Abstract
Magnetic separation is a versatile technique used in sample preparation for diagnostic purpose. For such application, an external magnetic field is applied to drive the separation of target entity (e.g. bacteria, viruses, parasites and cancer cells) from a complex raw sample in order to ease the subsequent task(s) for disease diagnosis. This separation process not only can be achieved via the utilization of high magnetic field gradient, but also, in most cases, low magnetic field gradient with magnitude less than 100 T m−1 is equally feasible. It is the aim of this review paper to summarize the usage of both high gradient magnetic separation and low gradient magnetic separation (LGMS) techniques in this area of research. It is noteworthy that effectiveness of the magnetic separation process not only determines the outcome of a diagnosis but also directly influences its accuracy as well as sensing time involved. Therefore, understanding the factors that simultaneously influence the efficiency of both magnetic separation process and target detection is necessary. Moreover, for LGMS, there are several important considerations that should be taken into account in order to ensure its successful implementation. Hence, this review paper aims to provide an overview to relate all this crucial information by linking the magnetic separation theory to biomedical diagnostic applications.
Keywords: low gradient magnetic separation, high gradient magnetic separation, magnetophoresis, magnetic particles, biomedical diagnostic, disease detection
1. Introduction
Fast, selective and accurate detection of diseases is critical in clinical diagnosis as it allows physicians to provide more precise and preliminary medical assessments to patients. However, biological samples are usually exceptionally complex due to the presence of multiple components. In order to perform disease diagnosis, it is essential to isolate the specific target entity (which is the infectious agent to be detected) from the complex raw samples. This sample preparation step is needed prior to analysis in order to speed up the screening process [1] as well as to enhance the detection limit [2].
Separation of targeted entity for diagnostic purpose is possible through the application of magnetophoretic force. The idea implemented here is to magnetically isolate the target entity, either those with or without intrinsic magnetic responsive characteristic (target entity without magnetic property must be magnetically labelled with magnetic particles) [3], from a complex mixture by the application of an external magnetic field (figure 1). Owing to the presence of magnetic field gradient, magnetic materials will be magnetically aligned and driven to the region with the highest magnetic field strength by magnetophoretic force [4]. This phenomenon is known as magnetophoresis which involves the motion of magnetic particles relative to their non-magnetic surrounding medium under a non-homogeneous magnetic field [5]. The magnetically trapped and concentrated samples can then be used for the subsequent routine analysis of target identification. This technique, commonly known as ‘magnetic separation’ (MS), has gained popularity in the preparation of samples for diagnostic purpose. There are many advantages offered by the utilization of this technique. First and foremost, physical contact between the magnetic source and the biological samples can be omitted throughout the entire separation process which in turn makes MS to be biologically non-invasive and does not exert a detrimental effect on the biological components [6]. In addition, MS technique is also known to be high-throughput, low-cost [7] and less energy intensive when a permanent magnet is employed as magnetic source [8].
Figure 1.
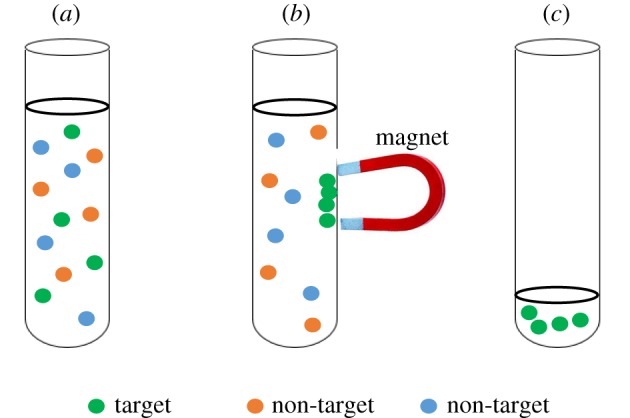
(a) Sample with a mixture of various components containing both target and non-target entities, (b) isolation of target entities using magnetic field, (c) concentrated target after removal of the non-targets. Note that the targets must possess intrinsic magnetic responsiveness, or have been pre-labelled with magnetic responsive particles.
Owing to the benefits offered by MS technique, a great deal of effort has been allocated in the design of different types of magnetic separator, which allow the MS process to occur either in a batch or continuous manner [9]. In addition, MS technique is feasible in wide-ranging length scales as it is not only workable in a laboratory test tube but also displays excellent performance in miniaturized devices [10,11]. Nam and co-workers employed microfluidic device with two outlets to separate late stage malaria-infected red blood cells (RBCs) which possessed relatively higher magnetic susceptibility in comparison with healthy RBCs [12]. Pamme & Wilhelm [13] demonstrated continuous MS of magnetically labelled cancer cells in a microfluidic separator with multiple outlets leading to the fractionation of the cancer cell–magnetic particle complexes according to their magnetic dipole moment. In addition, Chen and co-workers filled up their magnetic separation chamber with irregular-shaped iron particles with diameter ranging from 25 to 75 µm, in order to generate highly localized and strong magnetic field gradient upon exposure to an external magnetic field to allow the effective separation of HIV virions that had been labelled with magnetic particles [14].
In spite of all the sophisticated applications mentioned earlier, MS itself is a subject of research with many open-ended questions yet to be answered. Particularly, MS technique can be further classified into high gradient magnetic separation (HGMS) and low gradient magnetic separation (LGMS) according to the magnitude of magnetic field gradient employed in the separation process. In comparison with HGMS, the design rules for LGMS are ill defined [15], and hence, more theoretical and experimental works must be dedicated to its development in order to realize the true potential of LGMS technique for biomedical diagnostic as well as in other applications. Moreover, the underlying mechanism of magnetophoresis also determines the LGMS rate and the effectiveness of this separation method [4]. Concurrently, the time consumed to separate target entity from a complex sample is one of the key factors that decides the duration of the diagnostic process. Therefore, a thorough understanding of the theoretical background of the LGMS process is imperative in the design of magnetic separator used in a biomedical diagnostic application. Other factors, such as magnetic particle size, magnetic particle concentration and magnetization, also play inevitable roles in determining the effectiveness of LGMS for target detection. In this regard, it is expected that there will be several trade-offs originating from the complex interplay of all these factors and they are yet to be revealed and explored in depth.
Hence, in this paper, it is our goal (i) to review the utilization of MS technique in sample preparation for diagnosis of diseases, (ii) to highlight the feasibility of LGMS (less than 100 T m−1) in biomedical diagnostic application, (iii) to discuss important factors that simultaneously affect the effectiveness of LGMS and target detection, and (iv) to discuss some criteria that are worth considering for future implementation of LGMS technique in biomedical diagnostic application.
2. Magnetic separation in isolation of target entity for diagnostic purpose
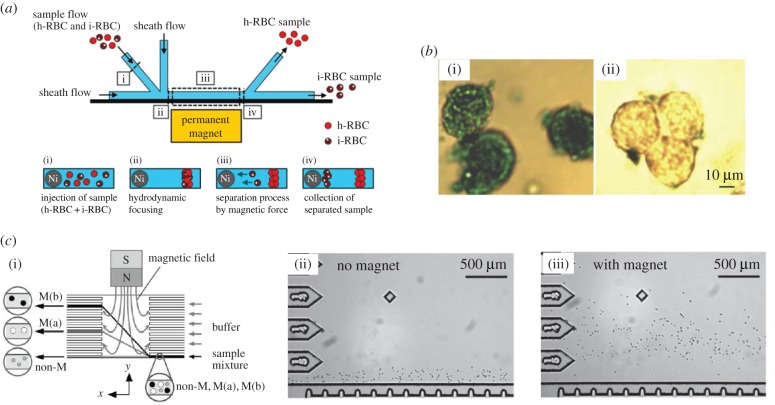
As mentioned in the Introduction, MS technique has been used in sample preparation for disease diagnosis. One of the active researches in this area is the magnetic isolation of malaria-infected red blood cells (i-RBCs) for clinical diagnosis of malaria. Malaria, a life-threatening disease, is triggered by Plasmodium parasites [16]. The malaria parasites convert haemoglobin in healthy cells to haemozoin [17] which contains iron(III) (Fe3+) that exhibits a relatively stronger magnetic susceptibility compared with iron(II) (Fe2+) in haemoglobin [12]. Owing to the presence of iron(III), i-RBCs display stronger paramagnetic effect than healthy red blood cells (h-RBCs) [12]. Thus, by taking advantage offered by the difference of their intrinsic magnetic susceptible property, fractionation of i-RBCs and h-RBCs can be achieved through the application of external magnetic field (figure 2a) [12]. This technique serves as an alternative to the conventional isolation method that relies on the density of i-RBCs [19]. By applying a similar concept, the separation of deoxyhemoglobin RBCs (paramagnetic) from white blood cells (diamagnetic) also can be conducted by MS technique [20]. In this regard, the blood cell sorting can be achieved solely depending on the difference in intrinsic magnetic properties. For instance, the absence of bound oxygen to Fe atom causes deoxygenated RBCs to possess relatively higher magnetic susceptibility in comparison with that of oxygenated RBCs [3]. In fact, the feasibility of MS technique in the isolation of RBCs from whole blood samples has been discussed way back in the 1970s by Melville et al. [21] in their work published in Nature.
Figure 2.
(a) Schematic diagram showing the principle of i-RBC separation from a blood sample using a microfluidic device containing ferromagnetic wire. An external magnetic field was applied normal to the ferromagnetic nickel (Ni) wire in order to create high magnetic field gradient. Paramagnetic i-RBCs were drawn towards the Ni wire and isolated out from the h-RBCs, reprinted with permission from [12], copyright © 2013 American Chemical Society. (b) SK-BR3 cells incubated (i) with IO that had been conjugated with Ab (dark blue), and (ii) with carboxyl-functionalized IO that had not been conjugated with Ab (less blue) (note: Prussian blue staining was implemented to confirm the presence of Fe ions on the cell surface), reprinted with permission from [18], copyright 2011 Elsevier. (c)(i) Schematic diagram illustrating a free-flow MS technique at which laminar flow was applied in x-direction, whereas magnetic field was applied in y-direction. Note that the non-magnetic material moved along the laminar flow, while those with magnetic responsiveness were deflected from the direction of laminar flow. This separation technique was used to separate HeLa cells in which HeLa cells that had been incubated with magnetic particles (ii) flowing straight through the channel when there was no applied magnetic field and (iii) deflected in their flow direction upon application of magnetic field, reprinted with permission from [13], copyright © 2006 Royal Society of Chemistry.
