Abstract
Purpose: Psylliumseeds are used in traditional herbal medicine to treat various disorders. Moreover, as a soluble fiber, psyllium has potential to stimulate bacterial growth in digestive system. We aimed to substitute alkali-extractable polysaccharides of psyllium for alginate in beads with second coat of poly-l-lysine to coat Lactobacillus acidophilus.
Methods: Beads were prepared using extrusion technique. Poly-l-lysine as second coat was incorporated on optimum alginate/psyllium beads using immersion technique. Beads were characterized in terms of size, encapsulation efficiency, integrity and bacterial survival in harsh conditions.
Results: Beads with narrow size distribution ranging from 1.85 ± 0.05 to 2.40 ± 0.18 mm with encapsulation efficiency higher than 96% were achieved. Psyllium concentrations in beads did not produce constant trend in bead sizes. Surface topography by SEM showed that substitution of psyllium enhanced integrity of obtained beads. Psyllium successfully protected the bacteria against acidic condition and lyophilization equal to alginate in the beads. Better survivability with beads of alginate/psyllium-poly-l-lysine was achieved with around 2 log rise in bacterial count in acid condition compared to the corresponding single coat beads.
Conclusion: Alginate/psyllium (1:2) beads with narrow size distribution and high encapsulation efficiency of the bacteria have been achieved. Presence of psyllium produced a much smoother and integrated surface texture for the beads with sufficient protection of the bacteria against acidic condition as much as alginate. Considering the health benefits of psyllium and its prebiotic activity, psyllium can be beneficially replaced in part for alginate in probiotic coating.
Keywords: Lactobacillus acidophilus, Alginate, Psyllium, Poly-L-Lysine, Coating
Introduction
Probiotics are defined as live microorganisms, which when administrated in sufficient dosage give one or more specific health benefits for the host.1 The most significant role of probiotics in health system can be their function to maintain normal intestinal microflora and defense against enteropathogen infections. Furthermore beneficial microorganisms have been shown to control the cholesterol levels of serum, improve utilization of lactose in lactose maldigesters, and have anticarcinogenic and antimutagenic activities.2 However, probiotics to show their potential abilities need to survive in the challenging conditions of gastrointestinal tract colonize and multiply on the epithelium of colon in appropriate population (more than 107 cfu/g of finished product). To improve viability and stability of probiotics and efficient delivery of the cells to their active sites, a number of techniques have been utilized including encapsulation of probiotics in a variety of polymers.3 Alginate, a commonly used polymer to encapsulate probiotics, is a natural, biocompatible and biodegradable linear anionic polysaccharide. Alginate beads encapsulating bacteria in their matrix can be prepared by using extrusion or emulsion techniques.4,5
Herbal medicine has been commonly used over the years for treatment and prevention of diseases.6,7 Psyllium is generally referred to seeds from some members of plants genus Plantago including but not limited to Plantago ovata, Plantago psyllium, and Plantago indica. It is native plant to Indian subcontinent and Iran, although psyllium is now commercially cultivated in many parts of the world.8 Psyllium composed of a highly branched arabinoxylan forming gel mucilage. It is structurally consists of xylose units with arabinose and xylose in the side chains.9 Psyllium seeds are primarily used in traditional herbal medicine to treat various disorders and some of its claimed health benefits have been scientifically approved now. There are several reports regarding the application of psyllium for treatment of constipation, diarrhea, irritable bowel syndrome, inflammatory bowel disease, ulcerative colitis, colon cancer, diabetes, and hypercholesterolemia.8,10,11 Moreover, psyllium as a soluble fiber with the potential to stimulate bacterial growth in digestive system has been used as prebiotic.12-14 Prebiotics are defined as “non-digestible food ingredients that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon, and thus improves host health”.15
Considering the health benefits of psyllium in digestive system and its prebiotic activity, we have successfully incorporated psyllium (up to 0.5% w/v) in alginate beads containing probiotic bacteria Lactobacillus acidophilus in our previous work.4 In the current study, we aimed to prepare alginate beads with maximum possible concentration of psyllium with a second layer of poly l lysine on top to improve the stability and protective capability. The physicochemical properties and viability of the bacteria in the prepared beads after exposing to acid, bile and freeze drying conditions were evaluated.
