Abstract
Nowadays, thanks to the successful discoveries in the biomedical field achieved in the last two decades, a deeper understanding about the complexity of mechanistic aspects of different pathological processes has been obtained. As a consequence, even the standard therapeutic protocols have undergone a vast redesign. In fact, the awareness about the necessity to progress towards a combined multitherapy in order to potentially increase the final healing chances has become a reality. One of the crucial elements of this novel approach is that large amounts of detailed information are highly needed and in vivo imaging techniques represent one of the most powerful tools to visualize and monitor the pathological state of the patient. To this scope, due to their unique features, nanostructured materials have emerged as attractive elements for the development of multifunctional tools for diagnosis and therapy. Hence, in this review, the most recent and relevant advances achieved by applying multifunctional nanostructures in multimodal theranosis of different diseases will be discussed. In more detail, the preparation and application of single multifunctional nano-radiotracers based on iron oxides and enabling PET/MRI dual imaging will be firstly detailed. After that, especially considering their highly promising clinical potential, the preparation and application of multifunctional liposomes useful for multimodal imaging and therapy will be reviewed. In both cases, a special focus will be set on the application of such a multifunctional nanocarriers in cancer as well as cardiovascular diseases.
Keywords: multimodal imaging, liposome, PET/MRI, molecular imaging, theranosis, iron oxide nanoparticles
1. Introduction
Nowadays, as a consequence of the brilliant progress in biomedical technology achieved in the past decades, it is undoubtedly clear that the heterogeneity of the disease and patients is one of the most crucial factors impacting on the final favourable evolution of a pathological process. In other terms, there is no panacea and each patient needs optimized therapy based on the differences in genetic factors, physical conditions, environmental factors and the disease characteristics (personalized medicine) [1–3]. In this novel concept, large amounts of detailed information about the disease and patients are much needed. With this aim, non-invasive diagnosis of patients, in vivo imaging techniques result in one of the most powerful tools to visualize the pathological state of the body and monitor biological evolution at the target site [4].
For clinical application, the most useful imaging modalities generally include optical imaging, magnetic resonance imaging (MRI), computed tomography (CT), ultrasound (US) and positron emission tomography (PET) or single photon emission computed tomography. Each single imaging modality shows unique advantages along with intrinsic limitations, such as insufficient sensitivity or spatial resolution. This circumstance makes it difficult getting accurate and reliable information at the disease site [5]. In order to improve the final diagnostic image and to characterize and quantify biological processes at the cellular and subcellular level in intact living subjects, the above-cited imaging modalities require the use of small molecules as probes (molecular imaging) [5]. For example, gadolinium complexes or iodinated compounds are used as contrast agents for T1-MRI or CT imaging, respectively. However, if not properly engineered, these small molecules generally present different limitations such as very short blood circulation time and non-specific biodistribution, which may cause many unwanted side effects.
In order to overcome many of these limitations, nanostructured materials can be employed [6]. In fact, it is a well-established reality the tremendous impact that nanotechnology development has had on society and especially in medicine. By virtue of their size-dependent physical properties and nanometre-scale dimensions, nanomaterials possess enormous synthetic design potential along with the ability to access biological features at the subcellular level. Hence, nanomaterials can be easily combined for multiple targeting, sensing, diagnostic and therapeutic functions [7]. This higher level of functional sophistication (not possible with small molecules) is the major driver for the development of nanomedicine, one of the fastest growing areas in nanotechnology and poised to revolutionize healthcare and medicine through the development of transformative new diagnostic and therapeutic tools [8].
The result of such a rational combination of different nanomaterials will then generate a novel multifunctional nanocarrier showing the best characteristic of their parental constituents and reducing their intrinsic limitations. In this way, different imaging and therapeutic strategies may be promoted at the same time (multimodal strategy), enhancing the final theranostic (therapeutic + diagnostic) effect. For example, the combination of MRI contrast agent and fluorescent organic dye on the same targeted nanocarrier allows detecting cancer through non-invasive MRI and the optical guide of surgery. Or, the encapsulation of MRI contrast agent and anti-cancer drug in a nanostructured matrix modified with a specific peptide or antibody on its surface has the potential to allow for simultaneous targeted diagnosis and chemotherapy [9].
