Abstract
Purpose: For the past few decades central nervous system disorders were considered as a major strike on human health and social system of developing countries. The natural therapeutic methods for CNS disorders limited for many patients. Moreover, nanotechnology-based drug delivery to the brain may an exciting and promising platform to overcome the problem of BBB crossing. In this review, first we focused on the role of the blood-brain barrier in drug delivery; and second, we summarized synthesis methods of nanomedicine and their role in different CNS disorder.
Method: We reviewed the PubMed databases and extracted several kinds of literature on neuro nanomedicines using keywords, CNS disorders, nanomedicine, and nanotechnology. The inclusion criteria included chemical and green synthesis methods for synthesis of nanoparticles encapsulated drugs and, their in-vivo and in-vitro studies. We excluded nanomedicine gene therapy and nanomaterial in brain imaging.
Results: In this review, we tried to identify a highly efficient method for nanomedicine synthesis and their efficacy in neuronal disorders. SLN and PNP encapsulated drugs reported highly efficient by easily crossing BBB. Although, these neuro-nanomedicine play significant role in therapeutics but some metallic nanoparticles reported the adverse effect on developing the brain.
Conclusion: Although impressive advancement has made via innovative potential drug development, but their efficacy is still moderate due to limited brain permeability. To overcome this constraint,powerful tool in CNS therapeutic intervention provided by nanotechnology-based drug delivery methods. Due to its small and biofunctionalization characteristics, nanomedicine can easily penetrate and facilitate the drug through the barrier. But still, understanding of their toxicity level, optimization and standardization are a long way to go.
Keywords: Blood brain barrier, CNS therapeutics, Drug delivery, Nanotechnology, Nanomedicine, Nanocarrier
Introduction
At present, the large spectrum of brain disorders classified as deficits in bot neurological and psychiatric chapters with short and long-term disabilities.1 These deficits are the results of intrinsic brain dysfunction or environmental interaction with brain.2 CNS disorders affect 1.5 million people worldwide and responsible for 1% deaths.3 Out of any other disease, 11% brain disorder burden is reported3 which might be increased to 14.7% by 2020.4
A variety of potential drugs has discovered to treat several neuronal disorders.5-8 But, the therapeutic success of these pharmaceuticals is still limited due to the presence of (i) Blood-brain barrier (BBB), and (ii) Blood-cerebrospinal fluid barrier (BCSFB). It acts as anatomical and biochemical dynamic barriers in the brain.9-11 BBB has made up by specific vascular endothelial cells that tightly bound with neurons, pericytes, and astrocytes.12-14 Less than 1% of the traditional drug can cross this barrier,15 therefore, BBB protects the brain from systematic circulatory molecules as well as externally injected molecules and poses a key challenge for drug delivery.9,16 Although, there are several endogenous transporters are present in the nervous system, BBB makes treatment ineffective by interacting with enzymes and restricts the entry of neuropharmaceutical agents.17 Hence, large dose of the drug requires to treat CNS disorders and neurotoxic effects observed in the form of physical or mental deformations.11
Several researchers are working on a multidisciplinary approach to nanotechnology to overcome these major obstacles in CNS therapeutics. Nanoparticles and combination with therapeutic agents may consider as an effective tool in brain drug targeting for safer therapies in future.9,18
In first decades PNPs, SLNS, liposomes, and micelles have used as nanocarriers in the medical field. But, now this nanotechnology approach has shifted towards newer and more advance nano-system e.g. dendrimers, nanoemulsions, nano gels and nanosuspensions.10 Traditional therapies have very little capacity to penetrate the BBB as well as null capacity for neuronal repair and neuronal regeneration.19 Hence, functionalized nanomaterial may serve as a potential drug delivery vehicle. It can use as both in-vivo and in-vitro viz, polysorbate coated poly (butyl cyanoacrylate) (PBCA) nanomaterial interact with endothelial cells of cerebral vessels and stimulate drug delivery via endocytosis.20 Nanotechnology combined with stem cell therapy is being increasingly used to rebuild the neural circuit and to induce specific cellular response.18,20-22
Recently, biofunctionalized carbon nanotubes (CNTs) have become a promising tool due to its cell-penetrating ability, surface chemistry diversity, structural, and mechanical properties.23 In contrast, instead of having larger structure than CNT, functionalized fullerenes have identified as more efficient in CNS drug delivery24,25 due to its higher permeability and less excitotoxity.26
Normal drug delivery to CNS and their challenges
For effective traditional therapy, the drug should lipid soluble with small molecular weight (400-600 Dalton's).27 This transport can perform by invasive, non-invasive and miscellaneous techniques,3,28 but, BBB allows restricted entry of potential drugs.9,15,16,29 Major reasons for therapeutic failures in the brain are slow drug action, association or conversion of the drug into non-transporting legends and less neuronal absorption.13 Some catalytic mechanisms in the nervous system also degrade the drug which performs a non-specific action or stay in inactive form in the brain.29
Strategies of drug delivery in brain
BBB acts as a capillary endothelial interface, that facilitates transport of essential chemical and ion to the brain.30 Crossing BBB is always a key obstacle for drug delivery system. Hydrophilic molecules reported transferring via specific carrier-mediated endocytosis, transporter, and paracellular pathway. Lipophilic molecules have transported by diffusion and P-glycoprotein.31 Routes of drug delivery include:
Invasive approach
This physically breached technique penetrates BBB and directly injects the drug into the brain. It requires craniotomy for intracerebroventricular (ICV) infusion and intracerebral drug administration.31,32 BBB disruption for drug delivery performs via breaking down the tight junction of endothelial cells.31,33 This can administer through osmotic disruption30,34,35 or disruptive plasma solutes.36,37 ICV drug delivery considered as a very poor approach, because the drug transported in the peripheral blood stream, less to the targeted tissues.38 Instead of having the advancement of high molecular drug transport, ICV also restricted to limited drug distribution and loss of desired CNS action due to high intracranial pressure during direct drug administration.39
Pharmacological approach
This observational approach based on the free passive movement of drugs through BBB.31,32 These molecules can cross BBB unassisted due to their small molecular size, low hydrogen bonding capacity and lipophilicity.40 This approach also consists chemical change, e.g. reduction in number of polar groups, which increases drug transfer across the BBB.41 But, the modified molecule may act as P-glycoprotein efflux pump, if lipophilicity increases by many folds.31
Physiological approach
Receptor-mediated and carrier-mediated drug delivery to the brain considered as a most advanced technique in pharmacology.30,31 Transferrin and insulin receptors are commonly found on the BBB.32 Hence, the drug adjoins with the ligand of these receptors might transport drugs to the targeted brain area. In the case of transporter mediated delivery, the drug needs to mimic to the endogenous carrier substrate.42 But kinetics and binding capacity of transporter molecule limit the CNS drug delivery through physiological approach.
Nano-formulated drug delivery in CNS
Conventional drug delivery strategies are unable to restore cytoarchitecture and connection pattern in CNS disorders.43 Nanotechnologies overcome these problems due to its nanoscale quantum effect, small and high surface area to volume ratio.44,45 Basically, nanotechnology is a convergence of science and engineering, which needs one-dimensional designing and characterization at the nanometric scale.21 Nanoparticles used in CNS drug delivery should have following promising features:
They should biodegradable, non-toxic and biocompatible.46,47
Their physical properties should easily manipulate according to mode of delivery.48
Different nanoparticles with modified chemical properties should achieve organ- or cell- specific drug delivery.49
The formulation should cost-effective.
In summary, all these beneficial considerations enhance CNS drug delivery.
Nano-formulation strategies
For an affecting drug delivery system in CNS treatment, nanoparticle alters the pharmacokinetics of drug48 and enhances drug loading capacity.50 Drugs need to chemically modify and transported to the brain via loading with different nanomaterial-based vehicles.45 It also received in the brain via transcytosis through the BBB.31 Nanobiotechnology has made a revolutionary progress in drug delivery system. We have mentioned the properties, nanotechnology-based drug delivery, and drug release mechanism with few example of patent nanomedicine in Table 1.51
Table 1. Properties of different nanocarriers, drug delivery and drug release mechanism with example of patents (partially adapted from reference 51).
| Type | Size (nm) | Synthesis technique | Mode of administration | Mechanism for delivery | Drug release mechanism | Example | ||
| Drug | disease | Patent | ||||||
| PNP | 10-1000 |
|
Subcutaneous, intravenous and oral |
|
|
Chitosan-coated erythropoietin (HMG-Co-A reductase inhibitors) | Brain targeting | US20070237827 |
| PLGA encapsulated NMDA-NR1 vaccine | Alzheimer’s disease | US20100173004 | ||||||
| SLN | 50-1000 |
|
Nasal, oral, parenteral, rectal and respiratory |
|
|
LDL-cholesterol conjugates | AD, PD, and cancer | US7682627 |
| LDL nanoparticles | Epilepsy, stroke, Trauma and AD | US20060222716 | ||||||
| Micelles | 80-100 |
|
Pulmonary delivery | Receptor-mediated transport, absorption, and endocytosis | Bursting, diffusion, and cleavage | doxorubicin, vincristine sulphate loaded poly (L-histidine)-poly(ethylene glycol) block copolymer and PLEG poly micelles | Cancer | US7659314 |
| Paclitaxel-loaded copolymer micelle | Lung cancer | NCT01023347 | ||||||
| Nanoliposomes | Less than 100 |
|
Pulmonary delivery, intravenous, | Adsorption, fusion and diffusion/ endocytosis | Endocytosis and Adsorption to cell surface Bursting due to environmental stimuli | Glutathione encapsulated liposomes | Myoclonus | US20100166846 |
| Tempamine loaded liposome | Multiple sclerosis and PD | US20110027351 | ||||||
| CNTs | Diameter of 3.5-70nm |
|
mainly intraperitoneal and intravenous | Endocytosis, diffusion, penetration | Electrically or chemical controlled | Streptavidin-HRP (Horseradish peroxidase) bounded SWCNT-annexin conjugates | Breast cancer | US201001846691A1 |
| Stem cell loaded CNT | AD, PD, and ischemia | US20090148417A1 | ||||||
| Dendrimers | Diameter range 1.5-13.5 |
|
Oral, transdermal, topical, IV | Transcytosis and endocytosis | Degradation and environmental stimuli | Anxiolytic and antipsychotic agents | Psychotic disorder | US20100160299 |
Nanotechnology-based drug delivery vehicles
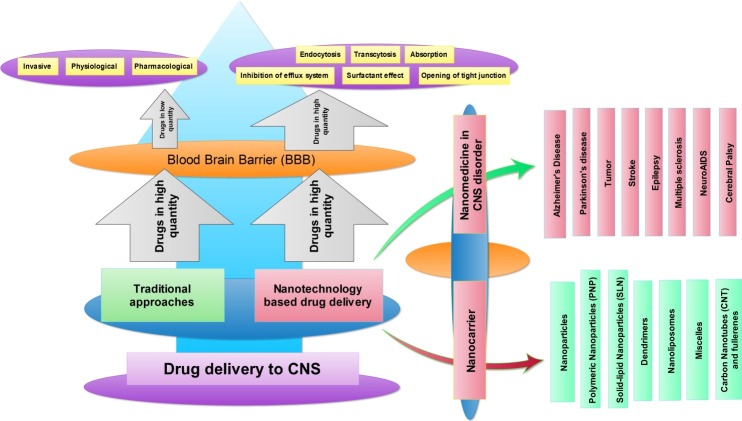
The nanotechnology-based drug administration has shown significant advantages over traditional drug delivery. The different nanoformulation carrier has used for targeted drug delivery, some of them are Nanoparticles (NP), lipid-based vehicle, carbon nanostructure-based vehicle and polymer based vehicle; as shown in Figure 1. We are discussing important nano drug carrier in the following section.
