Abstract
Objective
End-stage renal disease (ESRD) is a major consequence of lupus nephritis, but how this risk has changed over time is unknown. We conducted this systematic review to examine changes in ESRD among adults with lupus nephritis from 1971 to 2015 and to estimate risks of ESRD among contemporary patients.
Methods
We searched PubMed, Embase, and the Cochrane Database of Systematic Reviews for cohort studies and clinical trials on ESRD in adults with lupus nephritis. We analyzed studies from developed and developing countries separately. The outcome was probability of ESRD at 5, 10, and 15 years of lupus nephritis.
Results
We included 187 articles that reported on 18,309 patients. In developed countries, the 5-year risk of ESRD decreased from 16% (95% confidence interval [95% CI] 14–17%) in 1970–1979 to 11% (95% CI 10–12%) in the mid-1990s and then plateaued. ESRD risks at 10 years and 15 years showed steeper declines in the 1970s and 1980s but also plateaued in 1993–1997, with a notable increase in the late 2000s. The decrease in risk after 1980 coincided with increased use of cyclophosphamide. The 15-year ESRD risk was higher in developing countries than in developed countries. Patients with class IV lupus nephritis had the greatest risk of ESRD, with a 15-year risk of 44% during the 2000s.
Conclusion
Risks of ESRD in lupus nephritis improved between the 1970s and the mid-1990s and then plateaued, with an increase in the late 2000s. This pattern suggests limitations in the effectiveness of, or access to, current treatments.
Lupus nephritis affects more than one-half of patients with systemic lupus erythematosus (SLE) and is the most common serious manifestation of the disease (1). End-stage renal disease (ESRD) is the most important and costly complication of lupus nephritis. Patients with lupus nephritis have a 26-fold increased risk of death, and annual health care costs in 2006 were estimated to be between $43,000 and $107,000 per patient (2,3). The impact of ESRD due to lupus nephritis is particularly high because 73% of patients are younger than age 50 (4). ESRD has been reported to develop in 10–30% of patients with lupus nephritis, but these estimates are based on selected studies (5,6). Contemporary aggregate estimates of the risk of ESRD are needed to provide patients with accurate prognostic information.
Examination of trends in ESRD risks can also indicate whether advances in treatment strategies have resulted in improved long-term health outcomes. The treatment of lupus nephritis has changed greatly over the past 40 years, after identification of proliferative histologic subtypes as a key prognostic factor and with the adoption of more aggressive immunosuppressive regimens (7–10). Following influential clinical trials in the 1980s, corticosteroid monotherapy was replaced with combination treatment with corticosteroids and either azathioprine or cyclophosphamide (11). High-dose intravenous cyclophosphamide became the standard therapy for severe lupus nephritis (12). In the late 1990s, mycophenolate mofetil was shown to be efficacious, and new induction and maintenance regimens were introduced (13–15). Whether these advances have affected the risk of ESRD is unclear, as progression to ESRD often takes many years and is not commonly tested in short-term clinical trials. Despite the availability of efficacious treatments, limitations in access to care and intermittent adherence to treatment are important economic, social, and behavioral factors that can result in delayed diagnosis and poor outcomes (4,16–18). While these barriers exist in developed countries, they may be more pronounced in developing countries.
We conducted a systematic review and Bayesian meta-analysis to provide contemporary estimates of the risk of ESRD in adults with lupus nephritis, and to investigate how this risk has changed over the past several decades, given the treatment advances that have occurred. We hypothesized that with the increased use of immunosuppressive medications, the risks of ESRD at 5 years, 10 years, and 15 years of lupus nephritis will have progressively decreased over time, and to a greater degree in developed than in developing countries.
MATERIALS AND METHODS
Data sources and searches
We performed a systematic literature review to identify all published studies on the risk of ESRD in adults (age ≥18 years) with lupus nephritis. We followed a prespecified protocol and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (19) and Meta-analysis of Observational Studies in Epidemiology recommendations (20). We searched PubMed, Embase, and the Cochrane Database of Systematic Reviews from their inceptions to April 7, 2015. The search strategy was developed with a medical informationist (see Supplementary Appendix A, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39594/abstract). We also searched the reference lists of these studies and review articles. The study was exempted from human subjects review by the National Institutes of Health Office of Human Subjects Research.
Study selection
Two authors (MGT and MMW) independently reviewed the titles and abstracts and, when necessary, full texts to determine eligibility. We included articles without date or language restrictions. We excluded case series, reviews, and abstracts, studies of children, studies with fewer than 10 patients or 12 months of follow-up or less, studies focused on patients with advanced kidney disease (ESRD or rapidly progressive glomerulonephritis) at study entry, studies with incomplete data on the number of patients with ESRD, the number of patients at risk, or follow-up, and studies reporting only composite end points, such as ESRD and death. For studies published since 2000 that had incomplete data on ESRD or follow-up, we contacted study authors for this information. When more than one article on a cohort was available, we included the article with the longest follow-up or largest sample.
