Abstract
Purpose: Statin is an effective factor for promoting osteogenesis. The aim of the present study was to evaluate the effect of simvastatin (SIM) and/or HA addition on changes in osteogenesis levels by human DPSCs transferred onto three-dimensional (3D) nanofibrous Poly (ε-caprolactone) (PCL)/Poly lactic acide (PLLA) polymeric scaffolds.
Methods: For this purpose, a 3D nanofibrous composite scaffold of PCL/PLLA/HA was prepared by electrospinning method. SIM was added to scaffolds during DPSCs culturing step. Cell proliferation and osteogenic activity levels were assessed by using MTT assay and Alizarin Red assay methods. In addition, the expression of genes responsible for osteogenesis, including BMP2, Osteocalcin, DSPP and RUNX2, were determined before and 2 weeks after incorporation of SIM.
Results: The MTT assay showed that PCL/PLLA/HA scaffolds seeded with DPSCs has significant (p<0.05) more proliferative effect than PCL/PLLA or DMEM cultured cells, additionally SIM administration improved this result over the PCL/PLLA/HA scaffolds without SIM treatment. SEM imaging revealed improved adhesion and probably osteogenic differentiation of DPSCs on PCL/PLLA/HA nanofibers treated with SIM, moreover the alizarin red assay ensured significant (p<0.05) higher mineralization of this group. Finally, real time PCR confirmed the positive regulation (P<0.05) of the expression of osteo/odontogenesis markers BMP2, Osteocalcin, DSPP and RUNX2 genes in PLLA-PCL-HA (0.1)-SIM group.
Conclusion: As a result, addition of simvastatin with incorporation of hydroxyapatite in PCL-PLLA scaffolds might increase the expression of osteogenesis markers in the DPSCs, with a possible increase in cell differentiation and bone formation.
Keywords: Simvastatin, Hydroxyapatite, Co-Administration, Nanofibrous scaffold, Bioengineering
Introduction
In recent decades the medical sciences have been revolutionized due to the introduction of treatment modalities with the application of stem cells and the synthesis of various tissues and organs. Stem cells are progenitor cells that can be differentiated into cells with specific functions under some physiologic or laboratory conditions. These cells have indefinite potential for proliferation, can remain undifferentiated and preserve their capacity to differentiate into every cell type in the body.1
Stem cells have been isolated from the human primary tooth pulps and periodontal ligaments in various studies to date.2,3 Human dental pulp is one of the most available sources for stem cells. Availability of this source and the high rate of proliferation of these cells have made the dental pulp a proper source for tissue regeneration by stem cells.4 It appears that the stem cells isolated from dental pulp are therapeutically valuable similar to the bone marrow derived stem cells. These cells have the capacity to differentiate into adipose tissue, cartilage, neurons, bone and smooth muscles.5
The effects of different materials have been evaluated on the proliferation and differentiation of these cells, and the role of different materials in the induction or inhibition of these cells has been studied.
The structures of teeth and bones have very important roles in the health of the oral cavity and the periodontium. Loss of teeth and resorption of the alveolar bone create many problems for the individuals affected. Therefore, attempts are underway to regenerate lost teeth and osseous structures.5
Statins (such as simvastatin) are inhibitors of the HMG-COA reductase and are widely used to decrease cholesterol levels.6 Mundy et al showed that statins have a positive effect on the metabolism of bone and its regeneration; they exert such an effect by increasing the expression and synthesis of BMP2 protein and angiogenesis.7 There are conflicting results in relation to the effect of statins on regeneration of bone. However, some studies have shown that statins have a positive effect on the metabolism of bone and induction of its regeneration.7,8
Although some studies have not confirmed the effect of statins on the initiation and formation of bone.9,10 Von stechow et al evaluated the effect of statins on elimination of bony defects in the mandible of rabbits and reported that the amounts of regenerated bone were not significantly different with and without exposure to statins.10 Therefore, further studies are necessary to determine the role of this material in the induction of bone regeneration.
Scaffolds might be biologic or synthetic, and degradable or non-degradable. Natural polymers, degradable synthetic polymers and non-degradable synthetic polymers are tissue engineering biomaterials. It is possible to synthesize polymeric scaffolds with proper characteristics in relation to strength, the rate of degradation, porosity, microstructure, shape and size. Synthetic polymers are considered more suitable biomaterials due to their properties in relation to porosity, a definite time for degradation and good mechanical properties. These polymers are cheaper than biopolymers, can be synthesized in large quantities with a uniform quality and have longer durability. Polylactic acid (PLLA), polyglycolic acid (PGA) and lactic-glycolic acid copolymer (PLGA) are some of the most commonly used biodegradable synthetic polymers in tissue engineering procedures.11 Various polymeric nanofibers with high surface–area-to-volume ratio and interconnected pores which was similar to the natural extracellular matrix (ECM) have been produced by electrospinning methods.12 These nanofibrous scaffolds can potentially provide similar support to cell adhesion, proliferation, and migration which could be used in tissue engineering application.
Considering the discrepancies in the results of studies that represented the effect of stains on stem cells and lack of studies available on their effect on the transferred hDPSCs on polymeric scaffolds, revealed the need of further studies to determine the role of this material in the induction of bone regeneration. The present study was undertaken to evaluate the effect of simvastatin on changes in osteogenesis levels by human DPSCs transferred onto polymeric scaffolds.
Materials and Methods
DL-lactide (LA) was purchased from Sigma–Aldrich (Co., Steinem, Germany), and purified by recrystallization two times from ethyl acetate, and dried under high vacuum at room temperature before use. Stannous 2-ethyl hexanoate (stannous octoate, SnOct), and glutaraldehyde (25%) and dimethylformamide (DMF) were obtained from Sigma-Aldrich Chemical Co. (St. Louis,MO, USA). Dichloromethane (CH2Cl3)(DCM) and ɛ-caprolacton (CL) were purchased from Merck Chemical Co. Dulbecco Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS), trypsin–EDTA and phosphate buffered saline (PBS) were purchased from Gibco, Singapore. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide) was purchased from Invitrogen (Carlsbad, CA). All other materials were used as received.
Preparation of PCL/PLLA/HA nanocomposite
The synthesis protocol and characterization of PCL, PLLA and HA were described in detailed by our team in previous work.13,14
Fabrication of PCL/PLLA/HA nanofibrous scaffolds by the electrospinning method
PCL/PLLA (2:1 w/w) and HA nanoparticles were dissolved in four and one mL DCM: DMF (3:1 v/v ratio), respectively to prepare 20 wt% solutions of PCL/PLLA/HA with the ratio of 2:1:0, 2:1:0.1, 2:1:0.5 (w/w). The optimum electrospinning condition for preparation of PCL/PLLA/HA nanofibers on the electrospinning device (Fanavaran Nano-Meghyas, Iran) was reported in detailed in our team previous work.14
Isolation and characterization of dental pulp stem cells
In a previous study, the authors discussed the protocol of isolation and characterization of dental pulp stem cells.15 Dental pulp stem cells were isolated from primary and permanent teeth. They were characterized by FACS (Fluorescence-activated cell sorting). These cells were positive for CD90, CD166, CD105, and CD73 and negative for CD11b, CD34, CD133, CD64, CD106, CD31 and CD 45 markers. Current study is following the regeneration of teeth and bones using these cells seeded on suitable scaffold and useful stimulators. The current study investigated the regeneration of teeth and bones using these cells seeded on suitable scaffolds and with useful stimulators.