One of the most notable consequences related to the advent of these multifunctional nanomaterials is the possibility of combining different imaging modalities with a unique contrast agent. Starting from its early dawn, multimodal imaging was revealed to be a powerful methodology able to provide more accurate detection and analysis of disease sites [8,10,11]. For example, the combination of PET with CT or MRI techniques has generated a strong interest due to the highly synergistic improvement of currently used imaging instruments for diagnosis. In fact, PET images provide functional information about the disease with high sensitivity. On the other hand, CT and MRI offer high-resolution images for anatomical information. Therefore, a combination of these different imaging modalities can accomplish high sensitivity and high resolution simultaneously and provide more detailed anatomical or biological information about the target disease. The theoretical combinations to produce multifunctional probes for multimodal imaging are directly related to the engineered nanomaterials reported until now. Consequently, such combinations could be considered unlimited [8,9,12,13]. Nevertheless, from a practical point of view, these combinations are limited in number because they finally must provide more precise and detailed information for clear diagnosis than the constituting individual modality. For this reason, the rational selection of each modality enabling the multimodal strategy is crucial. In this sense, researchers should rationally avoid the overlapping of advantages and compensate for the flaws of each selected strategy in order to maximize the synergistic effect. This is the reason why imaging modalities with high sensitivity (PET, optical, etc.) are frequently combined with other imaging modalities with high spatial resolution (MR, CT, etc.) [5,8,9].
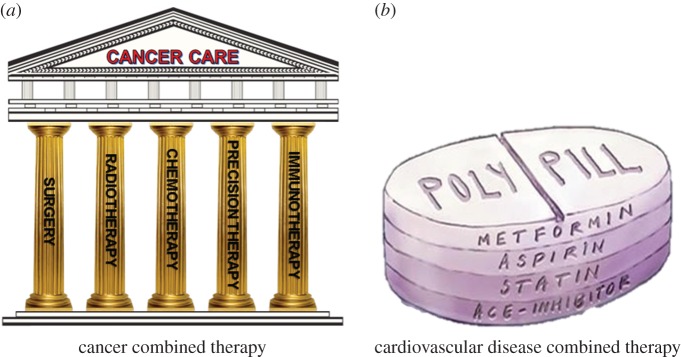
Even from a therapeutic point of view, in parallel to the growing knowledge of the complexity of each pathological process, the awareness about the necessity to progress towards a combined multitherapy in order to potentially increase the final healing chances has become a reality. Combined therapy of two or more strategies promotes synergism among each individual constituent and targets the pathology through distinct mechanisms of action (figure 1). Within the different clinical applications where this rational concept has been successfully applied (i.e. antiviral and antimicrobial therapies), cancer therapy represents one of the clearest examples. In fact, dating from its early successful evidences, immunotherapy quickly became the fifth pillar of cancer treatment together with the well-known more traditional options including radiation, surgery, chemotherapy and targeted therapy (figure 2a) [14]. Scientists have shown repeatedly that using just one therapeutic modality, poor results would be achieved. Only combination of the above-mentioned modalities will produce a sustained and lasting response in a wider number of patients. No pillar alone holds up the architrave to keep the temple intact (figure 2a).
Figure 1.
