Summary
A substantial proportion of patients with type 2 diabetes mellitus do not reach glycemic targets, despite treatment with oral anti‐diabetic drugs and basal insulin therapy. Several options exist for treatment intensification beyond basal insulin, and the treatment paradigm is complex. In this review, the options for treatment intensification will be explored, focusing on drug classes that act via the incretin system and paying particular attention to the short‐acting glucagon‐like peptide‐1 receptor agonists exenatide and lixisenatide. Current treatment guidelines will be summarized and discussed. © 2016 The Authors. Diabetes/Metabolism Research and Reviews Published by John Wiley & Sons Ltd.
Keywords: GLP‐1 receptor agonist, intensification, exenatide, lixisenatide, algorithms, latent autoimmune diabetes in adults (LADA)
Introduction
An estimated 40–50% 1 of patients with type 2 diabetes mellitus (T2DM) do not achieve optimal glycemic control when treated with oral medications and basal insulin. In other patients, control is maintained until disease progression brings about the need for additional pharmacotherapeutic management 2. When basal insulin offers insufficient control, a common course of action is the intensification of insulin therapy with the addition of prandial insulin, which can be effective but presents drawbacks such as the requirement for patient training in self‐monitoring of blood glucose (SMBG), weight gain and increased risk of hypoglycemia. Therefore, it is important to consider the individual patient's characteristics when deciding how to intensify therapy 2 and to consider options other than prandial insulin. Some patients will benefit from a different therapeutic approach, such as incretin‐based therapy with a glucagon‐like peptide‐1 receptor agonist (GLP‐1 RA), dipeptidyl peptidase‐4 (DPP‐4) inhibitor or a sodium‐glucose co‐transporter 2 (SGLT2) inhibitor as add‐on to basal insulin.
Glucagon‐like peptide‐1 receptor agonists exert an important action on glycemic control, show favourable effects on body weight and are associated with reduced risk of hypoglycemia; some have also demonstrated a complementary mechanism of action when combined with basal insulin 3. There are, however, several GLP‐1 RAs available, each with particular pharmacologic characteristics, which presents significant challenges for the clinician, including their correct positioning in the therapeutic algorithm. This review will discuss the rationale behind, and evidence for, the use of GLP‐1 RA therapies, in particular that of lixisenatide, versus other possible combination treatments, in patients who do not achieve glycemic control with basal insulin alone. Figure 1 shows a possible algorithm based on current guidelines 2 for intensification of basal insulin therapy, with particular regard to the placement of GLP‐1 RAs.
Figure 1.
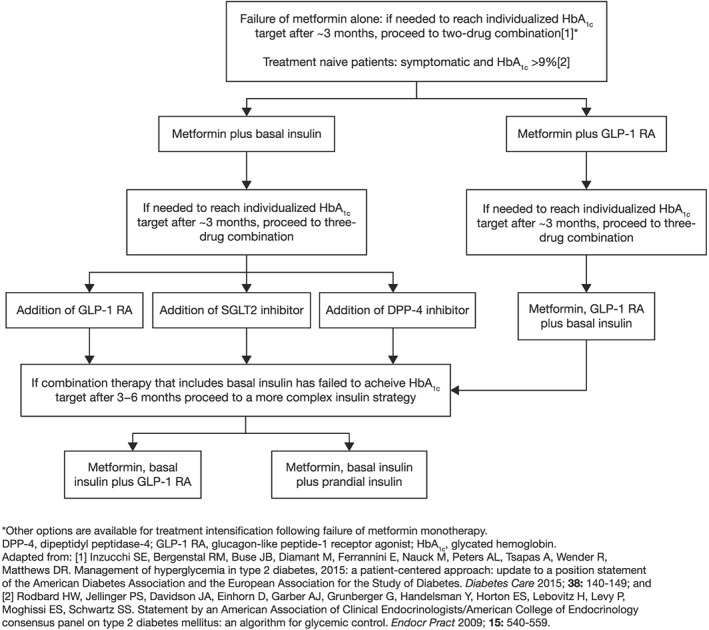
Options for intensification of basal insulin treatment and strategies for the use of glucagon‐like peptide‐1 receptor agonists
GLP‐1 receptor agonists
Endogenous GLP‐1 is normally secreted after glucose ingestion. It lowers postprandial glucose levels by stimulating insulin secretion and inhibiting release of glucagon in a glucose‐dependent manner and by slowing gastric emptying and the rate of intestinal glucose absorption 4. These effects make the augmentation of the effects of GLP‐1 an attractive therapeutic target in T2DM. Endogenous GLP‐1 is rapidly degraded by DPP‐4. GLP‐1 RAs are injectable therapeutic molecules that mimic the insulinotropic actions of endogenous GLP‐1. They have been developed to resist degradation by DPP‐4, prolonging their biological effects. GLP‐1 RA therapies lower hyperglycemia and have a low propensity for inducing hypoglycemia because their effects are glucose dependent. In addition to their effects on glycemic control, they are also associated with appetite suppression, reduced food intake, slowing of gastric emptying and favourable weight control 5. The most common adverse events (AEs) reported with GLP‐1 RAs therapies are gastrointestinal side effects, but these are generally mild to moderate and transient in nature 6, 7, 8, 9.
There are a variety of different GLP‐1 RAs available, which are characterized by different pharmacokinetic and clinical profiles. These agents can be broadly separated into two sub‐classes, long‐acting and short‐acting, or predominantly basal or prandial (Table 1).
Table 1.
Comparison of physiologic effects of short‐acting and long‐acting glucagon‐like peptide‐1 receptor agonists
| Parameter | Short‐acting GLP‐1 RAs | Long‐acting GLP‐1 RAs |
|---|---|---|
| Molecule | Lixisenatide Exenatide BID | Liraglutide |
| Exenatide LAR | ||
| Albiglutide | ||
| Dulaglutide | ||
| Half‐life | 2–5 h | 12 h to several days |
| Effects | ||
| Fasting blood glucose | Modest reduction | Marked reduction |
| Postprandial glucose excursion | Marked reduction | Modest reduction |
| Fasting insulin secretion | Modest stimulation | Marked stimulation |
| Postprandial insulin secretion | Reduction | Modest stimulation |
| Glucagon secretion | Reduction | Reduction |
| Gastric emptying rate | Marked deceleration | No effect or mild deceleration |
| Blood pressure | Reduction | Reduction |
| Heart rate | No effect or mild increase (0–2 bpm) | Moderate increase (2–9 bpm) |
| Reduction of body weight | 1–5 kg | 2–5 kg |
| Induction of nausea | 20–50%, slow attenuation (from weeks to months) | 20–40%, rapid attenuation (about 4–8 weeks) |
BID, twice daily; bpm, beats per minute; GLP‐1 RA, glucagon‐like peptide‐1 receptor agonist; LAR, long‐acting release. Reprinted by permission from Macmillan Publishers Ltd: Nat Rev Endocrinol (Meier JJ. GLP‐1 receptor agonists for individualized treatment of type 2 diabetes mellitus. 8:728–742), copyright (2012).
Exenatide is a GLP‐1 RA that is available in short‐acting twice daily (BID) and long‐acting once weekly (QW) formulations. It has a half‐life of approximately 2.4 h, regardless of the dose 7. The QW formulation is a prolonged‐release suspension 7. Both formulations are effective in lowering glycated haemoglobin (HbA1c) and inducing weight loss 10. The effects of exenatide on fasting plasma glucose (FPG) and postprandial glucose (PPG) levels differ between the formulations 10 – this difference will be discussed along with the findings of other head‐to‐head studies.
Liraglutide is an analogue of human GLP‐1, administered once daily (QD). It is a long‐acting GLP‐1 RA with a half‐life of approximately 13 h 11. At a daily dose of 1.8 mg QD, liraglutide reduces HbA1c through increased insulin levels and significant effects on FPG. Liraglutide treatment reduces body weight but has limited effects on gastric emptying and meal‐related glucose fluctuations 12.
Lixisenatide is a prandial GLP‐1 RA administered QD. It has a half‐life of approximately 3 h and is referred to as a short‐acting agent. QD dosing at 20 µg reduces PPG levels and prandial glucose fluctuations throughout the day to confer overall glucose control 13 and gives the optimal benefit/AE ratio 14, 15. Several mechanistic studies have demonstrated that lixisenatide is associated with a significant delay in gastric emptying 5, 13, 16. This results in a reduction of the rate at which glucose is absorbed from the small intestine, which in turn causes pronounced reductions in the level of PPG 17. This effect has been demonstrated in clinical trials of lixisenatide, which have shown significant reductions in PPG and HbA1c, with high proportions of treated patients achieving HbA1c targets 18, 19, 20. Treatment with lixisenatide is also associated with beneficial weight‐lowering effects 21.
Albiglutide is a long‐acting GLP‐1 RA administered QW with a half‐life of 6–8 days 22, 23. In a comparative clinical trial in patients on oral antidiabetic drug (OAD) therapy, albiglutide demonstrated a lesser effect on glycemic control than that seen with liraglutide. Although patients in both groups lost weight, the effect was less pronounced with albiglutide than with liraglutide 24.
With a half‐life of approximately 90 h 25, dulaglutide is a long‐acting GLP‐1 RA administered QW. A study published recently found the efficacy of dulaglutide to be noninferior to that of daily liraglutide, with similar rates of AEs 26. Reported effects of dulaglutide include lowering of FPG and PPG 27, and weight loss 28 through glucose‐dependent potentiation of insulin secretion and inhibition of glucagon secretion 25.