However, it should be highlighted that most biological compounds lack inherent magnetic properties in most situations. Thus, it is necessary to artificially enhance the magnetic property of the target entity so that it can be separated from the other non-magnetic components under an externally applied magnetic field. This intention can be accomplished by labelling the target entity with magnetic particles before subjecting to MS. Magnetite (Fe3O4), maghemite (γ-Fe2O3) and commercially available Dynabeads are some of the commonly used magnetic particles for this purpose. This approach has been used in the detection of various biological components such as bacteria, viruses and parasites [10,14,22–25]. In addition, early diagnosis of cancer can also be achieved by magnetic separation of circulating tumour cells (CTCs) that had been specifically labelled with magnetic particles [18].
Owing to the reason stated above, surface modification of the magnetic particles with specific molecules that only bind to the target entity, but not on other non-target components, is the main prerequisite. For instance, Xu and co-workers [18] surface modified their iron oxide nanoparticles with a water-soluble polymer which was then followed by bio-conjugation with anti-HER2 antibody. The as-prepared iron oxide-antibody conjugates (IO-Ab) were then incubated in a fresh human whole blood that was spiked with SK-BR3 (a human breast cancer cell line that is HER2-positive). The cells labelled with IO-Ab were then magnetically isolated by using a SuperMag™ separator before analysis on the captured cells was conducted. It was reported that IO-Ab were preferentially labelled to SK-BR3 cells in comparison with normal cells found in the blood sample. As illustrated in figure 2b, it can be seen that the amount of IO-Ab attached onto the cell is overwhelming (figure 2b(i)) in comparison to that of IO without Ab functionalization (figure 2b(ii)). This result has directly confirmed that the antibody (attached on magnetic particles) plays an essential role in the specific binding of magnetic particles onto the targets. Likewise, Chen and co-workers have also shown the high selectivity of their antibody-conjugated magnetic beads towards Salmonella enterica over other species of bacteria (Escherichia coli, Shigella spp., Staphylococcus aureus and spirillumcholera) due to the specific antibody–antigen recognition feature [22]. In other words, magnetic particles that serve as magnetic carriers in the MS process can be made more versatile to label different targets based on the different strategies/compounds used to functionalize their surface.
Besides attaching onto the target cell surface, magnetic particles might be further internalized into the cell body. For instance, Pamme & Wilhelm [13] found the internalization of γ-Fe2O3 nanoparticles with diameter less than 10 nm into the target cells (i.e. mouse macrophages and human ovarian cancer (HeLa) cells) in large quantity can later be used to facilitate the cell sorting via MS technique. Here, the MS process was performed in continuous mode within a customized microfluidic chip where the magnetic particle-labelled cells were deflected from their original pathway and driven towards the region with the strongest magnetic field gradient (figure 2c).
More interestingly, diagnosis can also be performed by measuring the amount of magnetic particles that are still remained in the sample solution after the MS process. This approach has been demonstrated in a recent work published by Chen and co-workers in the detection of Salmonella enterica, which is a food pathogen that affect human gastrointestinal tract. In this work, antibody-conjugated magnetic particles of size 250 nm (MP250) and 30 nm (MP30) were first dispersed into the biological sample in order to promote the formation of MP30-target-MP250 complex. Upon the application of an external magnetic field, the magnetophoretic separation rates of MP250 and MP30-target-MP250 complex are considerably higher compared with that of MP30 owing to their greater magnetic dipole moment. Accordingly, the amount of the target entity in the initial biological sample can be inferred from the transverse relaxation time of the water molecules (T2 value) surrounding the MP30 which remained within the biological sample after the MS process [22]. The positive outcomes obtained from this novel approach definitely encourage more exciting exploration in this research area in the future.
The MS process can also be conducted in a negative selection manner. In this regard, the undesired entities are labelled with magnetic particles and thus being separated during the MS process. On the contrary, the target entity, which is unlabelled and magnetically irresponsive, is still suspended in the biological sample after the MS process [26]. This approach is appealing as the target entity remains unaltered throughout the MS process. However, it might suffer low target yield due to non-specific loss of the target entity or inadequate removal of undesired biological components [27].
One of the factors that determines the successful implementation of all aforementioned MS applications is the magnetic separator design. Hence, in the next section, design and principle of MS technique are thoroughly discussed.
3. Strategies of magnetic separator design
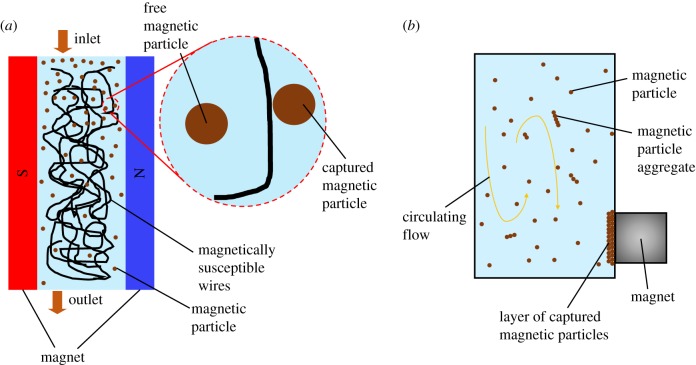
Basically, MS technique can be classified into two distinct classes, namely HGMS and LGMS, according to the magnitude of magnetic field gradient employed in the separation process. In fact, HGMS has widely been implemented in the isolation of magnetically responsive particles from their suspension [28]. To date, HGMS has been proved to be feasible in mineral processing [29–31], waste removal [32–34], biotechnology [35], as well as drug delivery [36,37]. In general, the operation of HGMS involves a column which is filled with randomly entangled magnetically susceptible wires and surrounded by an electromagnet (figure 3a) [38]. By turning on the electromagnet around/besides the column, the magnetically susceptible wires tend to dehomogenize the magnetic field such that high magnetic field gradient (more than 100 T m−1) is produced in the vicinity of the magnetizable wires. As the suspension of magnetic particles is channelled through the column, the magnetic particles are captured on the magnetically susceptible wires and hence being isolated from the initial solution. In contrast, LGMS involves only a simple set-up in which the separation of magnetic materials from the solution is performed typically by a hand held permanent magnet without any kind of packing. Under a non-homogeneous magnetic field produced by the permanent magnet, magnetic particles are driven to migrate towards the magnetic source by magnetophoretic force and therefore being isolated out of the solution (figure 3b). Because the magnitude of magnetic field gradient involved in this set-up is generally less than 100 T m−1, this type of MS technique is denoted as LGMS.
Figure 3.
Illustration of HGMS and LGMS processes. (a) The HGMS column is packed with randomly entangled magnetizable wires, which act as magnetic field ‘dehomogenizer’ to produce strong and localized magnetic field gradient near to it. Magnetic particles which are flowing through the column will experience an exceptionally strong magnetophoretic force in the vicinity of the magnetizable wires and be captured on them. (b) Illustration of LGMS process. Permanent magnet is used to create magnetic field gradient across the magnetic particle solution in which magnetic particles are going to be separated. The magnetic particles migrate towards the region with highest magnetic field gradient (which is usually the region adjacent to the magnet) and are separated from the solution. Particle aggregation (due to the magnetic dipole–dipole interaction) and continuous circulating flow (as a consequence of hydrodynamic effect) occur throughout the entire process and contribute to the distortion of the magnetophoretic pathway of magnetic particles.
3.1. Working principle of high gradient magnetic separation
Owing to the remarkable difference between magnetic susceptibility of magnetizable wires and that of suspending medium, the magnetic field within close range of the wires is significantly distorted which sequentially induces an intense magnetic field gradient in that region [28,39]. Magnetic field gradient as high as 1260 T m−1 can be created near the wires within the HGMS column when the electromagnet is switched on [29]. As magnetophoretic attraction force experienced by a magnetic particle is directly proportional to the magnetic field gradient (see equation (3.1)), the magnetic particle experience a huge magnetophoretic force as it passes through the region adjacent to the magnetizable wire [40]:
| 3.1 |
Here, Fm is magnetophoretic force exerted on single magnetic particle, r is radius of magnetic particle, μo is the permeability of free space, M is volumetric magnetization of magnetic particle and 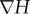 is magnetic field gradient. Such a gigantic magnetophoretic force is able to overcome viscous drag and Brownian fluctuation which oppose the magnetophoretic motion of magnetic particles. Finally, the magnetic particles will be captured on the wires and isolated from the solution.
is magnetic field gradient. Such a gigantic magnetophoretic force is able to overcome viscous drag and Brownian fluctuation which oppose the magnetophoretic motion of magnetic particles. Finally, the magnetic particles will be captured on the wires and isolated from the solution.
Even though HGMS has been well established and widely implemented in various applications, there are several drawbacks associated with the utilization of HGMS. First and foremost, the installation and operation cost of the HGMS is extremely high as huge amount of energy is required for the electromagnet to generate a magnetic field that is sufficiently intense to induce a successful collection of magnetic particles [32]. Apart from that, the highly complex and inhomogeneous magnetic field within the HGMS column prevents the further development of analytical model to accurately describe the HGMS process which in turn renders theoretical study of the given process exceptionally strenuous [28]. Last but not least, magnetic particles which have been attached on the wires are difficult to be removed due to the complicated set-up of the column. The retention of magnetic particles on the wires will greatly reduce the separation efficiency of the HGMS column [41].