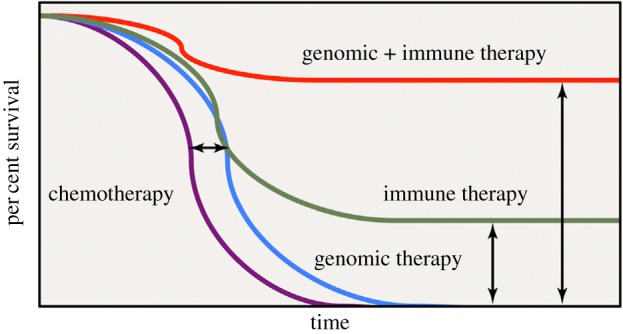
Improved overall cancer survival as a result of combination therapy. Adapted from [14].
Figure 2.
Schematic of combined multitherapy in (a) cancer disease and (b) cardiovascular disease.
Even for the treatment of cardiovascular diseases (CVDs), the multitherapy approach has demonstrated its high potential. In fact, in the past decade, a multiactive all-in-one pill (polypill) composed of four drugs (aspirin, a cholesterol lowering statin and two blood pressure drugs (ACE inhibitor and a diuretic) that are known to effectively treat CVD) has been proposed as a simple, cost-effective and innovative public health strategy to combat the CVD epidemic on a global scale (figure 2b). Several randomized clinical trials have consistently demonstrated the effects of polypills on cadiovascular risk factors [15]. Moreover, besides the clinical potential, several other studies have shown the polypill to be well tolerated and superior in terms of patients' adherence to standard of care [15–17].
In the literature, countless examples of novel engineered nanomaterials with biomedical potential in human health have been published [13,17–19]. However, the vast majority of existing reviews tend to focus only on a specific application of nanomaterials in biomedicine (one therapeutic strategy or one imaging technique). Only some of them target the multimodal theranostic activity of composed nanosystems in different pathological processes. For this reason, in the present review, more detailed insights concerning the most recent and relevant achievements obtained by applying the multifunctional nanostructures in multimodal theranosis of different diseases will be discussed. This special focus has been carefully selected considering the highly promising results obtained to date by the combined use of different engineered smart nanomaterials in multimodal theranosis (synergistic effect) if compared with the same results achieved when the same nanomaterials were used individually (additive effect). Beside this aspect, among the plethora of existing nanosystems described in the literature, the focus will be set on the most promising ones in multimodal imaging as much as in multitherapy of cancer and CVDs [20].
In more detail, as a representative example of a single multifunctional nanoprobe promoting multimodal imaging, the preparation and application of hybrid nano-radiotracers enabling the PET/MRI dual imaging of different pathological processes will be described. This particular theme was selected considering the rapidly growing interest in clinical application of such a multimodal imaging strategy. The different strategies enabling the preparation of these nanocarriers and their application in cancer and cardiovascular imaging and therapy will be discussed.
Subsequently, as a representative example of most promising nanomaterials useful in multimodal theranosis, the preparation and application of multifunctional liposomes will be reviewed. Indeed, within all the studied nanomaterials, these nanocarriers are the most used in clinical trials, showing the most promising commercial and clinical potential [20]. For example, in this direction, very recently a liposome-based system has been used to delivery RNA in three human patients with melanoma. As final result, a systemic immune response targeting melanoma cancer cells was triggered [21].
2. Nano-radiochemistry for multimodal imaging
The novel field of nano-radiochemistry tries to conjoin the best attributes of two branches of science that, in spite of all their common features, have traditionally ignored each other. The combination of the size-dependent properties of nanomaterials and the exquisite sensitivity of nuclear techniques is creating a new paradigm in molecular imaging. These new features extend to most of the typical issues when developing tracers for biomedical imaging like the concept of multifunctionality, biodistribution, pharmacokinetics and the administered dose.
High surface/volume ratio is a well-known characteristic in almost all nanomaterials. This feature is particularly suitable for its conjugation with multiple molecules that provide multifunctionality. Apart from enabling bimodal imaging experiments, combination of nanomaterials with a radioisotope allows to obtain larger ligand payload in further bioconjugations, when compared with the common radiotracers, due to the multifunctionality of nanomaterials [22,23].