While both short‐acting and long‐acting GLP‐1 RAs increase insulin secretion and reduce glucagon levels 3, they have different mechanistic properties, which account for their different clinical profiles. Long‐acting GLP‐1 RAs have a predominantly insulinotropic mechanism of action 3, which provides glycemic control primarily through reduction in FPG and hyperglycemia throughout the day 10, 12, 24. While short‐acting GLP‐1 RAs have insulinotropic effects, they are also particularly efficacious in slowing gastric emptying, an effect that is associated with pronounced reductions in PPG and meal‐related glucose fluctuations 5. Long‐acting GLP‐1 RAs do not delay gastric emptying to the same extent as short‐acting agents, which may be due to tachyphylaxis caused by sustained plasma concentrations of the GLP‐1 RA 29. Chronic occupation of GLP‐1 receptors by long‐acting GLP‐1 RAs may lead to downregulation of receptor expression and/or signalling; the phasic nature of exposure to short‐acting GLP‐1 RAs could instead preserve receptor expression and the effect on gastric emptying deceleration 30. The effect on delayed gastric emptying is vagally mediated. In contrast, the other effects of GLP‐1 RAs, such as that on insulin secretion, do not appear to be susceptible to attenuation by continuous exposure 31.
Differences in mechanism of action and clinical profiles between short‐acting and long‐acting GLP‐1 RAs have been demonstrated in several head‐to‐head studies: a study comparing the BID and QW formulations of exenatide as adjunct therapy with OADs found that the reduction from baseline in 2‐h PPG with exenatide BID was significantly greater than with exenatide QW – this was accompanied by significantly greater delay of gastric emptying with exenatide BID. In contrast, exenatide QW showed significantly greater reductions versus exenatide BID in FPG and HbA1c 10. In a comparative study of liraglutide QD versus exenatide BID in addition to metformin, sulfonylurea, or both, reductions in FPG and HbA1c levels were greater with liraglutide QD than with exenatide BID. Conversely, exenatide BID was significantly more effective than liraglutide QD in reducing PPG after breakfast and dinner – the times at which exenatide was administered 12.
A pharmacodynamic study of lixisenatide QD and liraglutide QD in addition to metformin showed that lixisenatide treatment was associated with greater reductions in PPG than liraglutide, an effect accompanied by greater reductions in postprandial glucagon and insulin with lixisenatide 32. These results were similar to those obtained with lixisenatide QD versus liraglutide QD as add‐on to basal insulin: lixisenatide demonstrated significantly greater reductions in PPG versus liraglutide, and these reductions were associated with greater delays in gastric emptying with the short‐acting prandial agent. It should be noted that HbA1c change from baseline was a secondary endpoint in this study, and that the 8‐week treatment period may not have been sufficient to detect between‐treatment differences in this endpoint. However, final HbA1c levels were comparable between lixisenatide and liraglutide 5, 16, suggesting that overall glycemic control is similar between the two agents, although reached through different mechanisms – delay of gastric emptying and glucagon suppression in the case of lixisenatide, and predominantly increased insulin release in the case of liraglutide.
The recent AWARD‐1 study compared QW dulaglutide at doses of 1.5 or 0.75 µg with exenatide 10 µg BID added on to metformin and pioglitazone. All treatments lowered HbA1c over 52 weeks, but the reduction was significantly greater in both dulaglutide groups than in the exenatide group. While exenatide BID reduced prandial glucose excursions to a greater extent than dulaglutide, larger reductions in FPG and overall hyperglycemia throughout the day were observed with dulaglutide 33.
Rationale for combining GLP‐1 RAs with basal insulin
Glucagon‐like peptide‐1 receptor agonists have demonstrated efficacy at different points in the treatment paradigm, as evidenced by clinical recommendations 2. Current guidelines recommend that, in patients with inadequate control on metformin monotherapy alone, escalation to a two‐drug combination is indicated, with the choice of addition of a thiazolidinedione, a sulfonylurea, a DPP‐4 inhibitor, an SGLT2 inhibitor, a GLP‐1 RA, or basal insulin. Subsequently, when a combination regimen including basal insulin fails to achieve the glycemic target after 3 months, the recommendation is to add either multiple daily insulin doses or a GLP‐1 RA (Figure 1) 2.
Glucagon‐like peptide‐1 receptor agonists are an important candidate class for addition to basal insulin because of the differing mechanisms of action and, particularly in the case of the short‐acting GLP‐1 RAs, complementary physiologic effects between the two agents. The principal effect of basal insulin is on FPG via inhibition of hepatic glucose production 2. The GLP‐1 RAs can also lower FPG (particularly the long‐acting formulations), but they also affect PPG and meal‐related glucose oscillations, which is particularly true for the short‐acting formulations 3. While basal insulin may induce ‘rest of β‐cell function’ 34, GLP‐1 RAs stimulate endogenous insulin secretion and inhibit glucagon secretion in a glucose‐dependent manner without causing β‐cell overload 3. They also have a significant potential for inducing β‐cell survival and protection from pro‐apoptotic actions of cytokines and fatty acids 35, 36, 37. Preclinical investigation has shown that lixisenatide may protect pancreatic β cells from apoptosis – when administered alone, it reduced the number of apoptotic cells by 50–60%. An 80% reduction was seen when lixisenatide and insulin glargine were added together, suggesting a synergistic effect on β‐cell survival 38, 39.
Importantly, insulin therapy is known to increase the risk of hypoglycemia 40, whereas treatment with GLP‐1 RAs is associated with a relatively low propensity to hypoglycemia because of glucose‐dependent effects on α and β cells 2. Furthermore, insulin therapy is associated with weight gain 41, while GLP‐1 RA therapy is associated with favourable weight effects 10, 21.
As both FPG level and PPG excursions contribute to a patient's glycemic profile 42, 43, it is logical to use a therapeutic approach that targets both to maintain overall glucose control, such as combining a prandial GLP‐1 RA and basal insulin. However, the relative degree to which FPG and PPG each contribute to overall HbA1c varies, being influenced by lifestyle, disease duration, specific etiologic and pathophysiologic features, and treatment 42, 43. While some patients will still require further FPG control after initiation of basal insulin, PPG excursions have been shown to provide an important contribution to persistent hyperglycemia in this population 43, making short‐acting GLP‐1 RAs a valuable option for treatment intensification in patients receiving basal insulin.
Combining short‐acting GLP‐1 RAs and basal insulin has been shown to be beneficial in clinical trials
Data from clinical trials demonstrate the efficacy of short‐acting GLP‐1 RAs when used in combination with basal insulin.
In a 30‐week randomized study, mean change from baseline in HbA1c was –1.74% (95% confidence interval [CI] –1.91, –1.56) with exenatide BID added to basal insulin, compared with –1.04% (–1.22, –0.86) in patients treated with basal insulin alone (between group difference: –0.69% [–0.93, –0.460]; p < 0.001). Weight loss occurred in the exenatide group compared with weight gain in the insulin‐only group (Table 2; Figure 2). There was no significant difference between the exenatide and insulin‐only groups in incidence of hypoglycemia 44.
Table 2.
Glycemic parameters from clinical studies combining glucagon‐like peptide‐1 receptor agonists and basal insulin
| Patients (%) achieving HbA1c ≤7.0% | Mean FPG change from baseline, mmol/L | Mean PPG change from baseline, mmol/L | Mean body weight change from baseline, kg | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Treatments added to basal insulin | GLP‐1 RA | Comparator | Between‐group difference | GLP‐1 RA | Comparator | Between‐group difference | GLP‐1 RA | Comparator | Between‐group difference | GLP‐1 RA | Comparator | Between‐group difference |
| Buse et al., 2011 44 | Exenatide 10 µg BID versus placebo; 30 weeks | 60 (51, 69) | 35 (25, 45) | 25 (12, 39); p < 0.001 | –1.6 (–1.9, –1.3) | –1.5 (–1.8, –1.2) | –0.1 (–0.52, 0.32); p = 0.63 | NR | NR | NR | –1.78 (–2.48, –1.08) | 0.96 (0.23, 1.70) | –2.74 (–3.74, –1.74); p < 0.001 |
| GetGoal‐L Asia; Seino et al., 2012 20 | Lixisenatide 20 µg QD versus placebo; 24 weeks | 35.6 | 5.2 | 30.4; p < 0.0001 | –0.42 | 0.25 | –0.67; p = 0.0187 | –7.96 | –0.14 | –7.83 (–8.89, –6.77); p < 0.0001 | –0.38 | 0.06 | –0.44 (–0.925, 0.061); p = 0.0857 |
| GetGoal‐Duo1; Riddle et al., 2013b 19 | Lixisenatide 20 µg QD versus placebo; 24 weeks | 56 | 39 | 17; p = 0.0001 | 0.3 (0.2) | 0.5 (0.2) | –0.1 (–0.5, 0.2); p = 0.5142 | –3.1 (0.5) | 0.1 (0.5) | –3.2 (–4.0, –2.4); p < 0.0001 | 0.3 (0.3) | 1.2 (0.3) | –0.89 (–1.4, –0.4); p = 0.0012 |
| GetGoal‐L; Riddle et al., 2013a 18 | Lixisenatide 20 µg QD versus placebo; 24 weeks | 28.3 | 12.0 | 16.3; p < 0.0001 | –0.6 (0.2) | –0.6 (0.3) | –0.1 (–0.6, 0.4); p = 0.7579 | –5.5 (0.5) | –1.7 (0.5) | –3.8 (–4.7, –2.9); p < 0.0001 | –1.8 (0.2) | –0.5 (0.3) | –1.3 (–1.8, –0.7); p < 0.0001 |
| BEGIN: VICTOZA ADD‐ON; Mathieu et al., 2014 48 | Liraglutide ≥1.8 mg QD versus insulin as part QD; 28 weeks | 58.0 | 44.9 | p = NS | –0.14 | –0.04 | –0.1; p = NS | –0.8* | –1.0* | p = NS | –2.8 (3.8) | 0.9 (2.5) | –3.75 (–4.70, –2.79); p < 0.0001 |
Values in parentheses are 95% confidence intervals or standard error. These were not reported for all studies.