3.2. Working principle of low gradient magnetic separation
In order to overcome the disadvantages of HGMS described above, another MS scheme was introduced to induce the magnetophoretic collection of magnetic particles from suspension. This separation technique is able to perform the magnetic particle collection without the intricate set-up of magnetizable wires within the HGMS column. On the contrary, the only part required is a permanent magnet which produces non-uniform magnetic field all over the magnetic particle solution subjected to MS [42]. Owing to the non-homogeneity of the magnetic field throughout the magnetic particle solution, magnetophoretic motion is initiated in which the magnetic particles migrate towards the region where the magnetic field gradient is the highest (figure 3b) [43]. In such a way, magnetic particles are removed from the suspending medium and the separation is accomplished. Because magnetic field strength declines rapidly with the displacement from the magnet, magnetic field gradient across the entire volume of magnetic particle solution subjected to separation is usually lower than 100 T m−1 even though a strong neodymium ferrite boron magnet (abbreviated as NdFeB magnet and is a permanent magnet with extremely strong remanent magnetization) is employed [44,45].
Yet, one of the most pronounced disadvantages of LGMS is originated from the weak magnetophoretic force (as a consequence of low magnetic field gradient) encountered by the magnetic particles to be separated [15]. There are two factors which oppose the deterministic magnetophoretic migration of magnetic particles, namely viscous drag and thermal motion [43]. Viscous drag is the resistance encountered by an object that performs a relative motion with respect to the surrounding fluid. The magnitude of viscous drag can be evaluated by the Stokes equation [46]:
| 3.2 |
where Fd is viscous drag experienced by magnetic particle, η is viscosity of the suspending fluid and ν is magnetophoretic velocity of magnetic particle relative to fluid. Besides, thermal motion is random fluctuation of magnetic particles that disrupts the deterministic pathway of magnetic particles along the magnetic field gradient. The intensity of thermal fluctuation can be reflected by the magnitude of diffusion coefficient D of the given particle in the suspension as demonstrated in the Stokes–Einstein equation [47]:
| 3.3 |
where k is Boltzmann constant and T is absolute temperature.
According to equations (3.1) and (3.2), magnetophoretic force Fm varies linearly with respect to r3 while the opposing viscous drag Fd is directly proportional to r. Hence, as the magnetic particle (and hence r) is small, viscous drag dominates the magnetophoretic force and thus the magnetic particles migrate slowly towards the magnet pole. Consequently, more time is required to execute a successful separation. Apart from that, the thermal fluctuation of magnetic particle is more intense as its size is small (see equation (3.3)). This motion randomizes the magnetophoretic pathway of magnetic particle and this has caused the difficulty in controlling the motion of magnetic particles. The relevant dimensionless numbers include Reynolds number (Re) and Péclet number (Pe) which characterize the ratio of inertia force (magnetophoretic force in this case) to viscous drag force and diffusive force, respectively [15]. According to this dimensionless analysis, magnetophoretic force is more dominant than viscous drag and diffusion in defining the motion of magnetic nanoparticle providing that Re and Pe are larger than unity. Consequently, these criteria must be fulfilled prior to the more comprehensive design of the given LGMS process.
Despite the arguments mentioned above, LGMS is still a feasible separation technique in the removal of magnetic particles from their suspension. Yavuz et al. [42] reported that magnetic nanoparticles as tiny as 12 nm can be collected within a reasonable timescale by applying low magnetic field gradient. This phenomenon is mainly due to the self-aggregation of magnetic particles under an external magnetic field [48]. For instance, when a magnetic particle is exposed to an external magnetic field, it will be magnetized and acquire net magnetic dipole moment. In order to lower the magnetic interaction potential energy, magnetic particles with net magnetic dipole moment tend to align themselves in the direction of the magnetic field lines and being driven towards the regions with higher magnetic field [49]. In the course of the magnetophoretic migration of magnetic particles, some particles will come close to one another. Because each magnetic particle with net magnetic dipole moment is behaving as a ‘small’ magnet, magnetic particles which are sufficiently near will experience magnetic attraction among each other and finally ‘stick’ together [50]. Accordingly, magnetic particles tend to collapse with one another forming larger aggregate [51]. Owing to their higher magnetic volume, magnetic particle aggregate acquires much larger magnetophoretic force which is able to overcome the aforementioned opposing forces (i.e. viscous drag force and thermal fluctuation) and move collectively towards the magnetic source with much magnetophoretic velocity [42]. Hence, by the aid of aggregation, magnetic particles can be separated by low magnetic field gradient generated by a hand-held permanent magnet within a reasonable timescale.
3.3. Application of high and low gradient magnetic separations in biomedical diagnostic
Application of both HGMS and LGMS in biomedical diagnostic has been proved to be feasible. Several published works in this area of research are summarized in table 1. The concept originated from HGMS has been used to separate E. coli bacteria from whole blood sample [10] as well as to concentrate and purify HIV viral product in human plasma [14]. In order to generate localized and strong magnetic field gradient which enables rapid and efficient separation of magnetically labelled E. coli bacteria, magnetizable substance is employed to dehomogenize the magnetic field created by the magnetic source. In the work reported by Xia and co-workers, magnetic field gradient as high as 290 T m−1 can be generated by NiFe microcomb that is acting as magnetic field dehomogenizer (table 1) [10]. This recorded magnetic field gradient value is much higher than the one that can be achieved within a microfluidic channel (approx. 20 T m−1) in the absence of NiFe microcomb. In the purification of human immunodeficiency virus type 1 virions, irregular iron oxide spheres were placed within the microfluidic separator to act as magnetic field gradient concentrator. Under this configuration, the solution containing viral product can be concentrated within 20 min and yields a solution that is 40–80 times more concentrated in comparison with the initial solution [14]. On the other hand, Pivetal and co-workers designed a micro-magnet array which can develop magnetic field gradient as high as 2.5 × 105 T m−1 to position and immobilize magnetic particle-labelled E. coli (row 11 in table 1) [57].
Table 1.
Details of magnetic particles (MPs) or cells with intrinsic magnetic property used in biomedical diagnosis based on magnetic separation concept.
| magnetic particlesa, or cells with intrinsic magnetic responsiveness | size of MPs (diameter) | magnetic properties | functionalizing layer | concentration of MPs | types of magnetic separator (batch/continuous) | magnetic field strength | magnetic field gradient | application in biomedical diagnostic | ref. |
|---|---|---|---|---|---|---|---|---|---|
| deoxygenated RBCs | 7.7 µm | Δχb = 0.265 × 10−6 | — | not counted | batch | mean = 1.40 T | mean = 131 T m−1 | determine magnetophoretic mobility of the cells | [52] |
| methemoglobin RBCs | Δχb = 0.301 × 10−6 | 0.5 × 106 cells ml−1 | |||||||
| γ-Fe2O3 | 8.4 nm | 3.1 × 105 A m−1c | carboxyl groups | 1015 particles ml−1 | continuous | 0.4 T | 50 T m−1 | sorting of mouse macrophages and human ovarian cancer cells (HeLa cells) | [13] |
| superparamagnetic green fluorescent bead (43% iron oxide) | 1.6 µm | n.a. | streptavidin | 3.1 × 109 beads ml−1 | continuous | 0.048 Td | 290 T m−1d | separation of living E. coli bacteria | [10] |
| parasite-infected erythrocytes | n.a. | n.a. | — | n.a. | continuous | 1.426 T | 804 T m−1 | concentrate erythrocytes parasitized by human malaria species | [53] |
| Fe3O4 | 27 ± 5 nm | n.a. | oleic acid + amphiphilic polymer + antibodies against human epithelial growth factor receptor 2 (anti-HER2 or anti-HER2/neu) | 2 mg Fe ml−1 | batch | n.a. | 100 T m–1 | separation of tumour cells from fresh whole blood | [18] |
| fluorescent–magnetic bifunctional nanoparticles | 211 nm | 8.18 emu g−1c | monoclonal antibody (mAb) | 10 × 1010 nanobioprobes ml−1 | batch | 0.4 T | 0–100 T m−1e | detection and extraction of leukaemia cells and prostate cancer cells | [54] |
| γ-Fe2O3 | 8.7 nm | 6.6 × 10−14 A m2f (for 1 pg of irong) | carboxylate functional groupsh | 1 mM (extracellular iron concentration) | continuous | 0.15– 0.26 T (from middle of the separation chamber to surface of the magnet) | 30–80 T m−1 | sorting of monocytes and macrophages based on amount of MPs internalize into the cells | [55] |
| deoxygenated RBCs | 6–8 µm | n.a. | — | 5 × 107 cells ml−1 | semi | 0–1.68 T | 1750 T m−1 | RBC debulking | [56] |
| methemoglobin RBCs | n.a. | ||||||||
| Fe3O4 | 30 nm | n.a. | streptavidin + antibodies against E. coli | 40 µg ml−1 for 30 nm MPs | batch | 1.15–1.65 T (mean = 1.35 T) | 0–300 T m−1 (mean = 90 T m−1) | isolate E. coli O157:H7 cells | [1] |
| 180 nm | carboxyl functional groups + streptavidin + antibodies against E. coli | 100 µg ml−1 for 180 nm MPs | |||||||
| magnetic beads | 30 nm, 250 nm | n.a. | capture antibody (Ab1), detection antibody (Ab2) | 0.2 g l−1i | batch | 0.01 T | n.a. | detection of S. enterica | [22] |
| microbeads | 50 nm | n.a. | streptavidinj | n.a. | batch | ∼ 0.12 to∼0.29 T | ∼ 2.89 × 104–2.5 × 10 T m−1 | selective arraying of E. coli bacteria by micro-magnet | [57] |
aMagnetic particles that were used to label on target entity which is originally non-magnetically responsive.
bΔχ = net magnetic susceptibility of the RBC in aqueous suspension=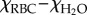 .
.
cSaturation magnetization.
dThe values of magnetic field strength and magnetic field gradient provided were the maximum (which occurs at the location closest to the magnet within the whole domain of flowing channel) under the influence of NiFe microcomb.
eSimulation based on the geometry configuration provided by using COMSOL Multiphysics (see electronic supplementary material for the simulation result).
fMagnetic moment.
g1 pg = 8 × 105 nanoparticles.
hThe carboxylate functional groups here are to stabilize the magnetic particles in aqueous medium.
iThe concentration of magnetic beads is estimated in the solution where magnetic separation occurs.
jDuring immunomagnetic labelling, the streptavidin-coated microbeads were mixed with bacterial cells that already pre-treated with biotinylated anti E. coli antibodies.
