 78/85) subjects in the therapy group completed the trial. A total of nine (10.8%, 9/83) subjects dropped out of the placebo group, and hence, 74 (89.2%, 74/83) subjects in the placebo group completed the trial. Thus, the per protocol analysis set (PPS) included a total of 152 subjects (Figure 1).
Demographic data and characteristics of the subjects prior to treatment
The conditions of the subjects before medication was provided were compared between the groups (Table 1). The mean ages were 43.63 years (SD = 11.79) in the therapy group and 43.72 years (SD = 10.36) in the placebo group. The number of male and female subjects were 68 (68/84, 80.95%) and 16 (16/84, 19.05%) in the therapy group and 70 (70/81, 86.42%) and 11 (11/81, 13.58%) in the placebo group. The mean BMI was 26.12 kg/m2 (SD = 2.56) in the therapy group and 26.27 kg/m2 (SD = 2.37) in the placebo group. Moreover, smoking (p = 0.7820), alcohol consumption (p = 0.5616) and caffeine consumption (p = 0.7466) were not significantly different between the two groups. The mean values of HADS score were 12.25 (SD = 4.58) in the therapy group and 11.86 (SD = 4.94) in the placebo group (p = 0.7161). The mean values of the CIS scores were 89.81 and 91.75 in the therapy and placebo groups, respectively. With regard to the other variables, no significant differences in the laboratory test results were observed between the two groups.
Table 1.
Baseline characteristics of the subjects in the full analysis set
| URSA‐S (n = 84) | Placebo (n = 81) | |
|---|---|---|
| Sex | ||
| Men | 68 (80.95) | 70 (86.42)* |
| Women | 16 (19.05) | 11 (13.58) |
| Age (years) | ||
| Average | 43.63 ± 11.79 | 43.72 ± 10.36† |
| < 30 | 5 (5.95) | 6 (7.41)* |
| 30–39 | 35 (41.67) | 29 (35.80) |
| 40–49 | 17 (20.24) | 22 (27.16) |
| > 50 | 27 (32.14) | 24 (29.63) |
| BMI (kg/m2) | 26.12 ± 2.56 | 26.27 ± 2.37† |
| Smoking | ||
| Non | 52 (61.90) | 48 (59.26)* |
| Ex | 15 (17.86) | 13 (16.05) |
| Current | 17 (20.24) | 20 (24.69) |
| Alcohol consumption | ||
| No | 71 (84.52) | 71 (87.65)* |
| Yes | 13 (15.48) | 10 (12.35) |
| Caffeine consumption | ||
| No | 6 (7.14) | 4 (4.94)‡ |
| Yes | 78 (92.86) | 77 (95.06) |
| HADS score | ||
| Anxiety | 5.58 ± 2.80 | 5.46 ± 2.80† |
| Depression | 6.67 ± 2.45 | 6.41 ± 2.70† |
| Total | 12.25 ± 4.58 | 11.86 ± 4.94† |
| CIS score | 89.81 ± 11.80 | 91.75 ± 12.10 |
| Liver function test | ||
| ALT (SGPT) | 47.58 ± 34.64 | 48.90 ± 27.08 |
| AST (SGOT) | 32.33 ± 16.27 | 32.47 ± 11.72 |
| γ‐GTP | 51.33 ± 40.74 | 58.54 ± 50.22 |
| Albumin | 4.59 ± 0.27 | 4.61 ± 0.22 |
| T‐bilirubin | 0.89 ± 0.44 | 0.82 ± 0.25 |
No significant differences in any variable. BMI, Body mass index; HADS, Hospital Anxiety and Depression Scale; CIS, checklist individual strength; ALT, alanine aminotransferase; AST, aspartate aminotransferase; r‐GTP, gamma glutamyl transpeptidase; T‐bilirubin, total bilirubin. Data showed number (%) or average ± SD. *Pearson's Chi‐squared test. †Wilcoxon rank‐sum test. ‡Fisher's exact test.