Meal with largest decrease from baseline.
BID, twice daily; FPG, fasting plasma glucose; GLP‐1 RA, glucagon‐like peptide‐1 receptor agonist; NS, nonsignificant; NR, not reported; PPG, postprandial plasma glucose; QD, once daily.
Figure 2.
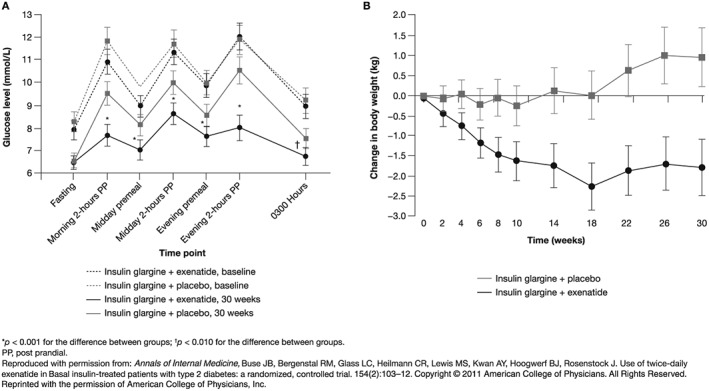
Effects on (A) the 7‐point glycemic profile and (B) body weight of the combination of exenatide twice daily and basal insulin compared with basal insulin alone
Four randomized controlled trials in the lixisenatide GetGoal Phase III clinical programme have assessed prandial lixisenatide 20 µg versus placebo in combination with basal insulin. The GetGoal‐L Asia trial included Asian patients with T2DM uncontrolled on ongoing basal insulin with/without a sulfonylurea. At week 24, mean HbA1c change from baseline was –0.77 in patients receiving lixisenatide added to basal insulin and 0.11 with basal insulin alone (between‐group difference [95% CI]: −0.88% [−1.116, −0.650]; p < 0.0001) 20. The GetGoal‐Duo1 trial included patients with T2DM uncontrolled on OADs newly initiating basal insulin. Mean (SD) change in HbA1c from baseline to week 24 was −0.71 (0.1) in patients treated with lixisenatide and –0.40 (0.1) in patients treated with basal insulin only (between‐group difference [95% CI]: –0.32 [–0.46, –0.17]; p < 0.0001) 19. GetGoal‐L studied patients with T2DM uncontrolled on basal insulin and found that mean (SD) change in HbA1c from baseline to week 24 was –0.7% (0.1) in patients treated with lixisenatide added to basal insulin and –0.4% (0.1) in patients receiving basal insulin alone (between‐group difference [95% CI]: –0.4 [–0.6, –0.2]; p = 0.0002) 18. Further glycemic and body weight outcomes for all four studies are shown in Table 2. Preliminary results were recently reported for GetGoal‐Duo2, which included predominantly obese patients with T2DM who were uncontrolled after 6 months’ basal insulin treatment, with or without 1–3 OADs. Following 12 weeks’ optimization of basal insulin glargine, treatment was intensified with either lixisenatide QD, insulin glulisine QD, or insulin glulisine thrice daily (TID). Mean HbA1c fell to a similar extent from baseline to week 26 in all three groups, with lixisenatide non‐inferior to both prandial insulin treatments. Mean body weight decreased with lixisenatide and increased with prandial insulin (p < 0.0001 for treatment difference versus insulin glulisine TID; a pre‐specified co‐primary endpoint) 45.
A post hoc analysis of data from GetGoal‐L showed that the response to treatment was more pronounced in patients whose baseline FPG was controlled by basal insulin 46. In GetGoal‐Duo1, body weight increased with basal insulin alone and fell with lixisenatide 19. In GetGoal‐L, body weight fell in patients treated with basal insulin alone, but fell to a greater extent in patients with lixisenatide added 18. No weight effect was seen in GetGoal‐L Asia, with the absence likely due to low patient body weight at baseline in this study 20.
In GetGoal‐L, rates of symptomatic documented hypoglycemia did not differ between groups; in GetGoal‐Duo1, hypoglycemia was reported in a higher proportion of patients receiving lixisenatide than in those receiving insulin alone during the first 6 weeks of treatment – after this point, the incidence was similar between groups; in GetGoal‐L Asia, lixisenatide was associated with a higher incidence of hypoglycemia than placebo in the overall population, although there was no difference between groups when patients receiving a sulfonylurea were excluded from the analysis 18.
Data from three studies of the GetGoal programme have also been assessed further in a meta‐analysis, which confirmed the significant reductions in HbA1c, PPG, and body weight with lixisenatide seen in the individual studies. Patients treated with basal insulin plus lixisenatide were more than 2.5 times more likely than those receiving basal insulin alone to achieve the composite endpoint of HbA1c levels <7% and no symptomatic hypoglycemia and no weight gain (p = 0.0007; Figure 3).
Figure 3.
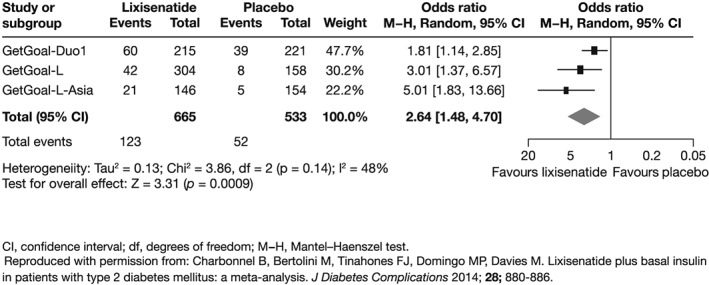
Meta‐analysis showing the likelihood of patients reaching the composite endpoint of glycated haemoglobin levels <7% with no symptomatic hypoglycemia and no weight gain
A fixed‐ratio combination of insulin glargine and lixisenatide is under investigation. A 24‐week study found that HbA1c was significantly lower with the combination than with insulin glargine alone (least squares mean difference –0.17%; p = 0.013). Two‐hour PPG was significantly lower with the combination than with glargine alone (least squares mean difference –57.07 mg/dL; p < 0.0001). Mean weight increased in the insulin group and fell in the combination group, which resulted in a significant difference. Composite endpoints of reaching target HbA1c <7% with no weight gain, or no weight gain and no hypoglycemia were met in the combination group more frequently than in the insulin alone group 47.
Long‐acting GLP‐1 RAs plus basal insulin also improve outcomes as treatment intensification
The 28‐week BEGIN: VICTOZA ADD‐ON trial 48 compared the efficacy of liraglutide added to insulin degludec versus a single daily dose of insulin aspart added to degludec. The mean change in HbA1c from baseline was –0.74% in patients treated with add‐on liraglutide and –0.39% in those treated with add‐on insulin aspart (between group difference [95% CI]: –0.32 [–0.53, –0.12]; p = 0.0024).
As shown in Table 2, a significantly larger proportion of liraglutide patients reached the composite endpoint of HbA1c <7% without confirmed or severe hypoglycemia. Mean body weight fell in liraglutide patients but increased in patients treated with degludec alone 48. An ongoing study is investigating the efficacy and safety of adding liraglutide to the regimens of patients who are receiving large (>50 units) daily doses of insulin 49. A novel combination of insulin degludec and liraglutide has been approved recently in Europe. A recent study examined the efficacy of this combination versus that of insulin degludec alone in patients receiving basal insulin. The combination was associated with superior glycemic control compared with insulin degludec 50. To date, no studies have investigated the addition of long‐acting exenatide or dulaglutide to basal insulin.
Safety considerations when treating with short‐ or long‐acting GLP‐1 RAs
Gastrointestinal events, including nausea and vomiting, are known AEs associated with GLP‐1 RAs. These tend to be mild, and incidence diminishes over time on treatment. Their frequency differs between agents 6, 7, 8, 9: the incidence of nausea was higher and more persistent with exenatide BID (34.5% patients) than with exenatide QW (26.4% patients) in an open‐label study comparing the two formulations 10. A head‐to‐head study found that the incidence of nausea was initially similar between patients treated with exenatide BID and liraglutide QD, but was less persistent with liraglutide (estimated treatment rate ratio 0.448; p < 0.0001) 12. In a head‐to‐head study, gastrointestinal events were more frequent with exenatide BID than with lixisenatide: at least one event was reported in 50.6% of exenatide patients and in 43.1% of lixisenatide patients. There was a significantly higher rate of nausea in exenatide than lixisenatide patients (35.1 vs 24.5%, respectively; p < 0.05) 51. A 28‐day study comparing the pharmacodynamics of lixisenatide with those of liraglutide in addition to metformin found that gastrointestinal tolerability was superior with lixisenatide: the rate of gastrointestinal AEs was 36% with lixisenatide and 46% with liraglutide. The most striking difference in the rate of individual events was with diarrhoea, reported in 3% of lixisenatide patients and in 15% of liraglutide patients 32. Similar results were obtained in a recent head‐to‐head pharmacokinetic/pharmacodynamic study of lixisenatide or liraglutide added to basal insulin – overall reported rates of gastrointestinal AEs were: lixisenatide 20 µg 35.4%; liraglutide 1.2 mg 44.7%; liraglutide 1.8 mg 46.8% 5.