Meanwhile, there are plenty of biomedical diagnostic applications which have employed the concept of LGMS in their operation unwittingly and resulted in positive outcomes. For instance, in the continuous sorting of monocytes and macrophages by magnetic nanoparticles, permanent magnet (NdFeB) was used to create the magnetic field throughout the microfluidic chip which generates magnetic field gradient within the range of 30–80 T m−1 (row 7 in table 1) [55]. Despite the low magnetic field gradient (less than 100 T m−1), excellent purification (more than 88%) and high-throughput (10–100 cells/s) could be accomplished in this magnetophoresis set-up. Apart from that, in the separation of cancer cells from fresh whole blood as reported by Xu and co-workers, SuperMag™ separator (Ocean NanoTech) was used to trigger the magnetophoretic motion of the magnetically labelled tumour cells (row 5 in table 1) [18]. Enrichment factor of cancer cell to normal cell as high as 1 : 10 000 000 has been achieved under the low magnetic field gradient (100 T m−1) created by permanent magnets within the magnetic separator. Furthermore, in the work done by Song and co-workers, magnetic scaffold was used to create magnetic field gradient below 100 T m−1 for the isolation and detection of particular target cell from analytical samples (see table 1 for detail) [54]. For instance, the magnetophoretic capture efficiencies of leukaemia cells and prostate cancer cells under the given experimental condition were reported as 96% and 97%, respectively. It should be noted that the magnetic field gradient can be high at the location near to the surface of the magnetic source and decay with the distance away from the magnetic source. As an example, Lin and co-workers designed a magnetic bioseparator by using two NdFeB magnet columns [1]. A small space was left between the two magnet columns and being employed as the MS zone. Even though the magnetic field gradient in the separation zone can be as high as approximately 300 T m−1 at the surface of the magnet columns, it decays to 0 T m−1 at the middle of the separation zone. This phenomenon has caused the average magnetic field gradient within the separation zone to be approximately 90 T m−1 (table 1). By using this set-up, it only took around 2 min to separate the E. coli that had been labelled with 180 nm diameter magnetic particles. Hence, LGMS appears as a beneficial and effective tool in separation of particular cells, bacteria or viruses for diagnostic purpose.
Nevertheless, in order to design and optimize the diagnosis process by incorporating LGMS technique, it is extremely vital to understand some of the control parameters that influence the performance of the process in the first place. This will be discussed in the next section.
4. Effectiveness of low gradient magnetic separation in biomedical diagnostic application
4.1. Factors that influence low gradient magnetic separation rate
Size of magnetic particles plays a very important role in controlling the LGMS kinetics. As depicted in equation (3.1), the magnitude of Fm experienced by a magnetic particle is directly proportional to r3. Here, force balance between Fm and Fd reveals that the magnetophoretic velocity, which determines the separation time, varies linearly with respect to r2. Thus, in an LGMS process, particles with larger size are separated first before the small ones when they are subjected to the same magnetic field [58].
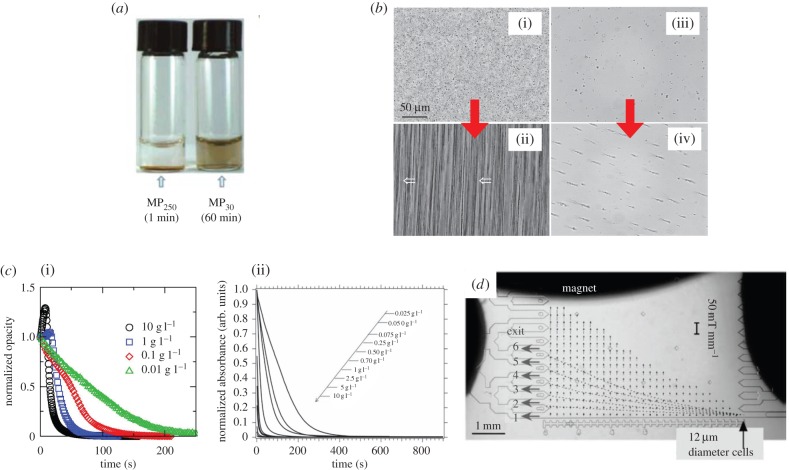
There exists plenty of literature in relating the MS efficiency to the magnetic particle size. Lately, Lin and co-workers showed that E. coli O157:H7 which had been labelled with 180 nm magnetic particles can be successfully isolated in less than 2 min within a magnetic bioseparator of mean magnetic field gradient approximately 90 T m−1 [1]. Meanwhile, when the same experiment was conducted by using magnetic particles of size 30 nm, the optimal MS duration was notably increased to 60 min, which is about 30 times slower in comparison with the MS process that employed 180 nm magnetic particles. Thus, magnetic particles of larger size are preferential in fast detection/screening applications. By reversing this concept, Chen and co-workers used a mixture of magnetic particles of two different sizes (i.e. 30 nm (MP30) and 250 nm (MP250)) in bacteria and virus detection [22]. Their preliminary study showed that MP250 was successfully precipitated in 1 min upon subjecting to a magnetic field of 0.01 T; while MP30 remains suspended even after prolonging the MS time to 1 h under the same magnetic field (figure 4a). By employing the advantage offered by such a huge distinction in MS time, the concentration of the target entity within a biological sample can be measured by dispersing the magnetic particle mixture into the given solution. Under this scenario, the interaction between antibody (from magnetic particles) and antigen (from targets) would cause the formation of large MP250-target-MP30 complex. The target entities (bacteria or virus) which were embedded in MP30-target-MP250 complex can then be rapidly removed upon exposure to a weak magnetic field due to the gigantic size of MP250-target-MP30 complex in comparison with MP30. The unreacted MP30 which were still suspended in the solution after the MS will change the T2 value of the surrounding water molecules. The change of T2 values was then correlated to the concentration of the target (bacteria or virus) in the initial biological sample. Results from the studies mentioned above have highlighted the importance of selecting magnetic particles of suitable size for successful employment of MS technique in biomedical diagnosing.
Figure 4.
(a) MP250 was separated in 1 min by using magnetic field of 0.01 T. Meanwhile, MP30 remains well dispersed even after 60 min subjected to the same magnetic field, reprinted with permission from [22], copyright 2015 American Chemical Society. (b) Optical micrographs of solution with 1 g ml−1 of 0.41 µm superparamagnetic microsphere (Estapor® M1–030/40) are demonstrated in (i) and (ii). Figure (i) was observed under the absence of magnetic field, while figure (ii) is the image taken after 120 s of magnetic field exposure. The alignment of superparamagnetic microsphere along the magnetic field was observed in (ii) and this is an indication of magnetic particle aggregation under low gradient magnetic field, reprinted with permission from [48], copyright © 2008 American Chemical Society. Optical micrograph of 425 nm magnetic nanoparticles in deionized water (iii) before the application of magnetic field and (iv) a few seconds after the exposure to magnetic field generated by NdFeB magnet, reprinted with permission from [45], copyright © 2008 American Institute of Physics Publishing LLC. Chain formation (particle aggregation) was observed in (iv) and this phenomenon has proven the occurrence of cooperative magnetophoresis. (c) The magnetophoresis profile for (i) Estapor® M1–030/40 particles, reprinted with permission from [48], copyright © 2008 American Chemical Society, and (ii) 30 nm bare iron oxide magnetic nanoparticles, reprinted with permission from [58], copyright © 2014 American Chemical Society. Both (i) and (ii) show that the magnetophoresis process is accelerated as magnetic particle solution with higher concentration is employed. The variation of magnetophoresis rate with the concentration of the magnetic particle solution indicates the significance of particle aggregation throughout the magnetophoresis. (d) Illustration of magnetic separation chamber used for the fractionation of mouse macrophages or human ovarian cancer cells (HeLa cells) according to the cell magnetic moment, reprinted with permission from [13], copyright © 2006 Royal Society of Chemistry.
In addition to particle size, the separation kinetic of MS is also strongly dependent on the concentration of the magnetic particles. This phenomenon is attributed to the cooperative nature of the magnetic particle system in which magnetic particles tend to aggregate and move collectively towards the magnetic source upon exposure to an external magnetic field [50,51]. Particle aggregation is initiated by the magnetic interaction between magnetic dipole moment possessed by each magnetic particle. As suggested by De Las Cuevas and co-workers, the significance of magnetic interaction between two magnetic particles can be estimated by magnetic Bjerrum length λB which is formulated as [48]
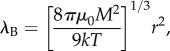 |
4.1 |
where μ0 is the permeability of free space. Particularly, magnetic interaction is dominant over the thermal random motion and particle aggregation is initiated as the separation distance between both magnetic particles is smaller than the magnetic Bjerrum length. Because the average distance between magnetic particles suspended in the solution decreases with the particle concentration of the solution, magnetic interaction between particles (which induces particle aggregation) is exceptionally substantial in the LGMS of highly concentrated magnetic particle solutions. In addition, according to thermodynamic self-assembly theory as proposed by Andreu et al. [59], the onset of particle aggregation in magnetic particle solution subjected to magnetophoresis can be predicted by aggregation parameter N* which is defined as
| 4.2 |
where ϕo is the volume fraction of magnetic particles in the given solution and Γ is the magnetic coupling parameter which is given by
| 4.3 |
Here, ms is the total magnetic dipole moment possessed by one magnetic particle at saturation and d is the diameter of magnetic particle. As N* is larger than unity, particle aggregation is initiated by the magnetic interaction between neighbouring magnetic particles [60]. Besides, the magnitude of N* is also representing the average number of particles within a particle aggregate in the cooperative regime. Both magnetic Bjerrum length λB [48] and aggregation parameter N* [59] analysis suggests that the particle aggregation is more significant at higher particle concentrations. The direct consequence of particle aggregation is the formation of larger-sized magnetic particle clusters with much stronger magnetic response in comparison to individual magnetic particles. In the work reported by De Las Cuevas et al. [48], they observed instantaneous alignment of Estapor M1–030/40 particles (the diameter of which is 0.41 µm) upon the application of an external magnetic field (figure 4b(i)(ii)). Meanwhile, Schaller et al. [45] also reported the similar result in their attempt to investigate the motion of magnetic particles under an external magnetic field (figure 4b(iii)(iv)).