Data extraction and quality assessment
Two authors (MGT and MMW) independently performed data extraction and quality assessment using a dedicated protocol. Discrepancies were resolved by consensus. We extracted data on study design, calendar years of enrollment and follow-up, patient characteristics, SLE duration (time between SLE diagnosis and either renal biopsy or study entry), duration of lupus nephritis (time between clinical diagnosis of lupus nephritis and either renal biopsy or study entry), nephritis characteristics, and immunosuppressive treatment.
The outcome was ESRD, defined as renal dialysis or renal transplantation. If studies reported ESRD outcomes separately by renal histologic class or calendar years, we extracted these data.
We assessed study quality using 10 items in 3 domains, adapted from the Newcastle-Ottawa scale: representativeness of the cohort, adequacy of follow-up, and completeness of description of enrollment criteria and treatment (21) (see Supplementary Appendix B, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39594/abstract). We considered representativeness as the most relevant indicator of study quality for this analysis, and we therefore rated as high quality studies of inception cohorts that did not require histologic diagnosis of lupus nephritis and did not exclude patients with renal failure at study entry.
Data synthesis and analysis
In the main analysis, we included all studies that enrolled patients based on a clinical diagnosis of lupus nephritis or that required a histologic diagnosis of lupus nephritis but included patients with at least 2 histologic classes of lupus nephritis. These studies reflected a spectrum of patients with lupus nephritis, in contrast to studies of patients in a single histologic class. We also performed a second analysis of studies that reported histologic class–specific results. Here, we computed ESRD risks separately for patients with class III, class IV, and class V lupus nephritis, omitting other classes because of their very low risk of ESRD.
Risks of ESRD were reported in the primary studies in 3 different ways: as Kaplan-Meier curves, as proportions with ESRD at specific follow-up times (without including a time-to-event figure), and as the proportion with ESRD over the period of observation. These different types of data required different approaches to obtain summary estimates amenable to pooling. For studies with Kaplan-Meier curves and time-to-event proportions, we used the published information to generate individual patient data, from which 5-year, 10-year, and 15-year risks could be estimated (22) (see Supplementary Appendix C, available at http://onlinelibrary.wiley.com/doi/10.1002/art.39594/abstract). We validated this approach by comparing estimates of ESRD risks obtained using this method to observed risks in a study for which we had primary data on individual patients (23). In this validation, Kaplan-Meier curves from the reconstructed data differed from the original Kaplan-Meier curves by at most 3 percentage points over the range of the data, and were within 1 percentage point for more than 95% of the follow-up. For studies that reported only the proportion with ESRD, we used this proportion.
Each primary study contributed a single summary of ESRD risk. We assumed that the time to ESRD followed a Weibull distribution. We used Bayesian estimation using Markov chain Monte Carlo methods to derive posterior distributions of the Weibull estimates (24). Studies with different follow-up times, including those shorter than 5 years, could therefore be included because each study contributed to the likelihood function for the parameters. We used these Weibull estimates to obtain 5-year, 10-year, and 15-year ESRD risks. We accounted for left truncation, where the start of patient follow-up was some time after the onset of SLE or lupus nephritis, in the estimation of time to ESRD. Detailed statistical methods are provided in Supplementary Appendix C, online at http://onlinelibrary.wiley.com/doi/10.1002/art.39594/abstract.
To investigate changes in risk over time, we segregated the calendar time covered by the studies into overlapping 5-year intervals. We included a given study in each interval from the calendar year of the midpoint of its enrollment period to the calendar year of the study end, and we fit the Bayesian model to data from studies included in each interval. We then sequentially re-estimated the model with data from subsequent intervals. This analysis produced 5-, 10-, and 15-year risks and corresponding 95% confidence intervals (95% CIs) for each calendar window, analogous to a moving average. For example, a study with an enrollment midpoint of 1990 and an end date of 1996 would contribute to 11 calendar windows (i.e., 1986–1990 through 1996–2000). Small variations in the curves resulted from individual studies entering or leaving a given calendar window. Because time-to-event data were needed to provide stable posteriors, we omitted years that did not have at least one study with either Kaplan-Meier or summary survival data.
In a sensitivity analysis, we set the maximum time that studies contributed to ESRD risks to 10 years after the midpoint of enrollment, rather than the end of follow-up. This analysis reduced the number of years that studies with very long follow-up continued to contribute to ESRD risk estimates, when treatment practices may have changed.
We separately analyzed studies from developed countries (high income) and developing countries (low to middle income), as classified by World Bank criteria at the midpoint year of patient enrollment, because differences in access to care may affect ESRD risks (25). This classification has been used previously in studies of global differences in health outcomes (26,27).
