Abstract
Purpose: Recurrent aphthous stomatitis is a disease with unknown etiology that’s mostly treated symptomatically and has no definite cure. Pomegranate (Punica granatum) flowers have been used as medicinal herb that due to its antimicrobial, antioxidant, anti-inflammatory, analgesic and healing effects, has been useful in treatment of oral aphthous. Therefore, we decided to formulate a mucoadhesive gel with pomegranate flower extract to reduce the need for corticosteroid therapy in patients.
Methods: Pomegranate flowers are extracted by percolation method. Several formulations with different amounts of carbomer 934, sodium carboxymethylcellulose (SCMC) and hydroxypropyl methylcellulose K4M were prepared and the condensed extract was dispersed in polyethyleneglycol (PEG) 400 and added to gel bases. Then the formulations underwent macroscopic and microscopic studies. The formulations that passed these tests successfully were studied through assay tests using spectrophotometry in 765 nm, drug release from mucoadhesive gel using cell diffusion method, viscosity test, mucoadhesion test and accelerated stability test.
Results: The phenolic content of pomegranate flower dried extract was found to be 212.3±1.4 mg/g in dried extract. The F4–F6 formulations contains carbomer 934, SCMC, pomegranate flower extract, PEG 400, potassium sorbate and purified water passed all above tests.
Conclusion: The F4 formulation had higher viscosity and mucoadhesion values due to its higher carbomer 934 and SCMC content. Since F4, F5 and F6 had no significant variation in drug release, the F4 formulation was chosen as the superior formulation because of proper appearance and uniformity, acceptable viscosity, mucoadhesion and stability in different temperatures.
Keywords: Aphthous stomatitis, Pomegranate flower, Gallic acid, Oral mucoadhesive gel
Introduction
Recurrent aphtous stomatitis is a rather common disease with unknown etiology that’s identified by one or more sore ulcers with red margins on the mucous membrane of mouth and is self-limiting in one or two weeks but can reoccur monthly or a few times a year. Since this disease has an unknown cause, its diagnosis is based on clinical signs.1 Aphthous patients can be categorized into three groups based on clinical status: minor, major and herpetifom; the most common of which is the minor type. The disease prevalence was reported differently in studies; for example in some surveys its prevalence in the general population was reported to be between 5 and 50 % and in specific populations such as students and military soldiers was said to be about 50 to 60 % because of daily stressful tasks like exams, etc.2
There is no definite etiology and pathology known for aphthous; although some factors are considered important such as topical trauma, bacterial and viral infections, genetics, nutrition, immunological, hormonal and psychological factors, allergies, medications and etc. Triamcinolone paste (Triadent), Irsha mouthwash, Persica and chlorhexidine mouthwash are medications for aphthous treatment in Iranian pharmaceutical market. Some generally suggested aphthous treatments are: antibiotics and antiseptics, herbal treatments, local analgesics, immunological mediators, both steroidal and non-steroidal anti-inflammatory drugs.1,2
Because of higher compatibility of herbal treatments with biological systems, these treatments are considered to have fewer side effects compared to synthetic medications. Many herbal compounds have been found in the past to be effective in buccal diseases. A preliminary study has demonstrated that a 5% solution of Myrtus communis and Melissa officinalis extracts can treate the sore and lead to elimination of ulcer.2 It has been shown in another study that a 2% mouth wash of Zataria mutiflora has a significant effect on minor aphthous compared to placebo.3 Anthemis nobelis is another herbal treatment suggested to be effective in aphthous ulcer improvement.4 The Hypericum perforatum extract used as a mouth wash may also reduce pain and shorten disease period in minor aphthous.5
Another suggested herb is pomegranate flowers of (Punica granatum L.) from the punicacea family. This plant grows as a 2 to 5 m tall tree or shrub that is indigenous to Iran, Afghanistan, China and Indian subcontinent.6 Pomegranate is found abundantly in parts of Iran with semi-warm climate. Different parts of pomegranate tree have had several applications in Iranian folk and traditional medicine. Pomegranate flowers have been used as for oral and anal ulcers, intra-nasal ulcers, peptic ulcer, sores between toes and ear pain. In Iranian medicine pomegranate flower is believed to have strengthening effect on gums and can be useful for loose teeth. Applying it to scrape or wounds can rapidly heal them.7