Efficacy evaluations
Primary outcome variables: fatigue recovery rate of the CIS score
In the FAS, the fatigue recovery rate in subjects, with improved CIS scores beyond the cut‐off of 76 points, was 79.76% and 45.68% in the therapy and placebo groups, respectively (p < 0.05; Table 2). In PPS, the fatigue recovery rate in subjects, with improved CIS scores beyond the cut‐off of 76 points, was 82.05% and 43.24% in the therapy and placebo groups, respectively (p < 0.05). The fatigue recovery rate of the CIS score was higher in the therapy group than in the placebo group. The difference in the fatigue recovery rate of the CIS score between the therapy group and placebo group was not statistically significant after 4 weeks, although this difference was statistically significant at the end‐point (8 weeks) (Figure 2).
Table 2.
The fatigue recovery rate of CIS scores based on a cut‐off of 76 points at 8 weeks
| CIS points | URSA‐S | Placebo | p‐value |
|---|---|---|---|
| FAS | |||
| N | 84 | 81 | < 0.01 |
| ≤ 76 | 67 (79.76) | 37 (45.68) | |
| 95% CI | 69.59–87.75 | 34.56–57.13 | |
| PPS | |||
| N | 78 | 74 | < 0.01 |
| ≤ 76 | 64 (82.05) | 32 (43.24) | |
| 95% CI | 71.72–89.83 | 31.77–55.28 | |
Values are expressed as n (%). Cochran–Mantel–Haenszel test (adjusted covariate: alcohol consumption within 2 years). CIS, checklist individual strength; FAS, full analysis set; PPS, per protocol analysis set.
Figure 2.
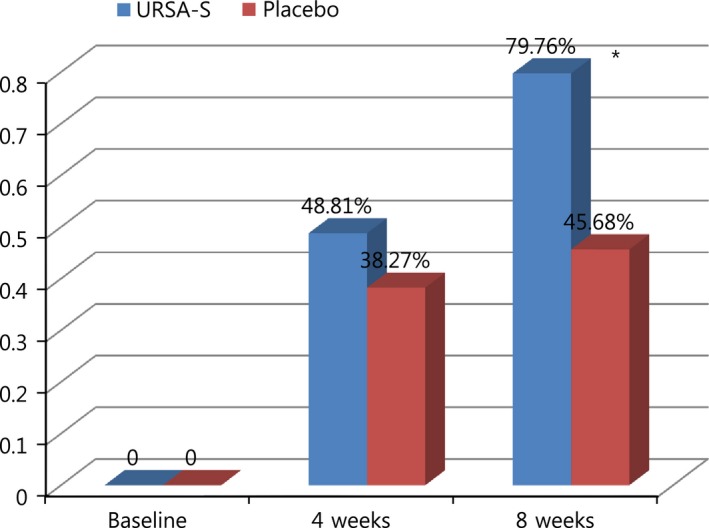
Proportion of subjects with improved CIS scores (≤ 76 points) at 4 and 8 weeks (full analysis set). CIS, checklist individual strength. *Statistically significant difference
When analysed separately in the patients with abnormal liver function tests and fatty liver disease, the fatigue recovery rate of the CIS score at 8 weeks was higher in the therapy group than in the placebo group (p < 0.05) (Table 3).
Table 3.
The fatigue recovery rate of the CIS scores at 8 weeks in the full analysis set of patients with abnormal liver function test (LFT) results and fatty liver
| CIS score | URSA‐S | Placebo | p‐value |
|---|---|---|---|
| Abnormal LFT | |||
| N | 36 | 43 | < 0.05 |
| ≤ 76 | 28 (77.78) | 23 (53.49) | |
| 95% CI | 60.85–89.88 | 37.65–68.82 | |
| Fatty liver | |||
| N | 64 | 65 | < 0.01 |
| ≤ 76 | 54 (84.38) | 30 (46.15) | |
| 95% CI | 73.14–92.24 | 33.70–58.97 | |
Values are expressed as n (%). Cochran–Mantel–Haenszel test (adjusted covariate: alcohol consumption within 2 years). LFT, liver function test; CIS, checklist individual strength.
Secondary outcome variables: change in the fatigue‐associated scale during the study
The fatigue scores of the CIS were decreased in both groups at 4 and 8 weeks after the start of the study (Table 4). A statistically significant change in the fatigue scores was observed in the therapy group at the end‐point (8 weeks), but not at 4‐week stage. In the FAS, the mean change in the CIS score over the 8‐week study period was −25.44 in the therapy group and −16.59 in the placebo group. Moreover, in the PPS, the mean change in the CIS score over the 8‐week study period was −25.83 in the therapy group and −15.43 in the placebo group. The test for normal distribution of CIS score was done by Shapiro–Wilk test. It showed normal distribution because the p‐values were more than 0.05. The mean CIS score in the therapy group at the study end‐point (8 weeks) was < 70.