We examined trends in the mean serum creatinine level at study entry and mean time between lupus nephritis diagnosis and study entry as potential sources of variation in ESRD risks over time. Lower serum creatinine levels or shorter delays would be expected to be associated with lower risks of ESRD. Too few studies reported data on creatinine clearance (n = 45) or estimated glomerular filtration rate (n = 21) to use these measures as markers of treatment delay.
Analyses were done using R version 3.0.2 and Just Another Gibbs Sampler version 3.0 (24).
RESULTS
Study characteristics
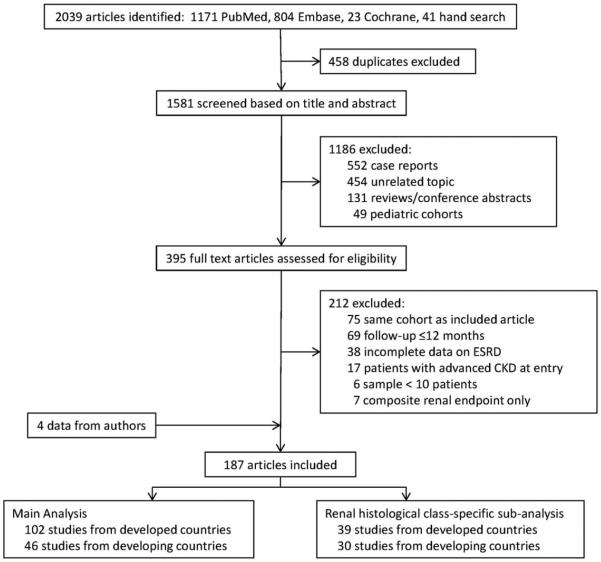
We included 187 studies that had investigated 18,309 patients (Figure 1) (see Supplementary Appendices D and E, online at http://onlinelibrary.wiley.com/doi/10.1002/art.39594/abstract). These included 16 prospective observational studies, 128 retrospective observational studies, and 43 clinical trials published from 1971 to 2015. Thirty-six percent of studies were from Europe, 26% from North America, 25% from Asia, 7% from Africa or the Mideast, 5% from Latin America, and 0.05% from Australia. Across all studies, 86% of patients were women, with a mean age of 31.2 years and a mean duration of lupus nephritis of 2.7 years. The median patient follow-up was 5.0 years (range 1.0–20.0 years). At study entry, 32% of patients had an elevated serum creatinine level (defined individually by study), and proteinuria averaged 3.6 gm daily. Forty-six studies (25%) specifically excluded patients with chronic kidney disease at entry. The ethnic composition of study cohorts in developed countries did not vary over time (see Supplementary Figure 1, online at http://onlinelibrary.wiley.com/doi/10.1002/art.39594/abstract).
Figure 1.
Flow diagram of the literature search and study inclusion. ESRD = end-stage renal disease; CKD = chronic kidney disease.
ESRD risks
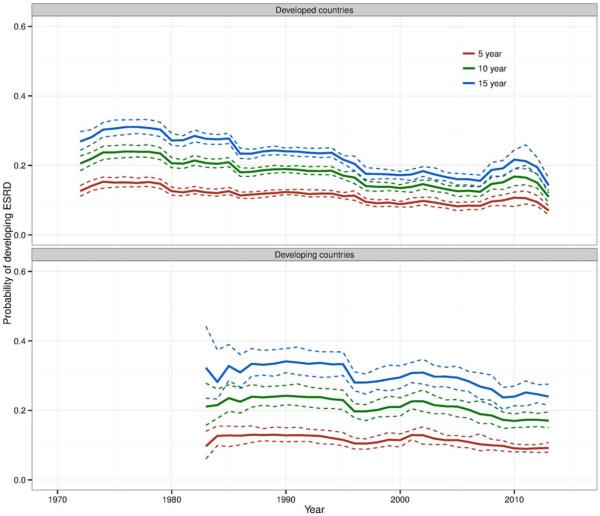
The main analysis of ESRD risk included 102 studies from developed countries and 46 studies from developing countries. The contribution of each study to ESRD risk estimates over time is shown in Supplementary Figures 2 and 3, online at http://onlinelibrary.wiley.com/doi/10.1002/art.39594/abstract. Renal biopsy confirmation of lupus nephritis was reported in 84% of patients in studies from developed countries and in 83% of patients in studies from developing countries. Among studies in developed countries, the estimated risk of ESRD at 5 years of lupus nephritis in 1970–1979 was 16% (95% CI 14–17%) (Figure 2). This risk decreased gradually until the mid-1990s, when it plateaued at 11% (95% CI 10–12%). ESRD risks at 10 years and 15 years showed steeper declines in the 1970s and 1980s, but also plateaued in 1993–1997 at 17% (95% CI 16–18%) and 22% (95% CI 20–23%), respectively. There was a notable increase in risks in the late 2000s, particularly in the 10-year and 15-year estimates, but these did not appear to persist after 2011.
Figure 2.