Materials and Methods
Materials
L. acidophilus was obtained from Pasteur institute (Iran), sodium alginate, poly l lysine, oxgall from Sigma-Aldrich (Germany), MRS broth and MRS agar, sodium hydrogen phosphate, calcium chloride, sodium hydroxide and hydrochloric acid from Merck (Germany), and psyllium seed husk was supplied from company Herbi Darou Tabriz-Iran.
Methods
Preparation of inoculum
L. acidophilus was cultured in MRS broth at 37°C for 18 h. Culture was harvested by centrifugation at 3000 rpm at 4°C for 7min and washed twice with saline and collected by centrifugation as above. The washed bacterial cells were resuspended in 7 mL saline, and the cell count was determined using pour plate technique in MRS agar in triplicate. The cell suspension was divided in some equal parts and consequently was used to prepare different formulations.16
Extraction of psyllium
Gel-forming fraction of the alkali-extractable polysaccharides of psyllium seed was extracted by a method described by17 with some modifications which is depicted in Figure 1. First, 30 g of whole psyllium seed was grinded and then dispersed in 1000 mL water and placed it over night at 80°C water bath which led to swelling and gel formation. The gel phase was separated from solution and dissolved in 2 M NaOH solution at room temperature for 2 h; alkaline extract was separated from the residue by centrifugation (12000 rpm for 1h) and accordingly neutralized with 2 M HCl. During the neutralization, gel-like white precipitate was produced and separated by centrifugation (12000 rpm for 1 h) from the soluble fraction and washed three times with distilled water. The gel precipitate was evaporated for 3 h and then dried at 40°C oven for 48 h and after grinding was used for beads preparation. The photographs of the extraction process are shown in Figures 1 and 2.
Figure 1.
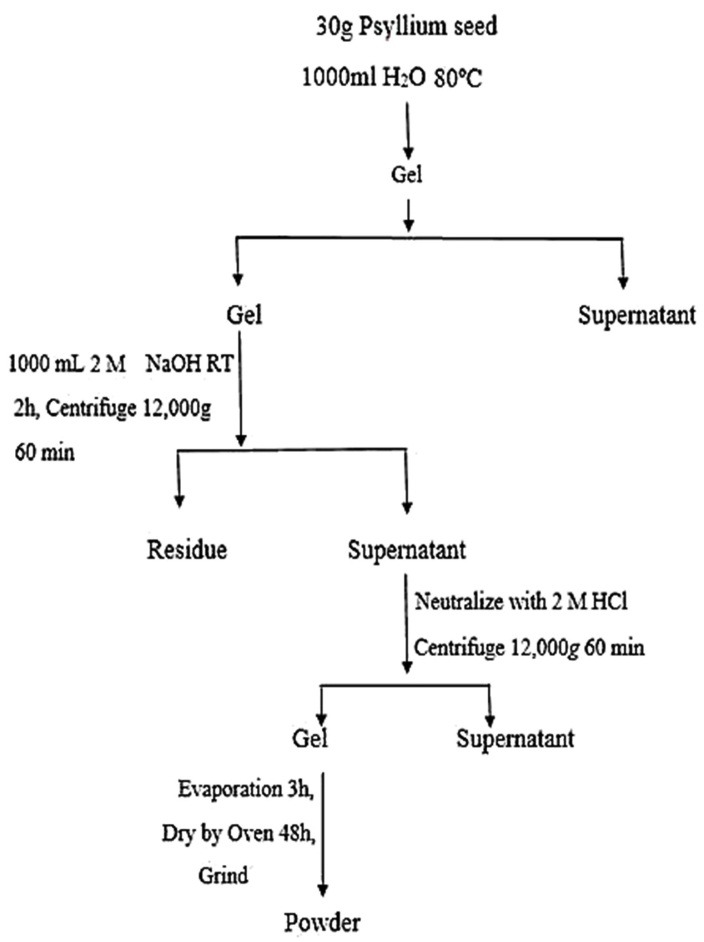
Diagram of psyllium gel extraction process.
Figure 2.
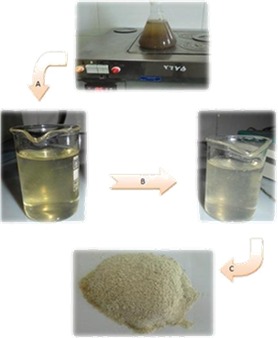
Procedure of psyllium gel extraction. A) psyllium gel and seed were separated from supernatant. NaOH was added to dissolve psyllium gel; alkaline solution was separated from the residue by centrifugation. B) Alkaline solution was neutralized by HCl 2M. C) White gel was harvested by centrifugation from supernatant; dried and grinded.