Tanins are the major compounds in pomegranate flowers. Tannins are a great group of natural compounds with complex phenolic structures. Hydrolysable tannins like Ellagic acid, Gallic acid and Punicalagin are the most important tannins in different parts of pomegranate plant.6 These compounds have an astringent effect on live tissues and can be used as digestive system astringent and treatment of burns and wounds. In burns and wounds, tissue proteins are precipitated by tannins and form a protective anti septic coating. Under this coating, new cells can grow and heal the wound.8
Free radicals have lately been suggested to play an important role in the etiology of aphthous. An increase in production of free radicals or the weakening of anti-oxidative defense systems causes a condition called the oxidative stress that can lead to tissue injury.9 Due to the established role of oxidative stress in inflammation and the inflammatory nature of recurrent aphthous stomatitis, this oxidative stress seems to be one of the factors causing this condition.10 According to this finding, the use of anti-oxidants might be effective in aphthous healing. The pomegranate flower seems to have a positive effect on treatment of aphthous symptoms due to its high antioxidant content.11 Pomegranate has an antimicrobial effect too. Machado et al. have studied the effects of pomegranate fruits on a bacterial species and have found most of the anti-bacterial properties to be due to Ellagitannin and Punicallagin in the fruit.12 Also in another study, antimicrobial effects of different extracts in methanol, water, chloroform and petroleum were analyzed. All extracts had anti-microbial properties which was significant in methanol extracts.13
Studies on effect of pomegranate flower extract on wound healing demonstrated positive effects on shortening the healing period.8 Pomegranate flower may also act as an analgesic to ameliorate aphthous symptoms.14
Since antioxidant and immune system activity disturbances are considered important factors in aphthous etiology and because this disease is accompanied by inflammation and pain, pomegranate flower can be used for exoneration of symptoms and decreasing disease period due to antioxidative, antimicrobial, antiinflammatory, analgesic and wound healing properties. Moreover, the existing tannins form a protective layer by precipitating proteins and preventing the ulcer from getting infected or exacerbated.8
Ointments, creams, pastes, emulsions and gels are topical formulations for diseases such as aphtous. Patient compliance and acceptance is extremely important for oral topical products. Ointments, creams and some emulsions are rarely used for oral topical treatment while the patients have lower acceptance for application of ointments in mouth. Formulation base must have acceptable mucoadhesion so that the medication remains on the spot of application for a longer time. Emulsions have low mucoadhesion and are rapidly washed away by saliva. Pastes and gels allow longer adhesion and allow both the protection of lesion and release of drug. Pomegranate flower extract seems to be more compatible with hydrogels because of its hydroalcoholic phase. Gel bases have high water content and low surface friction and have better application on mucous membranes and burned or injured tissues.
Topical steroid such as triamcinolone and prednisolone are the most important medication for aphthous treatment, but these medications have many side effects like adrenal suppression, immune suppress, osteoporosis, digestive disturbances, blood glucose elevation, etc.15 Considering these side effects and based on preliminary studies on pomegranate flower effectiveness on aphthous ulcers.16 We prepare a stable mucoadhesive formulation of pomegranate flower that can be used as an alternative treatment for aphthous stomatitis.
Materials and Methods
Punica granatum flowers were collected in Isfahan in May and June 2013 and underwent a validation process by Pharmacognosy specialists in department of pharmacognosy; then the flowers were dried at room temperature in shade. The flowers were then powdered and prepared for extracting.
In this study Carbomer 934, Sodium carboxymethylcellulose (SCMC), Hydroxypropyl
methylcellulose (HPMC), potassium sorbate, Triethanolamine, polyethylen glycol 400 (PEG 400), ethanol, gallic acid, sodium carbonate were prepared from Merck Company (Germany) and Folin- ciocalteu's indicator was purchased from Sigma Aldrich Company (Germany).
Extracting
Percolation method was used for extracting. 500 grams flower powder was soaked in ethanol 70% (as solvent) for 2 hours. The extraction was completed by letting the percolator drip 4 to 6 drops per minute (for every 100 grams of powder) for 48 hours. The remaining solvent was added on top of the powder so that rate of input and output drops are equal.17 After finishing extraction process, the extract was condensed by rotary evaporator (Heidolph VV 2000) with 70 rpm and 50°C to achieve sufficient viscosity.
pH determination
The pH of extract was measured by digital pH meter (Metrohm 827, Switzerland) right after extracting, in 48 hours, 1 week, 2 weeks, 1 month, 3 months and 6 months. The pH meter was calibrated with standard buffers before measurement and each time the measuring was repeated 3 times and the mean was calculated.