Estimated risk (solid lines) of end-stage renal disease (ESRD) at 5 years, 10 years, and 15 years of lupus nephritis in developed countries (105 studies) and developing countries (51 studies), by calendar year of observation, for studies of patients with a clinical diagnosis of lupus nephritis or reporting results for more than one renal histologic class of lupus nephritis. Each individual study contributed to risks starting at the median year of its enrollment period and extending to the end of its observation period. Dashed lines represent 95% confidence limits.
Data from developing countries did not extend prior to 1984, which limited the assessment of trends (Figure 2). ESRD risks at 5 years and 10 years were relatively stable over time at 12% (95% CI 11–13%) and 19% (95% CI 18–20%), respectively, while there was a slight decrease in the 15-year risk to 26% in the late 2000s. ESRD risks at 5 years were only slightly higher in developing countries than in developed countries during the 2000s, but 15-year risks were 10 percentage points higher in developing countries (see Supplementary Figure 4, online at http://onlinelibrary.wiley.com/doi/10.1002/art.39594/abstract).
ESRD risks were higher in clinical trials than in observational studies (see Supplementary Figure 5, online at http://onlinelibrary.wiley.com/doi/10.1002/art.39594/abstract). Because trials were of shorter duration and tended to include fewer patients, the overall results closely reflected those of the observational studies. Sixteen studies were designated high quality (see Supplementary Appendix F, online at http://onlinelibrary.wiley.com/doi/10.1002/art.39594/abstract), which provided too little data for meaningful analysis (see Supplementary Figure 6, online at http://onlinelibrary.wiley.com/doi/10.1002/art.39594/abstract).
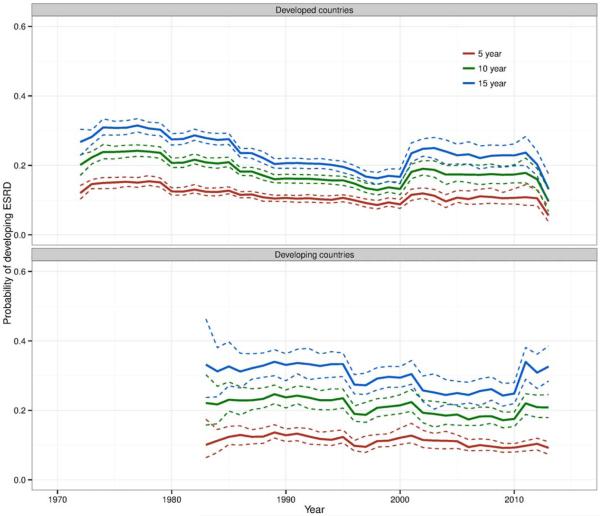
Results of the sensitivity analysis in which the contributions of long-term studies were truncated to 10 years after the midpoint of enrollment showed a similar pattern, but with a more pronounced decline in the 1990s and a more prominent increase after 2000 among studies from developed countries (Figure 3). This suggests that including long-term studies in windows after 10 years tended to attenuate changes in ESRD risks over time.
Figure 3.
Estimated risk (solid lines) of end-stage renal disease (ESRD) at 5 years, 10 years, and 15 years in developed and developing countries, including data for given studies from the midpoint of enrollment to the end of observation, truncating the study’s contribution at 10 years after the midpoint of enrollment for any study for which follow-up longer than 10 years was reported. Dashed lines represent 95% confidence limits.
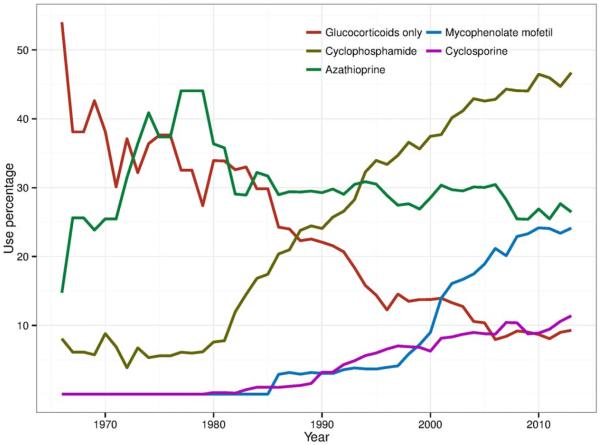
Over time, the proportion of patients treated with glucocorticoids alone in these studies decreased from 54% in 1966 to 9% in 2010, while the proportion treated with immunosuppressive agents increased (Figure 4). The decrease in ESRD risk after 1980 corresponded to increased use of immunosuppressive medications, particularly cyclophosphamide. Use of azathioprine remained stable in recent years, while studies reporting the use of mycophenolate mofetil and cyclosporine increased. The serum creatinine level at study entry and the time between onset of lupus nephritis and study entry were stable over time, suggesting no substantial differences in the time to presentation or treatment initiation among early and later studies (see Supplementary Figure 7, online at http://onlinelibrary.wiley.com/doi/10.1002/art.39594/abstract).