As previously stated, one critical issue related to the use of nanomaterials is their biodistribution. In fact, the biodistribution study of the nanomaterials is sometimes complicated and is frequently performed through ex vivo techniques using highly sensitive techniques [24,25]. In such a case, radiochemistry can clearly lend a hand to nanotechnology. By incorporating a radioisotope within the structural framework of the nanomaterial, the biodistribution can be studied effortlessly with in vivo and/or ex vivo radioactive techniques [26–30].
On the other hand, in the case of nano-radiotracers, nanotechnology comes to the aid of radiochemistry. From the point of view of pharmacokinetics, radiotracers sometimes present short circulation time in blood. To this scope, there are different strategies to improve the in vivo behaviour of radiotracers. One of the most used solutions is the attachment of PEGylated chains [31,32]. Even though PEGylated radiotracers usually show longer circulation times, this methodology needs at least one additional step, which increases synthetic cost and time. A clever solution to increase circulation time of the tracer is to incorporate it into a nanoplatform with an intrinsic long circulation time in blood [33,34]. Nano-radiochemistry also benefits from the possibility of including specific ligands on the surface of nanoparticles in order to direct nanoplatforms to desired sites, increasing specificity and efficiency of radiotracers.
Furthermore, the possibility of combining not only one, but several labels for different imaging modalities allows signal amplification. This special feature combined with high sensitivity of nuclear techniques and enhanced surface area presented by nanoparticles allows significant dosage reduction if compared with the single modality acquisition strategy.
2.1. Iron oxide nanoparticles for imaging
Iron oxide nanoparticles (IONPs) are the most common NP-based contrast agent for MRI [35]. MRI offers excellent soft-tissue contrast resolution and pathological discrimination. On the other hand, PET, one of the most typical molecular imaging techniques, presents poor probe anatomical localization potential [36]. Consequently, the fusion of these two techniques in a single measurement (PET/MRI) may help to overcome in some applications the lack of anatomical details offered by PET and poor sensitivity of MRI. IONPs have for long been studied as MRI probes for T2-weighted (dark or negative contrast) imaging. The T2 (the transverse relaxation time) based contrast from these IONPs is an intrinsic effect of their crystalline core and high magnetic moment. As an effect, they finally damp the signal of the nearby water molecules. The list of researches focusing upon this nanocarrier is enormous [11,13,37–41]. The interest normally lies in the improvement of the core crystal or surface functional structure, so as to make it as biocompatible as possible, while modifying the surface so as to enhance the target specificity to the maximum.
However, this marked effect on T2 can be a problem for many imaging applications. For example, it is crucial when the signal intensity is intrinsically low due to physiological and magnetic susceptibility characteristics of the organ/tissue (lungs, trabecular bone, paranasal sinus, etc.) or due to the presence of low-proton contents (necrosis, calcifications, etc.) in some pathologies. In these cases, the large negative contrast promoted by superparamagnetic iron oxide is not the best option. Because of that, in the last years there has been an intense development of extremely small (2–3 nm core size), superparamagnetic, IONPs as T1 contrast agents [42,43]. In this case, the effect observed here is the shortening of longitudinal relaxation time (T1). This T1-based (bright or positive) contrast is usually of more practical use for physicians when compared with its T2 counterpart. In one of our works, we illustrated this possibility using a microwave assisted one-pot synthesis of extremely small, FITC-CM dextran (4 kDa) capped iron oxide nanoparticles (fdIONP) for T1-based MRI contrast [42]. The particles were biocompatible, showed a long circulatory half-life and multimodal imaging ability due to the presence of FITC, especially for histological validation (figure 3) [11].
Figure 3.
Magnetic resonance angiography of a mouse at increasing times after intravenous injection of fdIONP. Adapted from [42].