Determination of dried extract
One gram of the condensed extract was heated to 40°C in vacuum oven and weighted daily till its weight was stabilized for 3 consecutive days. To find the weight percentage of dried extract, the container weight was subtracted and then the percentage of dried extract weight to total weight of the extract of calculated three times and the mean was chosen.18
Determination of total polyphenols
We used folin-ciocalteu,s method and gallic acid as standard. To prepare gallic acid stock solution, 0.5 gram gallic acid was dissolved in 10 ml ethanol 96% in a 100 ml volumetric flask and then filled to the etched line with water. Then volumes of 1, 2, 3, 5 and 10 ml of this stock solution were diluted with water in 100 ml volumetric flasks. These solutions had phenolic contents of 50, 100, 150, 250 and 500 mg/l of gallic acid. To prepare sample solution, 5 g of concentrated pomegranate flower extract was weighted and mixed with 10 ml of ethanol 96% and then diluted to 100 ml by water. 5 ml of this sample was then diluted to 100 ml by water in a volumetric flask.
20 µl of each of the samples, blank (purified water) and calibration solutions were added to 1.58 ml of purified water in test tubes. Then 100 µl of folin-ciocalteu,s indicator was added and the test tubes were shaken till mixed. After 30 sec to 8 minutes, 300 µl of 20% w/v sodium carbonate solution was added to test tubes and again mixed by shaking. At last the UV absorbance of solutions was measured against the blank sample by spectrophotometry (UV mini 1240) and standard curve of gallic acid absorption was prepared. Using this standard diagram, concentration the test sample was found.19
Gel preparation methods
For different gels, the concentrated pomegranate flower extract as the active ingredient and carbomer 934, SCMC and HPMC K4M were used as gelling polymers. Table 1 demonstrates each formulation and their contents.
Table 1. Composition of gel formulations with different polymers (Carbomer 934, SCMC and HPMC K4M) .
| Ingredients (g) | Formulations | |||||||||
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | |
| Carbomer 934 | 0.5 | 1 | - | 1 | 0.5 | 0.75 | 0.5 | 0.75 | - | - |
| Sodium CMC | - | - | 3 | 3 | 3 | 2 | 2 | 1 | - | - |
| HPMC K4M | - | - | - | - | - | - | - | - | 2 | 3 |
| Golnar extract | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 |
| PEG 400 | 13 | 13 | 13 | 13 | 13 | 13 | 13 | 13 | 13 | 13 |
| Potassium Sorbate | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Triethanolamin | qs | qs | - | - | - | - | - | - | - | - |
| Purified water to | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Preparation of carbomer 934 gel
Using the amounts on Table 1, first potassium sorbate (as preservative) was dissolved in 40°C purified water and then a specific amount of carbomer 934 was mixed with it till homogenous using a magnetic stirrer with 1200 rpm for 30 minutes. A determined amount of concentrated punica extract was weighed and mixed well with PEG 400. This mixture was slowly added to the gel and mixed to achieve a uniform gel. While monitoring the pH, triethanolamine was added to the gel for it to reach a pH of about 6.20
Preparation of SCMC gel
Pottasium sorbate was dissolved in 50°C purified water. Then an exact amount of SCMC was slowly added to it while being mixed with magnetic stirrer in 1200 rpm for 30 minutes till it was completely homogenous. Concentrated punica extract was weighted and added to PEG 400. The extract was added slowly to the gel and mixed till uniform.20
Preparation of HPMC gel
Potassium sorbate was dissolved in about one third of the formulation’s water heated to 80°C and then a specific amount of HPMC was slowly added and mixed using magnetic stirrer in 1200 rpm. The remaining water was cooled and slowly added and mixed till a uniform gel was achieved. The gel remained in refrigerator overnight (hot/cold technique). Then an exact amount of concentrated pomegranate flower extract was separately added to PEG 400 and mixed and then gradually added to the gel.20
Preparation of carbomer 934 and SCMC gel
For each of these formulations, the listed powders were separately made into transparent gels as said above and then gels were added to each other and mixed well. Finally the extract was dispersed in PEG 400 and added to the gel.20
Determination of polyphenols in formulations
48 hours after preparation of the formulations, 1 grams of each gel were diluted to 10 ml with water. The polyphenol contents were determined by folin-ciocalteu,s method using gallic acid standard curve.