Preparation of beads
The extrusion technique was used to prepare alginate and alginate/psyllium beads (Lotfipour et al., 2012). Sodium alginate and psyllium were weighted, added to distilled water in different ratios and sterilized by steam at 121°C for15 min. The cooled alginate or alginate/psyllium gels (4.5 mL) were mixed with bacterial inoculum and gently stirred for 30 min to obtain a homogeneous suspension. The suspensions were extruded drop wise through a 27 gage nozzle into sterile hardening solution (CaCl2) under shaking at 250 rpm for 15 min. The obtained beads were isolated, washed twice with sterile water, and kept in 0.5% w/v peptone solution at 4°C. Poly-l-lysine–(0.5% w/v) as the second coat was incorporated on the surface of the selective formulation, using immersion technique. The compositions of prepared formulations are shown in Table 1.
Table 1. Compositions of the studied formulation.
| Formulation | Alginate/Psyllium | CaCl2 (%w/v) |
| (Total polymer 2.5% w/v) | ||
| A1 | 3:0 | 2 |
| A2 | 3:0 | 4 |
| A3 | 3:0 | 6 |
| A4 | 2:1 | 2 |
| A5 | 2:1 | 4 |
| A6 | 2:1 | 6 |
| A7 | 1:2 | 2 |
| A8 | 1:2 | 4 |
| A9 | 1:2 | 6 |
Size and topographic analysis
The particle size of beads was assessed using optical microscopy (Dinolite, Taiwan) by scion image analyzer software. Data were collected from 50 beads in each sample, and mean particle size was reported. The topographical properties of prepared beads were investigated by scanning electron microscopy (SEM) (Philipse XL30, Holland) at an accelerating voltage of 20 KV. Prior to examination, samples were prepared on aluminum stubs and coated with gold under argon atmosphere by means of a sputter coater.
Encapsulation efficiency
To determine the encapsulation efficiency (EE), firstly prepared beads were mechanically disintegrated in phosphate buffer (pH = 6.8), then the number of entrapped cells after adequate dilution were measured by pour plate method, and counts were expressed as number of colony forming units (CFU), and calculated as:
EE= (Log10N /Log10 N0)
Where N is the number of viable entrapped cells released from the beads and N0 is the number of free cells added to the biopolymer mixture immediately before the production procedure.
Viability of encapsulated and Free L. acidophilus at acid Condition
Acid conditions were produced using 50 mL of 14.91 g/L potassium chloride and 3.0 g/L of pepsin and pH adjusted to 2 with hydrochloric acid (Chávarri et al., 2010). 100 mg beads with entrapped bacteria or 1 mL of cell suspension were mixed in 10 mL of acid solution and incubated for 2 h at 37°C with constant agitation at 50 rpm. After incubation, beads were disintegrated in phosphate buffer (pH = 6.8), then 1.0 mL aliquot of the mixture removed and assayed using pour plate method.
The survival (%) of the bacteria was calculated as follows:
%Survival= (log CFU/g beads after 2 h exposure to acidic condition/log CFU/g beads initial count) ×100
Viability of encapsulated and free L. acidophilus at bile salt condition
100 mg beads with entrapped bacteria or 1 mL of cell suspension were mixed in 10 mL of Bill condition containing 2% w/v oxgall for 2 h at 37°C with constant agitation at 50 rpm. After incubation, beads were disintegrated in phosphate buffer (pH = 6.8), samples were then taken, and bacterial growth was assayed using pour plate method.18
Incorporation poly l lysine as second coat
Selective formulation was washed by using sterile peptone. Poly l lysine (0.05% w/v) as a second coat was incorporated on the surface of alginate/psyllium beads using immersion technique. Hence, beads were dipped in poly l lysine (0.05% w/v) on stirrer at 250 rpm for 30 min.
Lyophilization of the prepared beads
Selective formulations were dipped in sucrose 10% and freezed for 24 h at -80°C. Accordingly, the samples were lyophilized using suction and second drying in a lyophilizer.
Statistical analyses
Statistical testing was carried out using SPSS19. All of the experiments were performed in triplicates. Data are presented as mean±SD. The One-Way ANOVA test was performed to assess the differences between beads and control groups and P<0.05 considered as a statistically significant difference.