Figure 1.
Overview of traditional and nanotechnology based drug delivery in CNS disorders
Nanoparticle
The inorganic nanoparticle of size 10-1000nm recently elicited much interest due to their chemical and biological properties. Several features of nanoparticles show significant advantages to overcome problems associated with traditional drug delivery, which includes: high drug carrying capacity, high stability, controlled release, high specificity and hydrophilic and hydrophobic molecules transportability.52 Drug-loaded nanoparticles release to target site via diffusion, degradation, erosion or due to external energy input.53 Protein and ceramic NP are most commonly used in targeted drug delivery.54
Easy functionalization property and good biocompatibility of modified molecule are the key requirements to select an effective route for prepare different sized NP.55 Drug delivery through gold nanoparticles (AuNP) gives a versatile platform for effective drug delivery. Doxorubicin coated AuNP have reported making enhanced drug accumulation by overcoming multidrug resistance (MDR) in cancer treatment.56 Similarly, curcumin conjugated AuNP also shows haemocompatibility, resulting in antitumor activity in leukemia.57 Recent research on imatinib mesylate (IM) encapsulated, layer by layer coated functionalized AuNP, demonstrated rapid delivery into murine melanoma cells in mice.58 This topical application for iontophoretic IM delivery shows effective cancer treatment. Chitosan derived mitochondrial targeted multifunctional NP (MNPs) performs lysosomal escape, multistage pH response, and mitochondrial and hepatocyte targeting for safe and targeted anticancer drug delivery.59
Polymeric nanoparticles (PNPs)
Polymeric nanoparticles are a particulate dispersion of biodegradable and biocompatible polymers with size 10-1000nm. The core-shell structure of PNP varies with hydrophilic and hydrophobic blocks present in the polymer chain.60 The core of these PNP made up of a dense polymer matrix to encapsulate the hydrophobic drug and hydrophilic polymers in corona to serve steric stability and stealth properties to NP.61 Drug delivery through PNP were also performed via drug encapsulation, absorption or chemically linked to surface.62
Availability of polymer choice and drug release from nanoparticle makes them unique candidates for drug delivery. Biologically inert polymers PEG (Polyethylene glycol), PLGA (poly-L- glutamic acid),43 poly(alkyl cyanoacrylate), and poly(butyl) cyanoacrylate are most common used formulated nanopolymers. Level of drug release is not only controlled by molecular weight & polymer composition, drug-to-polymer ratio also affects as well.63
The role of PNP's in drug delivery can also consider non-replaceable. Doxorubicin loaded nanoparticles used to treat glioblastoma64 and quinoline derivatives loaded polymeric nanoparticles used in Alzheimer’s disease (AD).65 Similarly, nano gels, a crosslinked polymer66 and nanosuspensions, mixture of crystalline drug and non-ionic surfactants67 provide excellent pharmacokinetics control in CNS disorders.68
Solid-lipid nanoparticles (SLN)
SLN is surfactant stabilized lipid oily droplet, which is generally solid at room temperature.54 It considered as a colloidal nano drug carrier that synthesized by homogenization of melted lipid at high pressure while dispersing in water at 70 0C with a nanometric range of 50-1000nm.69,70 It also exhibits physical stability and easy manufacturing; hence it replaces liposomal technology in drug delivery.71 SLN particles conjugate with lipid emulsions that can stabilize by high-level surfactant inclusions and protect from degradation.72 The active part or drug to transported is administrated via loading or coating with nanoparticles.73
Recently, self-amplifying RNA in SLN nanoparticles has demonstrated the importance of lipid nanoparticle in nucleic acid vaccine development.74 The effect of different SLN conjugated drug is widely investigated in CNS treatment. Quercetin loaded SLN shows the antioxidant property to treat AD75 and diminazene aceturate loaded SLN particles used to treat human African trypanosomiasis (HAT).76 Similarly, 3’,5’-dioctanoyl-5-fluoro-2’-deoxyuridine (DO-FUdR) incorporated SLN used to treat neurological disorders.77 (3H)-atazanavir loaded SLN also crossed the BBB in HIV-encephalitis treatment.78
Dendrimers
Highly branched dendrimers made up of a focal core, building blocks with repetitive units in interior layers and peripheral functional units.79 Other than synthesis routes, the functionality, and efficacy of dendrimers depend on upon the used monomer and targeted polymer structure.80 Low dispersity and high functionality of these dendrimers offer themselves as a useful therapeutic tool in biomedical and pharmaceutical science.81 High penetration ability, high density, and peripheral functional group reactivity also considered as featured advantages as a drug vehicle.82 The terminal surface group, biocompatibility, and multivalency of three-dimensional dendrimers have displayed their importance in emerging with Nanomedicine.83 Polyamidoamine (PAMAM), polypropylene imine (PPI), and polylysine dendrimers are the most commonly used dendrimeric drug carrier for both hydrophobic and hydrophilic drug molecule.84 Drug either physically entraps with dendrimer, or covalently bound with peripheral functionalized molecules of dendrimer to form dendrimer-drug conjugates.79 The complexities of their bounding keep the chemical integrity and pharmaceutical properties of the drug.
In further research, cholesterol loaded poly (amidoamine) dendrimers reported neuroinflammation treatment.85 Similarly, multi-functionalized CMCht/PAMAM dendrimer nanoparticles incorporation with antibody also played an important role in specific CNS targeting.86
Different dendrimers such as PAMAM, polyester-copolyester (PEPE) and PPI, shows anticancer and anti-inflammatory properties to treat several neurological disorders.87
Nanoliposome
These lipid nanoparticles are the most studied bilayer vehicle, developed in drug delivery in the 70’s.88 Less than 100nm sized nanoliposomes may consider as an advanced form of SLN that includes nanostructured lipid carrier (NLC), nanoemulsions and lipid nanocapsules (LNC).54 The distorted structure of NLC provides enough space to accommodate active drug molecule which can develop by mixing lipid droplet into solid media at very high temperature.54 Combination of liposomes and nanoemulsion particle gives rise to LNCs (less than 100nm) with thicker outer wall that allows more functionalization and controlled targeted drug delivery.89
The lipid, oily core of LNCs surrounded by lipophilic and hydrophobic surfactant that improves therapeutic drug delivery.42 Liposomal technique emerged with pegylation for targeted brain drug delivery41 which optimized the plasma pharmacokinetics. Neurotrophic agents loaded liposomes used in brain disorders.90 Pegylated liposomes loaded with doxorubicin and (3H)-Prednisolone treats brain tumors91 and autoimmune encephalitis92 respectively. OX26 monoclonal antibody-mediated antineoplastic agent, (3H) daunomycin, conjugate with a liposome and exhibit brain drug delivery.93 Similarly, heat shock protein (HSP) encapsulated liposomes also used in the stroke treatment.94
Micelles
Micelles are monolayered spherical lipid nanostructures with inwards facing hydrophobic ends and outwards facing hydrophilic ends with a range of 80-100nm.95 Due to its small, the micelles shows short circulation time in body compares to liposomes that make them easily transportable elements.54 Polymeric micelles considered as more stable with longevity and good biodistribution compare to traditional micelles.96 These modified micelles show improved target penetration due to their nanoscale size, easy transportation to target location, and low critical association concentration (CMC).97 Physically entrapped and covalently bonded micelles drug conjugate play an important role in controlled drug release system.98 Drug loading to micelles generally depends on upon the physiochemical property of drug, the chemical composition of core forming polymers, and physical state of micelles core.99 The release is generally affected by temperature, pH, and environment.100
Carbon Nanotubes and fullerenes
CNT exhibits advanced physical, mechanical property, and high aspect ratio at the nanometer scale of less than 100nm.101 Functionalized CNT shows high solubility and high biocompatibility which generally depends on upon surface property, size and shape of modified molecules.102 These parameters greatly influence the internalization of therapeutic molecules inside the cell. CNT functionalization strategies include the addition of an organic group at sidewall/tip of CNTs and carboxyl group coupling after oxidation process.103 Polymers and dendrimer conjugated CNTs also reduces aggregation, increases their solubility and biocompatibility.104 Very few studies of the CNT in CNS treatment have been reported, yet acetylcholine loaded SWCNT (Single wall carbon nanotube) studied in the AD treatment105 and CNT with stem cell therapy used in stroke treatment.106Amphotericin B loaded CNT showed lower aggregation, high solubility with reduced toxicity, and anti-fungal activity compares to administration of amphotericin B alone.107
Carbon nano horns and nanodiamonds modified the form of CNT which reported enhancing the nanotechnology application in biosciences and pharmaceutical industry.54 Diamond nanoparticles also used as an important therapeutic tool in tumor patches and wound healing.108 Fullerene has uniquely identified a class of carbon allotropes which described as 60 linked carbon with 60 vertices and 32 faces.109 The extensive research on nanosized C60 have identified its use in drug delivery.110 Their antioxidant and radical oxygen quenching character made them more promising than any other nanomaterial.111 Hydrated C60 fullerene prevents astrocytes and glial fibrillary acidic proteins (GFAP) damage which caused by oxidative stress and improves cognitive function.112
Nanoparticle-mediated drug transport mechanism
For effective drug treatment, nanomedicine needs to cross the BBB without losing its properties. There are several possibilities for this translocation: Absorption, opening of tight junctions, endocytosis, transcytosis, surfactant effect, and inhibition of efflux system.113
Polysorbate coated dalargin nanoparticle reported to induce an antinociceptive effect (surfactant effect) and created high concentration gradient which helps to transport nanomedicine.47
Polysorbate-80 coated nanoparticle also unfolded the tight junction and increases inulin space without disrupting BBB.114
At present, endocytosis is considered as the most likely mechanism of nanomedicine transport. Polysorbate-80 coated PBCA nanoparticle endocytotic transport studied by laser confocal microscopy and significant and rapid uptake of coated nanoparticles were observed, rather than uncoated nanoparticle.115
Dipalmitoyl phosphatidyl choline cholesterol-coated malto-dextrin nanoparticle transcytosis through BBB and upregulated the LDL receptor expression in a cholesterol-depleted model system.116
CNS disorder and nanomedicine
Recent trends of nano-therapeutics advance over traditional drug therapy in CNS disorders via its proper property to cross the BBB.19,117 Nanotechnology used in for both diagnoses (imaging) and treatment, here we will discuss in-vivo drug delivery system in CNS disorders.