Evaluation of physicochemical properties
These tests are used to study the formulation physicochemical properties and their stability in a long period of time. During formulations’ stability tests, they are undergone abnormal stress conditions and if these conditions are tolerated for a short time, this is an indicator of stability in normal conditions for a longer time. These tests include: microscopic and macroscopic appearance uniformity tests, centrifuge test, temperature change test, cooling and heating test, melting and freezing test, pH test, release test, viscosity determination and mucoadhesion tests.20
Macroscopic and microscopic tests
The formulations were studied 48 hours after preparation for macroscopic (lumps, color and transparency) and microscopic (by optic microscope with magnification of 10 and 40 for uniformity, gel texture and bubbles.20
Centrifuge test
Each of the chosen formulations were separately centrifuged in a test tube of 10 cm long and 1 cm width for 5, 15, 30 and 60 minutes with 2000 rpm (Hettich Universal Centrifuge) and then studied for sedimentation and gel stability.20
Temperature change test
To control the formulation stability in different seasons and different temperature conditions, tubes containing formulations were put in temperatures of 2-8°, 25°C and 40-45°C and then their appearance quality was controlled after 48 hours, 1 week, 2 weeks, 1 month, 3 months and 6 months.20
Melting, freezing, cooling and heating tests
The goal of this test is to study the formulation stability in extreme temperatures. Two sets of formulation test tubes were prepared. One set underwent 6 consecutive periods of temperature changes each including 48 hours in -8°C and 48 hours in 25°C (freezing and melting). The second set underwent 6 periods of temperature changes too but this time each period included 48 hours in 45°C and then 48 hours in 4°C (heating and cooling). After these 6 periods, the formulations’ qualities were analyzed.20
pH determination test
1 gram of each formulation was dispersed in 10 ml purified water. pH was measured after 48 hours, 1 week, 2 weeks, 1 month, 3 months and 6 months after preparation and each time three repeats were done.20
Viscosity test
Viscosities were measured by Brookfield (DV-III) viscometer. Each gel was poured into the container and the proper spindle (number 74) was attached. Then the viscosities were measured in 25°C and 50-250 rpm.20
Release test
This test is done with cell diffusion and synthetic membrane. 1g of the sample was spread over the membrane and the membrane was fixed on top of the cell. The receiving phase of the cell was filled with 37°C purified water and constantly stirred. For 6 hours, every 30 minutes a sampling of 1 ml was done and each time 1 ml purified water of 37°C was added to keep the volume constant. UV absorption of each sample was measured and the concentrations were found using the foline-ciocalteu,s method. To calculate the actual concentration of the sample, the following equation was used:20
| eq.1 |
Cn: Actual concentration of drug in sample n
C: Pseudo-concentration of drug in sample n
Cn-1: Actual concentration of drug in sample n-1
Vt: Total volume
V: Sample volume
Release of the active ingredient from each formulation has its own specific kinetics which is the rate of release based on the time variation. To study the release kinetics, three models of zero order, first order and Higuchi model were studied and their constants were calculated.
Mucoadhesion test
The tensiometer (fisher) was calibrated and then the gel came in contact with sodium alginate (substitute for mucin) for 5 minutes. Then the required force to detach the gels from solution surface (speed of 0.2 inch/min) were determined in dyne/cm2. This test was done 6 times for each formulation.20,21
Results
51.2 grams concentrated extract was obtained from every 100 g of dried flower powder, 81% of which is dried extract and the rest is water. According to the standard curve and the dilution factor, phenolic content of Pomegranate flower dried extract was found to be 212.3±1.4 mg/g in dried extract.
The pH of Pomegranate flower extract was about 3.7 and constant throughout 6 months. The results of extracts pH are demonstrated in Table 2.
Table 2. Determination of extracts pH .
| Time | pH (mean ± SD ) |
| 24 h after preparation | 0.01± 3.75 |
| 48 h later | 3.76 ± 0.02 |
| 1 week | 3.78 ± 0.01 |
| 2 weeks | 3.77 ± 0.01 |
| 1 month | 3.75 ± 0.00 |
| 3 months | 3.71 ± 0.01 |
| 6 months | 3.70 ± 0.02 |
48 hours after preparation, the formulations were studied for macroscopic and microscopic properties and appearance. The F9 and F10 formulations with HPMC lost uniform gel characteristics right after the addition of extract. F1 and F2 formulations had darkened. F3, F7 and F8 did not have proper thickness and viscosity. Other formulations (F4, F5 and F6) were completely uniform with no bubbles and no lumps when touched. These three formulations underwent further tests.
The results for polyphenol assays 48 hours after preparation are shown in Table 3.
Table 3. Determination of total polyphenols (mean ± SD) according to mg GAE/g in formulations F4-F6 .
| Formulations | Total polyphenols (mg GAE/g) |
| F4 | 21.2 ± 0.2 |
| F5 | 20.0 ± 0.1 |
| F6 | 22.1 ± 0.1 |
There were no observable sediment in centrifuge tests and the gels kept their uniformity.
In temperature change test (heating, cooling, melting and freezing), there were no appearance changes observed.
pH of formulations were fairly constant and about 4-5 in 6 months. The results of pH measurements are shown in Table 4.