Culturing human DPSCs, transferring the cells onto scaffolds and addition of simvastatin to the cell culture media
In this in vitro study, the human DPSCs were cultured in 2 flasks for 10 days to achieve a cell count of 2,000,000 in each flask. Cells counts were determined in 1 mL of the cellular content the flasks on a microscopic haemocytometer. Before hDPSCs seeding to the scaffolds, scaffolds were sterilized then pre-treated with media as follows. First 70% alcohol was transferred into each well and after 20‒30 minutes the scaffolds were rinsed 3 times with PBS and incubated for 24 hours with culturing media. The stem cells were trypsinated. Then 20000‒30000 cells were added to each well. Simvastatin powder was dissolved in ethanol at a concentration of 1 μmol/L and added to the cell cultures.12,16 Two wells were selected as the controls without SIM addition.
Evaluation of biocompatibility of scaffolds for hDPSCs in the presence of SIM
The hDPSCs were seeded in each scaffold including P PLLA A-PCL, PLLA-PCL-HA (0.1) and PLLA-PCL-HA (0.5) scaffolds before and after SIM addition to media. hDPSCs seeded on Tissue Culture Polystyrene (TCPs) in routine DMEM-FBS media as non-treated control. After 5 days, the upper media in each group was replaced with 2mg/ml MTT solution, incubated for 4 hours in 37 °C, 5% CO2 and dark place. Next, the MTT solution was aspirated and DMSO was added to each well of 6 well plates for dissolving furmazan crystals. The absorbance was measured by transferring the DMSO from each well into 96-well format ELISA reader.
Evaluation of the Alizarin Red Activity after 14 days
The potential of the hDPSCs to differentiate into osteo/odontogenic inductions was examined by Alizarin red (AR) S staining to measure calcium amounts. Alizarin red staining protocol and calcium mineral content quantization was described in our previous work14 according to method was done previously by Stanford et al.17 In summary, the supernatant on the cells was removed and the scaffolds were rinsed twice with calcium and phosphate-free saline solution, and fixed with ice-cold 70% ethanol for 1 h. After a brief wash with water, the cells were tainted with 40 mM Alizarin red solution (pH 4.2) at ambient temperature for 15 min. The cells were washed with water for six times followed washing for 20 min with PBS (with rotation) to remove excess Alizarin red stain. Stained cultures were scanned followed by a quantitative distaining procedure using 10% (w/v) cetylpyridinium chloride in 10 mM sodium phosphate (pH 7.0), for 15 min at ambient temperature. Afterwards, Alizarin Red S concentrations were assessed by absorbance measurement at 570 nm on Victor3TMV (PerkinElmer) using an Alizarin red standard curve in the similar solution. The color change of the cells to red was proportional to their osteogenic activity.
Total RNA isolation and complementary DNA synthesis
Fourteen days after cultivation of the hDPSCs on aforementioned scaffolds as well as treatment with SIM, the media were aspirated then washed with PBS. The cellular total RNAs were extracted using Trizol reagent (Invitrogen), according to the manufacturer recommendations. For evaluation of isolated RNA yield and quality, the gel electrophoresis and Nanodrop (Thermo scientific, Waltham, MA, USA) were used. The Revert Aid cDNA synthesis kit (Fermentase, Life Science, and USA) was employed for first strand cDNA synthesis. Finally, the Syber green Master Mix (Hot Equa master mix- sinaclone) was mixed with cDNA and gene specific primers of BMP2, DSPP, Osteocalcin and RUNX2 genes (Table 1) for qRT-PCR reaction on a Rotorgen 6000 system (Qiagene). The GAPDH gene was used as housekeeping gene. The fold changes were calculated by 2^ (-∆∆CT).
Table 1. Sequences of oligonucleotide primers used for qPCR .
| H-BMP2 F | GCTGTGATGCGGTGGACTGC |
| H-BMP2 R | CGCTGTTTGTGTTTGGCTTGA |
| H-Osteocalcin F | CCCATTGGCGAGTTTGAGAAG |
| H-Osteocalcin R | CAAGGCCCGATGTAGTCCAG |
| H-DSPP F | CCCAATAGCAGTGATGAATCTAA |
| H-DSPP R | TGTCATCATTCCCATTGTTACC |
| H-RUNX2 F | CTCACTGCCTCTCACTTGCC |
| H-RUNX2 R | CTGTACACACATCTCCTCCCTTC |
| H-GAPDH F | CAAGATCATCAGCAATGCCTCC |
| H-GAPDH R | GCCATCACGCCACAGTTTCC |
SEM Imaging
The prepared of nano HA and nanofibrous scaffolds were characterized by scanning electron microscopy (SEM) (ChamScan MV2300). Air-dried samples of nano HA, PCL-PLLA nanofibrous scaffold and HA-coated PCL-PLLA nanofibers were putted on aluminum stubs and coated with gold layer. An average HA particle size and fiber diameter of each image were calculated by random choosing of at least 60 different sample segments with an image analyzer (Image-Proplus, Media Cybernetics). After different times of in vitro implantation on the scaffolds, cells were rinsed twice with PBS, fixed in 2.5% glutaraldehyde in PBS buffer pH 7.4, rinsed in PBS, at 4 °C for about 24 h, dehydrated in a graded series of ethanol (25–100%), and finally air-dried at room temperature. The samples were then kept under vacuum at 30 °C to ensure complete drying and a nanometer-thick gold coating was applied. Scanning electron microscopy was used for the characterization of the sheet morphology.
Statistical analysis
Data were analyzed with descriptive statistics (frequencies, percentages, means and standard deviations) and by comparing the means. When the differences were significant, proper post hoc tests were used. McNemara test was used to evaluate qualitative variables. Statistical significance was set at P<0.05.
Results
Fabrication of PCL/PLLA/HA nanofiber
Nanofibers were prepared from PCL and PLLA that both of them are well known biocompatible and biodegradable polymers enable the successful cultivation of hDPSCs and osteogenic cells. To improve the osteo/odontogenic induces character of PCL and PLLA for hDPSCs seeding, HA nanoparticles were added to the PCL/PLLA blend before electrospinning. The control over different electrospinning parameters for example concentration, distance of source electrode from the substrate, applied voltage, and flow rate have been optimized for good spinnability and investigated in detiled at previous work conducted with our team.18 A mixture of dichloromethane and dimethylformamide in the ratio 3/1 (v/v) was chosen as a medium to dissolve PCL/PLLA/HA and this solvent mixture is also highly volatile. In order to gain smooth, droplet and bead free PCL/PLLA/HA nanofibers, the optimum polymer nanocomposite blend concentration was 20% that lead to porous nanofibrous structure with the average fiber diameters of approximately 120-300 nm (Figure 1a) with highly porous structure of the interior of a scaffold sample. Scaffold porosity is one of the necessities for tissue engineering because it provides sufficient cell growth space, and nutrient, water as well as growth factors transportation in the whole scaffold compared to the PCL/PLLA scaffolds. It is worth noting that the morphologies and structures of the prepared HA nanoparticle was nanorod with uniformity in size/shape and mean diameter in the range of approximately 20-40 nm (Figure 1b).
Figure 1.