Alzheimer’s disease
Alzheimer’s disease (AD) recognized as a progressive neurodegenerative disorder, which characterized by memory loss and dementia.118 Pieces of evidence support inclined graph of AD patients with prevalence rate 0.62% and 1.07% in people with age +55 and +65 years respectively. Estimated data are much scaring as 24.3 million people globally affected by dementia and each year 4.6 million cases reported.119,120 Amyloid-β aggregation considered as hallmarks of AD.121 Other than this, wide spectrum of AD pathology covers genetic change of EpoE protein, mitochondrial abnormalities, oxidative stress, and dysfunction of D-serine.122-124
Insufficient use of oral administrated drugs for AD, such as tacrine, memantine, rivastigmine etc, pulls the door open for nanomedicine in neurodegenerative disorders.125,126 Cerium oxide nanoparticles,127 SLN of ferulic acid,128 tempol loaded PLGA nanoparticles,129 and epigallocatechin-3-gallate (EGCG) phenol coated nanolipids130 reported to show antioxidant property and degrade amyloid-β.131 Thioflavin-T (ThT), charged and fluorescent biomarker, detect Aβ in senile plaques. Therefore, ThT encapsulated polymerized but cyanoacrylate NP injected directly into intrahippocampal space, and light microscopy and TEM analysis confirmed Aβ in AD brain.132 Cu (I) chelator and MBP-PE induced D- penicillamine nanoparticles were also used tauopathies detection in AD brain.133 Nanofabricated quinoline derivative, clioquinol (5-chloro-7-iodo-8-hydroxyquinoline,CQ), was reported to inhibit Aβ when it was functionalized with n-butyl cyanoacrylate and PBCA nanoparticle.65 Imbalance in Ach of the cholinergic nervous system also reported in AD and free Ach could not inject into the brain directly, because it is easier to decompose in the blood and high polarities.134 Curcumin nanoparticles have been also identified as important finding in AD treatment.135
Parkinson Disease
Increasing lifespan and demographic changes in population demonstrates increased prevalence of Parkinson disease (PD).136 50+ people in world’s most 10 populous countries have around 4.6 million PD patients, which might be 9.3 million by 2030 with a rate of 1 per 100.137,138 A hallmark of PD is gliosis and degeneration of dopaminergic neurons in the substantia nigra are not the only features of PD. It also involves selective denervation,139 dysfunctions in the mitochondrial and ubiquitin-proteosome system, and oxidative and nitrosative stress.140 Available drugs for PD neither surpass nor reverse disease progression141 and BBB causes additional challenge in drug delivery.142
Nanotechnologies control and manipulate the drug delivery in PD to overcome these problems. Recent research has demonstrated that nerve growth factor (NGF) bound poly butyl cyanoacrylate nanoparticles143 and L-Dopa encapsulated nanoparticles48 crosses BBB and reduces basic symptoms of PD. Physically modified saline RNS60 with charged-stabilized nanobubbles, suppresses the proinflammatory molecules in MPTP-induced animal model of PD.144 Similarly, coumarin-6 loaded lactoferrin conjugated PEG-PLGA nanoparticle show important role in neuroprotection in Parkinson disease.145
Tumor
Upward trends of brain tumor show increased incident rate with 6/100,000 for malignant brain tumors in the adult.146 Male shows higher susceptibility than female with increasing age at a rate of 8.5 v/s 7.9 per 100,000 that have increased 5-6 folds by now.147,148 Drug therapy is less effective in brain tumor because of less infiltration of tumor cells from normal cells149 and less microvascular permeability of BBB.150
To overcome these problems, nanoformulation drug therapy is widely used an alternative approach. Gold porphyrin or camptothecin encapsulated lipid nanoparticles enhanced drug delivery to tumor tissue with a low side effect to the liver.151 Nanotechnology-based drug delivery used in cancer treatment with a combination of gene and radiotherapy.152 Nanotechnology in chemotherapy enhances efficacy to treat glioblastoma. DOX-loaded nanodiamond exhibit excellent cell biocompatibility and increase apoptosis of glioma cell lines.153 MWCNTs (Multiwall carbon nanotube) showed a high level of internalization of macromolecules in microglial cells and their molecular modulation helped in immunotherapy of cancer.154 Folic acid (as targeting agent) and methotrexate conjugated PAMAM dendritic polymers bind to tumor cell which overexpressed for folate receptor in cancer treatment.155 Boron-enriched nanocomposites of copolymerized acetal-poly(ethylene glycol)-block-poly(lactide)-methacrylate with 4-vinylbenzyl substituted closo-carborane demonstrated high incorporation and hemocompatibility.156
NeuroAIDS
NeuroAIDS drags both infectious and neurological pathophysiologic pathways under one umbrella, in which HIV1 (Human Immunodeficiency Virus 1) enters in the CNS in the early stage of infection.157 Approximately 15-30% of AIDS patients experiences several neurological and neurocognitive complications in which 7.3-11.3% and 30-60% experienced dementia and encephalopathy respectively.158,159 BBB disruption is not the only mechanism in neuroAIDS, activated endothelial cells with decreased permeability of the barrier160 and CD 163, Glut5 & ISG15 genes161 are also shown deleterious effect. Currently, there are no effective vaccines or specific drug therapy for NeuroAIDS,162 therefore, multidisciplinary approach to nanotechnology shed light on potential therapeutic approaches in HIV infection.
Nanoformulated antiretroviral therapy (ART) reported increasing blood-brain penetration in neuroAIDS treatment. Indinavir (IDV) NP loaded murine bone marrow macrophages (BMM) cause reduced HIV-1 replication in HIVE (HIV-1 encephalitis) region of the brain.163 Their research also demonstrated the role of NP loaded BMM in studying targeted migration and antiretroviral responses. Nanotechnology-based, highly active antiretroviral therapy (HAART) also played a significant role in neurosis treatment.164 Several antiretroviral drugs, zidovudine, delavirdine, saquinavir, and lamivudine, were nanoformulation with PBCA, MMSPM (methylmethacrylate–sulfopropyl methacrylate), polylactide (PLA) and PLGA that increases BBP 10-20 folds.165 Liposome loaded AZT- myristate and zalcitabine were also reported with improved efficacy and longer half-life compare to traditional ARV drug treatment.166 SLN loaded ARV drugs recently come into a highlight. Large surface area and high efficacy of SLN coated delavirdine and saquinavir ARV drug replaced MMSPM coated ARV drug treatment in neuroAIDS.162
Stroke
With second place, stroke is affecting mortality rates of 6,000,000 deaths annually with estimated susceptibility of 8-10% of lifetime.167 1.2% deaths in India reported due to this in which 87% caused by ischemia and the remaining is due to hemorrhage.168 Glutamate excitotoxity, oxidative stress, lipid peroxidation, BBB dysfunction, leukocyte infiltration and brain injuries play an important role in the pathophysiology of stroke.169,170 BBB and blood-cerebrospinal-fluid barrier (BCSFB) are the main issues in stroke drug delivery,171 so optimization and efficiency of drug carriers are needed to improve.
The new, unusual perspective of nanotechnologies in stroke therapy is ‘jeevandayani’ (life protecting).37 One researcher used engineering triiodothyronine (T3) nanoparticle coated with PLGA-PEG and enhanced neuroprotection observed compared to glutathione alone.172 Cerium oxide nanoparticles also showed neuroprotective naturally in the rodent stroke model. Cerium oxide nanoparticle reduces the 3-nitrotyrosine level, which was generally induced by peroxynitrite radical during the stroke.173 Similarly, platinum nanoparticles showed their antioxidant property which reported lowering cerebral cortex volume and improved motor function in stroke animal model.174 Irreversible caspase-3 inhibitor loaded transferrin targeted nanospheres provide a reduction in infarct volume in ischemic brain.175 SiRNA loaded carbon nanotube also documented as potential therapeutics in stroke treatment.37 Transferrin-coupled liposomes promote vascular regeneration and neuroprotection via delivering vascular endothelial growth factor (VEGF) in stroke treatment.176 The stroke damage can also recover by progenitor stem cell differentiation when it impregnated with CNT.106
Cerebral palsy
Cerebral palsy (CP) is one of the major neurodevelopmental disorders in children that considered as chronic & non-progressive in nature.177 It causes motor dysfunction, serve paralysis178 and musculoskeletal problems in 2-3 per 1000 children179 with a male/female ratio of 1.4:1.180 Unfortunately, there is no effective cure available for CP due to unknown molecular and biochemical mechanisms involvement.181 But researchers show the wide interest to use Nanoscience used drug delivery in CP.
PAMAM dendrimers and dendrimer-based N-acetyl-L-cysteine administration suppress neuroinflammation & motor dysfunction in CP patients.182 Stem cell therapy with nanomedicine has also come in the limelight recently to cure CP via promoting repair and regeneration of injured neurons.183
Epilepsy
Epilepsy is leading in all CNS disorders with a rate of 57 per 1000 people184 which might increase as in 5.5 million patients by the year 2001 in India.185 Abnormal neuronal discharges considered linking with oxygen deprivation, trauma, tumors and infections that cause neuronal excitability186 and neuroinflammatory cytokine dysfunction in epilepsy.187 The adverse effect of anti-epileptic drugs,188 promotes the use of nanoparticle-loaded drugs with the ability to cross the BBB and direct drug delivery.189 Carbamazepine loaded solid lipid nanoparticles of chitosan reported to be more effective than nano emulged loaded carbamazepine.190 Similarly, poly (d,l-lactide-co-glycolide) nanoparticle loaded β-carotene anticonvulsant considered more effective when it coated with polysorbate-80.191
Multiple sclerosis
Multiple sclerosis (MS) considered as an autoimmune neurodegenerative disease with chronic inflammatory processes.192 Modification of myelin basic protein (MBP) and glial fibrillary acidic protein (GFAP) triggers lesions in white matter192 that causes MS. Advanced stage of MS causes demyelination and tissue damage due to oxidative stress are found higher in the patients.193 Ultra sized cerium oxide nanoparticles declines oxidative stress and alleviates motor deficits in MS brain.194
Challenges
Emerging nanotechnology with neurosciences is like a game of risk and gain. Currently, Nanomedicine considered as a successful tool in drug delivery via crossing the BBB.195,196 These nano drugs are in the process of clinical trials, but their proper transport and safety concerns are yet to be determined.1,45 The composition and properties of nanoparticles may lead to oxidative stress, amino acid disturbance and BBB disruption,196,197 that causes neurotoxicity in the brain. Although functionalized nanoparticles pose successful drug targeting, but their nano-size structure and the large surface area may result in particle aggregation and limited drug loading.65,198 State of aggregation and mechanical properties affects nanoparticles toxicity which basically depends on preparation and purification methods. Hence, one should select a proper method to reduce toxicity.
Toxicity concerns of nanomedicine delivery based on their mode of drug administration and a measure of the drug; which causes neuroinflammation, excitotoxicity, DNA damage and allergic responses.199 Therefore, biocompatibility and biodegradability of nano drug are also needed to understand.
As Nanomedicine need to interact with neurons at a systemic level to show their effect. But, multidimensional cellular interaction at neuronal level and restricted anatomical access increase the challenges in nano-drug delivery system.21 The primary function of CNS needed to preserve before drug administration which also a big challenge itself.200
Conclusion
CNS disorders are a most serious problem in this industrialized world. Nanotechnology has proven very advanced and promising science which provides easily targeted drug delivery to the brain. But, we still need to gain more knowledge about their properties and features to evaluate their dynamic behavior in biomedical science.201 At present, we don’t have any multidimensional drug for different CNS disorders that may result of several individual biochemical pathways.21 Nanodrugs may lead to solving this problem.
Sometimes, few diseases viz, diabetes, trauma or some of the psychotic diseases, also associated with the neurological disorder. Hence, nanomedicine requires achieving termination of these entire co-morbidity factors with fewer side effects. Other than this, Genetic manipulation in the neuronal cell is also considered as a difficult target, so nanotechnology-based drug delivery should potentially efficacious approach in CNS treatment.