Formation of particle aggregates, which possess larger size and more intense magnetic response, leads to accelerated MS such that magnetic particles can be segregated from the solution within a shorter timescale. Under this condition, magnetic particles interact along their magnetophoretic migration pathway; thus, this phenomenon is designated as cooperative magnetophoresis. In line with the same cooperative concept, MS is faster as the concentration of magnetic particles increased (figure 4c) [4,48,58]. Therefore, this principle can be used in biomedical diagnostic applications which employ MS technique to isolate a target entity. Even though the separation of the target entity by external magnetic field can be accelerated by labelling the target entity with larger magnetic particles, the utilization of larger magnetic particles offers some drawbacks. First and foremost, due to the fact that the ratio of surface area to volume is reducing with the increase in magnetic particle size, the available surface sites for labelling become fewer. Furthermore, the relative size between magnetic particle and target entity also has a significant effect on the sensitivity in certain biomedical detection which exerts extra constraint on the selection of the magnetic particle size (will be discussed in more detail in the next section) [61]. Henceforth, cooperative nature of magnetic particles throughout the LGMS process provides an alternative route to optimize the separation rate of magnetically labelled target entity in biomedical diagnostic application. By increasing the concentration of magnetic particles supplied to the sample filled with the target entity, the magnetophoretic separation rate of magnetically labelled target entity can be greatly enhanced upon cooperative movement of the aggregated magnetically responsive species.
In addition to the size and concentration effects, magnetization (which is the total magnetic dipole moment per unit mass or volume [62]) of magnetic particles is also an important criterion which affects the magnetophoretic separation rate of magnetic particles in any related biomedical diagnostic application. The linear correlation between magnetophoretic force experienced by magnetic particles and their magnetization has been demonstrated in equation (3.1). Magnetic particles with stronger magnetization (and hence more intense magnetic dipole moment) experience larger magnetophoretic force under the same external magnetic field and undergo more rapid MS. One related example which employs this principle is demonstrated in the work reported by Pamme & Wilhelm [13], in which target cells were fractionated according to their magnetic loading. The target cells were firstly incubated with nano-sized magnetic particles in order to allow the internalization of magnetic particles into the cells. Cells that were loaded with more magnetic particles possessed higher magnetic moment; while those loaded with fewer magnetic particles exhibited lower magnetic moment. When the mixture of cells (with different magnetic moments) was driven to flow through a microfluidic chamber where the magnetic field gradient was applied in the direction perpendicularly to the flow velocity, the cells start to deflect from their original flow direction under the influence of magnetophoretic force. Cells with higher magnetic moment were deflected to a greater extent towards the location where the external magnet was applied and vice versa (figure 4d). By this way, mixture of cells is fractionated according to their magnetic loading due to their different MS responsiveness. Similarly, this principle has also been successfully applied in the sorting of monocytes and macrophages based on the amount of magnetic particles that have been internalized into the cells [55].
4.2. Factors that simultaneously affect the performance of target detection
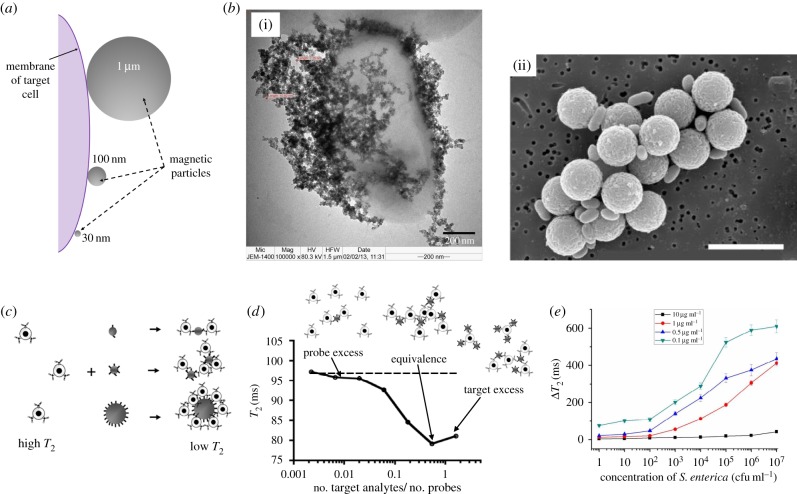
Apart from affecting the separation rate, particle size is also important in determining the efficiency of target detection. Generally, micrometre-sized (1–10 µm) and nanometre-sized (50–100 nm) magnetic particles are popular for the isolation of target entities from complex biological samples for diagnostic purpose [14]. Even though magnetic particles of larger size are preferable in terms of their shorter LGMS duration (as discussed in §4.1), they pose several shortcomings as compared with the nano-sized counterparts. First, the surface area to volume ratio of micrometre-sized particles is lower compared with nano-sized particles which in turn causes the reduction in the binding capacity [18] and capture efficiency [63] for magnetic particles with larger size. Second, gravitational sedimentation is the common issue encountered by large and dense particles [14,27], hence, continuous agitation is needed to keep them dispersed. On the contrary, small particles with nano-sized dimension are usually dispersed more uniformly within the suspension [1] and exhibit faster reaction kinetics [1,63]. Additionally, due to their less steric exclusion property, higher amount of nano-sized particles can be bound onto the surface of a target cell in comparison to that of micrometre-sized particles (as illustrated in figure 5a) [18]. A clear comparison between target cells that were attached with magnetic particles of different sizes is given in figure 5b. Figure 5b(i) shows immobilization of nano-sized magnetic particles on the surface of E. coli [64]; while figure 5b(ii) shows E. coli O157:H7 cells which were bound to antibody-coated magnetic beads (Dynabeads). In addition, Pamme & Wilhelm [13] predicted that the number of magnetic particles bound to the surface of a target cell can be significantly reduced from 2 × 106 to 102 and 100 when the diameter of the magnetic particles is enlarged from 9 nm to 400 nm and 2.8 µm, respectively. This prediction implies that smaller magnetic particles are more appropriate for use in the analysis of high-density cell surface markers; while for detection of rare cell surface markers, larger magnetic particles are more appropriate. Moreover, using smaller particles approaching the size of surface markers, the resolution of diagnostic process could be improved significantly.
Figure 5.
(a) A pictorial drawing (not drawn to scale) showing membrane of a target cell labelled with magnetic particles of various sizes (more nano-sized magnetic particles can be attached on the cell surface than the micrometre-sized counterparts due to less steric exclusion) [18]. (b)(i) Transmission electron microscopy image showing E. coli immobilized with 3-mercaptophenylboronic acid/1-decanethiol-modified magnetic nanoparticles, reprinted with permission from [64], copyright © 2013 Multidisciplinary Digital Publishing Institute; (ii) electron micrograph (scale bar, 5.14 µm) of E. coli O157:H7 cells captured by Dynabeads® M-280 Streptavidin (Dynal, Lake Success, NY, USA) coated with biotinylated antibodies, reprinted with permission from [65], copyright © 2002 John Wiley and Sons. (c) Solution with well-dispersed magnetic particles provides high T2 value. Once the target cell was mixed with the magnetic particle solution, interaction between antibody (from the functionalization layer of magnetic particles) and antigen (from the target cells) would induce the aggregation between magnetic particles and cells which results in large magnetic particles–cells complex. Owing to the presence of the larger-sized complex, T2 value of the solution becomes lower, reprinted with permission from [61], copyright © 2009 American Chemical Society. (d) Variation of T2 value with the number ratio of target cells to magnetic particle probes, reprinted with permission from [61], copyright © 2009 American Chemical Society. (e) The effect of magnetic particle (MP30) concentration (0.1, 0.5, 1 and 10 µg ml−1) on the sensitivity of the detection process. It can be observed that the sensitivity (change of T2 value) of the detection method is decaying with the concentration of MP30, reprinted with permission from [22], copyright © 2015 American Chemical Society.
Apart from acting as one of the non-trivial parameters which govern the dynamical behaviour of cooperative LGMS (separation rate), concentration of magnetic particle solution also affects the performance of LGMS-aided biomedical diagnostic application in term of other aspects. After dispersing antibody-conjugated magnetic particles into the biological sample containing the target entity, the binding between antibody (from magnetic particles) and antigen (from the target entity) fosters the aggregation of magnetic particles in the solution which in turn changes the spin–spin relaxation time (T2) of the surrounding water protons. In general case, T2 value declines with the degree of magnetic particle aggregation as particle aggregates (with larger size in comparison to individual magnetic particles) tend to cause magnetic field distortion around them and diphase the spins of water protons which are diffusing through the region more significantly (figure 5c) [61]. Thereby, the concentration of the target entity in the initial solution can be inferred from the percentage change of T2 value measured by a relaxometer [66]. However, the degree of magnetic particle aggregation (and hence change of T2 value) is highly dependent on the number ratio of the target entity to magnetic particles. For instance, when magnetic particles with a diameter of 70 nm were employed as the probe to analyse the concentration of 8 nm entity (BSA-(Tag)6) which has a smaller size than the magnetic particle probe, T2 value tends to decrease with increasing concentration of target entity until a minimum (denoted as equivalence point) is reached (as shown in figure 5d). However, beyond the equivalence point, T2 value increases with target entity concentration as the target entities which are present in excess have saturated the binding sites of magnetic particles and prevent the further aggregation of magnetic particles [61]. This phenomenon is known as prozone effect. The intervention of prozone effect has distorted the anti-correlation between concentration of the target entity and T2 value when the number ratio of target entity to magnetic particles exceeds the equivalence point [66]. Thereby, in order to obtain reliable measurements on concentration of the target entity, it is necessary to disperse sufficient amount of magnetic particles into the given solution so that the number ratio of target entity to magnetic particles is well below the equivalence point.