Figure 4.
Trends in corticosteroid and immunosuppressive treatment among studies of lupus nephritis.
Renal histologic class–specific ESRD risks
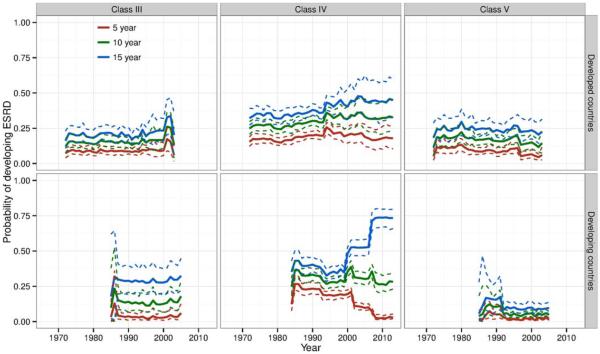
Histologic class–specific results were from 39 studies with 70 class-specific strata (17 class III, 34 class IV, and 19 class V) from developed countries, and from 30 studies with 51 class-specific strata (11 class III, 22 class IV, and 18 class V) from developing countries (see Supplementary Figure 8, online at http://onlinelibrary.wiley.com/doi/10.1002/art.39594/abstract). ESRD risks in these studies showed less evidence of improvement between the 1970s and 1990s (Figure 5) than did ESRD risks in studies in the main analysis. There was more volatility in risks from 1995 to 2010 in studies from developed countries, with slowly increasing risks in recent years in the class IV group. ESRD risks were highest among patients with class IV lupus nephritis, with 5-year, 10-year, and 15-year risks of 19% (95% CI 12–29%), 33% (95% CI 22–44%), and 44% (95% CI 32–56%), respectively, in studies from developed countries from 2000 to 2006 (Figure 5). Five-year risks in patients with class V (membranous) lupus nephritis were low and generally decreased over time. The 5-year, 10-year, and 15-year risks in patients with class V lupus nephritis in developed countries from 2000 to 2006 were 4% (95% CI 2–8%), 11% (95% CI 6–18%), and 20% (95% CI 13–28%), respectively.
Figure 5.
Estimated risk (solid lines) of end-stage renal disease (ESRD) at 5 years, 10 years, and 15 years of class III, class IV, and class V lupus nephritis in developed countries (17, 34, and 19 studies, respectively) and developing countries (11, 22, and 18 studies, respectively), by calendar year of observation. Each individual study contributed to risks starting at the median year of its enrollment period and extending to the end of its observation period. Dashed lines represent 95% confidence limits. Estimates at years in the tails of the data range in studies from developing countries reflect sparse data.
DISCUSSION
In this meta-analysis, the risks of ESRD in lupus nephritis in developed countries decreased substantially from the 1970s to the mid-1990s, but have since remained largely unchanged. Although historical data were limited, ESRD risks in developing countries also did not decrease during the 2000s. Results were similar when only observational studies were examined.
Although there is a clinical impression that ESRD risks in lupus nephritis have decreased over the past 40 years, few studies have examined changes in these risks. Of 5 single-center cohort studies that compared trends in ESRD between the 1970s or early 1980s and the 1990s or early 2000s, only 1 study showed improvement (6,17,28–30). In that study, 6 of 15 patients who presented with lupus nephritis in 1980–1989 had disease that progressed to ESRD, compared to none of 41 patients who presented with lupus nephritis in 1990–2000, although the follow-up in the latter group was only 2 years (30). National statistics from the US indicated that the incidence of treated ESRD due to lupus nephritis increased from 1982 to 1995, proportional to that of other causes of ESRD, and then plateaued but did not decrease between 1996 and 2004 (31).
Our analysis documents for the first time clear improvement in ESRD risk between the 1970s and mid-1990s in developed countries, with absolute decreases of 10% in 10-year and 15-year risks. Less marked improvement was seen in 5-year risks and in developing countries. The improvement coincided with increased use of immunosuppressive agents, particularly cyclophosphamide. While the use of immunosuppressive treatments likely played a major role in the decrease in ESRD risks, other factors may have contributed, including better control of hypertension and proteinuria. Delays in diagnosis and treatment are associated with risk of ESRD in patients with lupus nephritis, but we did not find temporal trends in baseline renal function or time to diagnosis that might have accounted for improvements in ESRD risk among these studies (17,30,32,33). These results should not imply a lack of improvement in time to diagnosis, the study of which would require assessment of patients in the same locality over decades. Attribution of temporal improvements in this study to any given factor, including individual medications, is difficult because these associations are necessarily ecological. However, examining outcomes in association with changes in treatment strategies over time provides a means to learn about long-term effectiveness.