Results
Characterization of prepared beads
Beads with different ratios of alginate: psyllium (3:0, 2:1, 1:2) and CaCl2 as hardening solution (2, 4 and 6% w/v) were prepared. Table 2 shows the results for diameters and encapsulation efficiencies of the prepared beads. As can be seen, beads with narrow size distribution ranging from 1.85 ± 0.05 to 2.40 ± 0.18 mm were achieved. The initial L. acidophilus count in the inocolum and polymers mix used for bead preparation was 9 ± 0.01 log CFU/mL. The bacterial counts in the prepared beads showed more than 96% encapsulation efficiency of the method.
Table 2. Size, encapsulation efficiency, and % survival in acid condition of prepared formulation .
| Formulation | Diameter (mm) | Encapsulation efficiency (%) | Count (CFU/g) after acid exposure | Count (CFU/g) after bile exposure |
| A1 | 2.09±0.18 | 98.1 ±3.4 | 5.8 ± 0.4 | 8.6±0.3 |
| A2 | 1.92±0.10 | 96.6± 5.7 | 5.6 ± 0.8 | 8.4±0.4 |
| A3 | 1.96±0.15 | 98 ±3.5 | 5.5 ± 0.3 | 8.5±0.3 |
| A4 | 2.03±0.09 | 97.4 ±2.6 | 5.4 ± 0.8 | 8.6±0.3 |
| A5 | 1.85±0.05 | 99.5±0.8 | 5.5 ± 0.4 | 8.5±0.8 |
| A6 | 1.92±0.12 | 98.5 ± 2.6 | 5.2 ± 0.1 | 8.5±0.5 |
| A7 | 2.04±0.19 | 99.7 ± 0.5 | 5.9 ± 0.5 | 8.6±0.6 |
| A8 | 2.23±0.15 | 99.8 ± 0.17 | 5.4 ± 0.2 | 8.9±0.1 |
| A9 | 2.40±0.18 | 99.6 ± 1.1 | 5.5 ± 0.2 | 8.4±0.5 |
| Untreated cells* | - | - | 3.01 ± 0.068 | 8.4±0.1 |
* Initial count: 9 ± 0.01 Log CFU/mL
SEM images of the beads showed wrinkle beads (×90, Figure 3a), with rough and approximately porous surface characteristics (×2000, Figure 3c). On the other hand, it can be seen in Figures 3b and 3d that incorporation of psyllium resulted in more wrinkle appearance in lower resolutions (×90) (Figure 3b) and smoother and integrated beads (×2000) than their alginate counterparts (Figure 3d).
Figure 3.
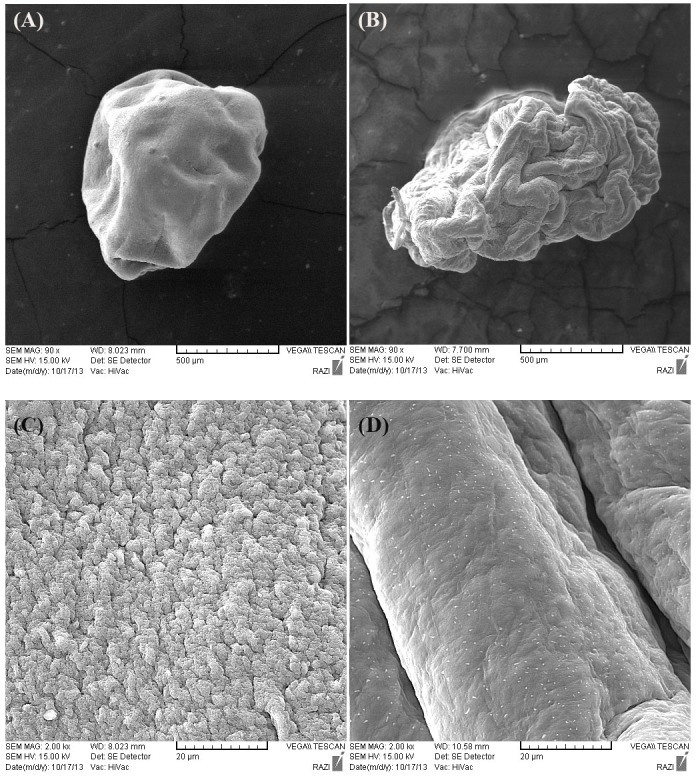
SEM pictures of A1(a) and A9(b) beads at a magnification of 90x , A1(c) and A4(d) at 2000x.