Polymer-based gold nanoparticles and CNT nano drugs have very few clinical trials, but due to their noble physical and mechanical strength, they may useful to carry the drug whose transport is still unidentified.
Although, the nanoparticle-based drug has several advantages, but many aspects are still matters of concern. So far there is no specific method to identify the toxicity level and targeted drug release in the CNS. Hence, the current nanotechnology application needs to improve further, so that it can be safe and target oriented.68
In recent years, some nanomedicine registered for patents in complex CNS treatment, which are following: Gold nanoparticle (US2011262546, US2011111040), lipid nanoparticle (WO2008024753, WO2008018932), chitosan nanoparticle (US2010260686) and SLN (US2011208161).1
Increasing population and increasing brain disorders are calling for the urgent need of new promising therapies. Involvements of nanotechnology in neurosciences will unmet medical need and give a hope to patients. The new generation nanomedicine might control prolonged and targeted drug delivery in a specific manner. Instead of reduced side effect and increased viability of nano drug, we still need to improve nanotechnological methods in pharmaceuticals for better comprehension and improved life quality. It can not be denied the potential benefit of nanomedicines, but their opportunity and risk formula also point towards hazardous effects. Due to the high ongoing emergence of nanotechnology in today’s research, one just cannot throw it away due to its negative points only. Specific guidelines should follow to avoid the most harmful effect of nanotechnology. It can also predict that nanotechnology-based drug delivery can revolutionize the era of traditional drugs delivery and that modified drug will be incredibly efficient from the current standard.
Ethical Issues
Not applicable.
Conflict of Interest
The authors report no conflicts of interest in this work.
References
- 1.Spuch C, Saida O, Navarro C. Advances in the Treatment of Neurodegenerative Disorders Employing Nanoparticles. Recent Pat Drug Deliv Formul. 2012;6(1):2–18. doi: 10.2174/187221112799219125. [DOI] [PubMed] [Google Scholar]
- 2. Hyman S, Chisholm D, Kessler R, Petal V, Whiteford H. Mental Disorders. In: Jamsion DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, et al, editors. Disease control priorities in developing countries. New York: Oxford University Press; 2006. P. 605-25.
- 3.Domínguez A, Álvarez A, Hilario E, Suarez-Merino B, Goñi-de-Cerio F. Central nervous system diseases and the role of the blood-brain barrier in their treatment. Neurosci Discov. 2013;1(3):1–11. doi: 10.7243/2052-6946-1-3. [DOI] [Google Scholar]
- 4.Menken M, Munsat TL, Toole JF. The global burden of disease study: implications for neurology. Arch Neurol. 2000;57(3):418–20. doi: 10.1001/archneur.57.3.418. [DOI] [PubMed] [Google Scholar]
- 5.Whiting PJ. GABA-A receptor subtypes in the brain: A paradigm for CNS drug discovery? A paradigm for CNS drug discovery? Drug Discov Today. 2003;8(10):Drug Discov Today. doi: 10.1016/S1359-6446(03)02703-X. [DOI] [PubMed] [Google Scholar]
- 6.Alguacil LF, Perez-Garcia C. Histamine H3 receptor: a potential drug target for the treatment of central nervous system disorders. Curr Drug Targets CNS Neurol Disord. 2003;2(5):303–13. doi: 10.2174/1568007033482760. [DOI] [PubMed] [Google Scholar]
- 7.Langmead CJ, Watson J, Reavill C. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol Ther. 2008;117(2):232–43. doi: 10.1016/j.pharmthera.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Sproule BA, Naranjo CA, Brenmer KE, Hassan PC. Selective serotonin reuptake inhibitors and CNS drug interactions. A critical review of the evidence. Clin Pharmacokinet. 1997;33(6):454–71. doi: 10.2165/00003088-199733060-00004. [DOI] [PubMed] [Google Scholar]
- 9.Jain KK. Nanobiotechnology-based drug delivery to the central nervous system. Neurodegener Dis. 2007;4(4):287–91. doi: 10.1159/000101884. [DOI] [PubMed] [Google Scholar]
- 10.Wong HL, Wu XY, Bendayan R. Nanotechnological advances for the delivery of CNS therapeutics. Adv Drug Deliv Rev. 2012;64(7):686–700. doi: 10.1016/j.addr.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Domínguez A, Álvarez A, Suárez-Merino B, Goñi-de-Cerio F. Neurological disorders and the blood-brain barrier. Strategies and limitations for drug delivery to the brain. Rev Neurol. 2014;58(5):213–24. [PubMed] [Google Scholar]
- 12. Pardridge WM. Strategies for Drug Delivery through the Blood-Brain Barrier. In: Borchardt RT, Repta AJ, Stella VJ, editors. Directed Drug Delivery: A Multidisciplinary Approach. Clifton, NJ: Humana Press; 1985. P. 83-96.
- 13.Tamai I, Tsuji A. Drug delivery through the blood-brain barrier. Adv Drug Deliv Rev. 1996;19(3):401–24. doi: 10.1016/0169-409X(96)00011-7. [DOI] [Google Scholar]
- 14.Tajes M, Ramos-Fernández E, Weng-Jiang X, Bosch-Morató M, Guivernau B, Eraso-Pichot A. et al. The blood-brain barrier: Structure, function and therapeutic approaches to cross it. Mol Membr Biol. 2014;31(5):152–67. doi: 10.3109/09687688.2014.937468. [DOI] [PubMed] [Google Scholar]
- 15.Stockwell J, Abdi N, Lu X, Maheshwari O, Taghibiglou C. Novel central nervous system drug delivery systems. Chem Biol Drug Des. 2014;83(5):507–20. doi: 10.1111/cbdd.12268. [DOI] [PubMed] [Google Scholar]
- 16.Yi X, Manickam DS, Brynskikh A, Kabanov AV. Agile delivery of protein therapeutics to CNS. J Control Release. 2014;190:637–63. doi: 10.1016/j.jconrel.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alam MI, Beg S, Samad A, Baboota S, Kohli K, Ali J. et al. Strategy for effective brain drug delivery. Eur J Pharm Sci. 2010;40(5):385–403. doi: 10.1016/j.ejps.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Halberstadt C, Emerich DF, Gonsalves K. Combining cell therapy and nanotechnology. Expert Opin Biol Ther. 2006;6(10):971–81. doi: 10.1517/14712598.6.10.971. [DOI] [PubMed] [Google Scholar]
- 19.Emerich DF, Orive G, Borlongan C. Tales of biomaterials, molecules, and cells for repairing and treating brain dysfunction. Curr Stem Cell Res Ther. 2011;6(3):171–89. doi: 10.2174/157488811796575350. [DOI] [PubMed] [Google Scholar]
- 20.Aliautdin RN, Kreuter J, Kharkevich DA. Drug delivery to the brain with nanoparticles. Eksp Klin Farmakol. 2003;66(2):65–8. [PubMed] [Google Scholar]
- 21.Silva GA. Nanotechnology approaches for the regeneration and neuroprotection of the central nervous system. Surg Neurol. 2005;63(4):301–6. doi: 10.1016/j.surneu.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Orive G, Anitua E, Pedraz JL, Emerich DF. Biomaterials for promoting brain protection, repair and regeneration. Nat Rev Neurosci. 2009;10(9):682–92. doi: 10.1038/nrn2685. [DOI] [PubMed] [Google Scholar]
- 23.Hwang JY, Shin US, Jang WC, Hyun JK, Wall IB, Kim HW. Biofunctionalized carbon nanotubes in neural regeneration: a mini-review. Nanoscale. 2013;5(2):487–97. doi: 10.1039/c2nr31581e. [DOI] [PubMed] [Google Scholar]
- 24.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–92. [PubMed] [Google Scholar]
- 25.Segal E, Satchi-Fainaro R. Design and development of polymer conjugates as anti-angiogenic agents. Adv Drug Deliv Rev. 2009;61(13):1159–76. doi: 10.1016/j.addr.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Chen YW, Hwang KC, Yen CC, Lai YL. Fullerene derivatives protect against oxidative stress in RAW 264.7 cells and ischemia-reperfused lungs. Am J Physiol Regul Integr Comp Physiol. 2004;287(1):R21–6. doi: 10.1152/ajpregu.00310.2003. [DOI] [PubMed] [Google Scholar]
- 27.Pardridge WM. Drug delivery to the brain. J Cereb Blood Flow Metab. 1997;17(7):713–31. doi: 10.1097/00004647-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Pathan SA, Iqbal Z, Zaidi SM, Talegaonkar S, Vohra D, Jain GK. et al. CNS drug delivery systems: novel approaches. Recent Pat Drug Deliv Formul. 2009;3(1):71–89. doi: 10.2174/187221109787158355. [DOI] [PubMed] [Google Scholar]
- 29. Crone C. The blood-brain barrier: a modified tight epithelium. In: Suckling AJ, Rumsby MG, Bradbury MWB, editors. The blood-brain barrier in health and disease. Chichester: Ellis Horwood Ltd; 1986. P. 17-40.
- 30.Upadhyay RK. Drug Delivery Systems, CNS Protection, and the Blood Brain Barrier. Biomed Res Int. 2014;2014:869269. doi: 10.1155/2014/869269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stenehjem DD, Hartz AM, Bauer B, Anderson GW. Novel and emerging strategies in drug delivery for overcoming the blood-brain barrier. Future Med Chem. 2009;1(9):1623–41. doi: 10.4155/fmc.09.137. [DOI] [PubMed] [Google Scholar]
- 32.Gabathuler R. Approaches to transport therapeutic drugs across the blood-brain barrier to treat brain diseases. Neurobiol Dis. 2010;37(1):48–57. doi: 10.1016/j.nbd.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 33.Inamura T, Black KL. Bradykinin selectively opens blood-tumor barrier in experimental brain tumors. J Cereb Blood Flow Metab. 1994;14(5):862–70. doi: 10.1038/jcbfm.1994.108. [DOI] [PubMed] [Google Scholar]
- 34.Neuwelt EA, Goldman DL, Dahlborg SA, Crossen J, Ramsey F, Roman-Goldstein S. et al. Primary CNS lymphoma treated with osmotic blood-brain barrier disruption: prolonged survival and preservation of cognitive function. J Clin Oncol. 1991;9(9):1580–90. doi: 10.1200/JCO.1991.9.9.1580. [DOI] [PubMed] [Google Scholar]
- 35.Rapoport SI. Osmotic opening of the blood-brain barrier: Principles, mechanism, and therapeutic applications. Cell Mol Neurobiol. 2000;20(2):217–30. doi: 10.1023/a:1007049806660. [DOI] [PubMed] [Google Scholar]
- 36.Erdlenbruch B, Schinkhof C, Kugler W, Heinemann DE, Herms J, Eibl H. et al. Intracarotid administration of short-chain alkylglycerols for increased delivery of methotrexate to the rat brain. Br J Pharmacol. 2003;139(4):685–94. doi: 10.1038/sj.bjp.0705302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee HJ, Park J, Yoon OJ, Kim HW, Lee DY, Kim do H. et al. Amine-modified single-walled carbon nanotubes protect neurons from injury in a rat stroke model. Nat Nanotechnol. 2011;6(2):121–5. doi: 10.1038/nnano.2010.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aird RB. A study of intrathecal, cerebrospinal fluid-to-brain exchange. Exp Neurol. 1984;86(2):342–58. doi: 10.1016/0014-4886(84)90192-4. [DOI] [PubMed] [Google Scholar]
- 39. Zhang Y, Miller DW. Pathways for drug delivery to the Central nervous system. In: Wang B, Siahaan TJ, Soltero R, editors. Drug delivery: Principles and applications. New Jersey: John Wiley and Sons; 2005. P. 29-56.