2.2. Nano-radiolabelling
2.2.1. Radioisotopes
There are many different radioisotopes that may be used as positron emitters in PET. They are usually classified according to their half-life time or production method. In the case of the half-life time, there are a large variety of radioisotopes covering all range spectra (table 1). Isotopes range from a very short half-life time like 15O (approx. 2 min) to long half-life time like 124I (4.18 days). Moreover, this half-life time of the radioisotope will determine its use in PET imaging. For long-time elimination tracers, the use of short half-life time radioisotopes is preferable for clinical purposes in order to reduce the patient's radioactivity exposure [44]. Despite this, sometimes there is no choice and a long half-life radioisotope has to be used, for instance, to visualize biological molecules with long circulation time in blood. Radioisotopes are commonly produced in a cyclotron (particle accelerator). This fact represents a disadvantage for radioisotopes with short half-life time due to the necessity of having a cyclotron close to the PET camera. Moreover, cyclotrons are expensive and require specific facilities. Another production method is through benchtop generators. These are portable armoured cylinders that contain a matrix with a father radioisotope embedded. Generators are small, usually cheap and easy to use, which is the main advantage of this methodology [45]. However, the use of generators for PET radioisotopes is limited to only two elements, 68Ga and 32Rb.
Table 1.
Main radioisotopes for molecular imaging.
| radioisotope | half-life | units | production route |
|---|---|---|---|
| 11C | 20.4 | minutes | 14N(p,α)11C (cyclotron) |
| 13N | 9.9 | minutes | 14O(p,α)13N (cyclotron) |
| 15O | 122 | seconds | 14N(d,n)18F (cyclotron) |
| 18F | 110 | minutes | 18O(p,n)18F (cyclotron) |
| 62Cu | 9.74 | minutes |
63Cu(p,n)62Zn (cyclotron) 62Zn → 62Cu (generator) |
| 64Cu | 12.7 | hours |
64Ni(p,n)64Cu (cyclotron) Zn(various)64Cu (cyclotron) |
| 68Ga | 67.7 | minutes |
69Ga(p,2n)68Ge (cyclotron) 68Ge → 68Ga (generator) |
| 76Br | 16.2 | hours | 76Se(p,n)76Br (cyclotron) |
| 124I | 4.15 | days | 124Te(p,n)124I (cyclotron) |
| 89Zr | 78.4 | hours | 89Y(p,n)89Zr (cyclotron) |
| 82Rb | 76 | seconds | 82Sr → 82Rb (generator) |
Because of its matched half-life for tagging many biological processes and production method in a generator, 68Ga is becoming an extremely important radioisotope in radiochemistry [46,47]. These generators contain a matrix with father isotope 68Ge that present a long half-life of 270 days allowing the elution of 68Ga for months, with the same generator. Half-life of 68Ga (67.7 min) is similar to circulation time in blood of many peptides, which makes this radioisotope very attractive for their in vivo visualization. There are some examples of the synthesis of 68Ga-based radiotracers. For example, within the most used radiotracers to assess somatostatin receptors, 68Ga-DOTATATE and 68Ga-DOTATOC were introduced for their high specificity towards different neuroendocrine tumours [48–50].
2.2.2. Radiolabelling of iron oxide nanoparticles:nano-radiochemistry
Different strategies for the synthesis of nano-radiotracers based on IONPs are available. The principal difference concerns the localization of the radioisotope with respect to the nanoparticle structure. In the first case, the radioisotope is tagged within the surfactant of the nanoparticle and, therefore, this approach is called surface labelling. The second option comprises doping the core of the nanoparticle with the radioisotope and is called core labelling. Both methods have some advantages and disadvantages that will be summarized in the following paragraphs.
2.2.3. Surface labelling
One of the most important features of nanoparticles is the high surface/volume ratio. This intrinsic characteristic provides the capability of multifunctionalization in the surface of the nanoparticle [51,52]. Surface labelling takes the advantage of this specific surface for the conjugation of the nanoparticle with the radioisotope.