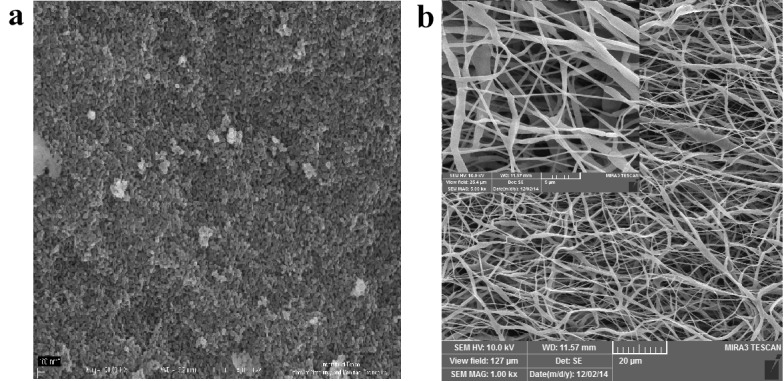
FESEM images of a) nano Hydroxyapatite, b) 20 wt % PCL/PLLA/HA (0.1)
Evaluation of proliferation and viability of hDPSCs seeded on scaffolds by MTT assay
MTT assay was performed to assess the cell survival rate upon attachment and proliferation in developed nanofibrous scaffold, as well as the biocompatibility of scaffolds. hDPSCs were seeded on the TCPS containing DMEM without scaffolds as control, and wells containing PCL/PLLA, PCL/PLLA/SIM, PCL/PLLA/HA as well as PCL/PLLA/HA/SIM. Figure 2 shows the cell viability percentage for hDPSCs grown on all wells after 5 days of cell culture. The similar cell viability values obtained for PCL/PLLA and TCPs cultured cells grown in routine DMEM-FBS media, which indicated that PCL/PLLA blended scaffolds matrix was non-toxic to hDPSCs and did not negatively affect the proliferation rate of hDPSCs which acknowledged the biocompatibility of scaffolds. The high cell viability values for cultured cells on PCL/PLLA/SIM and PCL/PLLA/HA groups in comparison to PCL/PLLA and TCPs groups (p<0.05) (Figure 2) implied more significant accelerating effects of HA and SIM on cell viability. PLLA-PCL-HA (0.1)-SIM group showed significant highest (p<0.01) cell viability and proliferation rate as compared to other treatments suggested that HA and SIM had positive effects on cell survival. Therefore, we concluded that SIM/HA played a decisive role in boosting cell viability (Figure 2).
Figure 2.
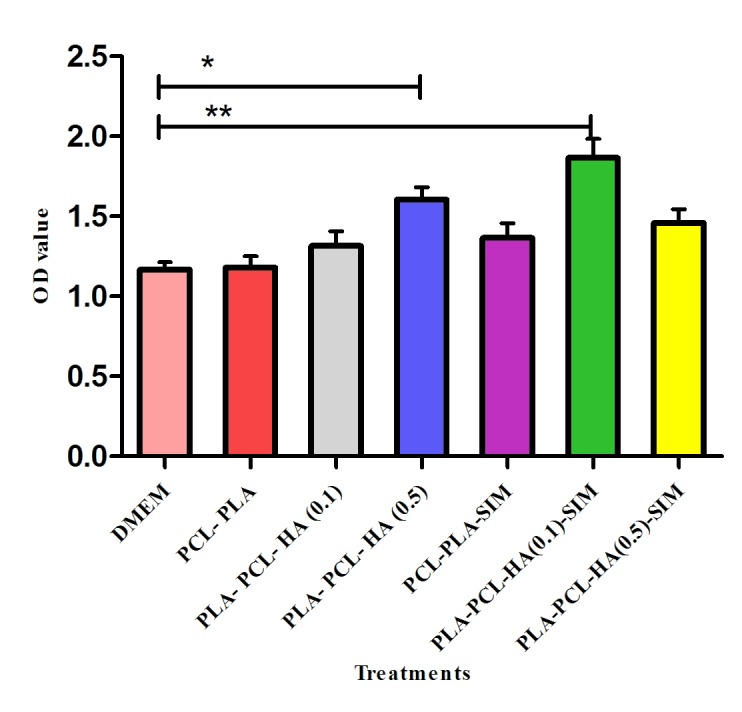
Cell viability study of hDPSCs grown on the TCPS plate and six scaffolds, control PCL/PLLA scaffold, PCL/PLLA/HA scaffolds (molar ratio of PCL to PLLA to HA of 2:1:0.1 and 2:1:0.5) with and without simvastatin and after 5 days.
Alizarin red
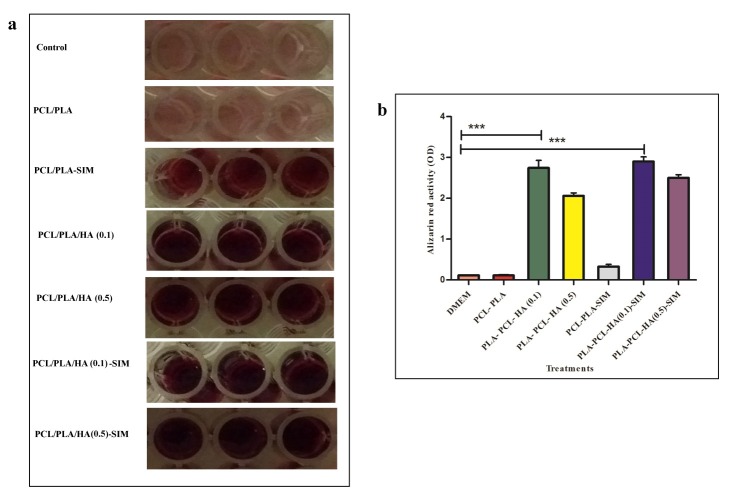
In order to investigate the effect of SIM content alone or in combination with HA on osteoblast maturation, Alizarin Red S staining assay was done in this study for determination of biomineralization in each group. Figure 3 represents the alizarin red staining results which reflected the biological function of osteoblast in hDPSCs in each group. hDPSCs were treated for 14 days in 3D PCL/PLLA, PCL/PLLA/HA (0.1), and PCL/PLLA/HA (0.5), PCL/PLLA/SIM, PCL/PLLA/HA (0.1)/SIM, and PCL/PLLA/HA (0.5)/SIM nanofibers and regular growth media (DMEM) used as control. It was obvious from results represented at Figure 3 that cells cultured in regular growth media (DMEM) (control cells) and 3D PCL/PLLA showed no alizarin red activity while a significantly higher degree of activity was observed in hDPSCs seeded on other scaffolds. hDPSCs cultured in scaffolds containing HA or SIM (both known as osteogenic supplement) alone or in combination in media for 14 days showed Alizarin red staining, commonly associated to the establishment of an osteo-like phenotype. Significantly superior alizarin red activity was observed in cells treated with HA and SIM (Figure 3a). The PLLA-PCL-HA (0.1)-SIM showed significant higher ODs (p<0.001) which proved osteogenesis potential of HA incorporated- SIM added conditions on hDPSCs (Figure 3b). Therefore the simultaneous addition of HA and SIM in scaffold and growth media might be have an important role in simulation of mineralization. Mineralization refers to cell-mediated deposition of extracellular calcium and phosphate ions, and finally leading to calcification, which is important for bone regeneration.17
Figure 3.
Effect of hydroxyapatite (HA) and simvastatin (SIM) on hDPSCs osteogenic differentiation, a) Cells was stained with Alizarin Red S at day 14 to visualize mineralized bone matrix. b) A bar graph showing quantitative results of osteoblast differentiated hDPSCs were stained with Alizarin Red S. Control (lower row) is slightly bright pink, whereas the show vast extracellular calcium deposits, stained in reddish (upper row). All experiments were performed a minimum of three. Alizarin red activity was significantly higher in PLLA-PCL-HA scaffolds than both PLLA-PCL and control (without scaffold) *** P<0.001.