- 40.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46(1-3):3–26. doi: 10.1016/S0169-409X(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 41.Pardridge WM. CNS drug design based on principles of blood-brain barrier transport. J Neurochem. 1998;70(5):1781–92. doi: 10.1046/j.1471-4159.1998.70051781.x. [DOI] [PubMed] [Google Scholar]
- 42.Allen TM, Cullis PR. Liposomal drug delivery systems: From concept to clinical applications. Adv Drug Deliv Rev. 2013;65(1):36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 43.Modi G, Pillay V, Choonara YE, Ndesendo VM, du Toit LC, Naidoo D. Nanotechnological applications for the treatment of neurodegenerative disorders. Prog Neurobiol. 2009;88(4):272–85. doi: 10.1016/j.pneurobio.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 44.Ochekpe NA, Olorunfemi PO, Ngwuluka NC. Nanotechnology and drug delivery part 1: Background and applications. Trop J Pharm Res. 2009;8(3):265–74. doi: 10.4314/tjpr.v8i3.44546. [DOI] [Google Scholar]
- 45.Dinda SC, Pattnaik G. Nanobiotechnology-based drug delivery in brain targeting. Curr Pharm Biotechnol. 2013;14(15):1264–74. doi: 10.2174/1389201015666140608143719. [DOI] [PubMed] [Google Scholar]
- 46.Desai MP, Labhasetwar V, Walter E, Levy RJ, Amidon GL. The mechanism of uptake of biodegradable microparticles in Caco-2 cells is size dependent. Pharm Res. 1997;14(11):1568–73. doi: 10.1023/A:1012126301290. [DOI] [PubMed] [Google Scholar]
- 47.Kreuter J. Influence of the surface properties on nanoparticle-mediated transport of drugs to the brain. J Nanosci Nanotechnol. 2004;4(5):484–8. doi: 10.1166/jnn.2003.077. [DOI] [PubMed] [Google Scholar]
- 48.Mohanraj K, Sethuraman S, Krishnan UM. Development of poly(butylene succinate) microspheres for delivery of levodopa in the treatment of parkinson's disease. J Biomed Mater Res B Appl Biomater. 2013;101(5):840–7. doi: 10.1002/jbm.b.32888. [DOI] [PubMed] [Google Scholar]
- 49.Miller G. Drug targetingBreaking down barriers. Science. 2002;297(5584):1116–8. doi: 10.1126/science.297.5584.1116. [DOI] [PubMed] [Google Scholar]
- 50.Khanbabaie R, Jahanshahi M. Revolutionary impact of nanodrug delivery on neuroscience. Curr Neuropharmacol. 2012;10(4):370–92. doi: 10.2174/157015912804143513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haque S, Md S, Intekhab Alam M, Kaur Sahni J, Ali J, Baboota S. Nanomedicines for brain targeting: A patent review. Recent Pat Nanomed. 2011;1(2):149–61. doi: 10.2174/1877912311101020149. [DOI] [Google Scholar]
- 52.Prabha S, Zhou WZ, Panyam J, Labhasetwar V. Size-dependency of nanoparticle-mediated gene transfection: Studies with fractionated nanoparticles. Int J Pharm. 2002;244(1-2):105–15. doi: 10.1016/s0378-5173(02)00315-0. [DOI] [PubMed] [Google Scholar]
- 53.Caldorera-Moore M, Peppas NA. Micro- and nanotechnologies for intelligent and responsive biomaterial-based medical systems. Adv Drug Deliv Rev. 2009;61(15):1391–401. doi: 10.1016/j.addr.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jain N, Jain R, Thakur N, Gupta BP, Jain DK, Banveer J. et al. Nanotechnology: A safe and effective drug delivery system. Asian J Pharm Clin Res. 2010;3(3):159–65. [Google Scholar]
- 55.Liu Q, Cao X, Wang T, Wang C, Zhang Q, Ma L. Synthesis of shape-controllable cobalt nanoparticles and their shape-dependent performance in glycerol hydrogenolysis. RSC Adv. 2015;5(7):4861–71. doi: 10.1039/c4ra13395a. [DOI] [Google Scholar]
- 56.Wang F, Wang YC, Dou S, Xiong MH, Sun TM, Wang J. Doxorubicin-tethered responsive gold nanoparticles facilitate intracellular drug delivery for overcoming multidrug resistance in cancer cells. ACS Nano. 2011;5(5):3679–92. doi: 10.1021/nn200007z. [DOI] [PubMed] [Google Scholar]
- 57.Manju S, Sreenivasan K. Gold nanoparticles generated and stabilized by water soluble curcumin-polymer conjugate: Blood compatibility evaluation and targeted drug delivery onto cancer cells. J Colloid Interface Sci. 2012;368(1):144–51. doi: 10.1016/j.jcis.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 58.Labala S, Mandapalli PK, Kurumaddali A, Venuganti VV. Layer-by-Layer Polymer Coated Gold Nanoparticles for Topical Delivery of Imatinib Mesylate To Treat Melanoma. Mol Pharm. 2015;12(3):878–88. doi: 10.1021/mp5007163. [DOI] [PubMed] [Google Scholar]
- 59.Chen Z, Zhang L, Song Y, He J, Wu L, Zhao C. et al. Hierarchical targeted hepatocyte mitochondrial multifunctional chitosan nanoparticles for anticancer drug delivery. Biomaterials. 2015;52:240–50. doi: 10.1016/j.biomaterials.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 60.Discher DE, Eisenberg A. Polymer vesicles. Science. 2002;297(5583):967–73. doi: 10.1126/science.1074972. [DOI] [PubMed] [Google Scholar]
- 61.Costantino L, Boraschi D. Is there a clinical future for polymeric nanoparticles as brain-targeting drug delivery agents? Drug Discov Today. 2012;17(7-8):367–78. doi: 10.1016/j.drudis.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 62.Parveen S, Misra R, Sahoo SK. Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine. 2012;8(2):147–66. doi: 10.1016/j.nano.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 63.Faraji AH, Wipf P. Nanoparticles in cellular drug delivery. Bioorg Med Chem. 2009;17(8):2950–62. doi: 10.1016/j.bmc.2009.02.043. [DOI] [PubMed] [Google Scholar]
- 64.Banks WA, Kastin AJ, Barrera CM. Delivering peptides to the central nervous system: dilemmas and strategies. Pharm Res. 1991;8(11):1345–50. doi: 10.1023/A:1015884603456. [DOI] [PubMed] [Google Scholar]
- 65.Roney C, Kulkarni P, Arora V, Antich P, Bonte F, Wu A. et al. Targeted nanoparticles for drug delivery through the blood-brain barrier for Alzheimer’s disease. J Control Release. 2005;108(2-3):193–214. doi: 10.1016/j.jconrel.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 66.Bronich TK, Bontha S, Shlyakhtenko LS, Bromberg L, Hatton TA, Kabanov AV. Template-assisted synthesis of nanogels from Pluronic-modified poly(acrylic acid) J Drug Target. 2006;14(6):357–66. doi: 10.1080/10611860600833781. [DOI] [PubMed] [Google Scholar]
- 67.Friedrich I, Reichl S, Muller-Goymann CC. Drug release and permeation studies of nanosuspensions based on solidified reverse micellar solutions (SRMS) Int J Pharm. 2005;305(1-2):167–75. doi: 10.1016/j.ijpharm.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 68.Dikpati A, Madgulkar AR, Kshirsagar SJ, Bhalekar MR, Singh Chahal A. Targeted Drug Delivery to CNS using Nanoparticles. J Adv Pharm Sci. 2012;2(1):179–91. [Google Scholar]
- 69.Hou D, Xie C, Huang K, Zhu C. The production and characteristics of solid lipid nanoparticles (SLNs) Biomaterials. 2003;24(10):1781–5. doi: 10.1016/S0142-9612(02)00578-1. [DOI] [PubMed] [Google Scholar]
- 70. Saupe A, Rades T. Solid Lipid Nanoparticles. In: Mozafari MR, editor. Solid Lipid Nanoparticles. Frontiers of Nanotherapy. Dordrecht, Netherlands: Springer; 2006. P. 41-50.