Table 4. Determination of formulations pH after preparation of formulations F4-F6 .
| Time | pH (mean ± SD) | ||
| F4 | F5 | F6 | |
| 24 h after | 4.43 ± 0.01 | 4.50 ± 0.02 | 4.56 ± 0.00 |
| 48 h after | 4.40 ± 0.00 | 4.60 ± 0.01 | 4.71 ± 0.02 |
| 1 week | 4.48 ± 0.00 | 4.56 ± 0.00 | 4.67 ± 0.03 |
| 2 weeks | 4.40 ± 0.00 | 4.48 ± 0.02 | 4.74 ± 0.00 |
| 1 month | 4.33 ± 0.02 | 4.45 ± 0.02 | 4.64 ± 0.01 |
| 3 months | 4.31 ± 0.00 | 4.55 ± 0.02 | 4.51 ± 0.01 |
| 6 months | 4.39 ± 0.02 | 4.45 ± 0.03 | 4.59 ± 0.01 |
Viscosities of the three best formulations were measured by Brookfield viscometer (DV-III). Figure 1 demonstrates the viscosity changes against rounds per minute of rotation. The faster the rotation, the more the increase in formulation viscosities. According to this figure, F4 viscosity is the highest.
Figure 1.
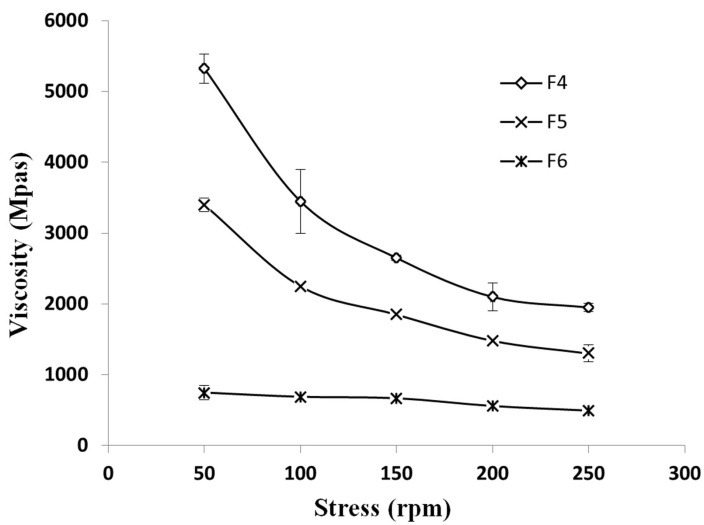
The viscosity changes against rpm, at 25°C
Results from mucoadhesion studies by tensiometery method are shown in Table 5. The F4 formulation has the highest mucoadhesion.
Table 5. Mucoadhesive strength (mean ± SD) of formulations F4- F7 according to tensiometry method .
| Formulations | Mucoadhesive strength (dyne/cm 2 ) |
| F4 | 54.6 ± 3.2 |
| F5 | 24.3 ± 3.8 |
| F6 | 31.3 ± 3.4 |
| F7 | 22.2 ± 3.6 |
The results of active ingredient release by cell diffusion method are displayed in Figure 2 as a curve of cumulative percentage of release in time.
Figure 2.
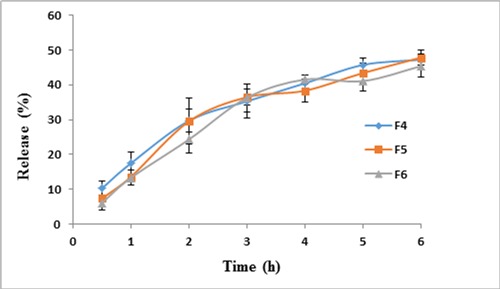
Percentage of cumulative drug release of formulations F4, F5 and F6 in Franz diffusion cell through a cellulose acetate membrance, at 37°C, during 6 h
To study the kinetic of release from synthetic membrane, the zero order, first order and Higuchi models were controlled. To study the zero order model, a diagram was prepared for percentage of released ingredient in time. For first order kinetic, the diagram for logarithm of remaining drug percentage in time; and for Higuchi model, the percentage of released drug was drawn against square root of time. Table 6 demonstrates the cumulative percentage of polyphenol release and the equation constants for zero order (K0), first order (K1) and Higuchi (Kh). In these equations, Qt is the percentage of release in time of t and Q0 is the percent of remaining drug.