Table 4.
Change in CIS and VAS scores during the study
| URSA‐S | Placebo | p value | |
|---|---|---|---|
| CIS score | |||
| FAS | N = 84 | N = 81 | |
| Baseline | 89.81 ± 11.80 | 91.75 ± 12.10 | |
| 4 weeks | 75.93 ± 13.12 | 78.90 ± 16.16 | |
| Change at 4 weeks | −13.88 ± 13.44 | −12.85 ± 13.93 | * |
| 8 weeks | 64.37 ± 15.97 | 75.16 ± 20.13 | |
| Change at 8 weeks | −25.44 ± 18.57 | −16.59 ± 17.29 | < 0.01† |
| PPS | N = 78 | N = 74 | |
| Baseline | 89.87 ± 12.08 | 92.07 ± 12.43 | |
| 4 weeks | 76.13 ± 13.46 | 79.55 ± 15.44 | |
| Change at 4 weeks | −13.74 ± 13.42 | −12.51 ± 13.72 | * |
| 8 weeks | 64.04 ± 16.14 | 76.64 ± 19.41 | |
| Change at 8 weeks | −25.83 ± 18.47 | −15.43 ± 16.80 | < 0.01† |
| VAS | |||
| FAS | N = 84 | N = 81 | |
| Baseline | 70.33 ± 13.46 | 71.89 ± 12.92 | |
| 4 weeks | 56.96 ± 15.34 | 57.96 ± 17.93 | |
| Change at 4 weeks | −14.40 ± 2.15 | −15.05 ± 2.24* | |
| 8 weeks | 44.25 ± 18.23 | 54.35 ± 21.04 | |
| Change at 8 weeks | −27.84 ± 2.70 | −19.46 ± 2.81 | < 0.01† |
| PPS | N = 78 | N = 74 | |
| Baseline | 70.09 ± 13.91 | 71.89 ± 13.18 | |
| 4 weeks | 57.44 ± 15.20 | 58.18 ± 16.73 | |
| Change at 4 weeks | −13.28 ± 2.17 | −14.35 ± 2.22* | |
| 8 weeks | 43.73 ± 18.43 | 54.62 ± 20.77 | |
| Change at 8 weeks | −28.31 ± 2.86 | −19.25 ± 2.92 | < 0.01† |
Values are expressed as mean ± SD. CIS, checklist individual strength; VAS, visual analogue scale; FAS, full analysis set; PPS, per protocol analysis set. *Rank transformed ANCOVA. †ANCOVA (adjusted covariate: alcohol consumption within 2 years). §Wilcoxon signed rank test.
The VAS scores had decreased in both groups at 4 and 8 weeks after the start of the study. Similar to the CIS scores, VAS scores for fatigue also exhibited a statistically significant change in the therapy group at the study end‐point (8 weeks), but not at the 4‐week stage.
Change in blood chemistry data during the study
No difference in the change in AST or gamma glutamyl transpeptidase (r‐GTP) and total bilirubin levels was observed between the therapy and placebo groups at 4 and 8 weeks; however, the change in ALT levels was significant (p = 0.0228) between the therapy group and placebo group at 4 weeks, but not at 8 weeks after the start of the study (p = 0.1278) (Table 5). The mean decreases in the serum ALT levels from the baseline value to after 4 weeks and 8 weeks were 7.45 and 8.31 IU/l, respectively, in the therapy group. The mean decreases in the serum AST levels from the baseline value to after 4 and 8 weeks were 2.74 and 2.81 IU/l, respectively, in the therapy group.
Table 5.