SEM
The attachment and proliferation of hDPSCs cultured over a 14-day incubation period on PCL/PLLA/SIM, PCL/PLLA/SIM/HA (HA; 0.1 wt %) and PCL/PLLA/SIM/HA (HA; 0.5 wt %) scaffolds were visualized by FESEM images (Figures 4-6). The attachment and proliferation of hDPSCs on PCL/PLLA and PCL/PLLA/HA (HA; 0.1 and 0.5 wt %), scaffolds were fully investigated in previous work conducted by our team.14
Figure 4.
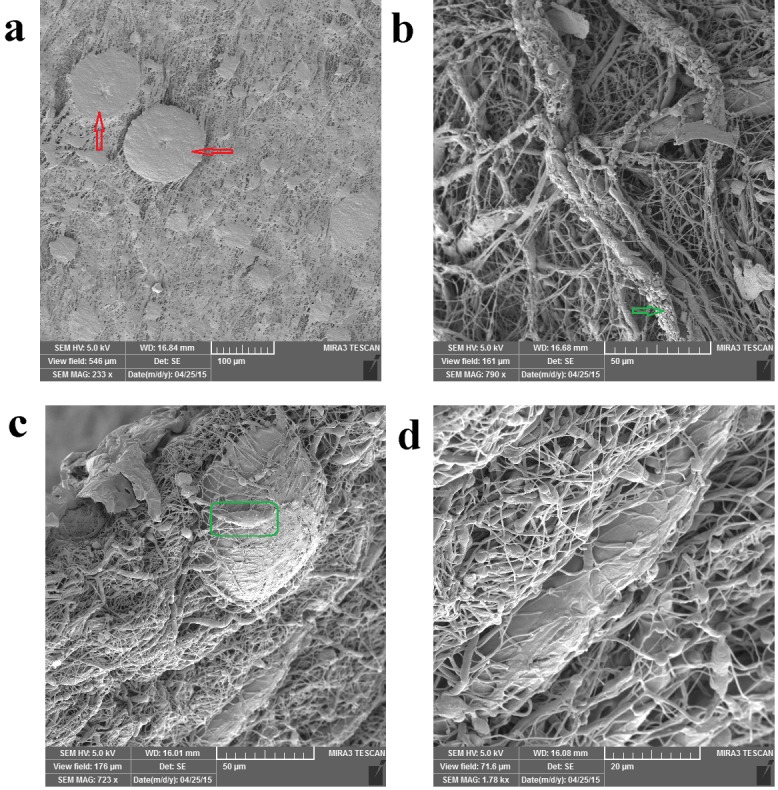
FESEM images of hDPSCs cell morphology on the PCL/PLLA/SIM scaffolds after 14 days of culture.
Figure 6.
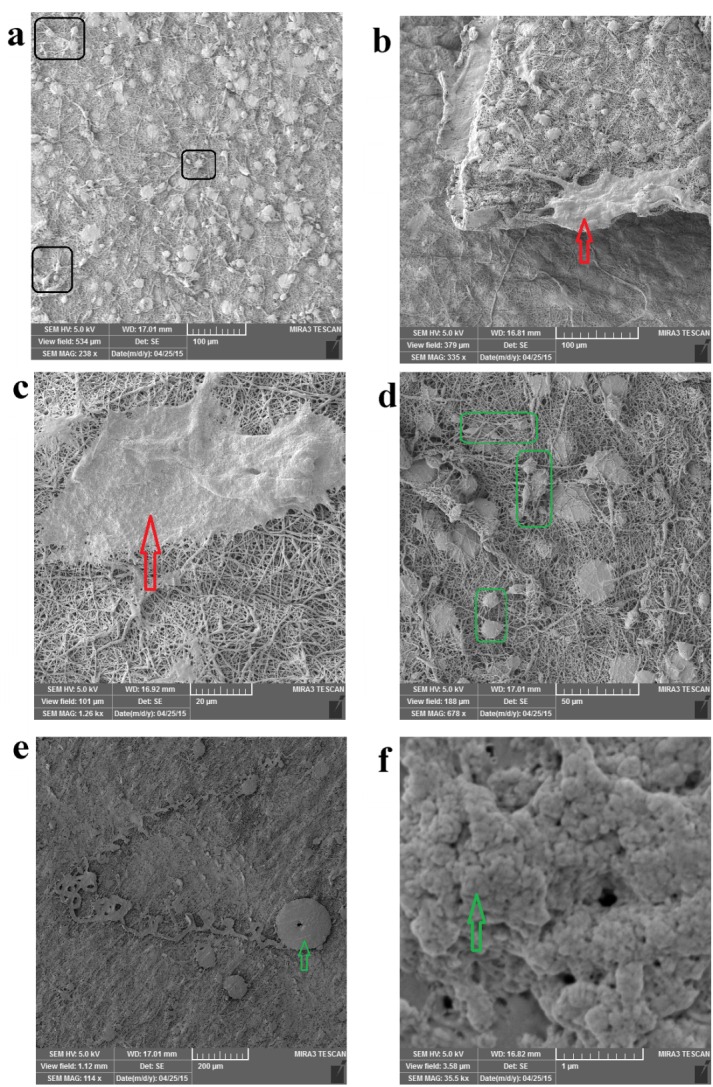
FESEM images of hDPSCs cell morphology on the PCL/PLLA/HA(0.5 wt%)/SIM scaffolds after 14 days of culture.