When intensifying treatment for T2DM by adding another agent to basal insulin, risk of hypoglycemia is an important consideration 2. The addition of a GLP‐1 RA to basal insulin carries only a minor additional risk of hypoglycemia 44, 48, 52, 53, 54.
A meta‐analysis of patients recruited to the lixisenatide GetGoal trial programme stratified patients by baseline renal function. Baseline renal impairment had no significant effect on the efficacy of lixisenatide. Furthermore, overall AEs were not significantly different between the subgroups of patients with differing renal function 55.
Increased heart rate has been reported with GLP‐1 RA therapy, with greater increases reported with long‐acting than short‐acting agents: a 26‐week trial of QW exenatide 2 mg treatment reported an increase in heart rate of 4 beats per minute (bpm) from baseline to study end 56, and a further study comparing the QW and BID formulations found that this increase over 24 weeks was larger in the QW formulation (4.1 versus 2.1 bpm) 57. In an assessment of liraglutide in the treatment of obesity, the US Food and Drug Administration acknowledged that placebo‐adjusted increases of 5.7–6.6 bpm in 24‐h heart rate and 7.0–8.9 bpm in 3‐h sleeping heart rate were seen with liraglutide 1.8 and 3 mg 58. Similar heart rate effects with liraglutide (an increase of approximately 9 bpm over 24 weeks) have also been seen in a Japanese study 59. A direct comparison of safety endpoints between lixisenatide and liraglutide showed that while both agents led to increased heart rate over 8 weeks’ treatment, the increase was significantly greater and clinically meaningful with liraglutide 1.2 and 1.8 mg than with lixisenatide 20 µg (9.3 and 9.2 bpm versus 3.3 bpm, respectively; p < 0.0001) 5.
Reduction in body weight following GLP‐1 RA therapy may reduce the risk of cardiovascular (CV) events, but theoretically, GLP‐1 RAs may also have direct beneficial effects on the CV system: GLP‐1 receptors are expressed in the heart, kidneys, and blood vessels, and their activation by endogenous GLP‐1 has favourable effects on endothelial and myocardial function, blood pressure, and sodium excretion. There is growing evidence that GLP‐1 RAs share these effects with endogenous GLP‐1 60, 61, 62. Several studies have shown that increased PPG is a risk factor for CV events 63, 64 and death 65, 66. Furthermore, a 14‐year follow‐up study in patients with T2DM showed that PPG was an independent risk factor for CV events, while FPG was not 67. The HEART2D study failed to show any effect on CV risk of lowering PPG by administration of prandial insulin 68. However, a subgroup analysis of this trial showed that elderly patients treated with prandial insulin had a lower risk of CV events than basal patients 69. It is thus postulated that reducing PPG in patients with T2DM can potentially improve CV outcomes. Several large randomized studies are underway, which will specifically examine CV outcomes in patients treated with short‐ and long‐acting GLP‐1 RAs (Table 3) 70, 71, 72, 73. However, since these clinical trials are designed to document safety, with non‐inferiority experimental methodologies, they are unlikely to show superiority of GLP‐1 RAs on CV endpoints, if any is indeed present.
Table 3.
Ongoing clinical trials investigating cardiovascular outcomes in patients treated with glucagon‐like peptide‐1 receptor agonists
| Identifier (study name) | Active treatment | Study type | Population | Estimated enrollment | Follow‐up duration | Primary endpoint | Estimated completion date |
|---|---|---|---|---|---|---|---|
| NCT01147250 (ELIXA) | Lixisenatide 20 µg QD | Randomized, placebo‐controlled | T2DM; acute coronary syndrome in previous 180 days | 6000 | ~204 weeks | CV death, nonfatal MI, nonfatal stroke, hospitalization for unstable angina, hospitalization for heart failure | February 2015 |
| NCT01144338 (EXSCEL) | Exenatide 2 mg QW | Randomized, placebo‐controlled | T2DM | 14 000 | ~7.5 years | CV death, nonfatal MI, or nonfatal stroke | April 2018 |
| NCT01179048 (LEADER) | Liraglutide 7.8 mg QD | Randomized, placebo‐controlled | T2DM; age ≥50 years and concomitant CV, cerebrovascular or peripheral vascular disease; chronic renal failure or chronic heart failure, or age ≥60 years and other specified risk factors for vascular disease | 9340 | ≤60 months | CV death, nonfatal MI, or nonfatal stroke | November 2015 |
| NCT01394952 (REWIND) | Dulaglutide 1.5 mg QW | Randomized, placebo‐controlled | T2DM; age ≥50 years with established clinical vascular disease, or age ≥55 years and subclinical vascular disease, or age ≥60 years and ≥2 cardiovascular risk factors | 9622 | ≤8 years | CV death, nonfatal MI, or nonfatal stroke | April 2019 |
CV, cardiovascular; MI, myocardial infarction; QD, once daily; QW, once weekly; T2DM, type 2 diabetes mellitus.
Clinical decision making – when to add a GLP‐1 RA to basal insulin
When considering which agent to add to basal insulin to improve patient outcomes, the differences in glycemic profiles associated with different GLP‐1 RAs may provide an opportunity to individualize therapy to suit individual patients’ needs and clinical characteristics. When the FPG target is achieved with well‐titrated basal insulin, residual hyperglycemia explaining the elevation of HbA1c should be mainly postprandial, and this could be effectively corrected using short‐acting GLP‐1 RAs. Through their effects on FPG and overall glycemic control, long‐acting GLP‐1 RAs have also demonstrated benefit in patients who are inadequately controlled with basal insulin. The use of long‐acting GLP‐1 RAs could be particularly considered when FPG target is not achieved with basal insulin, and improvements of both FPG and PPG are required
However, although the concept of ‘glycemic phenotyping’ appears attractive from the pathophysiologic and clinical point of view, consideration should be given to the feasibility of assessing glycemic alterations in clinical practice, taking into account the difficulties related to the implementation of SMBG, in particular in the initial phases of the disease and during treatment with OADs.
It has been demonstrated that the addition of a GLP‐1 RA offers body weight benefits when compared with basal insulin alone, making this an attractive treatment option in overweight and obese patients with T2DM, or those concerned about potential weight gain with intensification of insulin therapy.
As the efficacy of short‐acting GLP‐1 RAs relies predominantly on delay of gastric emptying rather than insulinotropic effects, maintenance of β‐cell function is not a necessity. Indeed, studies have shown that the prandial GLP‐1 RA lixisenatide confers glycemic control in patients regardless of β‐cell function 65, 66, 74, 75. This means that patients with T2DM with declined β‐cell function, such as those with long‐standing disease, may also benefit from the addition of a GLP‐1 RA to basal insulin. Potentially relevant to this concept, post hoc analyses of the GetGoal clinical trial programme have demonstrated the efficacy and tolerability of lixisenatide in elderly patients 76, 77. Conversely, because the long‐acting GLP‐1 RAs mediate their effects through predominantly insulinotropic effects, they could be most effective in patients with maintained β‐cell function. However, there are no published data exploring this hypothesis.
There is evidence of an underlying GLP‐1 insufficiency in Asian patients with T2DM 78. Therefore, the addition of GLP‐1 RA therapy to basal insulin may be especially advantageous in Asian patients. Indeed, a clinical trial has demonstrated the efficacy and safety of lixisenatide plus basal insulin in Asian patients 65, 77, 78. Similarly, a small (n = 84) study in obese Chinese patients found that the addition of liraglutide to existing insulin treatment conferred similar glycemic control to that seen with an increase in insulin dose, but with lower incidence of hypoglycemia 79.
It is noteworthy that the most recent update to the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) treatment guidelines place GLP‐1 RAs alongside prandial insulin in the final line of treatment of T2DM. This means that patients who do not achieve satisfactory glycemic control with other monotherapies or dual‐ or triple‐combination regimens should be treated with metformin plus basal insulin and either prandial insulin or a GLP‐1 RA 2.
Other options for treatment intensification
While the addition of a GLP‐1 RA to basal insulin is a viable option for treatment intensification, other strategies are available at this point in the treatment algorithm – addition of prandial insulin, DPP‐4 inhibitor, or SGLT2 inhibitor may also be considered. Mechanistic differences between these approaches are shown in Table 4.
Table 4.
Mechanistic effects of basal and prandial insulin, glucagon‐like peptide‐1 receptor agonists, dipeptidyl peptidase‐4 inhibitor, or a sodium‐glucose co‐transporter 2 (SGLT2) inhibitors and SGLT2 inhibitors
| Basal insulin | Prandial insulin | GLP‐1 RA | DPP‐4 inhibitor | SGLT2 inhibitor |
|---|---|---|---|---|
| Principal effect on FPG via the inhibition of hepatic glucose production | Reduces PPG | Principal effect on PPG (in particular with short‐acting GLP‐1 RAs) and FPG (in particular with long‐acting GLP‐1 RAs) | Acts on both PPG and FPG (PPG>FPG) | Increases urinary excretion of glucose |
| May induce ‘rest’ of pancreatic β‐cell function | β‐cell protective effect (evidence from preclinical studies) | β‐cell protective effect (evidence from preclinical studies) | ||
| Stimulation of insulin secretion and inhibition of glucagon secretion (glucose‐dependent) | Stimulation of insulin secretion and inhibition of glucagon secretion (glucose‐dependent) | Insulin independent | ||
| No effect on gastric emptying | No effect on gastric emptying | Slowing of gastric emptying (in particular with ‘short‐acting’ GLP‐1 RAs) | No effect on gastric emptying | No effect on gastric emptying |
| Increase in body weight | Increase in body weight | Weight reduction | No effect on weight | Weight reduction |
| Increased risk of hypoglycemia | Increased risk of hypoglycemia | Limited risk of hypoglycemia | Limited risk of hypoglycemia | Limited risk of hypoglycemia |
DPP‐4, dipeptidyl peptidase‐4; FPG, fasting plasma glucose; GLP‐1 RA, glucagon‐like peptide‐1 receptor agonist; PPG, postprandial plasma glucose.