Change in blood chemistry data, including the levels of liver enzymes during the study
| URSA‐S | Placebo | p value | |
|---|---|---|---|
| FAS | N = 84 | N = 81 | |
| ALT (SGPT) | |||
| Baseline (mean ± SD) | 47.58 ± 34.64 | 48.90 ± 27.08 | |
| Change at 4 weeks (LS mean ± SE) | −7.45 ± 2.24 | −0.11 ± 2.33 | < 0.05* |
| Change at 8 weeks (LS mean ± SE) | −8.31 ± 2.83 | −0.78 ± 2.96 | * |
| AST (SGOT) | |||
| Baseline (mean ± SD) | 32.33 ± 16.27 | 32.47 ± 11.72 | |
| Change at 4 weeks (LS mean ± SE) | −2.74 ± 1.29 | −0.77 ± 1.35 | * |
| Change at 8 weeks (LS mean ± SE) | −2.81 ± 3.75 | 3.12 ± 3.91 | * |
| γ‐GTP | |||
| Baseline (mean ± SD) | 51.33 ± 40.74 | 58.54 ± 50.22 | |
| Change at 4 weeks (LS mean ± SE) | −8.91 ± 2.44 | −4.10 ± 2.55 | * |
| Change at 8 weeks (LS mean ± SE) | −5.95 ± 2.67 | −2.12 ± 2.78 | * |
| Total bilirubin | |||
| Baseline (mean ± SD) | 0.89 ± 0.44 | 0.82 ± 0.25 | |
| Change at 4 weeks (LS mean ± SE) | 0.01 ± 0.04 | 0.06 ± 0.04 | * |
| Change at 8 weeks (LS mean ± SE) | −0.04 ± 0.04 | −0.04 ± 0.04 | * |
| PPS | N = 78 | N = 74 | |
| ALT (SGPT) | |||
| Baseline (mean ± SD) | 49.45 ± 35.21 | 49.19 ± 26.72 | |
| Change at 4 weeks (LS mean ± SE) | −8.25 ± 2.21 | −1.38 ± 2.26 | < 0.05* |
| Change at 8 weeks (LS mean ± SE) | −10.02 ± 3.00 | −0.77 ± 3.07 | * |
| AST (SGOT) | |||
| Baseline (mean ± SD) | 33.05 ± 16.60 | 32.30 ± 10.36 | |
| Change at 4 weeks (LS mean ± SE) | −3.29 ± 1.36 | −1.29 ± 1.39 | * |
| Change at 8 weeks (LS mean ± SE) | −3.83 ± 4.10 | 3.28 ± 4.19 | * |
| γ‐GTP | |||
| Baseline (mean ± SD) | 51.78 ± 40.47 | 57.86 ± 45.42 | |
| Change at 4 weeks (LS mean ± SE) | −9.07 ± 2.65 | −3.77 ± 2.71 | * |
| Change at 8 weeks (LS mean ± SE) | −6.30 ± 2.51 | −0.30 ± 2.56 | * |
| Total bilirubin | |||
| Baseline (mean ± SD) | 0.86 ± 0.39 | 0.83 ± 0.25 | |
| Change at 4 weeks (LS mean ± SE) | 0.02 ± 0.04 | 0.06 ± 0.04 | * |
| Change at 8 weeks (LS mean ± SE) | −0.02 ± 0.04 | −0.03 ± 0.04 | * |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; rGTP, gamma glutamyltranspeptidase. *Rank transformed ANCOVA (adjusted covariate: alcohol consumption within 2 years).
Summary of the safety results
The rates of adverse events in the therapy group and placebo group were 14.12% (12/85, 16 cases) and 14.46% (12/83, 15 cases), respectively (Table 6). There was no statistically significant difference in the rate of adverse effects between the groups (p = 0.9498).
Table 6.
Comparison of adverse events between the two groups
| URSA‐S (N = 85) | Placebo (N = 83) | p value | |||
|---|---|---|---|---|---|
| n (%) | Case | n (%) | Case | ||
| Adverse events | 12 (14.12) | 16 | 12 (14.46) | 15 | * |
| 95% CI | 7.51–23.36 | 7.70–23.89 | |||
| Adverse drug reactions | 0 (0.00) | 0 | 2 (2.41) | 2 | † |
| 95% CI | 0.00–4.25 | 0.29–8.43 | |||
| Serious adverse events | 0 (0.00) | 0 | 0 (0.00) | 0 | – |
| 95% CI | 0.00–4.25 | 0.00–4.35 | |||
*Pearson's Chi‐squared test. †Fisher's exact test.