- 71.Onoue S, Yamada S, Chan HK. Nanodrugs: Pharmacokinetics and safety. Int J Nanomedicine. 2014;9:1025–37. doi: 10.2147/IJN.S38378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Esposito E, Fantin M, Marti M, Drechsler M, Paccamiccio L, Mariani P. et al. Solid lipid nanoparticles as delivery systems for bromocriptine. Pharm Res. 2008;25(7):1521–30. doi: 10.1007/s11095-007-9514-y. [DOI] [PubMed] [Google Scholar]
- 73.McClements DJ, Decker EA, Weiss J. Emulsion-based delivery systems for lipophilic bioactive components. J Food Sci. 2007;72(8):R109–24. doi: 10.1111/j.1750-3841.2007.00507.x. [DOI] [PubMed] [Google Scholar]
- 74.Rodríguez-Gascón A, del Pozo-Rodríguez A, Solinís MÁ. Development of nucleic acid vaccines: Use of self-amplifying RNA in lipid nanoparticles. Int J Nanomedicine. 2014;9:1833–43. doi: 10.2147/IJN.S39810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dhawan S, Kapil R, Singh B. Formulation development and systematic optimization of solid lipid nanoparticles of quercetin for improved brain delivery. J Pharm Pharmacol. 2011;63(3):342–51. doi: 10.1111/j.2042-7158.2010.01225.x. [DOI] [PubMed] [Google Scholar]
- 76.Olbrich C, Gessner A, Kayser O, Müller RH. Lipid-drug-conjugate (LDC) nanoparticles as novel carrier system for the hydrophilic antitrypanosomal drug diminazenediaceturate. J Drug Target. 2002;10(5):387–96. doi: 10.1080/1061186021000001832. [DOI] [PubMed] [Google Scholar]
- 77.Wang JX, Sun X, Zhang ZR. Enhanced brain targeting by synthesis of 3’,5'-dioctanoyl-5-fluoro-2'-deoxyuridine and incorporation into solid lipid nanoparticles. Eur J Pharm Biopharm. 2002;54(3):285–90. doi: 10.1016/S0939-6411(02)00083-8. [DOI] [PubMed] [Google Scholar]
- 78.Chattopadhyay N, Zastre J, Wong HL, Wu XY, Bendayan R. Solid lipid nanoparticles enhance the delivery of the HIV protease inhibitor, atazanavir, by a human brain endothelial cell line. Pharm Res. 2008;25(10):2262–71. doi: 10.1007/s11095-008-9615-2. [DOI] [PubMed] [Google Scholar]
- 79.Cheng Y, Wang J, Rao T, He X, Xu T. Pharmaceutical applications of dendrimers: promising nanocarriers for drug delivery. Front Biosci. 2008;13:1447–71. doi: 10.2741/2774. [DOI] [PubMed] [Google Scholar]
- 80.Safari J, Zarnegar Z. Advanced drug delivery systems: Nanotechnology of health design A review. J Saudi Chem Soc. 2014;18(2):85–99. doi: 10.1016/j.jscs.2012.12.009. [DOI] [Google Scholar]
- 81.Bhadra D, Bhadra S, Jain S, Jain NK. A PEGylated dendritic nanoparticulate carrier of fluorouracil. Int J Pharm. 2003;257(1-2):111–24. doi: 10.1016/S0378-5173(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 82.Martinho N, Florindo H, Silva L, Brocchini S, Zloh M, Barata T. Molecular modeling to study dendrimers for biomedical applications. Molecules. 2014;19(12):20424–67. doi: 10.3390/molecules191220424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Duncan R, Izzo L. Dendrimer biocompatibility and toxicity. Adv Drug Deliv Rev. 2005;57(15):2215–37. doi: 10.1016/j.addr.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 84.Pan S, Wang C, Zeng X, Wen Y, Wu H, Feng M. Short multi-armed polylysine-graft-polyamidoamine copolymer as efficient gene vectors. Int J Pharm. 2011;420(2):206–15. doi: 10.1016/j.ijpharm.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 85.Boridy S, Soliman GM, Maysinger D. Modulation of inflammatory signaling and cytokine release from microglia by celastrol incorporated into dendrimer nanocarriers. Nanomedicine (Lond) 2012;7(8):1149–65. doi: 10.2217/nnm.12.16. [DOI] [PubMed] [Google Scholar]
- 86.Cerqueira SR, Silva BL, Oliveira JM, Mano JF, Sousa N, Salgado AJ. et al. Multifunctionalized CMCht/PAMAM dendrimer nanoparticles modulate the cellular uptake by astrocytes and oligodendrocytes in primary cultures of glial cells. Macromol Biosci. 2012;12(5):591–7. doi: 10.1002/mabi.201100294. [DOI] [PubMed] [Google Scholar]
- 87.Beg S, Samad A, Alam MI, Nazish I. Dendrimers as novel systems for delivery of neuropharmaceuticals to the brain. CNS Neurol Disord Drug Targets. 2011;10(5):576–88. doi: 10.2174/187152711796235023. [DOI] [PubMed] [Google Scholar]
- 88.Bilia AR, Guccione C, Isacchi B, Righeschi C, Firenzuoli F, Bergonzi MC. Essential oils loaded in nanosystems: A developing strategy for a successful therapeutic approach. Evid Based Complement Alternat Med. 2014;2014:651593. doi: 10.1155/2014/651593. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 89.Zucker D, Andriyanov AV, Steiner A, Raviv U, Barenholz Y. Characterization of PEGylated nanoliposomes co-remotely loaded with topotecan and vincristine: Relating structure and pharmacokinetics to therapeutic efficacy. J Control Release. 2012;160(2):281–9. doi: 10.1016/j.jconrel.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 90.Bawa R. Nanoparticle-based therapeutics in humans: A survey. Nanotechnol Law Bus. 2008;5(2):135–55. [Google Scholar]
- 91.Koukourakis MI, Koukouraki S, Giatromanolaki A, Kakolyris S, Georgoulias V, Velidaki A. et al. High intratumoral accumulation of stealth liposomal doxorubicin in sarcomas--rationale for combination with radiotherapy. Acta Oncol. 2000;39(2):207–11. doi: 10.1080/028418600430789. [DOI] [PubMed] [Google Scholar]
- 92.Schmidt J, Metselaar JM, Gold R. Intravenous liposomal prednisolone downregulates in situ TNF-alpha production by T-cells in experimental autoimmune encephalomyelitis. J Histochem Cytochem. 2003;51(9):1241–4. doi: 10.1177/002215540305100915. [DOI] [PubMed] [Google Scholar]
- 93.Huwyler J, Wu D, Pardridge WM. Brain drug delivery of small molecules using immunoliposomes. Proc Natl Acad Sci U S A. 1996;93(24):14164–9. doi: 10.1073/pnas.93.24.14164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ramos-Cabrer P, Campos F, Sobrino T, Castillo J. Targeting the ischemic penumbra. Stroke. 2011;42(1 Suppl):S7–11. doi: 10.1161/STROKEAHA.110.596684. [DOI] [PubMed] [Google Scholar]
- 95.Ahn J, Miura Y, Yamada N, Chida T, Liu X, Kim A. et al. Antibody fragment-conjugated polymeric micelles incorporating platinum drugs for targeted therapy of pancreatic cancer. Biomaterials. 2015;39:23–30. doi: 10.1016/j.biomaterials.2014.10.069. [DOI] [PubMed] [Google Scholar]
- 96.Mohamed S, Parayath NN, Taurin S, Greish K. Polymeric nano-micelles: versatile platform for targeted delivery in cancer. Ther Deliv. 2014;5(10):1101–21. doi: 10.4155/tde.14.69. [DOI] [PubMed] [Google Scholar]
- 97.Butun S, Ince FG, Erdugan H, Sahiner N. One-step fabrication of biocompatible carboxymethyl cellulose polymeric particles for drug delivery systems. Carbohydr Polym. 2011;86(2):636–43. doi: 10.1016/j.carbpol.2011.05.001. [DOI] [Google Scholar]
- 98.Akimoto J, Nakayama M, Okano T. Temperature-responsive polymeric micelles for optimizing drug targeting to solid tumors. J Control Release. 2014;193:2–8. doi: 10.1016/j.jconrel.2014.06.062. [DOI] [PubMed] [Google Scholar]
- 99.Zhao BX, Zhao Y, Huang Y, Luo LM, Song P, Wang X. et al. The efficiency of tumor-specific pH-responsive peptide-modified polymeric micelles containing paclitaxel. Biomaterials. 2012;33(8):2508–20. doi: 10.1016/j.biomaterials.2011.11.078. [DOI] [PubMed] [Google Scholar]
- 100.Vigderman L, Zubarev ER. Therapeutic platforms based on gold nanoparticles and their covalent conjugates with drug molecules. Adv Drug Deliv Rev. 2013;65(5):663–76. doi: 10.1016/j.addr.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 101.Sahoo NG, Rana S, Cho JW, Li L, Chan SH. Polymer nanocomposites based on functionalized carbon nanotubes. Prog Polym Sci. 2010;35(7):837–67. doi: 10.1016/j.progpolymsci.2010.03.002. [DOI] [Google Scholar]
- 102.Tran PA, Zhang L, Webster TJ. Carbon nanofibers and carbon nanotubes in regenerative medicine. Adv Drug Deliv Rev. 2009;61(12):1097–114. doi: 10.1016/j.addr.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 103.Dyke CA, Tour JM. Overcoming the insolubility of carbon nanotubes through high degrees of sidewall functionalization. Chemistry. 2004;10(4):812–7. doi: 10.1002/chem.200305534. [DOI] [PubMed] [Google Scholar]
- 104.Zeineldin R, Al-Haik M, Hudson LG. Role of polyethylene glycol integrity in specific receptor targeting of carbon nanotubes to cancer cells. Nano Lett. 2009;9(2):751–7. doi: 10.1021/nl8033174. [DOI] [PubMed] [Google Scholar]
- 105.Yang Z, Zhang Y, Yang Y, Sun L, Han D, Li H. et al. Pharmacological and toxicological target organelles and safe use of single-walled carbon nanotubes as drug carriers in treating alzheimer disease. Nanomedicine. 2010;6(3):427–41. doi: 10.1016/j.nano.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 106.Moon SU, Kim J, Bokara KK, Kim JY, Khang D, Webster TJ. et al. Carbon nanotubes impregnated with subventricular zone neural progenitor cells promotes recovery from stroke. Int J Nanomedicine. 2012;7:2751–65. doi: 10.2147/IJN.S30273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wu W, Wieckowski S, Pastorin G, Benincasa M, Klumpp C, Briand JP. et al. Targeted delivery of amphotericin b to cells by using functionalized carbon nanotubes. Angew Chem Int Ed Engl. 2005;44(39):6358–62. doi: 10.1002/anie.200501613. [DOI] [PubMed] [Google Scholar]
- 108.Passeri D, Rinaldi F, Ingallina C, Carafa M, Rossi M, Terranova ML. et al. Biomedical applications of nanodiamonds: An overview. J Nanosci Nanotechnol. 2015;15(2):972–88. doi: 10.1166/jnn.2015.9734. [DOI] [PubMed] [Google Scholar]
- 109.Nielsen GD, Roursgaard M, Jensen KA, Poulsen SS, Larsen ST. In vivo biology and toxicology of fullerenes and their derivatives. Basic Clin Pharmacol Toxicol. 2008;103(3):197–208. doi: 10.1111/j.1742-7843.2008.00266.x. [DOI] [PubMed] [Google Scholar]
- 110.Anilkumar P, Lu F, Cao L, Luo PG, Liu JH, Sahu S. et al. Fullerenes for applications in biology and medicine. Curr Med Chem. 2011;18(14):2045–59. doi: 10.2174/092986711795656225. [DOI] [PubMed] [Google Scholar]
- 111.Johnston HJ, Hutchison GR, Christensen FM, Aschberger K, Stone V. The biological mechanisms and physicochemical characteristics responsible for driving fullerene toxicity. Toxicol Sci. 2010;114(2):162–82. doi: 10.1093/toxsci/kfp265. [DOI] [PubMed] [Google Scholar]
- 112.Tykhomyrov AA, Nedzvetsky VS, Klochkov VK, Andrievsky GV. Nanostructures of hydrated c60 fullerene (c60hyfn) protect rat brain against alcohol impact and attenuate behavioral impairments of alcoholized animals. Toxicology. 2008;246(2-3):158–65. doi: 10.1016/j.tox.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 113.Kreuter J. Nanoparticulate systems for brain delivery of drugs. Adv Drug Deliv Rev. 2001;47(1):65–81. doi: 10.1016/s0169-409x(00)00122-8. [DOI] [PubMed] [Google Scholar]
- 114.Alyaudtin RN, Reichel A, Lobenberg R, Ramge P, Kreuter J, Begley DJ. Interaction of poly(butylcyanoacrylate) nanoparticles with the blood-brain barrier in vivo and in vitro. J Drug Target. 2001;9(3):209–21. doi: 10.3109/10611860108997929. [DOI] [PubMed] [Google Scholar]