To date, few head‐to‐head studies have examined the relative safety and efficacy of GLP‐1 RAs and prandial insulin added onto basal insulin. In those studies that have 80, 81, 82, both regimens provided similar overall glycemic control, with favourable weight benefits and lower risk of hypoglycemia with the GLP‐1 RA regimens.
Exenatide BID was compared with mealtime insulin lispro on a background of basal insulin in a 30‐week trial 80. Exenatide conferred a similar level of glycemic control as the prandial insulin (reductions in HbA1c of 1.13 and 1.10%, respectively). Mean body weight fell by 2.5 kg with exenatide and increased by 2.1 kg with prandial insulin lispro. Non‐nocturnal hypoglycemic events were less frequent with exenatide (103 events in 315 patients) than with insulin lispro (584 events in 312 patients; p < 0.001). Both treatments resulted in a positive change in patient satisfaction as measured by the Diabetes Treatment Satisfaction Questionnaire (DTSQ); the mean change from baseline in total DTSQ score was significantly greater in exenatide patients than in insulin lispro patients (2.19 versus 1.40, respectively; p < 0.01) 80.
A 26‐week direct comparison of albiglutide 30 mg QW versus prandial insulin lispro, each added to basal insulin, found similar levels of glycemic control with the two regimens: mean HbA1c fell by 0.82% with albiglutide and by 0.66% with insulin lispro. Mean body weight decreased by 0.73 kg in albiglutide patients and increased by 0.81 kg in insulin lispro patients. Documented symptomatic hypoglycemia was reported for 15.8% of albiglutide patients and 29.9% of insulin lispro patients 82.
An ongoing Phase III trial, GetGoal‐Duo2, will directly compare the efficacy and tolerability of lixisenatide plus basal insulin versus basal insulin plus prandial insulin QD or TID 83.
A propensity matching technique was used to perform an indirect analysis of pooled data on patients treated in several clinical trials with basal insulin with lixisenatide or QD rapid‐acting insulin. The lixisenatide population was twice as likely to reach the composite endpoint of HbA1c <7% with no weight gain and no symptomatic hypoglycemia – this endpoint was achieved by 29.2% of patients treated with lixisenatide group and by 15.3% in patients who received rapid‐acting insulin (p = 0.0046) 81.
These comparative data demonstrate that certain patients may be better suited to the addition of GLP‐1 RA therapy rather than prandial insulin, such as: (i) those concerned about potential weight gain with intensification of insulin therapy, or overweight and obese patients; (ii) patients at particular risk from hypoglycemia, including elderly or frail patients; and (iii) patients who are not comfortable with self‐administering multiple daily injections and unskilled at monitoring their plasma glucose levels. However, if a patient has a contraindication to GLP‐1 RAs, has an HbA1c much higher than target, or experiences a particular adverse reaction to GLP‐1 RA treatment, addition of prandial insulin may suit them better, although will usually require intense SMBG. Table 5 details some of the patient characteristics that the clinician should take into consideration when deciding whether to escalate basal insulin therapy with either a GLP‐1 RA or a prandial insulin.
Table 5.
Clinical features of a patient to consider when selecting either a glucagon‐like peptide‐1 receptor agonist or multiple daily insulin doses to escalate basal insulin therapy
| Basal insulin plus GLP‐1 RA | Basal insulin plus multiple daily insulin doses | |
|---|---|---|
| Body weight | Overweight/obese (BMI ≥ 28 kg/m2) | Normal weight/overweight (BMI < 28 kg/m2) |
| Duration of disease | Relatively short (<10 years) | Relatively long (>10 years) |
| Metabolic control | Closer to target (HbA1c < 8%/8.5%) | Further from target (HbA1c ≥ 8%/8.5%) |
| Residual β‐cell function | Maintained (C‐peptide ≥ 0.6–0.8 ng/mL) | Reduced (C‐peptide < 0.6–0.8 ng/mL) |
BMI, body mass index; GLP‐1 RA, glucagon‐like peptide‐1 receptor agonist.
A further option for intensifying basal insulin therapy is the addition of a DPP‐4 inhibitor. Inhibition of DPP‐4 prevents the degradation of GLP‐1, resulting in increased circulating levels of GLP‐1 and gastric inhibitory polypeptide, which in turn has the effect of increasing secretion of insulin and lowering that of glucagon 3. Addition of this class of drug to basal insulin can lower HbA1c levels, although to a more modest extent than GLP‐1 RAs 84, 85, 86, 87, 88, 89, 90, 91, 92. The principal DPP‐4 inhibitors currently available are sitagliptin, saxagliptin, vildagliptin, linagliptin, and alogliptin. All are indicated for T2DM in combination with basal insulin (± metformin) where this regimen alone, alongside diet and physical exercise, does not provide adequate glycemic control 2. All are taken orally QD; vildagliptin is also prescribed for BID use. The ADA and the EASD categorize DPP‐4 inhibitors as agents of intermediate efficacy, which are generally very well tolerated with neutral effects on body weight 2.
There is only one small published head‐to‐head study examining GLP‐1 RA versus DPP‐4 inhibitor therapy in combination with basal insulin. It found additional reductions in HbA1c with both exenatide BID and sitagliptin QD when added to insulin glargine and metformin, compared with insulin glargine and metformin only. A greater reduction was seen with exenatide than with sitagliptin 93. The addition of DPP‐4 inhibitor therapy to basal insulin may be of particular clinical utility to certain patients. In particular, for patients who are averse to multiple daily injections, an orally administered DPP‐4 inhibitor may be preferable. In addition, the DPP‐4 inhibitors display favourable renal tolerability and can be used at reduced dosages in patients with renal insufficiency 94, 95, 96. In the case of linagliptin, no dose adjustment is recommended in patients with renal impairment 97. This, coupled with the low risk of hypoglycemic episodes make this class of therapy potentially useful for treatment in elderly patients, for whom renal complications and hypoglycemia are particularly dangerous.
Finally, incretin‐based therapy may be considered for use in latent autoimmune diabetes in adults (LADA). Although it is thought to be a distinct autoimmune form of diabetes [98,99], LADA is often difficult to distinguish from early‐stage T2DM. As patients with LADA often do not become insulin dependent for some time after diagnosis, other treatment options are required in the early stages. Data from small studies suggest that the DPP‐4 inhibitors linagliptin [100], saxagliptin [101], and sitagliptin [102] may protect β‐cell function in patients with LADA as demonstrated by improved C‐peptide levels. Possible mechanisms that could explain a potential attenuation of decline in C‐peptide levels using incretin‐based therapies include a β‐cell protective effect through elevation of endogenous GLP‐1 [103], and/or nonGLP‐1‐related mechanisms through modulation of peptides involved in cell signalling and autoimmune pathways [104]. As the GLP‐1 RAs preserve β‐cell function, it is possible that they may have potential for the treatment of LADA, although studies have not yet confirmed this [105]. There is a clear unmet need for treatment of patients with LADA, and further research is needed to determine the role of incretin‐based therapies in this population.
SGLT2 inhibitors may also be added to basal insulin therapy. This class of agents promotes urinary excretion of glucose, thereby limiting the amount of glucose that is reabsorbed into the blood from the kidneys 106. Currently, dapagliflozin, empagliflozin, and canagliflozin are available for the treatment of T2DM. All three are indicated in combination with basal insulin with or without metformin, providing stabilization of insulin doses and weight loss.
Conclusions
When patients fail to reach glucose targets with basal insulin treatment, regimen adjustment is required 2, 94, 95, 96. The addition of prandial insulin to basal insulin can be effective, but is problematic to titrate optimally and may be burdensome to the patient owing to weight gain and increased risk of hypoglycemia. DPP‐4 inhibitors and SGLT2 inhibitors are well tolerated and can be moderately effective in addition to basal insulin, although the DPP‐4 inhibitors have no weight‐sparing effects. The GLP‐1 RAs discussed here can be added to basal insulin to improve glycemic control; the differing mechanisms of action and half‐lives of these agents allow personalization of the regimen to suit the patient's individual needs. Given the contribution of PPG to overall HbA1c levels in patients with T2DM treated with basal insulin, addressing uncontrolled PPG excursions is an important therapeutic target in many patients. Owing to their primary effect of reducing PPG levels, prandial or short‐acting GLP‐1 RAs (i.e., exenatide and lixisenatide) represent an efficacious and well‐tolerated treatment option for addition to basal insulin to treat patients who require additional glycemic control. Current guidelines recommend the addition of a GLP‐1 RA or prandial insulin to basal insulin treatment in patients who do not reach glycemic control with other monotherapies or combination regimens 2.
Author contributions
All authors contributed equally to the selection of content and to preparation, critical revision, and final approval of the content of the manuscript. As corresponding author, Francesco Giorgino takes responsibility for the accuracy of the material presented and for the opinions offered in this article.