Discussion
To our knowledge, this is the first study to show that URSA‐S can induce the recovery from fatigue in subjects with liver function abnormality and/or fatty liver. We observed that the fatigue recovery rate was higher in the URSA‐S therapy group than in the placebo group. This finding was observed independently in patients with abnormal liver function and fatty liver disease.
The term NAFLD describes a condition characterised by excess fat within the liver that affects individuals with minimal or no alcohol consumption. This condition may range from simple fatty liver (steatosis), through fat with necroinflammation and/or fibrosis – so‐called non‐alcoholic steatohepatitis (NASH) – to advanced fibrosis, cirrhosis and hepatocellular cancer 14. NAFLD is strongly associated with visceral obesity, insulin resistance, hypertension and dyslipidemia, and is considered to represent the manifestation of metabolic syndrome in the liver 15. Given the increase in the incidence of obesity and diabetes in Western countries, NAFLD has become a growing problem, and is currently recognised as the most common liver disease in these countries and the most common cause of incidental abnormal liver blood test results 16.
Fatigue is one of the most common symptoms in patients with liver disease, but it is easily overlooked or underestimated during treatment because it is hard to define and treat. However, worsening of fatigue may lead to increased inactivity or impairment of activities of daily living, which could ultimately lead to a lower quality of life. Hence, it requires appropriate treatment.
Dried black bear's bile, which is rich in ursodeoxycholic acid (URSA‐S), is recommended in China for the treatment of jaundice during the period of the Tang dynasty (618–907 AD), as documented in Tang MateriaMedica – the first state pharmacopoeia worldwide. URSA‐S was thereafter proposed as a potential therapeutic option for chronic cholestatic disorders with the following rationale: (1) the accumulation of toxic bile acids may be at least in part responsible for liver injury in chronic cholestasis; and (2) the replacement of endogenous bile acids by a non‐toxic bile acid (URSA‐S) could protect the liver and delay the progression of these disorders. This hypothesis was first tested in primary biliary cirrhosis 17. URSA‐S was shown to yield marked improvement in serum liver tests 17, 18. Placebo‐controlled trials showed that URSA‐S also improves the histological features and delays the progression to cirrhosis and the time to liver transplantation 19, 20, 21. At present, URSA‐S therapy is recommended for all patients with primary biliary cirrhosis, provided that they exhibit abnormal serum liver test results 22.
The mechanism underlying the anti‐fatigue action of URSA‐S is not known, but 5′‐AMP‐activated protein kinase (AMPK) activation may be involved. URSA‐S strongly increases AMPK phosphorylation, and AMPK is a key regulator of cellular and whole‐body energy balance 23. AMPK phosphorylates and regulates many proteins involved in nutrient metabolism, by largely acting to suppress anabolic ATP‐consuming pathways while stimulating catabolic ATP‐generating pathways 24. These observations suggest that URSA‐S has a beneficial effect on the regulation of energy production. The other proposed mechanism is that URSA‐S decreases hepatocyte sensitivity to hydrophobic bile acid‐induced oxidative stress 8, 25. Some studies found a significant association between lipid oxidation levels and fatigue 26, 27.
The fatigue recovery rate in CIS scores was approximately 46% in the placebo group, which is relatively higher as compared to that in other studies 13, 28. There are a few possible causes for this observation. First, the statement that URSA‐S is effective for detoxifying the liver was made widely known through advertising for several decades, and hence, Koreans had a high expectation that this drug would be effective to relieve fatigue. The placebo used in this study had a similar taste and smell and shape, and therefore, the high placebo effect may be because of the high expectation that the received drug would be URSA‐S. Second, fatigue may originate from multiple causes, and fatigue is difficult to assess objectively 3. Fatigue scales such as CIS are used to study fatigue and may not represent all the aspects of fatigue 29.
There was a limitation in our study. There was no validity study of the Korean version of CIS‐fatigue, but there were previous studies using the Korean version of CIS‐fatigue 13, 30, 31.
In this study, URSA‐S was not associated with any serious adverse events or adverse drug reactions, and the frequency of the adverse events was also low.