Additionally, concentration of magnetic particles also affects the sensitivity of target detection by MS. This phenomenon is observed in a bacteria and virus detection method proposed which involved strong magnetic responsive MP250-target-MP30 complex and poor magnetic responsive unreacted MP30 [22]. Here, unreacted MP30 will still remain suspended after being subjected to an external magnetic field. Because concentration of MP30 dispersed in the solution has a strong effect on the T2 value, the percentage change of T2 value of the solution after the MS process can be used to infer the concentration of the target entity in the initial solution. However, the sensitivity of this bacteria or virus detection method is strongly dependent on the concentration of MP30 supplied in the initial solution (figure 5e). As MP30 have been oversupplied, the fraction of MP30 involved in the formation of MP250-target-MP30 complex is too low to provide noticeable change in T2 value of the solution after undergoing MS. On the contrary, when the concentration of MP30 in the initial solution is too low, the magnetic signal obtained from T2 value measurement is not sufficiently intense to provide reliable result which accurately reflects the concentration of the target in the initial solution. Furthermore, the linear relationship between the change of T2 value and the target concentration can only be observed in a particular range of target concentration [22]. Therefore, in order to have an accurate measurement of the target concentration by using MS method, concentration of the magnetic particles and the target must be carefully tuned to certain range which provide reliable feedback from the analysis.
Furthermore, for MS-based in vivo diagnosis, the size and concentration of magnetic particles employed must be considered carefully as their nanotoxicity is unclear. In this regard, Nel et al. [67] demonstrated that nano-sized magnetic particles are biologically toxic due to their large exposed surface area that facilitates harmful interaction with biological systems. For instance, Kunzmann and co-workers showed the decay of cell viability of human monocyte derived dendritic cells upon exposure to CSNPs (silica coated iron oxide nanoparticles) with size of 30 and 50 nm. On the contrary, this phenomenon is not observed throughout their exposure to larger CSNPs (70 and 120 nm) [68]. In parallel, the same authors also observed that micro-sized titanium oxide (TiO2) caused relatively higher DNA damage compared with their nano-sized counterpart due to their higher surface reactivity [69]. Hence, the toxicity dependency on magnetic particle size cannot be solely coming from the trade-off between total exposure surface area and surface reactivity of the given magnetic particles. In addition, concentration is also one of the major factors that influences bio-compatibility of a magnetic particle solution. This is because magnetic particle solution which is highly concentrated (beyond certain threshold concentration) was found to be toxic to living organism [70]. The toxicity is mainly due to the induction of oxidative stress and generation of reactive oxygen species which will subsequently injure or kill the cells. For a cell–particle mixture, cell viability decreases with increasing concentration of magnetic particles [71]. For instance, it was reported that the viability of PC12 cells diminishes as they are exposed to a higher concentration of iron oxide (Fe2O3) nanoparticles [72]. Hence, even though the utilization of highly concentrated magnetic particle solution is capable of increasing the MS rate in biomedical diagnostic application, there exists certain constraint for us to select magnetic particle solution with a suitable concentration in the design of biomedical diagnostic process. Apart from optimizing the MS rate by increasing the concentration of magnetic particles, it is also necessary to ensure the concentration falls within the range that is characterized by excellent sensitivity and biological harmlessness.
To summarize all the above discussion, figure 6 is presented to highlight the complex interplay between the particle size, particle concentration and magnetization, which determine the design of LGMS for biomedical diagnostic application.
Figure 6.
Schematic diagram demonstrating the relationship between the three major factors (particle size, particle concentration and magnetization) that influence the performance of LGMS-aided biomedical diagnosis. Image components reprinted with permission from [13], Copyright © 2006 Royal Society of Chemistry, [22] Copyright © 2015 American Chemical Society, [45] Copyright © 2008 American Institute of Physics and [48] Copyright © 2008 American Chemical Society.
5. Concluding remarks and future considerations
Over the past few decades, tremendous efforts have been dedicated to the development of MS technique for sample purification prior to the diagnosis of diseases. In this regard, understanding the underlying principles that govern the dynamical behaviour of MS is critical in the optimization of separation efficiency. Concurrently, along with the progressive growth in research works related to magnetophoresis of magnetic particles under low magnetic field gradient, more findings directly related to its working principle have been discovered and disclosed. In the near future, those findings definitely should be taken into consideration for any design of LGMS technique for biomedical diagnostic application.
First and foremost, magnetic particles employed to magnetically label the specified target entity must possess good colloidal stability. This prerequisite is needed to ensure the particles present in well-dispersed condition so that more effective mixing and tagging with target entity can be promoted. Apart from imparting good colloidal stability to the functionalized magnetic particles in the initial suspending medium, it is paramount to ensure their colloidal stability is not disrupted by different biological matrices (e.g. DPBS, RPMI-1640, human plasma) throughout the magnetic labelling process [18]. Generally, stability of magnetic particles can be strengthened by imparting electrostatic repulsion and steric hindrance to the given particles. However, it was reported that enhanced colloidal stability compromised the MS rate of magnetic particles under low magnetic field gradient [73]. Hence, the conflicting effect between colloidal stability and MS efficiency must be carefully assessed in the design of LGMS technique for biomedical diagnostic application.
As discussed in §3.2, several limitations exist in the isolation of tiny size magnetic particles by employing LGMS technique. In order to perform a successful LGMS, magnetophoretic force exerted on magnetic particles must be sufficiently intense to overcome viscous drag, thermal motion and gravitational pulling. To accomplish these requirements, there are several well-established rules of thumb which can provide a fast screening of the suitability of any selected magnetic particle system in a particular LGMS process. Here, dimensionless number analysis (i.e. Reynolds number, Péclet number and Froude number) serves as a beneficial tool in determining whether the LGMS of a particular system can be successfully conducted [15].
Another particle size-related criterion that also must be carefully addressed is the impact of particle-size distribution (PSD). As demonstrated in our previous work, magnetophoresis profile of an electrosterically stabilized magnetic nanoparticle suspension under LGMS is greatly influenced by PSD of the given particle system [58]. Preparation of highly monodisperse magnetic particle system with narrow PSD is essential in order to have a high-quality control of the LGMS process. Yet, the synthesis of monodisperse magnetic particles is technically challenging as aggregation of the monodisperse particles, which results in polydisperse magnetic particles, is possible [74]. In addition, the aggregation of magnetic particles is further promoted by the magnetic attraction due to the induced magnetic dipole moment possessed by each magnetic particle in the presence of an external magnetic field. For ferro- and ferri-magnetic particles, magnetic dipole moment exists even without the external magnetic field. Also, particle size is exceptionally vital in determining the bio-compatibility of the magnetic particle system (as discussed in §4.2), and tight control of self-aggregation throughout the diagnosis process is indispensable. Thus, it is not only essential to synthesize highly monodispersed magnetic particles, the PSD of the magnetic particles throughout the diagnosis process must be closely monitored and controlled as well.
Timescale involved for disease diagnosis is influenced by the rate at which MS occurs. Therefore, it is crucial to optimize the separation rate of magnetic particles in any biomedical diagnostic application. In particular, self-aggregation of magnetic particles leading to cooperative magnetophoresis is able to accelerate the LGMS rate. In fact, there were high amount of magnetic particles in the cluster form, which bind to (or internalize into) a unit target entity [13,18,55]. Hence, the magnetophoresis of this magnetic particle-bound target is a result of synergistic movement of a large number of magnetic particles. This process operated under the cooperative magnetophoresis mode in which magnetic particles move collectively towards magnetic source. Additionally, the magnetic particle-bound targets might clump to each other during magnetophoretic migration which in turn further disturbs the dynamical behaviour of the LGMS process. For this case, examining the magnetophoretic migration of these magnetic particle-bound targets at a microscopic level is highly recommended.
Finally, our recent work has revealed that hydrodynamic effect is also playing a vital role in governing the dynamical behaviour of LGMS process. Owing to the momentum transfer between moving magnetic particles and the surrounding fluid, continuous sweeping flow is created within the entire magnetic particle solution that is subjected to LGMS [44]. The convective flow generated due to the particle motion is directed towards the area where the magnetic field gradient is lower. Simultaneously, this circulating flow drives magnetic particles located within the same area towards the magnet within a relatively shorter timescale in comparison with the motion which is purely driven by magnetophoretic force. In such a way, hydrodynamics engenders the fast magnetophoretic collection of magnetic particles from their suspension and makes LGMS more applicable for biomedical applications in which fast separation kinetics is needed. By taking advantage of the hydrodynamic interaction, it is believed that the efficiency of diagnosis process could be further enhanced. However, for LGMS that is conducted in continuous mode, the magnetophoretic pathway of particles might be considerably disrupted by the convective flow initiated from hydrodynamic effect. In this regard, the interference of hydrodynamically driven convection has caused difficulty in the theoretical prediction of deterministic magnetophoretic pathway of magnetic particles subjected to LGMS. To date, we are only able to calculate the macroscopic quantities of the LGMS system by solving the Navier–Stokes equation (which is a nonlinear and fourth-order partial differential equation in three-dimensional space [75]) numerically with the aid of computational simulation tools. By contrast, theoretical description of the microscopic picture of LGMS incorporating the hydrodynamic effect is still unexplored. Thus, it is imperative to develop a theoretical framework in this aspect, such that the incorporation of LGMS into a biomedical diagnostic application can be facilitated in the upcoming future.
Supplementary Material
Acknowledgement
Sim Siong Leong acknowledges the support from the MOHE Malaysia through the MyPhD Scholarship.
Authors' contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This project is financially supported by (i) International Foundation for Sciences (IFS) and co-financed by the Organisation for the Prohibition of Chemical Weapons (OPCW) (grant no. 6050324/I100) and (ii) Fundamental Research Grant Scheme (FRGS) grant from Ministry of Higher Education (MOHE) (grant no. 6071269).