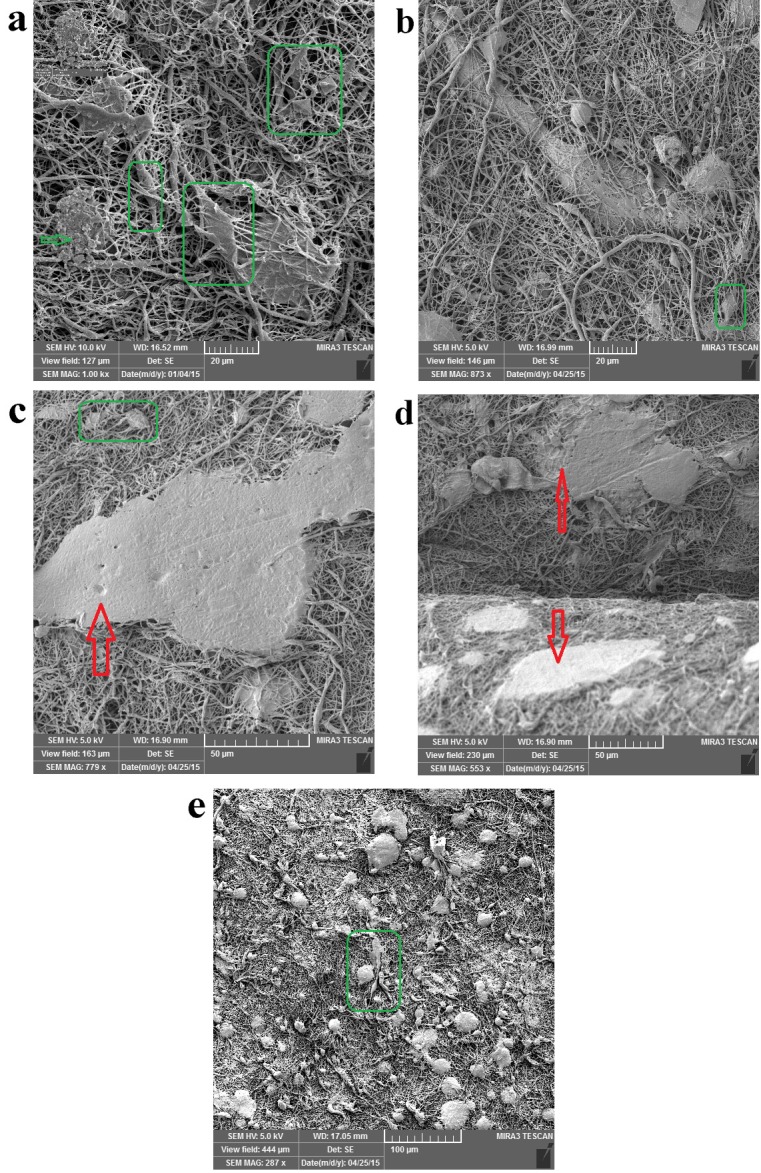
The hDPSCs are uniformly and abundantly distributed in the whole area of PCL/PLLA/SIM and PCL/PLLA/SIM/HA (HA; 0.1 and 0.5 wt %) scaffolds surface and exhibited normal cell adhesion (Figures 4a, 5a, 6e). Most of the cells appeared fibroblast-like morphology with multiple filopodia. High-magnification SEM images showed the formation of cytoplasmic extensions on PCL/PLLA/SIM and PCL/PLLA/SIM/HA (HA; 0.1 and 0.5 wt %) scaffolds (Figures 4-6). Their spread-out cytoplasm exhibited well-attachment onto scaffold. Their filopodia was anchored to the surface of PCL/PLLA/SIM and PCL/PLLA/SIM/HA (HA; 0.1 wt %) scaffolds (black box image in Figure 5a). The migrated DPSCs derived into bone-like tissue that was able to synthesis typical bone-like tissue, such as collagen (green arrow) and cortical (red arrow) structures, together with many mineralized nodules in the ECM (green arrow) (Figures 4, 5 and 6a). Cells aligned themselves on the scaffold, representing a very intense cell adhesion, which is indicated by the flat spread out cell bodies on the scaffold (red and green arrows on Figures 4-6). The whole area, surrounding the nanofibers was covered by multilayered cells.
Figure 5.
FESEM images of hDPSCs cell morphology on the PCL/PLLA/ HA(0.1wt%)/SIM scaffolds after 14 days of culture.
The cells showed morphology of osteoblast like cells, i.e. cube/spindle-shaped, big sized and exhibit long cytoplasmic prolongations to adhere to the surface (green box in Figures 4-6). These results indicate a good cyto-compatibility and close interactions of hDPSCs with the scaffolds prepared, independently of their chemical composition and microstructure. These cellular morphologies may be associated with the differentiation of the hDPSCs cultured on PCL/PLLA/HA/SIM toward the osteogenic lineage.
Cells were covering almost the entire scaffold and able to cover both side of scaffold surface and migrate inside the large pores, filled interconnected microspores and eventually embedded in a matrix (Figures 4b, c, d and 5b, d and 7b, d). hDPSCs could bridge the hDPSCs on both sides of the Nanofibrous scaffold (forward and backward sides of scaffolds) (Figures 5b and 6d). Multi-cell layers were formed where the underlying scaffold could not be observed at all and a continuous cell sheet can be clearly observed (Figures 4a, 5c, e and 6c). The cells grew by spreading on the scaffold surface and vertically penetration into the porous structure (Figure 4c, d and 5d and 6a, b).
Figure 7.
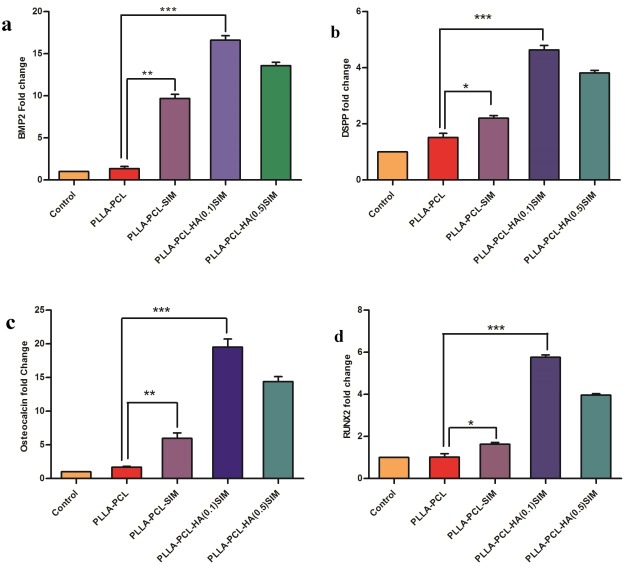
The expression of BMP2 (a), DSPP (b), Osteocalcin (c), and RUNX2 (d) genes were investigated by qRT-PCR in hDPSCs seeded in three different scaffolds (PCL/PLLA, PCL/PLLA/SIM, PCL/PLLA/HA(0.1)/SIM and PCL/PLLA/HA(0.5)/SIM) and control (without scaffold). GAPDH gene was used as internal control. All differentiation marker genes, BMP2, DSPP, Osteocalcin and RUNX2 are significantly up-regulated in PLLA/PCL/HA (0.1 wt %) /SIM group (p value<0.001). * p<0.05, ** p<0.01, *** p<0.001)
Evaluation of the expression of BMP2, Osteocalcin, DSPP and RUNX2 genes 14 days after incorporation of the inductive materials in different study groups
In order to assess the effect of SIM alone or in combination with HA on the differentiation of osteoblasts and osteogenesis, the expression levels of BMP2, Osteocalcin, DSPP and RUNX2, all osteo/odontogenic markers were detected on day 14 for all samples with the use of qPCR technique (Figure 7). The expression of all four candidate genes showed similar results at mRNA levels in three groups by qRT-PCR method. The effect of HA on PCL/PLLA nanofibrous scaffold was investigated in our team previous work.14
The expression of BMP2 gene in the PLLA-PCL scaffold, after incorporation of simvastatin, was significantly higher than that in the control group and PLLA-PCL scaffold without simvastatin (P<0.01). In addition, the expression of BMP2 in the PLLA-PCL-HA (0.1)-SIM group was significantly higher than that in the control group and PLLA-PCL scaffold without simvastatin (P<0.001). However, in the PLLA-PCL-HA (0.5)-SIM group the expression of BMP2 was slightly less than that in the group with 0.1 wt% HA. In general, the results showed that incorporation of simvastatin in association with hydroxyapatite resulted in an increase in the expression of genes responsible for osteogenesis and possibly cell differentiation and osteogenesis levels (Figure 7a).
The expression of DSPP gene in the PLLA-PCL-HA (0.1)-SIM group was significantly higher than that in the other groups (P<0.001). However, this increase was lower in the PLLA-PCL-HA (0.5)-SIM group. In addition, the expression of DSPP gene in the PLLA-PCL scaffold, after incorporation of simvastatin, was significantly higher than that in the control group and the PLLA-PCL scaffold without simvastatin (P<0.05). The results showed that incorporation of simvastatin in association with HA resulted in an increase in the expression of DSPP gene (Figure 7b).