We observed that the mean decrease in serum ALT levels from baseline to after 4 weeks was significantly higher in the therapy group than in the placebo group, although a significant difference was not observed between baseline and the 8‐week stage. No significant change in serum AST levels was observed. We included patients with fatty liver and/or abnormal liver function test results (which were not over five times of the normal limits) irrespective of the underlying liver diseases. The underlying liver disease may include alcoholic liver disease or viral hepatitis. Hence, another limitation of the study is the lack of appropriate assessments of the cause of hepatopathy prior inclusion.
This study is valuable as a multi‐centre, double‐blinded study that is the first to assess the efficacy and safety of the URSA‐S on fatigue in patients with abnormal liver function and/or fatty liver. We conclude that continuous URSA‐S administration up to 8 weeks can reduce fatigue in patients with liver function abnormality and/or fatty liver. We do not recommend extrapolating this result to severely ill patients. Further studies are needed to investigate the anti‐fatigue efficacy of URSA‐S in fatigued patients with diseases such as liver cirrhosis.
Conclusion
URSA‐S is found to be effective for alleviating fatigue in patients with abnormal liver function and/or fatty liver. However, no significant difference in the adverse effects is observed between patients administered URSA‐S and placebo.
Author contributions
WhanSeok Choi, Sat Byul Park, Yun Jun Yang, Eon Sook Lee designed the research; Bumjo Oh, WhanSeok Choi, Sat Byul Park, Belong Cho, Yun Jun Yang, Eon Sook Lee, Jun Hyung Lee performed the research and collected the date; Eon Sook Lee, Jun Hyung Lee analysed the data; Bumjo Oh, WhanSeok Choi made the draft of the article; Yun Jun Yang approved the article.
Acknowledgements
Sources of funding for the research and its publication: Daewoong Pharmaceutical Company. Protocol no. is DW_UDCA003. It helped the study design. The ‘C&R Research’ company helped the process of data collection and statistical analysis.
Disclosures
None of the authors has potential conflicts of interest in any company or institution that might benefit from the publication.
References
- 1. Krupp LB, Pollina DA. Mechanisms and management of fatigue in progressive neurological disorders. Curr Opin Neurol 1996; 9: 456–60. [DOI] [PubMed] [Google Scholar]
- 2. Berrios GE. Feelings of fatigue and psychopathology: a conceptual history. Compr Psychiatry 1990; 31: 140–51. [DOI] [PubMed] [Google Scholar]
- 3. Norheim KB, Jonsson G, Omdal R. Biological mechanisms of chronic fatigue. Rheumatology 2011; 50: 1009–18. [DOI] [PubMed] [Google Scholar]
- 4. Copaci I, Micu L, Iliescu L, Voiculescu M. New therapeutical indications of ursodeoxycholic acid. Rom J Gastroenterol 2005; 14: 259–66. [PubMed] [Google Scholar]
- 5. Saksena S, Tandon RK. Ursodeoxycholic acid in the treatment of liver diseases. Postgrad Med J 1997; 73: 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sato S, Miyake T, Tobita H, et al. A dose‐up of ursodeoxycholic acid decreases transaminases in hepatitis C patients. World J Gastroenterol 2009; 15: 2782–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xiang Z, Chen YP, Ma KF, et al. The role of ursodeoxycholic acid in non‐alcoholic steatohepatitis: a systematic review. BMC Gastroenterol 2013; 13: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buryova H, Chalupsky K, Zbodakova O, et al. Liver protective effect of ursodeoxycholic acid includes regulation of ADAM17 activity. BMC Gastroenterol 2013; 13: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ikegami T, Matsuzaki Y. Ursodeoxycholic acid: mechanism of action and novel clinical applications. Hepatol Res 2008; 38: 123–31. [DOI] [PubMed] [Google Scholar]
- 10. Woollett LA, Buckley DD, Yao L, et al. Effect of ursodeoxycholic acid on cholesterol absorption and metabolism in humans. J Lipid Res 2003; 44: 935–42. [DOI] [PubMed] [Google Scholar]
- 11. Nakagawasai O, Yamada K, Nemoto W, et al. Liver hydrolysate assists in the recovery from physical fatigue in a mouse model. J Pharmacol Sci 2013; 123: 328–35. [DOI] [PubMed] [Google Scholar]