Table 6. Drug release percent and drug release kinetics of gel formulations (F4, F5 and F6) .
| Zero-order release | First-order release | Higuchi equation | Cumulative drug release (%) | Formulations | |||
| R 2 | K h | R 2 | K 1 | R 2 | K 0 | ||
| 22.15 | 0.982 | 0.102 | 0.825 | 6.621 | 0.936 | 47.72 ± 4.2 (6 h) | F4 |
| 22.40 | 0.959 | 0.057 | 0.740 | 3.323 | 0.899 | 47.98 ± 2.5 (6 h) | F5 |
| 23.24 | 0.949 | 0.062 | 0.755 | 3.449 | 0.891 | 45.57 ± 1.7 (6 h) | F6 |
Qt = K0 t eq. 2
lnQt = lnQ0 - K1 eq. 3
Qt = Kh t1/2eq. 4
Discussion
Aphthous is an oral disease characterized by one or several recurrent ulcers on oral mucosa. The etiology of the disease is unknown and it is mostly symptomatically treated and the aim of the treatment is to decrease the period of the ulcers. The most frequently used drugs for treatment of aphthous are topical steroid which can cause several side effects on continuous application. The herbal medications have been widely used for different oral diseases. Due to its anti-microbial, anti-oxidant, analgesic and healing properties, the pomegranate flower can improve aphthous symptoms and because of the high amount of tannins, it can form a protective layer on the ulcers by precipitating proteins and hence accelerate the healing process. Considering these pharmacological effects of pomegranate flower extract and the preliminary studies indicating effectiveness of pomegranate flower extract on aphthous ulcers.16 We decided to utilize these effects and design a suitable, stable and easily accessible formulation of this herb.
The folin-ciocalteu,s method was used to indicate the amount of poly-phenols in dried extract. This determination can be done by the linear correlation between the concentration and absorbance of gallic acid after finding its standard curve using gallic acid standard solution. In this study, the poly-phenol content was about 212.3± 1.4 mg per gram of dried extract. In a study done by Mortezaei et al. in Iran’s Chahar-Mahal-e-Bakhtiari, this was reported to be 480.6 mg per gram of dried extract.22 The found difference might be due to the different drying and extracting methods, solvents and assay methods.
In this study the pomegranate flower extract was prepared as a mucoadhesive gel formulation. Considering the ease of application, good distribution and ability of adhesion and remaining on oral mucosa for a long enough time to release its drug, this formulation can be well accepted for treatment of oral ulcers and diseases such as aphthous.
To prepare a mucoadhesive gel, polymers like carbomer 934, SCMC and HPMC were used. These polyemers are all water soluble and useful in pharmaceutical industries.20 Many gels, specially the water-based ones are susceptible to microbial growth and hence the use of a suitable preservative decreases the chance of microbial contamination and the change in formulation properties21 In this study, potassium sorbate was applied as preservative agent. Sorbates are safe and effective against mold, yeast and bacteria. These materials are used to decrease the chance of contamination to a minimum.23
Formulations were prepared by different proportions of polymers and after 48 hours, the ones with superior microscopic and macroscopic properties were selected to undergo stability tests. The F9 and F10 formulations with HPMC base didn’t have suitable uniformity and physical properties, i.e. the gel base lost its homogenous texture right after the addition of extract. This may be because of pomegranate flower extract incompatibility with HPMC base. F1 and F2 formulations with Carbomer 934 base changed color after 48 hours. When only Carbomer is used to prepare the gel, we need to increase the pH and neutralize the mixture by triethanolamine so that the gel can swell and remain clear. Incompatibility of the extract with triethanolamine or its instability in neutral or basic pH can be the reason for its change of color. The F3, F7 and F8 formulations did not have sufficient thickness. This is due to lower amounts of polymer in these formulations compared to F4, F5 and F6 formulations. So it was made clear that polymers enhance thickness and the formulations with fewer polymers will not have a sufficient firmness. The F4, F5 and F6 formulations had acceptable macroscopic and microscopic properties and underwent further tests. A study was conducted on the same formulation which confirms these results.20
Generally the formulation pH must be constant during storage time and its fluctuations can indicate complications such as microbial growth, ingredient incompatibilities or decomposition of some ingredients.20 The F4, F5 and F6 formulations had a rather constant pH throughout a 6 months duration and could be accepted as stable. The condensed extract also has a pH between 3.6 and 3.7 in 6 months which is almost constant and this might be a reason for extract stability for a long period of time.
The phenolic compounds assay was operated by the folin-ciocalteu,s method and standard curve of gallic acid and the results show homogenous and uniform distribution of polyphenols in gel formulations.
The heat stress tests are applied to determine the formulation half-life. If the formulation can withstand 2 to 3 months of high temperature or 3 to 4 heat cycles, it is deemed to have an adequate half-life.20 Our formulations showed no change in visual quality after stability tests such as temperature change, heating and cooling, melting and freezing tests. Hence, the results indicate that the formulations can endure 6 months of undesirable temperature conditions. A study conducted by Tavakoli et al also noted that if the formulation accomplishes the stability studies (such as changes in temperature and pH) without any changes, thus a 2-years shelf life can be suggested for it.21
According to Figure 1, the F4 and F5 formulations have higher viscosities. This might be due to higher proportions of Carbomer to SCMC in these formulations. Gels with mucoadhesion and proper viscosity have a longer adhesion time and better durability on site. Studies by Mortazavi et al. demonstrated that carbomers specially carbomer 934 have the highest mucoadhesion and remain longer on mucous surface.24 This study confirms our findings concerning mucoadhesion of formulations.