- 115.Ramge P, Unger RE, Oltrogge JB, Zenker D, Begley D, Kreuter J. et al. Polysorbate-80 coating enhances uptake of polybutylcyanoacrylate (PBCA)-nanoparticles by human and bovine primary brain capillary endothelial cells. Eur J Neurosci. 2000;12(6):1931–40. doi: 10.1046/j.1460-9568.2000.00078.x. [DOI] [PubMed] [Google Scholar]
- 116.Fenart L, Casanova A, Dehouck B, Duhem C, Slupek S, Cecchelli R. et al. Evaluation of effect of charge and lipid coating on ability of 60-nm nanoparticles to cross an in vitro model of the blood-brain barrier. J Pharmacol Exp Ther. 1999;291(3):1017–22. [PubMed] [Google Scholar]
- 117.Hwang SR, Kim K. Nano-enabled delivery systems across the blood-brain barrier. Arch Pharm Res. 2014;37(1):24–30. doi: 10.1007/s12272-013-0272-6. [DOI] [PubMed] [Google Scholar]
- 118.Mucke L. Neuroscience: Alzheimer's disease. Nature. 2009;461(7266):895–7. doi: 10.1038/461895a. [DOI] [PubMed] [Google Scholar]
- 119.Sloane PD, Zimmerman S, Suchindran C, Reed P, Wang L, Boustani M. et al. The public health impact of alzheimer's disease, 2000-2050: Potential implication of treatment advances. Annu Rev Public Health. 2002;23:213–31. doi: 10.1146/annurev.publhealth.23.100901.140525. [DOI] [PubMed] [Google Scholar]
- 120.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M. et al. Global prevalence of dementia: A delphi consensus study. Lancet. 2005;366(9503):2112–7. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Drolle E, Hane F, Lee B, Leonenko Z. Atomic force microscopy to study molecular mechanisms of amyloid fibril formation and toxicity in alzheimer's disease. Drug Metab Rev. 2014;46(2):207–23. doi: 10.3109/03602532.2014.882354. [DOI] [PubMed] [Google Scholar]
- 122.Munoz DG, Feldman H. Causes of alzheimer's disease. CMAJ. 2000;162(1):65–72. [PMC free article] [PubMed] [Google Scholar]
- 123.Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS. et al. Mitochondrial abnormalities in alzheimer's disease. J Neurosci. 2001;21(9):3017–23. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hashimoto K, Fukushima T, Shimizu E, Okada S, Komatsu N, Okamura N. et al. Possible role of d-serine in the pathophysiology of alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(2):385–8. doi: 10.1016/j.pnpbp.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 125.Fazil M, Shadab Shadab, Baboota S, Sahni JK, Ali J. Nanotherapeutics for alzheimer's disease (ad): Past, present and future. J Drug Target. 2012;20(2):97–113. doi: 10.3109/1061186X.2011.607499. [DOI] [PubMed] [Google Scholar]
- 126.Goldsmith M, Abramovitz L, Peer D. Precision nanomedicine in neurodegenerative diseases. ACS Nano. 2014;8(3):1958–65. doi: 10.1021/nn501292z. [DOI] [PubMed] [Google Scholar]
- 127.D’Angelo B, Santucci S, Benedetti E, Di Loreto S, Phani RA, Falone S. et al. Cerium Oxide nanoparticles trigger neuronal survival in a human Alzheimer disease model by modulating BDNF pathway. Curr Nanosci. 2009;5(2):167–76. doi: 10.2174/157341309788185523. [DOI] [Google Scholar]
- 128.Picone P, Bondi ML, Montana G, Bruno A, Pitarresi G, Giammona G. et al. Ferulic acid inhibits oxidative stress and cell death induced by Ab oligomers: Improved delivery by solid lipid nanoparticles. Free Radic Res. 2009;43(11):1133–45. doi: 10.1080/10715760903214454. [DOI] [PubMed] [Google Scholar]
- 129.Carroll RT, Bhatia D, Geldenhuys W, Bhatia R, Miladore N, Bishayee A. et al. Brain-targeted delivery of tempol-loaded nanoparticles for neurological disorders. J Drug Target. 2010;18(9):665–74. doi: 10.3109/10611861003639796. [DOI] [PubMed] [Google Scholar]
- 130.Smith A, Giunta B, Bickford PC, Fountain M, Tan J, Shytle RD. Nanolipidic particles improve the bioavailability and alpha-secretase inducing ability of epigallocatechin-3-gallate (egcg) for the treatment of alzheimer's disease. Int J Pharm. 2010;389(1-2):207–12. doi: 10.1016/j.ijpharm.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Giordano C, Albani D, Gloria A, Tunesi M, Rodilossi S, Russo T. et al. Nanocomposites for neurodegenerative diseases: Hydrogel-nanoparticle combinations for a challenging drug delivery. Int J Artif Organs. 2011;34(12):1115–27. doi: 10.5301/IJAO.2011.8915. [DOI] [PubMed] [Google Scholar]
- 132.Hartig W, Paulke BR, Varga C, Seeger J, Harkany T, Kacza J. Electron microscopic analysis of nanoparticles delivering thioflavin-t after intrahippocampal injection in mouse: Implications for targeting beta-amyloid in alzheimer's disease. Neurosci Lett. 2003;338(2):174–6. doi: 10.1016/s0304-3940(02)01399-x. [DOI] [PubMed] [Google Scholar]
- 133.Cui D, Gao H. Advance and prospect of bionanomaterials. Biotechnol Prog. 2003;19(3):683–92. doi: 10.1021/bp025791i. [DOI] [PubMed] [Google Scholar]
- 134.Herholz K. Acetylcholine esterase activity in mild cognitive impairment and alzheimer's disease. Eur J Nucl Med Mol Imaging. 2008;35 Suppl 1:S25–9. doi: 10.1007/s00259-007-0699-4. [DOI] [PubMed] [Google Scholar]
- 135.Cheng KK, Yeung CF, Ho SW, Chow SF, Chow AH, Baum L. Highly stabilized curcumin nanoparticles tested in an in vitro blood-brain barrier model and in alzheimer's disease Tg2576 mice. AAPS J. 2013;15(2):324–36. doi: 10.1208/s12248-012-9444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fernandes C, Soni U, Patravale V. Nano-interventions for neurodegenerative disorders. Pharmacol Res. 2010;62(2):166–78. doi: 10.1016/j.phrs.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 137.Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K. et al. Projected number of people with parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68(5):384–6. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- 138.Singh N, Pillay V, Choonara YE. Advances in the treatment of parkinson's disease. Prog Neurobiol. 2007;81(1):29–44. doi: 10.1016/j.pneurobio.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 139.Moore RY. Organization of midbrain dopamine systems and the pathophysiology of parkinson's disease. Parkinsonism Relat Disord. 2003;9 Suppl 2:S65–71. doi: 10.1016/s1353-8020(03)00063-4. [DOI] [PubMed] [Google Scholar]
- 140.Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of parkinson's disease. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- 141.Lewitt PA. Levodopa for the treatment of parkinson's disease. N Engl J Med. 2008;359(23):2468–76. doi: 10.1056/NEJMct0800326. [DOI] [PubMed] [Google Scholar]
- 142.Pardridge WM. Blood-brain barrier delivery. Drug Discov Today. 2007;12(1-2):54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 143.Kurakhmaeva KB, Djindjikhashvili IA, Petrov VE, Balabanyan VU, Voronina TA, Trofimov SS. et al. Brain targeting of nerve growth factor using poly(butyl cyanoacrylate) nanoparticles. J Drug Target. 2009;17(8):564–74. doi: 10.1080/10611860903112842. [DOI] [PubMed] [Google Scholar]
- 144.Khasnavis S, Ghosh A, Roy A, Pahan K. Castration induces parkinson disease pathologies in young male mice via inducible nitric-oxide synthase. J Biol Chem. 2013;288(29):20843–55. doi: 10.1074/jbc.M112.443556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hu K, Shi Y, Jiang W, Han J, Huang S, Jiang X. Lactoferrin conjugated peg-plga nanoparticles for brain delivery: Preparation, characterization and efficacy in parkinson's disease. Int J Pharm. 2011;415(1-2):273–83. doi: 10.1016/j.ijpharm.2011.05.062. [DOI] [PubMed] [Google Scholar]
- 146.Ricard D, Idbaih A, Ducray F, Lahutte M, Hoang-Xuan K, Delattre JY. Primary brain tumours in adults. Lancet. 2012;379(9830):1984–96. doi: 10.1016/S0140-6736(11)61346-9. [DOI] [PubMed] [Google Scholar]
- 147.Walker AE, Robins M, Weinfeld FD. Epidemiology of brain tumors: The national survey of intracranial neoplasms. Neurology. 1985;35(2):219–26. doi: 10.1212/wnl.35.2.219. [DOI] [PubMed] [Google Scholar]
- 148.Wrensch M, Minn Y, Chew T, Bondy M, Berger MS. Epidemiology of primary brain tumors: Current concepts and review of the literature. Neuro Oncol. 2002;4(4):278–99. doi: 10.1093/neuonc/4.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bidros DS, Vogelbaum MA. Novel drug delivery strategies in neuro-oncology. Neurotherapeutics. 2009;6(3):539–46. doi: 10.1016/j.nurt.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhan C, Lu W. The blood-brain/tumor barriers: Challenges and chances for malignant gliomas targeted drug delivery. Curr Pharm Biotechnol. 2012;13(12):2380–7. doi: 10.2174/138920112803341798. [DOI] [PubMed] [Google Scholar]
- 151.Lee P, Zhang R, Li V, Liu X, Sun RW, Che CM. et al. Enhancement of anticancer efficacy using modified lipophilic nanoparticle drug encapsulation. Int J Nanomedicine. 2012;7:731–7. doi: 10.2147/IJN.S28783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jain KK. Nanotechnology-based drug delivery for cancer. Technol Cancer Res Treat. 2005;4(4):407–16. doi: 10.1177/153303460500400408. [DOI] [PubMed] [Google Scholar]
- 153.Xi G, Robinson E, Mania-Farnell B, Vanin EF, Shim KW, Takao T. et al. Convection-enhanced delivery of nanodiamond drug delivery platforms for intracranial tumor treatment. Nanomedicine. 2014;10(2):381–91. doi: 10.1016/j.nano.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 154.Kateb B, Van Handel M, Zhang L, Bronikowski MJ, Manohara H, Badie B. Internalization of mwcnts by microglia: Possible application in immunotherapy of brain tumors. NeuroImage. 2007;37 Suppl 1:S9–17. doi: 10.1016/j.neuroimage.2007.03.078. [DOI] [PubMed] [Google Scholar]
- 155.Kukowska-Latallo JF, Candido KA, Cao Z, Nigavekar SS, Majoros IJ, Thomas TP. et al. Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer Res. 2005;65(12):5317–24. doi: 10.1158/0008-5472.CAN-04-392. [DOI] [PubMed] [Google Scholar]
- 156.Yinghuai Z, Hosmane NS. Applications and perspectives of boron-enriched nanocomposites in cancer therapy. Future Med Chem. 2013;5(6):705–14. doi: 10.4155/fmc.13.47. [DOI] [PubMed] [Google Scholar]
- 157.Shapshak P, Chiappelli F, Commins D, Singer E, Levine AJ, Somboonwit C. et al. Molecular epigenetics, chromatin, and NeuroAIDS/HIV: translational implications. Bioinformation. 2008;3(1):53–7. doi: 10.6026/97320630003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mintz M. Clinical comparison of adult and pediatric NeuroAIDS. Adv Neuroimmunol. 1994;4(3):207–21. doi: 10.1016/S0960-5428(06)80259-7. [DOI] [PubMed] [Google Scholar]
- 159.Bloom FE, Rausch DM. HIV in the brain: pathology and neurobehavioral consequences. J Neurovirol. 1997;3(2):102–9. doi: 10.3109/13550289709015800. [DOI] [PubMed] [Google Scholar]
- 160.Banks WA, Ercal N, Price TO. The blood-brain barrier in neuroAIDS. Curr HIV Res. 2006;4(3):259–66. doi: 10.2174/157016206777709447. [DOI] [PubMed] [Google Scholar]