- 12. Bultmann U, de Vries M, Beurskens AJ, et al. Measurement of prolonged fatigue in the working population: determination of a cutoff point for the checklist individual strength. J Occup Health Psychol 2000; 5: 411–6. [DOI] [PubMed] [Google Scholar]
- 13. Lee KK, Choi WS, Yum KS, et al. Efficacy and safety of human placental extract solution on fatigue: a double‐blind, randomized, placebo‐controlled study. Evid Based Complement Alternat Med 2012; 2012: 130875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Day CP. Non‐alcoholic fatty liver disease: current concepts and management strategies. Clin Med 2006; 6: 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marchesini G, Marzocchi R, Agostini F, Bugianesi E. Nonalcoholic fatty liver disease and the metabolic syndrome. Curr Opin Lipidol 2005; 16: 421–7. [DOI] [PubMed] [Google Scholar]
- 16. Skelly MM, James PD, Ryder SD. Findings on liver biopsy to investigate abnormal liver function tests in the absence of diagnostic serology. J Hepatol 2001; 35: 195–9. [DOI] [PubMed] [Google Scholar]
- 17. Poupon R, Chretien Y, Poupon RE et al. Is ursodeoxycholic acid an effective treatment for primary biliary cirrhosis? Lancet 1987; 1: 834–6. [DOI] [PubMed] [Google Scholar]
- 18. Leuschner U, Fischer H, Kurtz W, et al. Ursodeoxycholic acid in primary biliary cirrhosis: results of a controlled double‐blind trial. Gastroenterology 1989; 97: 1268–74. [DOI] [PubMed] [Google Scholar]
- 19. Poupon RE, Balkau B, Eschwege E, Poupon R. A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. UDCA‐PBC Study Group. N Engl J Med 1991; 324: 1548–54. [DOI] [PubMed] [Google Scholar]
- 20. Poupon RE, Poupon R, Balkau B. Ursodiol for the long‐term treatment of primary biliary cirrhosis. The UDCA‐PBC Study Group. N Engl J Med 1994; 330: 1342–7. [DOI] [PubMed] [Google Scholar]
- 21. Pares A, Caballeria L, Rodes J, et al. Long‐term effects of ursodeoxycholic acid in primary biliary cirrhosis: results of a double‐blind controlled multicentric trial. UDCA‐Cooperative Group from the Spanish Association for the Study of the Liver. J Hepatol 2000; 32: 561–6. [DOI] [PubMed] [Google Scholar]
- 22. EASL Clinical Practice Guidelines . Management of cholestatic liver diseases. J Hepatol 2009; 51: 237–67. [DOI] [PubMed] [Google Scholar]
- 23. Grahame Hardie D. AMP‐activated protein kinase: a key regulator of energy balance with many roles in human disease. J Int Med 2014; 276: 543–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Viollet B, Guigas B, Leclerc J et al. AMP‐activated protein kinase in the regulation of hepatic energy metabolism: from physiology to therapeutic perspectives. Acta Physiol 2009; 196: 81–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Amaral JD, Viana RJ, Ramalho RM, et al. Bile acids: regulation of apoptosis by ursodeoxycholic acid. J Lipid Res 2009; 50: 1721–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kennedy G, Spence VA, McLaren M, et al. Oxidative stress levels are raised in chronic fatigue syndrome and are associated with clinical symptoms. Free Radic Biol Med 2005; 39: 584–9. [DOI] [PubMed] [Google Scholar]
- 27. Maes M, Twisk FN. Chronic fatigue syndrome: Harvey and Wessely's (bio)psychosocial model versus a bio(psychosocial) model based on inflammatory and oxidative and nitrosative stress pathways. BMC Med 2010; 8: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stulemeijer M, de Jong LW, Fiselier TJ, et al. Cognitive behaviour therapy for adolescents with chronic fatigue syndrome: randomised controlled trial. BMJ 2005; 330: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shahid A, Shen J, Shapiro CM. Measurements of sleepiness and fatigue. J Psychosom Res 2010; 69: 81–9. [DOI] [PubMed] [Google Scholar]
- 30. Kim YK, Cha NH. Correlations among occupational stress, fatigue, and depression in call center employees in Seoul. J Phys Ther Sci 2015; 27: 3191–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Park JS, Choi ER. A study on relationships between sleep disorder, fatigue and job stress in police shift‐workers. Police Sci J 2010; 5(1): 25–53. [Google Scholar]