Author disclosures
Francesco Giorgino has participated in advisory boards for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, MSD, NovoNordisk, Sanofi, and has received research funding from Sanofi, AstraZeneca and Lifescan. Riccardo Bonadonna has participated in speaker bureaus for Sanofi, MSD, BMS, Eli Lilly, AstraZeneca and Janssen and in advisory boards for MSD, Eli Lilly and Sanofi. Sandro Gentile has no conflicts to declare. Roberto Vettor has participated in speaker bureaus for Sanofi, Novo Nordisk, AstraZeneca and in advisory boards for Novo Nordisk and Sanofi. Paolo Pozzilli has received research and travel grants from Boehringer Ingelheim, Sanofi, AstraZeneca, Lilly, Novo Nordisk and Medtronic. He has also received consultancy fees from Takeda, Merck Sharp and Dohme, and Lilly.
Funding
Editorial assistance was funded by Sanofi.
Giorgino, F. , Bonadonna, R. C. , Gentile, S. , Vettor, R. , and Pozzilli, P. (2016) Treatment intensification in patients with inadequate glycemic control on basal insulin: rationale and clinical evidence for the use of short‐acting and other glucagon‐like peptide‐1 receptor agonists. Diabetes Metab Res Rev, 32: 497–511. doi: 10.1002/dmrr.2775.
References
- 1. Raccah D, Bretzel RG, Owens D, Riddle M. When basal insulin therapy in type 2 diabetes mellitus is not enough‐‐what next? Diabetes Metab Res Rev 2007; 23(4): 257–264. [DOI] [PubMed] [Google Scholar]
- 2. Inzucchi SE, Bergenstal RM, Buse JB, et al Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38(1): 140–149. [DOI] [PubMed] [Google Scholar]
- 3. Meier JJ. GLP‐1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 2012; 8(12): 728–742. [DOI] [PubMed] [Google Scholar]
- 4. Nauck MA, Heimesaat MM, Behle K, et al Effects of glucagon‐like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab 2002; 87(3): 1239–1246. [DOI] [PubMed] [Google Scholar]
- 5. Meier JJ, Rosenstock J, Hincelin‐Mery A, et al Contrasting effects of lixisenatide and liraglutide on postprandial glycemic control, gastric emptying, and safety parameters in patients with type 2 diabetes on optimized insulin glargine with or without metformin: a randomized, open‐label trial. Diabetes Care 2015; 38(7): 1263–1273. [DOI] [PubMed] [Google Scholar]
- 6. Sanofi‐Aventis . Lyxumia® (lixisenatide) Summary of Product Characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Information/human/002445/WC500140401.pdf. Last accessed 10 October 2014.
- 7. AstraZeneca AB. Byetta® (exenatide) Summary of Product Characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Information/human/000698/WC500051845.pdf. Last accessed 10 October 2014.
- 8. Novo Nordisk AS. Victoza® (liraglutide) Summary of Product Characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Information/human/001026/WC500050017.pdf. Last accessed 10 October 2014.
- 9. GlaxoSmithKline . Esperzan® (albiglutide) Summary of Product Characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Information/human/002735/WC500165117.pdf. Last accessed 10 October 2014.
- 10. Drucker DJ, Buse JB, Taylor K, et al Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open‐label, non‐inferiority study. Lancet 2008; 372(9645): 1240–1250. [DOI] [PubMed] [Google Scholar]
- 11. Knudsen LB, Nielsen PF, Huusfeldt PO, et al Potent derivatives of glucagon‐like peptide‐1 with pharmacokinetic properties suitable for once daily administration. J Med Chem 2000; 43(9): 1664–1669. [DOI] [PubMed] [Google Scholar]
- 12. Buse JB, Rosenstock J, Sesti G, et al Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26‐week randomised, parallel‐group, multinational, open‐label trial (LEAD‐6). Lancet 2009; 374(9683): 39–47. [DOI] [PubMed] [Google Scholar]
- 13. Lorenz M, Pfeiffer C, Steinstrasser A, et al Effects of lixisenatide once daily on gastric emptying in type 2 diabetes ‐ relationship to postprandial glycemia. Regul Pept 2013; 185: C1–C8. [DOI] [PubMed] [Google Scholar]
- 14. Werner U. Effects of the GLP‐1 receptor agonist lixisenatide on postprandial glucose and gastric emptying‐‐preclinical evidence. J Diabetes Complications 2014; 28(1): 110–114. [DOI] [PubMed] [Google Scholar]
- 15. Ratner RE, Rosenstock J, Boka G. Dose‐dependent effects of the once‐daily GLP‐1 receptor agonist lixisenatide in patients with Type 2 diabetes inadequately controlled with metformin: a randomized, double‐blind, placebo‐controlled trial. Diabet Med 2010; 27(9): 1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Menge BA, Kapitza C, Hincelin‐Mery A, et al Impact of baseline gastric emptying on effects of lixisenatide and liraglutide in type 2 diabetes mellitus as add‐on to insulin glargine. Diabetologia 2014; 54(Suppl. 1): OP75. [Google Scholar]
- 17. Edwards CM, Stanley SA, Davis R, et al Exendin‐4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab 2001; 281(1): E155–E161. [DOI] [PubMed] [Google Scholar]
- 18. Riddle MC, Aronson R, Home P, et al Adding once‐daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24‐week, randomized, placebo‐controlled comparison (GetGoal‐L). Diabetes Care 2013; 36(9): 2489–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Riddle MC, Forst T, et al Adding once‐daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: a 24‐Week, randomized, placebo‐controlled study (GetGoal‐Duo 1). Diabetes Care 2013; 36(9): 2497–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seino Y, Min KW, Niemoeller E, Takami A. Randomized, double‐blind, placebo‐controlled trial of the once‐daily GLP‐1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal‐L‐Asia). Diabetes Obes Metab 2012; 14(10): 910–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Raccah D. Efficacy and safety of lixisenatide in the treatment of type 2 diabetes mellitus: a review of Phase III clinical data. Expert Rev Endocrinol Metab 2013; 8: 105–121. [DOI] [PubMed] [Google Scholar]
- 22. Matthews JE, Stewart MW, De Boever EH, et al Pharmacodynamics, pharmacokinetics, safety, and tolerability of albiglutide, a long‐acting glucagon‐like peptide‐1 mimetic, in patients with type 2 diabetes. J Clin Endocrinol Metab 2008; 93(12): 4810–4817. [DOI] [PubMed] [Google Scholar]
- 23. Bush MA, Matthews JE, De Boever EH, et al Safety, tolerability, pharmacodynamics and pharmacokinetics of albiglutide, a long‐acting glucagon‐like peptide‐1 mimetic, in healthy subjects. Diabetes Obes Metab 2009; 11(5): 498–505. [DOI] [PubMed] [Google Scholar]
- 24. Pratley RE, Nauck MA, Barnett AH, et al Once‐weekly albiglutide versus once‐daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open‐label, multicentre, non‐inferiority phase 3 study. Lancet Diabetes Endocrinol 2014; 2(4): 289–297. [DOI] [PubMed] [Google Scholar]
- 25. Barrington P, Chien JY, Tibaldi F, Showalter HD, Schneck K, Ellis B. LY2189265, a long‐acting glucagon‐like peptide‐1 analogue, showed a dose‐dependent effect on insulin secretion in healthy subjects. Diabetes Obes Metab 2011; 13(5): 434–438. [DOI] [PubMed] [Google Scholar]
- 26. Dungan KM, Povedano ST, Forst T, et al Once‐weekly dulaglutide versus once‐daily liraglutide in metformin‐treated patients with type 2 diabetes (AWARD‐6): a randomised, open‐label, phase 3, non‐inferiority trial. Lancet 2014; 384(9951): 1349–1357. [DOI] [PubMed] [Google Scholar]
- 27. Barrington P, Chien JY, Showalter HD, et al A 5‐week study of the pharmacokinetics and pharmacodynamics of LY2189265, a novel, long‐acting glucagon‐like peptide‐1 analogue, in patients with type 2 diabetes. Diabetes Obes Metab 2011; 13(5): 426–433. [DOI] [PubMed] [Google Scholar]
- 28. Umpierrez GE, Blevins T, Rosenstock J, Cheng C, Anderson JH, Bastyr EJ III. The effects of LY2189265, a long‐acting glucagon‐like peptide‐1 analogue, in a randomized, placebo‐controlled, double‐blind study of overweight/obese patients with type 2 diabetes: the EGO study. Diabetes Obes Metab 2011; 13(5): 418–425. [DOI] [PubMed] [Google Scholar]
- 29. Nauck MA, Kemmeries G, Holst JJ, Meier JJ. Rapid tachyphylaxis of the glucagon‐like peptide 1‐induced deceleration of gastric emptying in humans. Diabetes 2011; 60(5): 1561–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Horowitz M, Rayner CK, Jones KL. Mechanisms and clinical efficacy of lixisenatide for the management of type 2 diabetes. Adv Ther 2013; 30(2): 81–101. [DOI] [PubMed] [Google Scholar]
- 31. Holmes GM, Browning KN, Tong M, Qualls‐Creekmore E, Travagli RA. Vagally mediated effects of glucagon‐like peptide 1: in vitro and in vivo gastric actions. J Physiol 2009; 587(Pt 19): 4749–4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kapitza C, Forst T, Coester HV, Poitiers F, Ruus P, Hincelin‐Mery A. Pharmacodynamic characteristics of lixisenatide once daily versus liraglutide once daily in patients with type 2 diabetes insufficiently controlled on metformin. Diabetes Obes Metab 2013; 15(7): 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wysham C, Blevins T, Arakaki R, et al Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD‐1). Diabetes Care 2014; 37(8): 2159–2167. [DOI] [PubMed] [Google Scholar]