In release studies with Franz cell diffusion method, as it is demonstrated on Table 6, there is little difference in release percentage from different formulation and these small differences are minor and non-significant. This might be because of the similar polymer proportions in remaining formulations since the ones with considerably diverse proportions were omitted on the primary screenings. Also water is used as the release environment, so the release is possibly different in oral pH and due to the buffer nature of saliva, the active ingredient might have an increased release profile. To determine the amount of released drug, the folin- ciocalteu,s method is used as a standard way to estimate the phenolic content. This method is based on reduction of folin-ciocalteu,s indicator by phenolic compounds in basic environment and formation of a blue complex with maximum absorbance in 765 nm.25 It was not possible to measure the amount of released extract in-vitro in a buffer environment. This may be due to the interaction between phosphate buffer and the folin-ciocalteu,s reagent and preventing the basic environment needed for the reaction. This is why we used only water as the receiving phase.
The release kinetics were studied by zero order, first order and Higuchi model and the kinetic was found to be following the Higuchi model in all formulations. So it is logical to conclude that the release kinetic in formulations is not independent from formulation base (cream, ointment, etc).
Conclusion
The ideal formulation for treatment of aphthous must have high mucoadhesion and suitable durability. The results showed that an increase in the amount of polymer in gel can increase mucoadhesion and lead to a longer durability in mouth. Hence, the F4 formulation has the highest mucoadhesion and viscosity because of its higher polymer content and it is able to remain on mucous surface long enough to release its active ingredient. So because of uniformity, proper appearance, stability and acceptable viscosity and mucoadhesion the F4 formulation was chosen as the superior formulation.
We suggest that the effectiveness of the superior formulation is determined through clinical studies.
Acknowledgments
We appreciate Isfahan University of Medical Science Vice Chancellery for Research that supported us financially through research project number 393066.
Ethical Issues
Not applicable.
Conflict of Interest
The authors report no conflicts of interest in this work.
References
- 1.Rad F, Yaghmaee R, MehdiAbadi P, Khatibi R. A comparative clinical trial of topical triamcinolone (adcortyle) and a herbal solution for the treatment of minor aphthous stomatitis. Armaghane-danesh. 2010;15(3):191–8. [Google Scholar]
- 2.Eslami Raveshty SS, Eslami Raveshty SB. The effect of combining essences of Myrtus Communis and Melissa Officinalis in the treatment of minor aphta. J Zanjan Univ Med Sci. 2011;19(76):76–83. [Google Scholar]
- 3.Mansoori P, Hadji Akhoondi A, Ghavami R, Shafiei A. Clinical evaluation of Zataria multiflora essential oil mouthwash in the management of recurrent aphthous stomatitis. DARU J Pharm Sci. 2002;10(2):74–7. [Google Scholar]
- 4.Jafari S, Amanlou M, Borhan-Mojabi K, Farsam H. Comparartive study of Zataria multiflora and Anthemis nobelis extracts with Myrthus communis preparation in the treatment of recurrent aphthous stomatitis. DARU J Pharm Sci. 2003;11(1):23–7. [Google Scholar]
- 5. Motallebnejad M, Moghadamnia A, Talei M. The efficacy of Hypericum perforatum extract on recurrent aphthous ulcers. J Med Sci. 2008;8(1):39–43. doi: 10.3923/jms.2008.39.43. [DOI] [Google Scholar]
- 6. Ismail T, Sestili P, Akhtar S. Pomegranate peel and fruit extracts: A review of potential anti-inflammatory and anti-infective effects. J Ethnopharmacol. 2012;143(2):397–405. doi: 10.1016/j.jep.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Al-rhazes (Rhazes) M. Persian translation by Afsharipour S. Tehran: Academy of medical sciences publication; 2005. [Google Scholar]