The expression of Osteocalcin gene was very similar to that of BMP2 gene, with higher expression of this gene in scaffolds containing simvastatin. In addition, the expression of Osteocalcin gene in the PLLA-PCL-HA (0.1)-SIM group was significantly higher than that in the other groups (P<0.001). The results showed that incorporation of simvastatin in association with HA increased cellular differentiation and the expression of this gene (Figure 7c).
The expression of RUNX2 gene was very similar to that of DSPP gene, with the results indicating that incorporation of simvastatin in association with HA resulted in an increase in the expression of RUNX2 gene. In addition, the expression of RUNX2 gene in the PLL-PCL scaffold, after incorporation of simvastatin, was significantly higher than that in the control group and the PLLA-PCL scaffold without simvastatin (P<0.05). The expression of RUNX2 gene in the PLLA-PCL-HA(0.1)-SIM group was significantly higher than that in the other groups (P<0.001) (Figure 7d).The high expression of all osteo/odontogenic markers meant that cell attachment was also increased in PCL/PLLA/HA/SIM groups.
Discussion
The present in vitro study was carried out to evaluate the effect of simvastatin on changes in osteogenesis levels in human DPSCs placed on polymeric scaffolds. The main goal of odontoblastic tissue engineering is producing odontoblasts which resemble as much as possible the body’s own native teeth structure. In this way, utilizing both scaffolds and other dental-differentiation supplementary materials has been investigated in recent researches.16,19-21
The ideal cell resource for tissue engineering should be proliferative enough, non-immunogenic, and have the ability to differentiate into desired functional cells. Therefore adult stem cells in particular, MSCs isolated from either bone marrow or other origins have shown promising outcomes in regenerative medicine and cell therapy.
The human DPSCs (hDPSCs) exhibit the postnatal characteristics of the stem cells, including multipotent differentiation, self-regeneration capacity, clonogenic potential and expression of various surface markers of mesenchymal stem cells.22-24 In addition, the stem cells of the human dental pulp have been recognized as heterogenic stem cells that are the analogs of mesenchymal stem cells of the bone marrow.22,24,25
One of the unique characteristics of the hDPSCs is their capacity to form pulp‒dentin-like tissue when transplanted into immunosuppressed rats with the use of a hydroxyapatite/tricalcium phosphate carrier.22,24,25 Under in vitro conditions, the capacity of the hDPSCs to differentiate into osteoblasts,26,27 in conjunction with the excretion of large amounts of extracellular matrix, resulting in the synthesis of woven bone, has been demonstrated.28 Furthermore, hDPSCs can differentiate into osteoblasts and endothelial cells to synthesize mature bone-like tissue in association with provision of internal blood supply in vitro.29 Statins are a group of widely used lipid lowering drugs that block the biosynthesis of cholesterol.30
Simvastatin(SIM) increased the proliferation of smaller MSCs,30 and can increase osteogenesis of them.31 The mechanism for enhancing osteogenesis is mediated by increasing both actin filament organization and cell rigidity.32 Also, SIM enhances the odontogenic plasticity of human DPSCs at both in vitro and in vivo conditions16 SIM loaded porous titanium oxide surface can accelerate osseointegration as well as peri-implant formation of bone.21 Simvastatin in combination with osteo-conductive biomaterials can produce synergistic effects for enhancing yield of tissue engineering. The odontoblastic differentiation of hDPSCs by the controlled release of SIM from gelatin hydrogel granules is possible.20 Calcium phosphate composite scaffolds containing simvastatin-loaded PLGA microspheres showed biocompatibility and osteogenesis potential applicable for bone tissue engineering.33 Simvastatin increases the proliferation and use of osteoprogenitor cells, which is an important and vital step in the initial stages of bone regeneration.34 There is strong evidence that statins increase the differentiation of osteoblasts and mineralization, resulting in osteogenesis.35,36 In addition, statins increase osteogenesis indirectly by inhibiting inflammation which is responsible for an imbalance in the metabolism of bone.37 Furthermore statins inhibit the activation of osteoclasts by inhibiting intracellular signaling pathways in osteoclasts38 and have a role in maintaining the skeletal integrity.39 In the present study, the expression of 4 genes, BMP2, Osteocalcin, DSPP and RUNX2, was evaluated with the qPRC technique in order to investigate the effect of incorporating simvastatin and the scaffold type on the differentiation of osteoblasts and osteogenesis level. In relation to changes in the expression of BMP2 gene, the results of the present study showed that incorporation of simvastatin into the scaffold resulted in a significant increase in the expression of this gene compared to that in the scaffold without simvastatin. In addition, incorporation of 0.1 µg of hydroxyapatite in association with simvastatin had a significant effect on increasing the expression of BMP2 and osteogenesis. Statin has been recognized as a factor accelerating the expression of BMP2 and increasing osteogenesis.8 Consistent with the results of the present study, Mundy et al, showed the positive effect of statins on the metabolism of bones and induction of their regeneration and reported that statins exert this effect by up-regulating BMP2 and inducing angiogenesis.7 In addition, Maeda et al, too, showed the effect of statins on promotion of osteoblastic differentiation and on increasing the production of alkaline phosphatase (ALP) and BMP2 in MC3T3-E1 cells, resulting in an increase in osteogenesis.40
The results of the present study in relation to the expression of Osteocalcin gene were very similar to those of BMP2 gene, with significantly higher expression of this gene in scaffolds containing simvastatin; in addition, incorporation of simvastatin in association with hydroxyapatite resulted in an increase in cellular differentiation and up-regulation of this gene. Osteocalcin, along with bone sialoprotein (BSP) are recognized as late-stage markers of osteogenic differentiation and the results of a study by Min et al, too, showed an increase in the production of this osteogenic marker when they were added to tooth pulp stem cells.41 Varalakshimi et al reported that when simvastatin is used in association with tricalcium phosphate (TCP) carrier, there is an increase in the expression of Osteocalcin in hDPSCs.42
Evaluation of changes in the expression of DSPP gene, too, showed that incorporation of simvastatin alone and in association with hydroxyapatite resulted in a significant increase in the expression of DSPP gene. Karanxha et al concluded in a similar study that incorporation of simvastatin in association with enamel matrix derivatives resulted in an increase in osteoblastic differentiation by increasing the expression of DSPP gene.43 Also Stanford et al reported that when simvastatin was added to the dental pulp stem cells, DSPP and Osteocalcin were up-regulated, subsequently resulting in an increase in osteogenesis in vitro and in vivo; it was also suggested that statins might be considered active and ideal compounds for accelerating the differentiation of these cells.17
The expression pattern of RUNX2 gene was very similar to that of DSPP gene. The results showed that incorporation of simvastatin in association with HA increased the expression of RUNX2 gene. In addition, the expression of RUNX2 gene in the PLLA-PCL scaffold when simvastatin was added to it was significantly higher than that in the control group and in the scaffolds without simvastatin. The transcription factors of RUNX2 are necessary and increase the expression of many genes related to teeth and bones.44 However, in a study by Junqueira et al no significant differences were observed between the control group and the group with simvastatin in relation to the expression of RUNX2 gene.9 Such discrepancy between the results of the present study and the study above might be attributed to differences in the methodologies between the two studies and use of different concentrations of the materials tested.