- 34. Brown RJ, Rother KI. Effects of beta‐cell rest on beta‐cell function: a review of clinical and preclinical data. Pediatr Diabetes 2008; 9(3 Pt 2): 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ferdaoussi M, Abdelli S, Yang JY, et al Exendin‐4 protects beta‐cells from interleukin‐1 beta‐induced apoptosis by interfering with the c‐Jun NH2‐terminal kinase pathway. Diabetes 2008; 57(5): 1205–1215. [DOI] [PubMed] [Google Scholar]
- 36. Natalicchio A, De Stefano F, Orlando MR, et al Exendin‐4 prevents c‐Jun N‐terminal protein kinase activation by tumor necrosis factor‐alpha (TNFalpha) and inhibits TNFalpha‐induced apoptosis in insulin‐secreting cells. Endocrinology 2010; 151(5): 2019–2029. [DOI] [PubMed] [Google Scholar]
- 37. Natalicchio A, Labarbuta R, Tortosa F, et al Exendin‐4 protects pancreatic beta cells from palmitate‐induced apoptosis by interfering with GPR40 and the MKK4/7 stress kinase signalling pathway. Diabetologia 2013; 56(11): 2456–2466. [DOI] [PubMed] [Google Scholar]
- 38. Tews D, Werner U, Eckel J. Enhanced protection against cytokine‐ and fatty acid‐induced apoptosis in pancreatic beta cells by combined treatment with glucagon‐like peptide‐1 receptor agonists and insulin analogues. Horm Metab Res 2008; 40(3): 172–180. [DOI] [PubMed] [Google Scholar]
- 39. Werner U, Haschke G, Herling AW, Kramer W. Pharmacological profile of lixisenatide: A new GLP‐1 receptor agonist for the treatment of type 2 diabetes. Regul Pept 2010; 164(2‐3): 58–64. [DOI] [PubMed] [Google Scholar]
- 40. Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care 2003; 26(6): 1902–1912. [DOI] [PubMed] [Google Scholar]
- 41. Russell‐Jones D, Khan R. Insulin‐associated weight gain in diabetes‐‐causes, effects and coping strategies. Diabetes Obes Metab 2007; 9(6): 799–812. [DOI] [PubMed] [Google Scholar]
- 42. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care 2003; 26(3): 881–885. [DOI] [PubMed] [Google Scholar]
- 43. Riddle M, Umpierrez G, DiGenio A, Zhou R, Rosenstock J. Contributions of basal and postprandial hyperglycemia over a wide range of A1C levels before and after treatment intensification in type 2 diabetes. Diabetes Care 2011; 34(12): 2508–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Buse JB, Bergenstal RM, Glass LC, et al Use of twice‐daily exenatide in basal insulin‐treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med 2011; 154(2): 103–112. [DOI] [PubMed] [Google Scholar]
- 45. Rosenstock J, Hanefeld H, Gentile S, et al. Advancing basal insulin glargine with prandial lixisenatide QD vs. insulin glulisine QD or TID in T2DM: the GetGoal‐Duo2 evidence‐based trial (NCT01768559). Diabetes 2015; 64(Suppl. 1): 107‐LB. [Google Scholar]
- 46. Vidal J, Aronson R, Giorgino F, et al. Therapeutic efficacy of lixisenatide added to basal insulin is greater when FPG is well‐controlled. Diabetologia 2013; 56(Suppl. 1): S9‐S10 (OP6). [Google Scholar]
- 47. Rosenstock J, Diamant M, Silvestre L, Souhami E, Zhou T, Fonseca V. Benefits of a fixed‐ratio formulation of once‐daily insulin glargine/lixisenatide (LixiLan) vs glargine in type 2 diabetes inadequately controlled on metformin. Diabetologia 2014; 57(Suppl. 1): 1–564. [Google Scholar]
- 48. Mathieu C, Rodbard HW, Cariou B, et al A comparison of adding liraglutide versus a single daily dose of insulin aspart to insulin degludec in subjects with type 2 diabetes (BEGIN: VICTOZA ADD‐ON). Diabetes Obes Metab 2014; 16(7): 3636–3644. [DOI] [PubMed] [Google Scholar]
- 49. ClinicalTrials.gov . Study of liraglutide in individuals with type 2 diabetes using insulin. Available from: https://clinicaltrials.gov/ct2/show/NCT01628445. Last updated 26 May 2015.
- 50. Buse JB, Vilsbøll T, Thurman J, et al Contribution of liraglutide in the fixed‐ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care 2014; 37(11): 2926–2933. [DOI] [PubMed] [Google Scholar]
- 51. Rosenstock J, Raccah D, Koranyi L, et al Efficacy and safety of lixisenatide once daily versus exenatide twice daily in type 2 diabetes inadequately controlled on metformin: a 24‐week, randomized, open‐label, active‐controlled study (GetGoal‐X). Diabetes Care 2013; 36(10): 2945–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Charbonnel B, Bertolini M, Tinahones FJ, Domingo MP, Davies M. Lixisenatide plus basal insulin in patients with type 2 diabetes mellitus: a meta‐analysis. J Diabetes Complications 2014; 28(6): 880–886. [DOI] [PubMed] [Google Scholar]
- 53. Gao Y, Yoon KH, Chuang LM, et al Efficacy and safety of exenatide in patients of Asian descent with type 2 diabetes inadequately controlled with metformin or metformin and a sulphonylurea. Diabetes Res Clin Pract 2009; 83(1): 69–76. [DOI] [PubMed] [Google Scholar]
- 54. Lahtela J, Ahmann A, Rodbard H, et al Efficacy and safety of liraglutide vs placebo when added to basal insulin analogues in subjects with type 2 diabetes (LIRA‐ADD2BASAL): a randomised, placebo‐controlled trial. Diabetologia 2014; 57(Suppl. 1): 1–564. [Google Scholar]
- 55. Hanefeld M, Ambos A, Arteaga J, et al Lixisenatide is effective and well tolerated in patients with type 2 diabetes mellitus and renal impairment. Diabetologia 2014; 57(Suppl. 1): P841. [Google Scholar]
- 56. Diamant M, Van Gaal L, Stranks S, et al Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION‐3): an open‐label randomised trial. Lancet 2010; 375(9733): 2234–2243. [DOI] [PubMed] [Google Scholar]
- 57. Blevins T, Pullman J, Malloy J, et al DURATION‐5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab 2011; 96(5): 1301–1310. [DOI] [PubMed] [Google Scholar]
- 58. Food and Drug Administration . Briefing document: Liraglutide injection, 3 mg. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM413317.pdf. Last accessed 3 February 2015.
- 59. Hara K, Aso Y, Nakamachi T, et al The effect of liraglutide in heart rate variability assessed by power spectral analysis on 24‐h electrocardiographic recording in patients with type 2 diabetes [abstract]. Diabetes 2014; 63(Suppl. 1): 1015‐P. [Google Scholar]
- 60. Laviola L, Leonardini A, Melchiorre M, et al Glucagon‐like peptide‐1 counteracts oxidative stress‐dependent apoptosis of human cardiac progenitor cells by inhibiting the activation of the c‐Jun N‐terminal protein kinase signaling pathway. Endocrinology 2012; 153(12): 5770–5781. [DOI] [PubMed] [Google Scholar]
- 61. Okerson T, Chilton RJ. The cardiovascular effects of GLP‐1 receptor agonists. Cardiovasc Ther 2012; 30(3): e146–e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocr Rev 2012; 33(2): 187–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 1999; 22(2): 233–240. [DOI] [PubMed] [Google Scholar]
- 64. Jackson CA, Yudkin JS, Forrest RD. A comparison of the relationships of the glucose tolerance test and the glycated haemoglobin assay with diabetic vascular disease in the community. The Islington Diabetes Survey. Diabetes Res Clin Pract 1992; 17(2): 111–123. [DOI] [PubMed] [Google Scholar]
- 65. Hanefeld M, Fischer S, Julius U, et al Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11‐year follow‐up. Diabetologia 1996; 39(12): 1577–1583. [DOI] [PubMed] [Google Scholar]
- 66. DECODE Study Group . Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Lancet 1999; 354(9179): 617–621. [PubMed] [Google Scholar]
- 67. Cavalot F, Pagliarino A, Valle M, et al Postprandial blood glucose predicts cardiovascular events and all‐cause mortality in type 2 diabetes in a 14‐year follow‐up: lessons from the San Luigi Gonzaga Diabetes Study. Diabetes Care 2011; 34(10): 2237–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Raz I, Wilson PW, Strojek K, et al Effects of prandial versus fasting glycemia on cardiovascular outcomes in type 2 diabetes: the HEART2days trial. Diabetes Care 2009; 32(3): 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Raz I, Ceriello A, Wilson PW, et al Post hoc subgroup analysis of the HEART2D trial demonstrates lower cardiovascular risk in older patients targeting postprandial versus fasting/premeal glycemia. Diabetes Care 2011; 34(7): 1511–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. ClinicalTrials.gov . Evaluation of cardiovascular outcomes in patients with type 2 diabetes after acute coronary syndrome during treatment with AVE0010 (lixisenatide) (ELIXA). Available from: http://clinicaltrials.gov/show/NCT01147250. Last updated 10 March 2015.
- 71. ClinicalTrials.gov . Researching cardiovascular events with a weekly incretin in diabetes (REWIND). Available from: http://clinicaltrials.gov/show/NCT01394952. Last updated 8 September 2014.