Viability of untreated and encapsulated bacteria in acid conditions
The protective effects of different coats of alginate and alginate/psyllium after 2 h exposure to acid conditions (pH=2) are compared to untreated bacteria, and results are expressed as log CFU/g in Table 2 and %survival in Figure 4.
Figure 4.
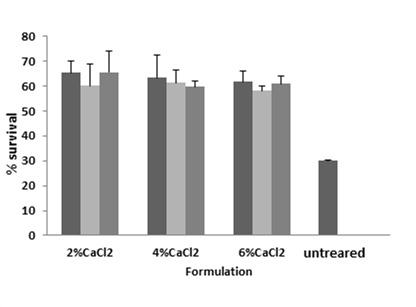
Percent of bacterial survival in the prepared beads after 2h acid exposure;
■: ALG; ■: ALG:PSL (2:1); ■: ALG:PSL (1:2)
As can be seen from Table 2, the initial inoculum count of untreated L. acidophilus was 9 ± 0.01 Log CFU/g which declined to 3.01 ± 0.068 Log CFU/g after acid exposure for 2 h (around 33% survival). On the other hand, in our prepared beads with the initial bacterial numbers of 9 ± 0.01 Log CFU/g, after 2 h acid exposure, the counts were 5.2 ± 0.15 to 5.9 ± 0.46 Log CFU/g indicating more than 58% survival in all the formulations.
Moreover, the effect of 2 h exposure to acid condition (pH=2) on the survival of L. acidophilus encapsulated in A8 with a second coat of poly l lysine (nominated as P8) are expressed as Log CFU/g and % survival in Table 3.
Table 3. % survival in acid condition of A8 and P8 .
| Formulation | Count (CFU/g) after acid exposure | %Survival |
| A8 | 5.4±0.2 | 59.6±2.6 |
| P8 | 7.5±0.3 | 83.3±1.2 |
Initial count: 9 ± 0.01 Log CFU/mL
Viability of untreated and Encapsulated Bacteria at High Bile Salt Concentration
The effect of 2 h exposure to 2% w/v oxgall on the survival of untreated L. acidophilus and in the prepared beads is demonstrated in Figure 5 and Table 2 as well. According to our results, viability of L. acidophilus after bile exposure was more than 90% in the case of untreated bacteria. Furthermore, the viability of the bacteria in all prepared beads was not significantly (P>0.05) different from those of untreated bacteria.
Figure 5.
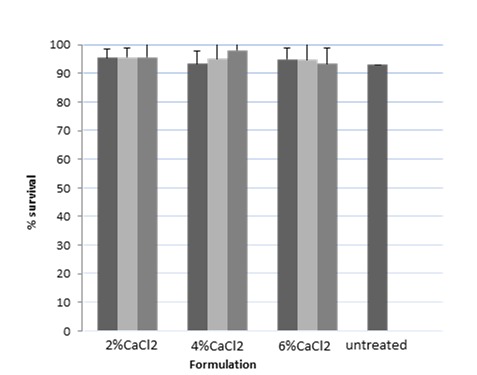
Percent of bacterial survival in the prepared beads after 2h bile exposure
■: ALG; ■: ALG:PSL (2:1); ■: ALG:PSL (1:2)
Effect of lyophilization in survival of L. acidophilus
A2, A5 and A8 beads with alginate/psyllium ratios of 3:0, 2:1 and 1:2 respectively and equal cross linker concentrations were subjected to lyophilization and the survival of the bacteria in the lyophilized beads were investigated. The results are expressed as log CFU/g and % survival in Table 4. According to the results, 2 log declines in bacterial count were observed. Our finding also concurs with the previous studies.19
Table 4. Viability of the bacteria before and after lyophilization .
| Formulation |
Count (CFU/g)
Before lyophilization |
Count (CFU/g) After lyophilization | %survival |
| A2 | 10.0±0.1 | 8.0±0.1 | 80 |
| A5 | 8.9±0.2 | 7.2±0.2 | 80.9 |
| A8 | 8.8±0.3 | 7.3±0.1 | 82.9 |
Discussion
In the present study, we aimed to prepare alginate beads with maximum possible concentration of psyllium with a second layer of poly l lysine on top to protect the probiotic bacteria and to use the beneficial properties of psyllium as well.