References
- 1.Lin J, Li M, Li Y, Chen Q. 2015. A high gradient and strength bioseparator with nano-sized immunomagnetic particles for specific separation and efficient concentration of E. coli O157:H7. J. Magn. Magn. Mater. 378, 206–213. ( 10.1016/j.jmmm.2014.11.039) [DOI] [Google Scholar]
- 2.He J, Huang M, Wang D, Zhang Z, Li G. 2014. Magnetic separation techniques in sample preparation for biological analysis: a review. J. Pharmaceut. Biomed. Anal. 101, 84–101. ( 10.1016/j.jpba.2014.04.017) [DOI] [PubMed] [Google Scholar]
- 3.Gijs MAM, Lacharme F, Lehmann U. 2010. Microfluidic applications of magnetic particles for biological analysis and catalysis. Chem. Rev. 110, 1518–1563. ( 10.1021/cr9001929) [DOI] [PubMed] [Google Scholar]
- 4.Lim J, Yeap SP, Leow CH, Toh PY, Low SC. 2014. Magnetophoresis of iron oxide nanoparticles at low field gradient: the role of shape anisotropy. J. Colloid Interface Sci. 421, 170–177. ( 10.1016/j.jcis.2014.01.044) [DOI] [PubMed] [Google Scholar]
- 5.Andreu JS, Camacho J, Faraudo J, Benelmekki M, Rebollo C, Martínez LM. 2011. Simple analytical model for the magnetophoretic separation of superparamagnetic dispersions in a uniform magnetic gradient. Phys. Rev. E 84, 021402 ( 10.1103/PhysRevE.84.021402) [DOI] [PubMed] [Google Scholar]
- 6.Pamme N. 2007. Continuous flow separations in microfluidic devices. Lab Chip 7, 1644–1659. ( 10.1039/b712784g) [DOI] [PubMed] [Google Scholar]
- 7.Probst CE, Zrazhevskiy P, Gao X. 2011. Rapid multitarget immunomagnetic separation through programmable DNA linker displacement. J. Am. Chem. Soc. 133, 17 126–17 129. ( 10.1021/ja2072324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yavuz CT, Prakash A, Mayo JT, Colvin VL. 2009. Magnetic separations: from steel plants to biotechnology. Chem. Eng. Sci. 64, 2510–2521. ( 10.1016/j.ces.2008.11.018) [DOI] [Google Scholar]
- 9.Kaminski MD, Chen H, Liu X, Rempfer D, Rosengart AJ. 2012. Removal of blood-borne toxin in the body using magnetic nanospheres. In Magnetic nanoparticles: from fabrication to clinical applications (ed. Thanh NT.), pp. 195–214. Boca Raton, FL: CRC press. [Google Scholar]
- 10.Xia N, Hunt TP, Mayers BT, Alsberg E, Whitesides GM, Westervelt RM, Ingber DE. 2006. Combined microfluidic-micromagnetic separation of living cells in continuous flow. Biomed. Microdevices 8, 299–308. ( 10.1007/s10544-006-0033-0) [DOI] [PubMed] [Google Scholar]
- 11.Zborowski M, Williams PS, Moore LR, Chalmers JJ, Zimmerman PA. 2007. New challenges and opportunities. In Laboratory techniques in biochemistry and molecular biology (eds Zborowski M, Chalmers JJ), pp. 331–412. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 12.Nam J, Huang H, Lim H, Lim C, Shin S. 2013. Magnetic separation of malaria-infected red blood cells in various developmental stages. Anal. Chem. 85, 7316–7323. ( 10.1021/ac4012057) [DOI] [PubMed] [Google Scholar]
- 13.Pamme N, Wilhelm C. 2006. Continuous sorting of magnetic cells via on-chip free-flow magnetophoresis. Lab Chip 6, 974–980. ( 10.1039/b604542a) [DOI] [PubMed] [Google Scholar]
- 14.Chen GD, Alberts CJ, Rodriguez W, Toner M. 2010. Concentration and purification of human immunodeficiency virus type 1 virions by microfluidic separation of superparamagnetic nanoparticles. Anal. Chem. 82, 723–728. ( 10.1021/ac9024522) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim J, Yeap SP, Low SC. 2014. Challenges associated to magnetic separation of nanomaterials at low field gradient. Sep. Purif. Technol. 123, 171–174. ( 10.1016/j.seppur.2013.12.038) [DOI] [Google Scholar]
- 16.Tangpukdee N, Duangdee C, Wilairatana P, Krudsood S. 2009. Malaria diagnosis: a brief review. Korean J. Parasitol. 47, 93–102. ( 10.3347/kjp.2009.47.2.93) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hackett S, Hamzah J, Davis TME, St Pierre TG. 2009. Magnetic susceptibility of iron in malaria-infected red blood cells. Biochim. Biophys. Acta 1792, 93–99. ( 10.1016/j.bbadis.2008.11.001) [DOI] [PubMed] [Google Scholar]
- 18.Xu H, Aguilar ZP, Yang L, Kuang M, Duan H, Xiong Y, Wei H, Wang A. 2011. Antibody conjugated magnetic iron oxide nanoparticles for cancer cell separation in fresh whole blood. Biomaterials 32, 9758–9765. ( 10.1016/j.biomaterials.2011.08.076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhakdi SC, Ottinger A, Somsri S, Sratongno P, Pannadaporn P, Chimma P, Malasit P, Pattanapanyasat K, Neumann HP. 2010. Optimized high gradient magnetic separation for isolation of Plasmodium-infected red blood cells. Malar. J. 9, 38 ( 10.1186/1475-2875-9-38) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qu B-Y, Wu Z-Y, Fang F, Bai Z-M, Yang D-Z, Xu S-K. 2008. A glass microfluidic chip for continuous blood cell sorting by a magnetic gradient without labeling. Anal. Bioanal. Chem. 392, 1317–1324. ( 10.1007/s00216-008-2382-4) [DOI] [PubMed] [Google Scholar]
- 21.Melville D, Paul F, Roath S. 1975. Direct magnetic separation of red cells from whole blood. Nature 255, 706 ( 10.1038/255706a0) [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Xianyu Y, Wang Y, Zhang X, Cha R, Sun J, Jiang X. 2015. One-step detection of pathogens and viruses: combining magnetic relaxation switching and magnetic separation. ACS Nano 9, 3184–3191. ( 10.1021/acsnano.5b00240) [DOI] [PubMed] [Google Scholar]
- 23.Olsvik O, Popovic T, Skjerve E, Cudjoe KS, Hornes E, Ugelstad J, Uhlen M. 1994. Magnetic separation techniques in diagnostic microbiology. Clin. Microbiol. Rev. 7, 43–54. ( 10.1128/CMR.7.1.43) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lien K-Y, Lin J-L, Liu C-Y, Lei H-Y, Lee G-B. 2007. Purification and enrichment of virus samples utilizing magnetic beads on a microfluidic system. Lab Chip 7, 868–875. ( 10.1039/b700516d) [DOI] [PubMed] [Google Scholar]
- 25.Gundersen SG, Haagensen I, Jonassen TO, Figenschau KJ, de Jonge N, Deelder AM. 1992. Quantitative detection of schistosomal circulating anodic antigen by a magnetic bead antigen capture enzyme-linked immunosorbent assay (MBAC-EIA) before and after mass chemotherapy. Trans. R. Soc. Trop. Med. Hyg. 86, 175–178. ( 10.1016/0035-9203(92)90559-U) [DOI] [PubMed] [Google Scholar]
- 26.Zborowski M. 2007. Commercial magnetic cell separation instruments and reagents. In Laboratory techniques in biochemistry and molecular biology (eds Zborowski M, Chalmers JJ), pp. 265–292. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 27.Šafařík I, Šafaříková M. 1999. Use of magnetic techniques for the isolation of cells. J. Chromatogr. B Biomed. Sci. Appl. 722, 33–53. ( 10.1016/S0378-4347(98)00338-7) [DOI] [PubMed] [Google Scholar]
- 28.Moeser GD, Roach KA, Green WH, Alan Hatton T, Laibinis PE. 2004. High-gradient magnetic separation of coated magnetic nanoparticles. AIChE J. 50, 2835–2848. ( 10.1002/aic.10270) [DOI] [Google Scholar]
- 29.Oberteuffer J. 1973. High gradient magnetic separation. IEEE Trans. Magn. 9, 303–306. ( 10.1109/tmag.1973.1067673) [DOI] [Google Scholar]
- 30.Oder R. 1976. High gradient magnetic separation theory and applications. IEEE Trans. Magn. 12, 428–435. ( 10.1109/tmag.1976.1059076) [DOI] [Google Scholar]
- 31.Kelland D. 1973. High gradient magnetic separation applied to mineral beneficiation. IEEE Trans. Magn. 9, 307–310. ( 10.1109/tmag.1973.1067683) [DOI] [Google Scholar]
- 32.Toh PY, Yeap SP, Kong LP, Ng BW, Chan DJC, Ahmad AL, Lim JK. 2012. Magnetophoretic removal of microalgae from fishpond water: feasibility of high gradient and low gradient magnetic separation. Chem. Eng. J. 211–212, 22–30. ( 10.1016/j.cej.2012.09.051) [DOI] [Google Scholar]
- 33.Franzreb M, Holl WH. 2000. Phosphate removal by high-gradient magnetic filtration using permanent magnets. IEEE Trans. Appl. Supercond. 10, 923–926. ( 10.1109/77.828382) [DOI] [Google Scholar]
- 34.Mariani G, Fabbri M, Negrini F, Ribani PL. 2010. High-gradient magnetic separation of pollutant from wastewaters using permanent magnets. Sep. Purif. Technol. 72, 147–155. ( 10.1016/j.seppur.2010.01.017) [DOI] [Google Scholar]
- 35.Hoffmann C, Franzreb M, Holl WH. 2002. A novel high-gradient magnetic separator (HGMS) design for biotech applications. IEEE Trans. Appl. Supercond. 12, 963–966. ( 10.1109/tasc.2002.1018560) [DOI] [Google Scholar]
- 36.Ritter JA, Ebner AD, Daniel KD, Stewart KL. 2004. Application of high gradient magnetic separation principles to magnetic drug targeting. J. Magn. Magn. Mater. 280, 184–201. ( 10.1016/j.jmmm.2004.03.012) [DOI] [Google Scholar]