- 161.Roberts ES, Zandonatti MA, Watry DD, Madden LJ, Henriksen SJ, Taffe MA. et al. Induction of pathogenic sets of genes in macrophages and neurons in NeuroAIDS. Am J Pathol. 2003;162(6):2041–57. doi: 10.1016/S0002-9440(10)64336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Doualla-Bell F, Turner D, Loemba H, Petrella M, Brenner B, Wainberg MA. HIV drug resistance and optimization of antiviral treatment in resource-poor countries. Med Sci (Paris) 2004;20(10):882–6. doi: 10.1051/medsci/20042010882. [DOI] [PubMed] [Google Scholar]
- 163.Dou H, Destache CJ, Morehead JR, Mosley RL, Boska MD, Kingsley J. et al. Development of a macrophage-based nanoparticle platform for antiretroviral drug delivery. Blood. 2006;108(8):2827–35. doi: 10.1182/blood-2006-03-012534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sagar V, Pilakka-Kanthikeel S, Pottathil R, Saxena SK, Nair M. Towards nanomedicines for neuroAIDS. Rev Med Virol. 2014;24(2):103–24. doi: 10.1002/rmv.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Omar RF, Dusserre N, Desormeaux A, Poulin L, Tremblay M, Beauchamp D. et al. Liposomal encapsulation of foscarnet protects against hypocalcemia induced by free foscarnet. Antimicrob Agents Chemother. 1995;39(9):1973–8. doi: 10.1128/AAC.39.9.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kuo YC, Su FL. Transport of stavudine, delavirdine, and saquinavir across the blood-brain barrier by polybutylcyanoacrylate, methylmethacrylate-sulfopropylmethacrylate, and solid lipid nanoparticles. Int J Pharm. 2007;340(1-2):143–52. doi: 10.1016/j.ijpharm.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 167.Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB. et al. The lifetime risk of stroke: estimates from the Framingham Study. Stroke. 2006;37(2):345–50. doi: 10.1161/01.STR.0000199613.38911.b2. [DOI] [PubMed] [Google Scholar]
- 168.Shcharbina N, Shcharbin D, Bryszewska M. Nanomaterials in stroke treatment: perspectives. Stroke. 2013;44(8):2351–5. doi: 10.1161/STROKEAHA.113.001298. [DOI] [PubMed] [Google Scholar]
- 169.Allen CL, Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke. 2009;4(6):461–70. doi: 10.1111/j.1747-4949.2009.00387.x. [DOI] [PubMed] [Google Scholar]
- 170.Martin HG, Wang YT. Blocking the Deadly Effects of the NMDA Receptor in Stroke. Cell. 2010;140(2):174–6. doi: 10.1016/j.cell.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 171.Weiss N, Miller F, Cazaubon S, Couraud PO. Blood-brain barrier part III: therapeutic approaches to cross the blood-brain barrier and target the brain. Rev Neurol (Paris) 2010;166(3):284–8. doi: 10.1016/j.neurol.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 172.Mdzinarishvili A, Sutariya V, Talasila PK, Geldenhuys WJ, Sadana P. Engineering triiodothyronine (T3) nanoparticle for use in ischemic brain stroke. Drug Deliv Transl Res. 2013;3(4):309–17. doi: 10.1007/s13346-012-0117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Estevez AY, Pritchard S, Harper K, Aston JW, Lynch A, Lucky JJ. et al. Neuroprotective mechanisms of cerium oxide nanoparticles in a mouse hippocampal brain slice model of ischemia. Free Radic Biol Med. 2011;51(6):1155–63. doi: 10.1016/j.freeradbiomed.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 174.Takamiya M, Miyamoto Y, Yamashita T, Deguchi K, Ohta Y, Abe K. Strong neuroprotection with a novel platinum nanoparticle against ischemic stroke- and tissue plasminogen activator-related brain damages in mice. Neuroscience. 2012;221:47–55. doi: 10.1016/j.neuroscience.2012.06.060. [DOI] [PubMed] [Google Scholar]
- 175.Karatas H, Aktas Y, Gursoy-Ozdemir Y, Bodur E, Yemisci M, Caban S. et al. A nanomedicine transports a peptide caspase-3 inhibitor across the blood-brain barrier and provides neuroprotection. J Neurosci. 2009;29(44):13761–9. doi: 10.1523/JNEUROSCI.4246-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhao H, Bao XJ, Wang RZ, Li GL, Gao J, Ma SH. et al. Postacute ischemia vascular endothelial growth factor transfer by transferrin-targeted liposomes attenuates ischemic brain injury after experimental stroke in rats. Hum Gene Ther. 2011;22(2):207–15. doi: 10.1089/hum.2010.111. [DOI] [PubMed] [Google Scholar]
- 177.Beckung E, Hagberg G. Neuroimpairments, activity limitations, and participation restrictions in children with cerebral palsy. Dev Med Child Neurol. 2002;44(5):309–16. doi: 10.1111/j.1469-8749.2002.tb00816.x. [DOI] [PubMed] [Google Scholar]
- 178.Li MH, Choi SK, Thomas TP, Desai A, Lee KH, Kotlyar A. et al. Dendrimer-based multivalent methotrexates as dual acting nanoconjugates for cancer cell targeting. Eur J Med Chem. 2012;47(1):560–72. doi: 10.1016/j.ejmech.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Odding E, Roebroeck ME, Stam HJ. The epidemiology of cerebral palsy: incidence, impairments and risk factors. Disabil Rehabil. 2006;28(4):183–91. doi: 10.1080/09638280500158422. [DOI] [PubMed] [Google Scholar]
- 180.Arneson CL, Durkin MS, Benedict RE, Kirby RS, Yeargin-Allsopp M, Van Naarden Braun K. et al. Prevalence of cerebral palsy: Autism and Developmental Disabilities Monitoring Network, three sites, United States, 2004. Disabil Health J. 2009;2(1):45–8. doi: 10.1016/j.dhjo.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 181.Robinson KG, Mendonca JL, Militar JL, Theroux MC, Dabney KW, Shah SA. et al. Disruption of basal lamina components in neuromotor synapses of children with spastic quadriplegic cerebral palsy. PLoS One. 2013;8(8):e70288. doi: 10.1371/journal.pone.0070288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kannan S, Dai H, Navath RS, Balakrishnan B, Jyoti A, Janisse J. et al. Dendrimer-based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model. Sci Transl Med. 2012;4(130):130ra46. doi: 10.1126/scitranslmed.3003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Huang DM, Chung TH, Hung Y, Lu F, Wu SH, Mou CY. et al. Internalization of mesoporous silica nanoparticles induces transient but not sufficient osteogenic signals in human mesenchymal stem cells. Toxicol Appl Pharmacol. 2008;231(2):208–15. doi: 10.1016/j.taap.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 184.Senanayake N, Roman GC. Epidemiology of epilepsy in developing countries. Bull World Health Organ. 1993;71(2):247–58. [PMC free article] [PubMed] [Google Scholar]
- 185.Sridharan R, Murthy BN. Prevalence and pattern of epilepsy in India. Epilepsia. 1999;40(5):631–6. doi: 10.1111/j.1528-1157.1999.tb05566.x. [DOI] [PubMed] [Google Scholar]
- 186.Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, Van Emde Boas W. et al. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51(4):676–85. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 187.Vezzani A, Balosso S, Ravizza T. The role of cytokines in the pathophysiology of epilepsy. Brain Behav Immun. 2008;22(6):797–803. doi: 10.1016/j.bbi.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 188.Lee HS, Wang SY, Salter DM, Wang CC, Chen SJ, Fan HC. The impact of the use of antiepileptic drugs on the growth of children. BMC Pediatr. 2013;13:211. doi: 10.1186/1471-2431-13-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Eskandari S, Varshosaz J, Minaiyan M, Tabbakhian M. Brain delivery of valproic acid via intranasal administration of nanostructured lipid carriers: in vivo pharmacodynamic studies using rat electroshock model. Int J Nanomedicine. 2011;6:363–71. doi: 10.2147/IJN.S15881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Samia O, Hanan R, Kamal el T. Carbamazepine mucoadhesive nanoemulgel (MNEG) as brain targeting delivery system via the olfactory mucosa. Drug Deliv. 2012;19(1):58–67. doi: 10.3109/10717544.2011.644349. [DOI] [PubMed] [Google Scholar]
- 191.Yusuf M, Khan RA, Khan M, Ahmed B. Plausible antioxidant biomechanics and anticonvulsant pharmacological activity of brain-targeted β-carotene nanoparticles. Int J Nanomedicine. 2012;7:4311–21. doi: 10.2147/IJN.S34588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bradford CM, Ramos I, Cross AK, Haddock G, McQuaid S, Nicholas AP. et al. Localisation of citrullinated proteins in normal appearing white matter and lesions in the central nervous system in multiple sclerosis. J Neuroimmunol. 2014;273(1-2):85–95. doi: 10.1016/j.jneuroim.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 193.Gironi M, Borgiani B, Mariani E, Cursano C, Mendozzi L, Cavarretta R. et al. Oxidative stress is differentially present in multiple sclerosis courses, early evident, and unrelated to treatment. J Immunol Res. 2014;2014:961863. doi: 10.1155/2014/961863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Heckman KL, Decoteau W, Estevez A, Reed KJ, Costanzo W, Sanford D. et al. Custom cerium oxide nanoparticles protect against a free radical mediated autoimmune degenerative disease in the brain. ACS Nano. 2013;7(12):10582–96. doi: 10.1021/nn403743b. [DOI] [PubMed] [Google Scholar]
- 195.Menon PK, Muresanu DF, Sharma A, Mössler H, Sharma HS. Cerebrolysin, a Mixture of Neurotrophic Factors Induces Marked Neuroprotection in Spinal Cord Injury Following Intoxication of Engineered Nanoparticles from Metals. CNS Neurol Disord Drug Targets. 2012;11(1):40–9. doi: 10.2174/187152712799960781. [DOI] [PubMed] [Google Scholar]
- 196.Sharma HS, Sharma A. Nanoparticles aggravate heat stress induced cognitive deficits, blood-brain barrier disruption, edema formation and brain pathology. Prog Brain Res. 2007;162:245–73. doi: 10.1016/S0079-6123(06)62013-X. [DOI] [PubMed] [Google Scholar]
- 197.Sharma HS, Ali SF, Tian ZR, Hussain SM, Schlager JJ, Sjöquist PO. et al. Chronic treatment with nanoparticles exacerbate hyperthermia induced blood-brain barrier breakdown, cognitive dysfunction and brain pathology in the rat. Neuroprotective effects of nanowired-antioxidant compound H-290/51. J Nanosci Nanotechnol. 2009;9(8):5073–90. doi: 10.1166/jnn.2009.gr10. [DOI] [PubMed] [Google Scholar]
- 198.Kaur IP, Bhandari R, Bhandari S, Kakkar V. Potential of solid lipid nanoparticles in brain targeting. J Control Release. 2008;127(2):97–109. doi: 10.1016/j.jconrel.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 199.Vega-Villa KR, Takemoto JK, Yáñez JA, Remsberg CM, Forrest ML, Davies NM. Clinical toxicities of nanocarrier systems. Adv Drug Deliv Rev. 2008;60(8):929–38. doi: 10.1016/j.addr.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 200.Krol S. Challenges in drug delivery to the brain: Nature is against us. J Control Release. 2012;164(2):145–55. doi: 10.1016/j.jconrel.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 201.Gloria A, De Santis R, Ambrosio L, Causa F, Tanner KE. A multi-component fiber-reinforced PHEMA-based hydrogel/HAPEXTM device for customized intervertebral disc prosthesis. J Biomater Appl. 2011;25(8):795–810. doi: 10.1177/088532820936093. [DOI] [PubMed] [Google Scholar]