According to ficsher et al. the extraction yield of alkali-extractable polysaccharides of psyllium was higher. Furthermore alkali-extractable polysaccharides of psyllium seem to have more potential for gel forming with Cacl2. Hence we used alkali-extractable polysaccharides of psyllium in this work.9
In the formulation experiments, total polymer concentration was selected to be 2.5% w/v according to our previous study.4 Beads with narrow size distribution ranging from 1.85 ± 0.05 to 2.40 ± 0.18 mm were achieved. Inclusion of psyllium in different ratios or using of different concentrations of CaCl2 as crosslinker, produced no constant trend in the sizes of obtained beads.
The results pertaining to EE indicated that more than 96% of cells were successfully entrapped in the beads. The obtained high EE indicated that there was no considerable loss of viability in the process of beads preparation, confirming the gentle propriety of the applied method.20 Furthermore, there were no significant differences (P>0.05) regarding the EE between different formulations.
The rough and approximately porous surface characteristics of alginate beads shown in SEM pictures is in good agreement with the other studies which can be explained by the egg-box structure of calcium alginate beads.21-24 Alginate is able to form gel by reaction with divalent cations such as Ca2+. Gelation of alginate is mainly achieved by the exchange of Na+ from the guluronic acids with Ca2+, and stacking of these guluronic groups to form the characteristic egg-box structure. The Ca2+ binds to the α-L guluronic acid blocks in a highly cooperative manner and the size of the cooperative unit is more than 20 monomers. Each alginate chain dimerizes to form junctions with many other chains and accordingly gel networks are formed.25,26
According to our results, substitution of psyllium in the bead formulations (Figure 3d) gives a smoother surface to the beads. It can be assumable that psyllium gel as a cement like agent may fill the cracks and pores of the calcium alginate egg-box structure and can enhance the apparent integrity of the beads surface.
Based on the results shown in Table 2, it is clear that count of survived bacteria after acid exposure, in all prepared beads were significantly (P<0.05) higher than those of untreated bacteria. In fact, 6 log reductions in bacterial count in the case of untreated L. acidophilus decreased to 3 log reduction among bacteria encapsulated in the beads, and it can be concluded that coating of the bacteria as alginate or alginate/psyllium beads can improve the viability of L. acidophilus in acid conditions. This is in line with the previous studies for the probiotic encapsulation27,28 Furthermore, replacement of alginate by psyllium, produced no significant changes in the bacterial viability (P>0.05) indicating an equal level of protection for the bacteria by using alginate/psyllium in comparison with alginate alone (Figure 4).
On the other hand, 2 h exposure to 2% w/v oxgall results in no significant difference in the case of untreated bacteria and bacteria encapsulated in the beads (P>0.05) indicating high resistance of our species against bile condition. The intrinsic resistance of L. acidophilus against bile condition has been reported previously.29
To evaluate the effect of second coat of poly l lysine, an optimum single coat formulation was selected. As the viability of the bacteria in acid and bile conditions were not significantly different between the formulations, the criteria for the selection of optimum formulation was based on the maximum replacement of alginate with psyllium (A7, A8 and A9). Among them A8 with 4% CaCl and maximum stability was chosen and subjected for incorporation of the second coat of poly l lysine by immersion technique and nominated as P8. The results of 2 h exposure to acid condition showed a better survivability with P8, as around 2 log rise was observed in bacterial count among P8 beads in comparison with A8 beads. It can be concluded that coating of the bacteria as alginate/psyllium-poly l lysine beads can improve the viability of L. acidophilus in harsh conditions. Better protection of live bacteria in double and triple coated beads compared to single one has been shown by other studies. Thickening of the protection layer and lowering the porosity of the obtained beads resulted in production of more stable and integrated beads. Incorporation of poly l lysine on the alginate beads as the second layer and its effectiveness in the probiotics coating has also been studied.30
Conclusion
In the present study, replacement of alginate by psyllium in high ratio (alginate/psyllium 1:2) for encapsulation of L. acidophilus was carried out. Alginate/psyllium Beads with narrow size distribution and high encapsulation efficiency of the bacteria have been achieved. Presence of psyllium in the prepared beads produced a much smoother and integrated surface texture than alginate. Moreover, psyllium sufficiently protected the bacteria against acidic condition as much as alginate. Considering the pharmacological benefits of psyllium in gastrointestinal system and its prebiotic activity, it can be beneficially replaced in part for alginate in probiotic coating.