- 72. ClinicalTrials.gov . Liraglutide effect and action in diabetes: evaluation of cardiovascular outcome results ‐ a long term evaluation (LEADER®). Available from: http://clinicaltrials.gov/show/NCT01179048. Last updated 7 May 2015.
- 73. ClinicalTrials.gov . Exenatide study of cardiovascular event lowering trial (EXSCEL): a trial to evaluate cardiovascular outcomes after treatment with exenatide once weekly in patients with type 2 diabetes mellitus. Available from: http://clinicaltrials.gov/show/NCT01144338. Last updated 29 May 2015.
- 74. Bonadonna RC, Blonde L, Antsiferov MB, et al. Lixisenatide as add‐on treatment among patients with different B‐cell function levels as assessed by HOMA‐B index. Diabetes 2014; 63(Suppl. 1): 1018‐P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yabe D, Ambos A, Cariou B, et al. Adjunctive lixisenatide treatment improves glycemic control in patients with T2DM irrespective of ß‐Cell function. Diabetes 2014; 63(Suppl. 2): 978‐P. [Google Scholar]
- 76. Hanefeld M, Berria R, Lin J, et al Lixisenatide as a treatment for older patients with type 2 diabetes mellitus insufficiently controlled on oral antidiabetics: a meta‐analysis of five randomized controlled trials. Adv Ther 2014; 31(8): 861–872. [DOI] [PubMed] [Google Scholar]
- 77. Raccah D, Miossec P, Esposito V, Niemoeller E, Cho M, Gerich J. Efficacy and safety of lixisenatide in elderly (>65 years) and very elderly (>75 years) patients with type 2 diabetes: an analysis from the Phase 3 GetGoal program. Diabetes Metab Res Rev 2015; 31(2): 204–211. [DOI] [PubMed] [Google Scholar]
- 78. Seino Y, Takami A, Boka G, Niemoeller E, Raccah D. Pharmacodynamics of the glucagon‐like peptide‐1 receptor agonist lixisenatide in Japanese and Caucasian patients with type 2 diabetes mellitus poorly controlled on sulphonylureas with/without metformin. Diabetes Obes Metab 2014; 16(8): 739–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Li CJ, Li J, Zhang QM, et al Efficacy and safety comparison between liraglutide as add‐on therapy to insulin and insulin dose‐increase in Chinese subjects with poorly controlled type 2 diabetes and abdominal obesity. Cardiovasc Diabetol 2012; 11: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Diamant M, Nauck MA, Shaginian R, et al Glucagon‐like peptide 1 receptor agonist or bolus insulin with optimized basal insulin in type 2 diabetes. Diabetes Care 2014; 37(10): 2763–2773. [DOI] [PubMed] [Google Scholar]
- 81. Raccah D, Lin J, Wang E, et al Once‐daily prandial lixisenatide versus once‐daily rapid‐acting insulin in patients with type 2 diabetes mellitus insufficiently controlled with basal insulin: analysis of data from five randomized, controlled trials. J Diabetes Complications 2014; 28(1): 40–44. [DOI] [PubMed] [Google Scholar]
- 82. Rosenstock J, Fonseca VA, Gross JL, et al Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP‐1 receptor agonist, versus thrice‐daily prandial insulin lispro. Diabetes Care 2014; 37(8): 2317–2325. [DOI] [PubMed] [Google Scholar]
- 83. ClinicalTrials.gov . Efficacy and safety of lixisenatide versus insulin glulisine on top of insulin glargine with or without metformin in type 2 diabetic patients (GetGoal Duo‐2). Available from: https://clinicaltrials.gov/show/NCT01768559. Last updated 19 January 2015.
- 84. Richter B, Bandeira‐Echtler E, Bergerhoff K, Lerch CL. Dipeptidyl peptidase‐4 (DPP‐4) inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev 2008; 2: CD006739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Doucet J, Chacra A, Maheux P, Lu J, Harris S, Rosenstock J. Efficacy and safety of saxagliptin in older patients with type 2 diabetes mellitus. Curr Med Res Opin 2011; 27(4): 863–869. [DOI] [PubMed] [Google Scholar]
- 86. Singh‐Franco D, Laughlin‐Middlekauff J, Elrod S, Harrington C. The effect of linagliptin on glycaemic control and tolerability in patients with type 2 diabetes mellitus: a systematic review and meta‐analysis. Diabetes Obes Metab 2012; 14(8): 694–708. [DOI] [PubMed] [Google Scholar]
- 87. Gerrald KR, Van Scoyoc E, Wines RC, Runge T, Jonas DE. Saxagliptin and sitagliptin in adult patients with type 2 diabetes: a systematic review and meta‐analysis. Diabetes Obes Metab 2012; 14(6): 481–492. [DOI] [PubMed] [Google Scholar]
- 88. Sherifali D, Nerenberg K, Pullenayegum E, Cheng JE, Gerstein HC. The effect of oral antidiabetic agents on A1C levels: a systematic review and meta‐analysis. Diabetes Care 2010; 33(8): 1859–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Deacon CF, Mannucci E, Ahren B. Glycaemic efficacy of glucagon‐like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors as add‐on therapy to metformin in subjects with type 2 diabetes‐a review and meta analysis. Diabetes Obes Metab 2012; 14(8): 762–767. [DOI] [PubMed] [Google Scholar]
- 90. Liu SC, Tu YK, Chien MN, Chien KL. Effect of antidiabetic agents added to metformin on glycaemic control, hypoglycaemia and weight change in patients with type 2 diabetes: a network meta‐analysis. Diabetes Obes Metab 2012; 14(9): 810–820. [DOI] [PubMed] [Google Scholar]
- 91. Aroda VR, Henry RR, Han J, et al Efficacy of GLP‐1 receptor agonists and DPP‐4 inhibitors: meta‐analysis and systematic review. Clin Ther 2012; 34(6): 1247–1258. [DOI] [PubMed] [Google Scholar]
- 92. Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta‐analysis. JAMA 2007; 298(2): 194–206. [DOI] [PubMed] [Google Scholar]
- 93. Arnolds S, Dellweg S, Clair J, et al Further improvement in postprandial glucose control with addition of exenatide or sitagliptin to combination therapy with insulin glargine and metformin: a proof‐of‐concept study. Diabetes Care 2010; 33(7): 1509–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chan JC, Scott R, Arjona Ferreira JC, et al Safety and efficacy of sitagliptin in patients with type 2 diabetes and chronic renal insufficiency. Diabetes Obes Metab 2008; 10(7): 545–555. [DOI] [PubMed] [Google Scholar]
- 95. Engel SS, Round E, Golm GT, Kaufman KD, Goldstein BJ. Safety and tolerability of sitagliptin in type 2 diabetes: pooled analysis of 25 clinical studies. Diabetes Ther 2013; 4(1): 119–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Williams‐Herman D, Engel SS, Round E, et al Safety and tolerability of sitagliptin in clinical studies: a pooled analysis of data from 10,246 patients with type 2 diabetes. BMC Endocr Disord 2010; 10: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ingelheim Boehringer. Tradjenta® (linagliptin) Prescribing Information. http://bidocs.boehringer‐ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing+Information/PIs/Tradjenta/Tradjenta.pdf. Last accessed 20 January 2015.
- 98. Hawa MI, Kolb H, Schloot N, et al Adult‐onset autoimmune diabetes in Europe is prevalent with a broad clinical phenotype: Action LADA 7. Diabetes Care 2013; 36(4): 908–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Mollo A, Hernandez M, Marsal JR, et al Latent autoimmune diabetes in adults is perched between type 1 and type 2: evidence from adults in one region of Spain. Diabetes Metab Res Rev 2013; 29(6): 446–451. [DOI] [PubMed] [Google Scholar]
- 100. Johansen OE, Boehm BO, Grill V, et al C‐peptide levels in latent autoimmune diabetes in adults treated with linagliptin versus glimepiride: exploratory results from a 2‐year double‐blind, randomized, controlled study. Diabetes Care 2014; 37(1): e11–e12. [DOI] [PubMed] [Google Scholar]
- 101. Pozzilli P, Buzzetti R, Frederich R, Iqbal N, Hirshberg B. Saxagliptin increases B‐cell function and improves HOMA index in patients with latent autoimmune diabetes in adults. Diabetes 2014; 63(Suppl. 1): OR152. [Google Scholar]
- 102. Zhao Y, Yang L, Xiang Y, et al Dipeptidyl peptidase 4 inhibitor sitagliptin maintains beta‐cell function in patients with recent‐onset latent autoimmune diabetes in adults: one year prospective study. J Clin Endocrinol Metab 2014; 99(5): E876–E880. [DOI] [PubMed] [Google Scholar]
- 103. Drucker DJ. Biologic actions and therapeutic potential of the proglucagon‐derived peptides. Nat Clin Pract Endocrinol Metab 2005; 1(1): 22–31. [DOI] [PubMed] [Google Scholar]
- 104. Ohnuma K, Hosono O, Dang NH, Morimoto C. Dipeptidyl peptidase in autoimmune pathophysiology. Adv Clin Chem 2011; 53: 51–84. [DOI] [PubMed] [Google Scholar]
- 105. Cernea S, Buzzetti R, Pozzilli P. Beta‐cell protection and therapy for latent autoimmune diabetes in adults. Diabetes Care 2009; 32(Suppl. 2): S246–S252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hardman TC, Dubrey SW. Development and potential role of type‐2 sodium‐glucose transporter inhibitors for management of type 2 diabetes. Diabetes Ther 2011; 2(3): 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]