We did not observe a similar decrease in ESRD risks between 1970 and 1990 in the histologic class–specific analyses, principally because ESRD risks in the class-specific analyses were low in the early years. For example, in developed countries, the 5-year ESRD risk in patients with class IV lupus nephritis in 1970–1979 was 15%. This risk was similar to the 16% risk in the main analysis that included all histologic classes, despite class IV being the subclass with the highest risk. This pattern suggests that in the early years, class-specific studies with good outcomes may have been selectively reported.
Despite extensive use of immunosuppressive medications through the 2000s, we did not find continued improvement in ESRD risks; instead, we found a slight increase in risks in the late 2000s. The increase did not appear to be attributable to increased representation of ethnic minorities, who may be more likely to develop ESRD, in recent studies (34–36). In the main analysis, trends suggest that this increase may have been temporary, but further follow-up will be needed to determine if this is sustained. Among the histologic class–specific studies, the increase was most notable in the class IV group, where it was persistent. This pattern suggests that changes specific to patients with this class of lupus nephritis may be responsible for the trends seen in the overall group. The most prevalent recent change in this subset of patients has been a shift in treatment away from high-dose cyclophosphamide, raising the question of whether these new treatment approaches result in different long-term outcomes.
More broadly, the plateau in risk since the mid-1990s might be interpreted to mean that the limits of effectiveness of current treatments have been reached, and that further improvements in lupus nephritis outcomes will require new treatments. However, it is also possible that the plateau primarily reflects lack of progress in the way currently available and effective treatments are deployed. This includes health system factors that result in delays in treatment initiation and patient and health system factors that result in treatment interruptions and reduced adherence (4,18,34,37). Other potential explanations for the recent increase in ESRD risk, specifically among patients with proliferative nephritis, include an increased number of clinical trials in recent years or the inclusion of more severely affected patients generally. However, in developed countries, the increase was most prominent in observational studies, and the serum creatinine level at study entry and the time between onset of lupus nephritis and study entry were stable over time.
We anticipated that health system barriers would be more prominent in developing countries and may have been responsible for the higher 10-year and 15-year ESRD risks in developing countries. For example, in 2 recent studies, ESRD prevalence was 21% after a mean of 2 years in a Moroccan cohort and 30% after a mean of 4.7 years in a South African cohort (38,39). High serum creatinine levels at baseline in both cohorts suggested delays in diagnosis. We did not find differences in ESRD risks between developed and developing countries in studies of patients who had undergone renal biopsies, likely because access to a biopsy in developing countries was an indicator of access to high-quality care. In recent studies, the largest proportion of risk occurred in the first 5 years of lupus nephritis. This pattern suggests that response to treatment early in the course of lupus nephritis is critical in the preservation of renal function. Later risk may be a consequence of scarring, recurrent renal inflammation, and transformation to more aggressive subtypes.
Our study is limited in that we did not estimate risks beyond 15 years, because few studies included longer follow-up. We included both clinical trials and observational studies to provide a broader assessment of outcomes, but findings were similar when only observational studies were considered. We did not examine outcomes other than ESRD. However, ESRD is arguably the most important renal consequence of lupus nephritis. Improvements in survival over time could have increased the proportion of patients living with lupus nephritis and therefore at risk of ESRD. However, decreases in ESRD risks were seen during the 1970s and early 1980s, a period during which survival improved in patients with SLE (40). Short-term survival in SLE has been largely stable since the late 1980s, suggesting that changes in patient survival are not responsible for the trends in ESRD that we observed (41). Changes in ESRD risks due to improved survival would be expected to have been more gradual than those that we found. Due to limited data, we did not examine other factors that have been associated with ESRD in lupus nephritis, including primary treatment responses, renal flares, and uncontrolled hypertension. Few studies were judged to be of high quality. We also do not know the extent to which publication bias may have influenced the results.
These results can be used to counsel patients on risks of ESRD. They also provide benchmarks by which to judge the effectiveness of future treatments. Seventy-eight percent of contemporary patients with lupus nephritis do not develop ESRD, likely because of mild disease or access to effective treatments. However, for the 22% of patients overall and the 44% of patients with class IV lupus nephritis who develop ESRD within 15 years, the risk is greatest in the first 5 years. Knowing whether these early losses reflect primary treatment failures or ineffectiveness due to delayed treatment initiation or poor adherence will indicate the strategies needed to reduce these risks.
Supplementary Material
Acknowledgments
Supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH.
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Ward had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Tektonidou, Dasgupta, Ward.
Acquisition of data. Tektonidou, Ward.
Analysis and interpretation of data. Tektonidou, Dasgupta, Ward.