The mechanism of the effect of simvastatin on up-regulating genes responsible for osteogenesis and odontogenesis has not been completely elucidated. However, Min et al reported that the effect of simvastatin on up-regulating odontogenic and angiogenic factors might be implemented through mechanisms that involve hemoxygenase-1 (HO-1) and its product, i.e. carbon monoxide (CO).41 In addition, Chen et al reported that ERK is one of the signaling pathways that might have a role in the simvastatin-mediated differentiation of osteoblasts in rats.45 ERK is one of the principal mediators in growth factor-mediated cellular differentiation in different cells. In this context, the results of a study by Kim et al showed that simvastatin might induce osteoblastic differentiation of PDL cells by regulating ERK pathways.46
In general, the results of the present study revealed that incorporation of simvastatin along with HA resulted in up-regulation of osteogenic genes and possibly in increasing the cellular differentiation and osteogenesis. Moreover, in this study only one concentration of simvastatin was used and the effect of different concentrations on the viability of cells and expression of the genes in question was not evaluated.
Conclusion
Nanofibrous PCL-PLLA-HA scaffolds with higher surface areas and porosities treated with SIM were evaluated as supports for hDPSCs cell growth for their potential use in bone/dentin tissue engineering. An adequate interaction of hDPSCs was observed with all the scaffolds. Based on the results of the present study, incorporation of simvastatin might up-regulate genes responsible for Osteogenesis, including BMP2, Osteocalcin, DSPP and RUNX2 in the hDPSCs and might increase cellular differentiation and osteogenesis. However, PCL/PLLA/HA(0.1wt%)/SIM scaffolds exhibited higher cell viability, proliferation and differentiation in comparison to other groups. These results suggest a better response of cells treated with HA in combination with SIM in blended PCL-PLLA polymeric nanofibrous matrices as an osteo/odonto-conductive scaffold.
Acknowledgments
The authors are grateful for the continuing financial support of this research project by the Stem Cell Research Center of Tabriz University of Medical Sciences.
Ethical Issues
Not applicable.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Gurtner GC, Callaghan MJ, Longaker MT. Progress and potential for regenerative medicine. Annu Rev Med. 2007;58:299–312. doi: 10.1146/annurev.med.58.082405.095329. [DOI] [PubMed] [Google Scholar]
- 2.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG. et al. Shed: Stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100(10):5807–12. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J. et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364(9429):149–55. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 4.Casagrande L, Cordeiro MM, Nor SA, Nor JE. Dental pulp stem cells in regenerative dentistry. Odontology. 2011;99(1):1–7. doi: 10.1007/s10266-010-0154-z. [DOI] [PubMed] [Google Scholar]
- 5.Stadtfeld M, Hochedlinger K. Induced pluripotency: History, mechanisms, and applications. Genes dev. 2010;24(20):2239–63. doi: 10.1101/gad.1963910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armitage J, Bowman L, Wallendszus K, Bulbulia R, Rahimi K, Haynes R. et al. Intensive lowering of ldl cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: A double-blind randomised trial. Lancet. 2010;376(9753):1658–69. doi: 10.1016/S0140-6736(10)60310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G. et al. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286(5446):1946–9. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 8.Pauly S, Luttosch F, Morawski M, Haas NP, Schmidmaier G, Wildemann B. Simvastatin locally applied from a biodegradable coating of osteosynthetic implants improves fracture healing comparable to bmp-2 application. Bone. 2009;45(3):505–11. doi: 10.1016/j.bone.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Junqueira JC, Mancini MN, Carvalho YR, Anbinder AL, Balducci I, Rocha RF. Effects of simvastatin on bone regeneration in the mandibles of ovariectomized rats and on blood cholesterol levels. J Oral Sci. 2002;44(3-4):117–24. doi: 10.2334/josnusd.44.117. [DOI] [PubMed] [Google Scholar]
- 10.Von Stechow D, Fish S, Yahalom D, Bab I, Chorev M, Müller R. et al. Does simvastatin stimulate bone formation in vivo? BMC Musculoskelet Disord. 2003;4(1):1. doi: 10.1186/1471-2474-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marolt D, Knezevic M, Novakovic GV. Bone tissue engineering with human stem cells. Stem Cell Res Ther 2010;1(10). [DOI] [PMC free article] [PubMed]
- 12.Salehi R, Irani M, Eskandani M, Nowruzi K, Davaran S, Haririan I. Interaction, controlled release, and antitumor activity of doxorubicin hydrochloride from pH-sensitive p(NIPAAm-MAA-VP) nanofibrous scaffolds prepared by green electrospinning. Int J Polym Mater. 2014;63(12):609–19. doi: 10.1080/00914037.2013.854234. [DOI] [Google Scholar]
- 13.Valizadeh A, Bakhtiary M, Akbarzadeh A, Salehi R, Frakhani SM, Ebrahimi O. et al. Preparation and characterization of novel electrospun poly (ϵ-caprolactone)-based nanofibrous scaffolds. Artif Cells Nanomed Biotechnol. 2016;44(2):504–9. doi: 10.3109/21691401.2014.965310. [DOI] [PubMed] [Google Scholar]
- 14.Asghari F, Salehi R, Agazadeh M, Alizadeh E, Adibkia K, Samiei M. et al. The odontogenic differentiation of human dental pulp stem cells on hydroxyapatite-coated biodegradable nanofibrous scaffolds. Int J Polym Mater. 2016;65(14):720–8. doi: 10.1080/00914037.2016.1163564. [DOI] [Google Scholar]
- 15.Salehi R, Aghazadeh M, Rashidi M, Samadi N, Salehi S, Davaran S. et al. Bioengineering of dental pulp stem cells in a microporous PNIPAAm-PLGA scaffold. Int J Polym Mater. 2014;63(15):767–76. doi: 10.1080/00914037.2013.879449. [DOI] [Google Scholar]
- 16.Okamoto Y, Sonoyama W, Ono M, Akiyama K, Fujisawa T, Oshima M. et al. Simvastatin induces the odontogenic differentiation of human dental pulp stem cells in vitro and in vivo. J Endod. 2009;35(3):367–72. doi: 10.1016/j.joen.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 17.Stanford CM, Jacobson PA, Eanes ED, Lembke LA, Midura RJ. Rapidly forming apatitic mineral in an osteoblastic cell line (UMR 106-01 BSP) J Biol Chem. 1995;270(16):9420–8. doi: 10.1074/jbc.270.16.9420. [DOI] [PubMed] [Google Scholar]
- 18.Farshi Azhar F, Olad A, Salehi R. Fabrication and characterization of chitosan–gelatin/nanohydroxyapatite–polyaniline composite with potential application in tissue engineering scaffolds. Des Monomers Polym. 2014;17(7):654–67. doi: 10.1080/15685551.2014.907621. [DOI] [Google Scholar]
- 19.Liu Y, Zhang X, Liu Y, Jin X, Fan C, Ye H. et al. Bi-functionalization of a calcium phosphate-coated titanium surface with slow-release simvastatin and metronidazole to provide antibacterial activities and pro-osteodifferentiation capabilities. PloS One. 2014;9(5):e97741. doi: 10.1371/journal.pone.0097741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyazawa A, Matsuno T, Asano K, Tabata Y, Satoh T. Controlled release of simvastatin from biodegradable hydrogels promotes odontoblastic differentiation. Dent Mater J. 2015;34(4):466–74. doi: 10.4012/dmj.2014-272. [DOI] [PubMed] [Google Scholar]