- 8.Pirbalouti AG, Koohpayeh A, Karimi I. The wound healing activity of flower extracts of punica granatum and achillea kellalensis in wistar rats. Acta Pol Pharm. 2010;67(1):107–10. [PubMed] [Google Scholar]
- 9.Najafi SM, Mohammadzadeh M, Monsef Esfahani HR, Meighani Gh, Rezaei N. The effect of Purslane in the treatment of recurrent aphthous stomatitis. Tehran Univ Med J. 2013;71(2):102–8. [Google Scholar]
- 10.Caglayan F, Miloglu O, Altun O, Erel O, Yilmaz AB. Oxidative stress and myeloperoxidase levels in saliva of patients with recurrent aphthous stomatitis. Oral Dis. 2008;14(8):700–4. doi: 10.1111/j.1601-0825.2008.01466.x. [DOI] [PubMed] [Google Scholar]
- 11.Celik I, Temur A, Isik I. Hepatoprotective role and antioxidant capacity of pomegranate (punica granatum) flowers infusion against trichloroacetic acid-exposed in rats. Food Chem Toxicol. 2009;47(1):145–9. doi: 10.1016/j.fct.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 12.Machado TdB, Leal ICR, Amaral ACF, Santos KRN, Silva MG, Kuster RM. Antimicrobial ellagitannin of Punica granatum fruits. J Braz Chem Soc. 2002;13(5):606–10. [Google Scholar]
- 13.Prashanth D, Asha MK, Amit A. Antibacterial activity of punica granatum. Fitoterapia. 2001;72(2):171–3. doi: 10.1016/s0367-326x(00)00270-7. [DOI] [PubMed] [Google Scholar]
- 14.Chakraborthy GS. Analgesic activity of various extracts of Punica granatum (Linn) flowers. Int J Green Pharm. 2008;2(3):145. doi: 10.4103/0973-8258.42730. [DOI] [Google Scholar]
- 15.Katzung BG, Masters SB, Trevor AG. Basic and clinical pharmacology. 12th ed. London: McGraw-Hill Education; 2012. [Google Scholar]
- 16.Ghalayani P, Zolfaghary B, Farhad AR, Tavangar A, Soleymani B. The efficacy of punica granatum extract in the management of recurrent aphthous stomatitis. J Res Pharm Pract. 2013;2(2):88–92. doi: 10.4103/2279-042X.117389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghalayani P , Zolfaghary B , Farhad AR , Tavangar A , Soleymani B . Formulation and physicochemical evaluation of an herbal antihemorrhoid ointment from Quercus‚ Black cumin and Fenugreek for the treatment of internal anal hemorrhoids. Pharmaceut Sci. 2009;14(4):247–57. [Google Scholar]
- 18.Mohagheghi Samarin A, Poorazarang H, Akhlaghi H, Elhami Rad A, Hematyar N. Antioxidant activity of potato (Solanum tuberosum, raja) peel extracts. Iran J Nutr Sci Food Technol. 2008;3(3):23–32. [Google Scholar]
- 19.Mena P, Garcia-Viguera C, Navarro-Rico J, Moreno DA, Bartual J, Saura D. et al. Phytochemical characterisation for industrial use of pomegranate (punica granatum l.) cultivars grown in spain. J Sci Food Agric. 2011;91(10):1893–906. doi: 10.1002/jsfa.4411. [DOI] [PubMed] [Google Scholar]
- 20.Aslani A, Ghannadi A, Najafi H. Design, formulation and evaluation of a mucoadhesive gel from quercus brantii lAnd coriandrum sativum lAs periodontal drug delivery. Adv Biomed Res. 2013;2:21. doi: 10.4103/2277-9175.108007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tavakoli N, Minaiyan M, Saghaei E. Preparation of diltiazem topical gel for the treatment of anal fissure and In-vitro, Ex-vivo drug release evaluations. J Kerman Univ Med Sci. 2007;14(3):163–75. [Google Scholar]
- 22.Mortazaei S, Rafieian M, Ansary Samani R, Shahinfard N. Comparison of phenolic compounds concentrations and antioxidant activity of eight medicinal plants. J Rafsanjan Univ Med Sci. 2013;12(7):519–30. [Google Scholar]
- 23.Barzegar H, Azizi MH, Barzegar M, Hamidi Z. Preparation and evaluation of active starch-clay nanocomposite film containing cinnamon oil and potassium sorbate. J Res Innov Food Sci Technol. 2013;2(2):167–78. [Google Scholar]
- 24.Mortazavi A, Salehi A. In vitro assessment of the efficacy of various mucosa-adhesive materials for the preparation of a buccal mucosa-adhesive gel. Hakim Res J. 2002;4(1):31–8. [Google Scholar]
- 25.Salmanian SH, Sadeghi Mahoonak AR, Alami M, Ghorbani M. Evaluation of total phenolic, flavonoid, anthocyanin compounds, antibacterial and antioxidant activity of hawthorn (Crataegus Elbursensis) fruit acetonic extract. J Rafsanjan Univ Med Sci. 2014;13(1):53–66. [Google Scholar]