Ethical Issues
Not applicable.
Conflict of Interest
The authors declare that there is no conflict of interests regarding the publication of this article.
References
- 1.Pineiro M, Stanton C. Probiotic bacteria: Legislative framework-- requirements to evidence basis. J Nutr. 2007;137(3 Suppl 2):850S–3S. doi: 10.1093/jn/137.3.850S. [DOI] [PubMed] [Google Scholar]
- 2.Huq T, Khan A, Khan RA, Riedl B, Lacroix M. Encapsulation of probiotic bacteria in biopolymeric system. Crit Rev Food Sci Nutr. 2013;53(9):909–16. doi: 10.1080/10408398.2011.573152. [DOI] [PubMed] [Google Scholar]
- 3.Kailasapathy K. Microencapsulation of probiotic bacteria: Technology and potential applications. Curr Issues Intest Microbiol. 2002;3(2):39–48. [PubMed] [Google Scholar]
- 4.Lotfipour F, Mirzaeei S, Maghsoodi M. Preparation and characterization of alginate and psyllium beads containing lactobacillus acidophilus. Sci World J. 2012;2012:680108. doi: 10.1100/2012/680108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lotfipour F, Mirzaeei S, Maghsoodi M. Evaluation of the effect of cacl2 and alginate concentrations and hardening time on the characteristics of lactobacillus acidophilus loaded alginate beads using response surface analysis. Adv Pharm Bull. 2012;2(1):71–8. doi: 10.5681/apb.2012.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nazemiyeh H, Lotfipoor F, Delazar A, Razavi SM, Asnaashari S, Kasebi N. et al. Chemical composition, and antibacterial and free-radical- scavenging activities of the essential oils of a citronellol producing new chemotype of Thymus pubescens Boiss. & Kotschy ex Celak. Rec Nat Prod. 2011;5(3):184–92. [Google Scholar]
- 7.Khodaie L, Delazar A, Lotfipour F, Nazemiyeh H. Antioxidant and antimicrobial activity of pedicularis sibthorpii boiss. And pedicularis wilhelmsiana fisch ex. Adv Pharm Bull. 2012;2(1):89–92. doi: 10.5681/apb.2012.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu LL, Lutterodt H, Cheng Z. Beneficial health properties of psyllium and approaches to improve its functionalities. Adv Food Nutr Res. 2009;55:193–220. doi: 10.1016/S1043-4526(08)00404-X. [DOI] [PubMed] [Google Scholar]
- 9.Fischer MH, Yu N, Gray GR, Ralph J, Anderson L, Marlett JA. The gel-forming polysaccharide of psyllium husk (plantago ovata forsk) Carbohydr Res. 2004;339(11):2009–17. doi: 10.1016/j.carres.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 10.Marlett JA, Fischer MH. The active fraction of psyllium seed husk. Proc Nutr Soc. 2003;62(1):207–9. doi: 10.1079/pns2002201. [DOI] [PubMed] [Google Scholar]
- 11.Singh B. Psyllium as therapeutic and drug delivery agent. Int J Pharm. 2007;334(1-2):1–14. doi: 10.1016/j.ijpharm.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 12.Fujimori S, Tatsuguchi A, Gudis K, Kishida T, Mitsui K, Ehara A. et al. High dose probiotic and prebiotic cotherapy for remission induction of active crohn's disease. J Gastroenterol Hepatol. 2007;22(8):1199–204. doi: 10.1111/j.1440-1746.2006.04535.x. [DOI] [PubMed] [Google Scholar]
- 13.Fujimori S, Gudis K, Mitsui K, Seo T, Yonezawa M, Tanaka S. et al. A randomized controlled trial on the efficacy of synbiotic versus probiotic or prebiotic treatment to improve the quality of life in patients with ulcerative colitis. Nutrition. 2009;25(5):520–5. doi: 10.1016/j.nut.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Rishniw M, Wynn SG. Azodyl, a synbiotic, fails to alter azotemia in cats with chronic kidney disease when sprinkled onto food. J Feline Med Surg. 2011;13(6):405–9. doi: 10.1016/j.jfms.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J Nutr. 1995;125(6):1401–1. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 16.Farajnia S, Hassan M, Hallaj Nezhadi S, Mohammadnejad L, Milani M, Lotfipour F. Determination of indicator bacteria in pharmaceutical samples by multiplex PCR. J Rapid Meth Aut Mic. 2009;17(3):328–38. [Google Scholar]