REFERENCES
- 1.Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine (Baltimore) 2003;82:299–308. doi: 10.1097/01.md.0000091181.93122.55. [DOI] [PubMed] [Google Scholar]
- 2.Yap DY, Tang CS, Ma MK, Lam MF, Chan TM. Survival analysis and causes of mortality in patients with lupus nephritis. Nephrol Dial Transplant. 2012;27:3248–54. doi: 10.1093/ndt/gfs073. [DOI] [PubMed] [Google Scholar]
- 3.Li T, Carls GS, Panopalis P, Wang S, Gibson TB, Goetzel RZ. Long-term medical costs and resource utilization in systemic lupus erythematosus and lupus nephritis: a five-year analysis of a large Medicaid population. Arthritis Rheum. 2009;61:755–63. doi: 10.1002/art.24545. [DOI] [PubMed] [Google Scholar]
- 4.Ward MM. Medical insurance, socioeconomic status, and age of onset of end-stage renal disease in patients with lupus nephritis. J Rheumatol. 2007;34:2024–7. [PubMed] [Google Scholar]
- 5.Lateef A, Petri M. Unmet medical needs in systemic lupus erythematosus. Arthritis Res Ther. 2012;14(Suppl 4):S4. doi: 10.1186/ar3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Croca SC, Rodrigues T, Isenberg D. Assessment of a lupus nephritis cohort over a 30-year period. Rheumatology (Oxford) 2011;50:1424–30. doi: 10.1093/rheumatology/ker101. [DOI] [PubMed] [Google Scholar]
- 7.Ortega LM, Schultz DR, Lenz O, Pardo V, Contreras GN. Lupus nephritis: pathologic features, epidemiology and a guide to therapeutic decisions. Lupus. 2010;19:557–74. doi: 10.1177/0961203309358187. [DOI] [PubMed] [Google Scholar]
- 8.Appel GB, Cohen DJ, Pirani CL, Meltzer JI, Estes D. Long-term follow-up of patients with lupus nephritis: a study based on the classification of the World Health Organization. Am J Med. 1987;83:877–85. doi: 10.1016/0002-9343(87)90645-0. [DOI] [PubMed] [Google Scholar]
- 9.Donadio JV, Jr, Hart GM, Bergstralh EJ, Holley KE. Prognostic determinants in lupus nephritis: a long-term clinicopathologic study. Lupus. 1995;4:109–15. doi: 10.1177/096120339500400206. [DOI] [PubMed] [Google Scholar]
- 10.Najafi CC, Korbet SM, Lewis EJ, Schwartz MM, Reichlin M, Evans J, et al. Significance of histologic patterns of glomerular injury upon long-term prognosis in severe lupus glomerulone-phritis. Kidney Int. 2001;59:2156–63. doi: 10.1046/j.1523-1755.2001.00730.x. [DOI] [PubMed] [Google Scholar]
- 11.Felson DT, Anderson J. Evidence for the superiority of immunosuppressive drugs and prednisone over prednisone alone in lupus nephritis: a pooled analysis. N Engl J Med. 1984;311:1528–33. doi: 10.1056/NEJM198412133112402. [DOI] [PubMed] [Google Scholar]
- 12.Austin HA, III, Klippel JH, Balow JE, le Riche NG, Steinberg AD, Plotz PH, et al. Therapy of lupus nephritis: controlled trial of prednisone and cytotoxic drugs. N Engl J Med. 1986;314:614–9. doi: 10.1056/NEJM198603063141004. [DOI] [PubMed] [Google Scholar]
- 13.Henderson L, Masson P, Craig JC, Flanc RS, Roberts MA, Strippoli GF, et al. Treatment for lupus nephritis. Cochrane Database Syst Rev. 2102;12:CD002922. doi: 10.1002/14651858.CD002922.pub3. [DOI] [PubMed] [Google Scholar]
- 14.Hahn BH, McMahon MA, Wilkinson A, Wallace WD, Daikh DI, Fitzgerald JD, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken) 2012;64:797–808. doi: 10.1002/acr.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertsias GK, Tektonidou M, Amoura Z, Aringer M, Bajema I, Berden JH, et al. Joint European League against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann Rheum Dis. 2012;71:1771–82. doi: 10.1136/annrheumdis-2012-201940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jurkovitz CT, Li S, Norris KC, Saab G, Bomback AS, Whaley-Connell AT, et al. Association between lack of health insurance and risk of death and ESRD: results from the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2013;61(Suppl 2):S24–32. doi: 10.1053/j.ajkd.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faurschou M, Dreyer L, Kamper AL, Starklint H, Jacobsen S. Long-term mortality and renal outcome in a cohort of 100 patients with lupus nephritis. Arthritis Care Res (Hoboken) 2010;62:873–80. doi: 10.1002/acr.20116. [DOI] [PubMed] [Google Scholar]
- 18.Ward MM. Socioeconomic status and the incidence of ESRD. Am J Kidney Dis. 2008;51:563–72. doi: 10.1053/j.ajkd.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 21.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. URL: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 22.Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. doi: 10.1186/1471-2288-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ward MM, Studenski S. Clinical prognostic factors in lupus nephritis: the importance of hypertension and smoking. Arch Intern Med. 1992;152:2082–8. [PubMed] [Google Scholar]
- 24.Plummer M. rjags: Bayesian graphical models using MCMC. R package version 3-12. URL: http://CRAN.R-project.org/package=rjags.