- 21.Nyan M, Hao J, Miyahara T, Noritake K, Rodriguez R, Kasugai S. Accelerated and enhanced bone formation on novel simvastatin-loaded porous titanium oxide surfaces. Clin Implant Dent Relat Res. 2014;16(5):675–83. doi: 10.1111/cid.12045. [DOI] [PubMed] [Google Scholar]
- 22.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97(25):13625–30. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18(4):696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 24.Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A. et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81(8):531–5. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 25.Shi S, Robey PG, Gronthos S. Comparison of human dental pulp and bone marrow stromal stem cells by cdna microarray analysis. Bone. 2001;29(6):532–9. doi: 10.1016/S8756-3282(01)00612-3. [DOI] [PubMed] [Google Scholar]
- 26.Thaweboon S, Thaweboon B, Chunhabundit P, Suppukpatana P. Effect of fluoride on human dental pulp cells in vitro. Southeast Asian J Trop Med Public Health. 2003;34(4):915–8. [PubMed] [Google Scholar]
- 27.Kopp JB, Robey PG. Sodium fluoride lacks mitogenic activity for fetal human bone cells in vitro. J Bone Miner Res. 1990;5(Suppl 1):S137–41. doi: 10.1002/jbmr.5650051321. [DOI] [PubMed] [Google Scholar]
- 28.Laino G, Carinci F, Graziano A, d'Aquino R, Lanza V, De Rosa A. et al. In vitro bone production using stem cells derived from human dental pulp. J Craniofac Surg. 2006;17(3):511–5. doi: 10.1097/00001665-200605000-00021. [DOI] [PubMed] [Google Scholar]
- 29.d'Aquino R, Graziano A, Sampaolesi M, Laino G, Pirozzi G, De Rosa A. et al. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: A pivotal synergy leading to adult bone tissue formation. Cell Death Differ. 2007;14(6):1162–71. doi: 10.1038/sj.cdd.4402121. [DOI] [PubMed] [Google Scholar]
- 30.Zanette DL, Lorenzi JC, Panepucci RA, Palma PV, Dos Santos DF, Prata KL. et al. Simvastatin modulates mesenchymal stromal cell proliferation and gene expression. PloS One. 2015;10(4):e0120137. doi: 10.1371/journal.pone.0120137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang CZ, Fu YC, Jian SC, Wang YH, Liu PL, Ho ML. et al. Synthesis and characterization of cationic polymeric nanoparticles as simvastatin carriers for enhancing the osteogenesis of bone marrow mesenchymal stem cells. J Colloid Interface Sci. 2014;432:190–9. doi: 10.1016/j.jcis.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 32.Tai IC, Wang YH, Chen CH, Chuang SC, Chang JK, Ho ML. Simvastatin enhances rho/actin/cell rigidity pathway contributing to mesenchymal stem cells' osteogenic differentiation. Int J Nanomedicine. 2015;10:5881–94. doi: 10.2147/ijn.s84273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang HX, Xiao GY, Wang X, Dong ZG, Ma ZY, Li L. et al. Biocompatibility and osteogenesis of calcium phosphate composite scaffolds containing simvastatin-loaded plga microspheres for bone tissue engineering. J Biomed Mater Res A. 2015;103(10):3250–8. doi: 10.1002/jbm.a.35463. [DOI] [PubMed] [Google Scholar]
- 34.Nyan M, Miyahara T, Noritake K, Hao J, Rodriguez R, Kuroda S. et al. Molecular and tissue responses in the healing of rat calvarial defects after local application of simvastatin combined with alpha tricalcium phosphate. J Biomed Mater Res B Appl Biomater. 2010;93(1):65–73. doi: 10.1002/jbm.b.31559. [DOI] [PubMed] [Google Scholar]
- 35.Kaji H, Naito J, Inoue Y, Sowa H, Sugimoto T, Chihara K. Statin suppresses apoptosis in osteoblastic cells: Role of transforming growth factor-beta-Smad3 pathway. Horm Metab Res. 2008;40(11):746–51. doi: 10.1055/s-0028-1082051. [DOI] [PubMed] [Google Scholar]
- 36.Park JB, Zhang H, Lin CY, Chung CP, Byun Y, Park YS. et al. Simvastatin maintains osteoblastic viability while promoting differentiation by partially regulating the expressions of estrogen receptors α. J Surg Res. 2012;174(2):278–83. doi: 10.1016/j.jss.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka Y, Nakayamada S, Okada Y. Osteoblasts and osteoclasts in bone remodeling and inflammation. Curr Drug Targets Inflamm Allergy. 2005;4(3):325–8. doi: 10.2174/1568010054022015. [DOI] [PubMed] [Google Scholar]
- 38.Hughes A, Rogers MJ, Idris AI, Crockett JC. A comparison between the effects of hydrophobic and hydrophilic statins on osteoclast function in vitro and ovariectomy-induced bone loss in vivo. Calcif Tissue Int. 2007;81(5):403–13. doi: 10.1007/s00223-007-9078-1. [DOI] [PubMed] [Google Scholar]
- 39.Ahn KS, Sethi G, Chaturvedi MM, Aggarwal BB. Simvastatin, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, suppresses osteoclastogenesis induced by receptor activator of nuclear factor-kappaB ligand through modulation of NF-kappaB pathway. Int J Cancer. 2008;123(8):1733–40. doi: 10.1002/ijc.23745. [DOI] [PubMed] [Google Scholar]
- 40.Maeda T, Matsunuma A, Kawane T, Horiuchi N. Simvastatin promotes osteoblast differentiation and mineralization in mc3t3-e1 cells. Biochem Biophys Res Commun. 2001;280(3):874–7. doi: 10.1006/bbrc.2000.4232. [DOI] [PubMed] [Google Scholar]
- 41.Min KS, Lee YM, Hong SO, Kim EC. Simvastatin promotes odontoblastic differentiation and expression of angiogenic factors via heme oxygenase-1 in primary cultured human dental pulp cells. J Endod. 2010;36(3):447–52. doi: 10.1016/j.joen.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 42.Varalakshmi PR, Kavitha M, Govindan R, Narasimhan S. Effect of statins with α-tricalcium phosphate on proliferation, differentiation, and mineralization of human dental pulp cells. J Endod. 2013;39(6):806–12. doi: 10.1016/j.joen.2012.12.036. [DOI] [PubMed] [Google Scholar]
- 43.Karanxha L, Park SJ, Son WJ, Nör JE, Min KS. Combined effects of simvastatin and enamel matrix derivative on odontoblastic differentiation of human dental pulp cells. J Endod. 2013;39(1):76–82. doi: 10.1016/j.joen.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen S, Gluhak-Heinrich J, Wang YH, Wu YM, Chuang HH, Chen L. et al. Runx2, osx, and dspp in tooth development. J Dent Res. 2009;88(10):904–9. doi: 10.1177/0022034509342873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen PY, Sun JS, Tsuang YH, Chen MH, Weng PW, Lin FH. Simvastatin promotes osteoblast viability and differentiation via ras/smad/erk/bmp-2 signaling pathway. Nutr Res. 2010;30(3):191–9. doi: 10.1016/j.nutres.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Kim IS, Jeong BC, Kim OS, Kim YJ, Lee SE, Lee KN. et al. Lactone form 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitors (statins) stimulate the osteoblastic differentiation of mouse periodontal ligament cells via the ERK pathway. J Periodontal Res. 2011;46(2):204–13. doi: 10.1111/j.1600-0765.2010.01329.x. [DOI] [PubMed] [Google Scholar]