- 17.Guo Q, Cui SW, Wang Q, Young JC. Fractionation and physicochemical characterization of psyllium gum. Carbohyd Polym. 2008;73(1):35–43. doi: 10.1016/j.carbpol.2007.11.001. [DOI] [Google Scholar]
- 18.Ding WK, Shah NP. An improved method of microencapsulation of probiotic bacteria for their stability in acidic and bile conditions during storage. J Food Sci. 2009;74(2):M53–61. doi: 10.1111/j.1750-3841.2008.01030.x. [DOI] [PubMed] [Google Scholar]
- 19.Bergenholtz AS, Wessman P, Wuttke A, Hakansson S. A case study on stress preconditioning of a lactobacillus strain prior to freeze-drying. Cryobiology. 2012;64(3):152–9. doi: 10.1016/j.cryobiol.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Mokarram RR, Mortazavi SA, Habibi Najafi MB, Shahidi F. The influence of multi stage alginate coating on survivability of potential probiotic bacteria in simulated gastric and intestinal juice. Food Res Int. 2009;42(8):1040–5. doi: 10.1016/j.foodres.2009.04.023. [DOI] [Google Scholar]
- 21.Pluemsab W, Fukazawa Y, Furuike T, Nodasaka Y, Sakairi N. Cyclodextrin-linked alginate beads as supporting materials for sphingomonas cloacae, a nonylphenol degrading bacteria. Bioresour Technol. 2007;98(11):2076–81. doi: 10.1016/j.biortech.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Jahan ST, Islam MS, Sadat SMA, Islam MK, Jalil RU, Chowdhury JA. Surface morphology and release behaviors of theophylline loaded sodium alginate gel beads. Bangl Pharm J. 2010;13(2):41–6. [Google Scholar]
- 23.Rasel MAT, Hasan M. Formulation and evaluation of floating alginate beads of diclofenac sodium. Dhaka Univ J Pharm Sci. 2012;11(1):29–35. doi: 10.3329/dujps.v11i1.12484. [DOI] [Google Scholar]
- 24.Tzu TW, Tsuritani T, Sato K. Sorption of Pb (II), Cd (II), and Ni (II) toxic metal ions by alginate-bentonite. J Environ Prot Ecol. 2013;4(1):51–5. doi: 10.4236/jep.2013.41B010. [DOI] [Google Scholar]
- 25.Singhal P, Kumar K, Pandey M, Saraf SA. Evaluation of acyclovir loaded oil entrapped calcium alginate beads prepared by ionotropic gelation method. Int J Chem Tech Res. 2010;2(4):2076–85. [Google Scholar]
- 26.Verma A, Sharma M, Verma N, Pandit JK. Floating alginate beads: studies on formulation factors for improved drug entrapment efficiency and in vitro release. Farmacia. 2013;61(1):143–61. [Google Scholar]
- 27.Pan LX, Fang XJ, Yu Z, Xin Y, Liu XY, Shi LE. et al. Encapsulation in alginate-skim milk microspheres improves viability of lactobacillus bulgaricus in stimulated gastrointestinal conditions. Int J Food Sci Nutr. 2013;64(3):380–4. doi: 10.3109/09637486.2012.749841. [DOI] [PubMed] [Google Scholar]
- 28.Trabelsi I, Bejar W, Ayadi D, Chouayekh H, Kammoun R, Bejar S. et al. Encapsulation in alginate and alginate coated-chitosan improved the survival of newly probiotic in oxgall and gastric juice. Int J Biol Macromol. 2013;61:36–42. doi: 10.1016/j.ijbiomac.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 29.Ruiz L, Margolles A, Sanchez B. Bile resistance mechanisms in lactobacillus and bifidobacterium. Front Microbiol. 2013;4:396. doi: 10.3389/fmicb.2013.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaikham P, Apichartsrangkoon A, George T, Jirarattanarangsri W. Efficacy of polymer coating of probiotic beads suspended in pressurized and pasteurized longan juices on the exposure to simulated gastrointestinal environment. Int J Food Sci Nutr. 2013;64(7):862–9. doi: 10.3109/09637486.2013.799124. [DOI] [PubMed] [Google Scholar]