- 37.Forbes ZG, Yellen BB, Halverson DS, Fridman G, Barbee KA, Friedman G. 2008. Validation of high gradient magnetic field based drug delivery to magnetizable implants under flow. IEEE Trans. Biomed. Eng. 55, 643–649. ( 10.1109/tbme.2007.899347) [DOI] [PubMed] [Google Scholar]
- 38.Stephens JR, Beveridge JS, Williams ME. 2012. Analytical methods for separating and isolating magnetic nanoparticles. Phys. Chem. Chem. Phys. 14, 3280–3289. ( 10.1039/c2cp22982j) [DOI] [PubMed] [Google Scholar]
- 39.Ditsch A, Lindenmann S, Laibinis PE, Wang DIC, Hatton TA. 2005. High-gradient magnetic separation of magnetic nanoclusters. Ind. Eng. Chem. Res. 44, 6824–6836. ( 10.1021/ie048841s) [DOI] [Google Scholar]
- 40.Gerber R, Birss RR. 1983. High gradient magnetic separation, vol. 209 Research Studies Press Div. of John Wiley & Sons, Ltd. [Google Scholar]
- 41.Gómez-Pastora J, Bringas E, Ortiz I. 2014. Recent progress and future challenges on the use of high performance magnetic nano-adsorbents in environmental applications. Chem. Eng. J. 256, 187–204. ( 10.1016/j.cej.2014.06.119) [DOI] [Google Scholar]
- 42.Yavuz CT, et al. 2006. Low-field magnetic separation of monodisperse Fe3O4 nanocrystals. Science 314, 964–967. ( 10.1126/science.1131475) [DOI] [PubMed] [Google Scholar]
- 43.Lim J, Lanni C, Evarts ER, Lanni F, Tilton RD, Majetich SA. 2011. Magnetophoresis of nanoparticles. ACS Nano 5, 217–226. ( 10.1021/nn102383s) [DOI] [PubMed] [Google Scholar]
- 44.Leong SS, Ahmad Z, Lim J. 2015. Magnetophoresis of superparamagnetic nanoparticles at low field gradient: hydrodynamic effect. Soft Matter 11, 6968–6980. ( 10.1039/c5sm01422k) [DOI] [PubMed] [Google Scholar]
- 45.Schaller V, et al. 2008. Motion of nanometer sized magnetic particles in a magnetic field gradient. J. Appl. Phys. 104, 093918 ( 10.1063/1.3009686) [DOI] [Google Scholar]
- 46.McCabe WL, Smith JC, Harriott P. 2005. Unit operations of chemical engineering. New York, NY: McGraw-Hill. [Google Scholar]
- 47.Berg HC. 1993. Random walks in biology. Princeton, NJ: Princeton University Press. [Google Scholar]
- 48.De Las Cuevas G, Faraudo J, Camacho J. 2008. Low-gradient magnetophoresis through field-induced reversible aggregation. J. Phys. Chem. C 112, 945–950. ( 10.1021/jp0755286) [DOI] [Google Scholar]
- 49.Yellen BB, Hovorka O, Friedman G. 2005. Arranging matter by magnetic nanoparticle assemblers. Proc. Natl Acad. Sci. USA 102, 8860–8864. ( 10.1073/pnas.0500409102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Faraudo J, Camacho J. 2010. Cooperative magnetophoresis of superparamagnetic colloids: theoretical aspects. Colloid Polym. Sci. 288, 207–215. ( 10.1007/s00396-009-2107-z) [DOI] [Google Scholar]
- 51.Faraudo J, Andreu JS, Calero C, Camacho J. 2016. Predicting the self-assembly of superparamagnetic colloids under magnetic fields. Adv. Funct. Mater. 26, 3837–3858. ( 10.1002/adfm.201504839) [DOI] [Google Scholar]
- 52.Zborowski M, Ostera GR, Moore LR, Milliron S, Chalmers JJ, Schechter AN. 2003. Red blood cell magnetophoresis. Biophys. J. 84, 2638–2645. ( 10.1016/S0006-3495(03)75069-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zimmerman PA, Thomson JM, Fujioka H, Collins WE, Zborowski M. 2006. Diagnosis of malaria by magnetic deposition microscopy. Am. J. Trop. Med. Hyg. 74, 568–572. [PMC free article] [PubMed] [Google Scholar]
- 54.Song E-Q, Hu J, Wen C-Y, Tian Z-Q, Yu X, Zhang Z-L, Shi Y-B, Pang D-W. 2011. Fluorescent-magnetic-biotargeting multifunctional nanobioprobes for detecting and isolating multiple types of tumor cells. ACS Nano 5, 761–770. ( 10.1021/nn1011336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robert D, Pamme N, Conjeaud H, Gazeau F, Iles A, Wilhelm C. 2011. Cell sorting by endocytotic capacity in a microfluidic magnetophoresis device. Lab Chip 11, 1902–1910. ( 10.1039/c0lc00656d) [DOI] [PubMed] [Google Scholar]
- 56.Moore LR, Williams PS, Nehl F, Abe K, Chalmers JJ, Zborowski M. 2013. Feasibility study of red blood cell debulking by magnetic field-flow fractionation with step-programmed flow. Anal. Bioanal. Chem. 406, 1661–1670. ( 10.1007/s00216-013-7394-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pivetal J, Royet D, Ciuta G, Frenea-Robin M, Haddour N, Dempsey NM, Dumas-Bouchiat F, Simonet P. 2015. Micro-magnet arrays for specific single bacterial cell positioning. J. Magn. Magn. Mater. 380, 72–77. ( 10.1016/j.jmmm.2014.09.068) [DOI] [Google Scholar]
- 58.Yeap SP, Leong SS, Ahmad AL, Ooi BS, Lim J. 2014. On size fractionation of iron oxide nanoclusters by low magnetic field gradient. J. Phys. Chem C 118, 24 042–24 054. ( 10.1021/jp504808v) [DOI] [Google Scholar]
- 59.Andreu JS, Camacho J, Faraudo J. 2011. Aggregation of superparamagnetic colloids in magnetic fields: the quest for the equilibrium state. Soft Matter 7, 2336–2339. ( 10.1039/c0sm01424a) [DOI] [Google Scholar]
- 60.Faraudo J, Andreu JS, Camacho J. 2013. Understanding diluted dispersions of superparamagnetic particles under strong magnetic fields: a review of concepts, theory and simulations. Soft Matter 9, 6654–6664. ( 10.1039/c3sm00132f) [DOI] [Google Scholar]
- 61.Koh I, Hong R, Weissleder R, Josephson L. 2009. Nanoparticle−target interactions parallel antibody−protein interactions. Anal. Chem. 81, 3618–3622. ( 10.1021/ac802717c) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosensweig RE. 1997. Ferrohydrodynamics. New York, NY: Dover Publications. [Google Scholar]
- 63.Tamer U, Boyacı İH, Temur E, Zengin A, Dincer İ, Elerman Y. 2011. Fabrication of magnetic gold nanorod particles for immunomagnetic separation and SERS application. J. Nanopart. Res. 13, 3167–3176. ( 10.1007/s11051-010-0213-y) [DOI] [Google Scholar]
- 64.Tamer U, Cetin D, Suludere Z, Boyaci IH, Temiz HT, Yegenoglu H, Daniel P, Dinçer İ, Elerman Y. 2013. Gold-coated iron composite nanospheres targeted the detection of Escherichia coli. Int. J. Mol. Sci. 14, 6223–6240. ( 10.3390/ijms14036223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun W, Khosravi F, Albrechtsen H, Brovko LY, Griffiths MW. 2002. Comparison of ATP and in vivo bioluminescence for assessing the efficiency of immunomagnetic sorbents for live Escherichia coli O157:H7 cells. J. Appl. Microbiol. 92, 1021–1027. ( 10.1046/j.1365-2672.2002.01639.x) [DOI] [PubMed] [Google Scholar]
- 66.Kim GY, Josephson L, Langer R, Cima MJ. 2007. Magnetic relaxation switch detection of human chorionic gonadotrophin. Bioconjugate Chem. 18, 2024–2028. ( 10.1021/bc070110w) [DOI] [PubMed] [Google Scholar]
- 67.Nel A, Xia T, Mädler L, Li N. 2006. Toxic potential of materials at the nanolevel. Science 311, 622–627. ( 10.1126/science.1114397) [DOI] [PubMed] [Google Scholar]
- 68.Kunzmann A, et al. 2011. Efficient internalization of silica-coated iron oxide nanoparticles of different sizes by primary human macrophages and dendritic cells. Toxicol. Appl. Pharmacol. 253, 81–93. ( 10.1016/j.taap.2011.03.011) [DOI] [PubMed] [Google Scholar]
- 69.Karlsson HL, Gustafsson J, Cronholm P, Möller L. 2009. Size-dependent toxicity of metal oxide particles—a comparison between nano- and micrometer size. Toxicol. Lett. 188, 112–118. ( 10.1016/j.toxlet.2009.03.014) [DOI] [PubMed] [Google Scholar]
- 70.Häfeli UO, Riffle JS, Harris-Shekhawat L, Carmichael-Baranauskas A, Mark F, Dailey JP, Bardenstein D. 2009. Cell uptake and in vitro toxicity of magnetic nanoparticles suitable for drug delivery. Mol. Pharmaceut. 6, 1417–1428. ( 10.1021/mp900083m) [DOI] [PubMed] [Google Scholar]
- 71.Dobson J. 2006. Gene therapy progress and prospects: magnetic nanoparticle-based gene delivery. Gene Therapy 13, 283–287. ( 10.1038/sj.gt.3302720) [DOI] [PubMed] [Google Scholar]
- 72.Pisanic Ii TR, Blackwell JD, Shubayev VI, Fiñones RR, Jin S. 2007. Nanotoxicity of iron oxide nanoparticle internalization in growing neurons. Biomaterials 28, 2572–2581. ( 10.1016/j.biomaterials.2007.01.043) [DOI] [PubMed] [Google Scholar]
- 73.Yeap SP, Toh PY, Ahmad AL, Low SC, Majetich SA, Lim J. 2012. Colloidal stability and magnetophoresis of gold-coated iron oxide nanorods in biological media. J. Phys. Chem. C 116, 22 561–22 569. ( 10.1021/jp306159a) [DOI] [Google Scholar]
- 74.Costo R, Bello V, González-Carreño T, Veintemillas-Verdaguer S, Wang D. 2013. Size sorting of ultrasmall magnetic nanoparticles and their aggregates behaviour. Mater. Res. Bull. 48, 4294–4300. ( 10.1016/j.materresbull.2013.06.050) [DOI] [Google Scholar]
- 75.Bird RB, Stewart WE, Lightfoot EN. 2006. Transport phenomena, 2nd edn New York, NY: John Wiley & Sons, Inc. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.