- 25.World Bank How do we classify countries? URL: http://data.worldbank.org/about/country-classifications.
- 26.Teo K, Lear S, Islam S, Mony P, Dehghan M, Li W, et al. on behalf of the PURE Investigators Prevalence of a healthy lifestyle among individuals with cardiovascular disease in high-, middle-, and low-income countries: the Prospective Urban Rural Epidemiology (PURE) study. JAMA. 2013;309:1613–21. doi: 10.1001/jama.2013.3519. [DOI] [PubMed] [Google Scholar]
- 27.Wang PS, Aguilar-Gaxiola S, Alonso J, Angermeyer MC, Borges G, Bromet EJ, et al. Use of mental health services for anxiety, mood, and substance disorders in 17 countries in the WHO world mental health surveys. Lancet. 2007;370:841–50. doi: 10.1016/S0140-6736(07)61414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moroni G, Quaglini S, Gallelli B, Banfi G, Messa P, Ponticelli C. Progressive improvement of patient and renal survival and reduction of morbidity over time in patients with lupus nephritis. Lupus. 2013;22:810–8. doi: 10.1177/0961203313492576. [DOI] [PubMed] [Google Scholar]
- 29.Moroni G, Quaglini S, Gallelli B, Banfi G, Messa P, Ponticelli C. The long-term outcome of 93 patients with proliferative lupus nephritis. Nephrol Dial Transplant. 2007;22:2531–9. doi: 10.1093/ndt/gfm245. [DOI] [PubMed] [Google Scholar]
- 30.Fiehn C, Hajjar Y, Mueller K, Waldherr R, Ho AD, Andrassy K. Improved clinical outcome of lupus nephritis during the past decade: importance of early diagnosis and treatment. Ann Rheum Dis. 2003;62:435–9. doi: 10.1136/ard.62.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ward MM. Changes in the incidence of endstage renal disease due to lupus nephritis in the United States, 1996-2004. J Rheumatol. 2009;36:63–7. doi: 10.3899/jrheum.080625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esdaile JM, Abrahamowitz M, MacKenzie T, Hayslett JP, Kashgarian M. The time-dependence of long-term prediction in lupus nephritis. Arthritis Rheum. 1994;37:359–68. doi: 10.1002/art.1780370309. [DOI] [PubMed] [Google Scholar]
- 33.Ward MM. Access to care and the incidence of endstage renal disease due to systemic lupus erythematosus. J Rheumatol. 2010;37:1158–63. doi: 10.3899/jrheum.091199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petri M, Perez-Gutthann S, Longenecker JC, Hochberg M. Morbidity of systemic lupus erythematosus: role of race and socio-economic status. Am J Med. 1991;91:345–53. doi: 10.1016/0002-9343(91)90151-m. [DOI] [PubMed] [Google Scholar]
- 35.Dooley MA, Hogan S, Jennette C, Falk R, for the Glomerular Disease Collaborative Network Cyclophosphamide therapy for lupus nephritis: poor renal survival in black Americans. Kidney Int. 1997;51:1188–95. doi: 10.1038/ki.1997.162. [DOI] [PubMed] [Google Scholar]
- 36.Alarcon GS, McGwin G, Jr, Petri M, Ramsey-Goldman R, Fessler BJ, Vila LM, et al. for the PROFILE Study Group Time to renal disease and end-stage renal disease in PROFILE: a multiethnic lupus cohort. PLoS Med. 2006;3:e396. doi: 10.1371/journal.pmed.0030396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Julian LJ, Yelin E, Yazdany J, Panopalis P, Trupin L, Criswell LA, et al. Depression, medication adherence, and service utilization in systemic lupus erythematosus. Arthritis Rheum. 2009;61:240–6. doi: 10.1002/art.24236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haddiya I, Hamzaoui H, Tachfouti N, Hamany ZA, Radoui A, Zbiti N, et al. Features and outcomes of lupus nephritis in Morocco: analysis of 114 patients. Int J Nephrol Renovasc Dis. 2013;6:249–58. doi: 10.2147/IJNRD.S34299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ayodele OE, Okpechi IG, Swanepoel CR. Long-term renal outcome and complications in South Africans with proliferative lupus nephritis. Int Urol Nephrol. 2013;45:1289–300. doi: 10.1007/s11255-012-0376-9. [DOI] [PubMed] [Google Scholar]
- 40.Trager J, Ward MM. Mortality and causes of death in systemic lupus erythematosus. Curr Opin Rheumatol. 2001;13:345–51. doi: 10.1097/00002281-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Urowitz MB, Gladman DD, Tom BD, Ibanez D, Farewell VT. Changing patterns of mortality and disease outcomes for patients with systemic lupus erythematosus. J Rheumatol. 2008;35:2152–8. doi: 10.3899/jrheum.080214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.