2.2.3.1. Chelator strategy
A common way to produce the nano-radiotracer is to functionalize the nanoparticle with a chelate ligand, in order to form a coordination complex between the nanoparticle and the radioisotope in a final step.
Despite its easiness, there are two different important issues that must be taken into account with this approach. The first one is the stability of the bond between the nanoparticle and the chelator agent, and the second is the stability of the coordination complex between the chelator and the radioisotope.
Concerning the first aspect, a strong covalent bond between the nanoparticle and the chelator is preferred so as to avoid in vivo desorption. In PET imaging, the signal derives from the radioisotope. If the chelator is desorbed from the surface of the nanoparticle, the signal of the radioisotope will come from the chelator-radioisotope coordination complex and not from the nano-radiotracer. Therefore, the use of organic chemistry to obtain stable chelate-functionalized nanoparticles is mandatory, with many different options depending on the desired final formulation.
The second important aspect to take into account regarding this approach is the stability of the coordination complex between the chelator and the radioisotope. Owing to the presence of different cations in blood, transmetallation reactions involving the nano-radiotracer can occur. To avoid this process, the use of high affinity complex between the chelator and the radioisotope is required. The choice of the radioisotope determines the chelator. A very common chelator in the 68Ga radiolabelling of IONPs is 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid, also known as DOTA. The use of a chelating agent to build the nano-radiotracer presents, in a general way, some advantages and disadvantages in comparison with other strategies. The main advantage in the use of a chelator on the surface of the nanoparticle is that it allows multifunctionalization of the nano-radiotracer before the radiolabelling step. However, this technique requires a multiple-step synthesis and purification protocol.
To overcome the necessity of a multiple-step synthesis, other methods have appeared, called chelator-free synthesis.
2.2.3.2. Chelator-free strategy
Chelator-free approaches are mainly based on the addition of the radioisotope directly over the surface of the nanoparticle without the use of a chelator. Only a few examples based on this strategy have been reported. For example, one of them exploits the affinity of the magnetite towards arsenic for the synthesis of radioarsenic-labelled IONPs [53]. The mechanism of the sorption of the arsenic onto IONPs is known and it has been used in contaminated anoxic groundwaters to reduce the toxicity [54]. Another example of this approach is the synthesis of 69Ge radiolabelled IONPs. The incorporation of another germanium radioisotope such as 68Ge onto metal oxides is well known and it has been long used for the fabrication of 68Ge/68Ga generators [55,56]. In this case, the authors took the same idea as previously described in studies with 68Ge, to incorporate 69Ge directly on the surface of the nanoparticle without the help of chelators [57].
Albeit these methods are adequate for the radiolabelling of IONPs, there are some inherent drawbacks. Firstly, desorption of the radioisotope could be a problem for in vivo behaviour giving a low signal/noise ratio or toxicity problems when using isotopes like arsenic. Moreover, the existing methods are limited to radioisotopes without translational potential, which is the main disadvantage of the strategy.
2.2.4. Core labelling
Core labelling methodology is the newest approach for the production of chelator-free nano-radiotracers. This synthetic strategy combines non-radioactive precursor materials and a radioactive material to dope the core simultaneously with nanoparticle formation.
Hence, under appropriate conditions the radioisotope is entrapped inside the crystalline structure of the nanoparticle. Consequently, the location of the radioisotope (inside the core) ensures radiochemical stability, avoiding desorption or transmetallation reactions, making this approach very attractive, particularly in combination with isotopes of short half-life such as 68Ga. In fact, when the nanoparticle core starts to be degraded in vivo, the radioactivity has been long decayed.
Owing to the novelty of the method, as far as we know, there are only two examples of core-labelled IONPs described in the literature. Both of them expect microwave technology to be driving force of the synthesis. The reason why microwave technology is important in the process, lies in the reaction rate. Synthesis of IONPs by traditional methods like co-precipitation or thermal decomposition of organic precursors lasts from 3 to 24 h [58,59]. Common reaction times are unsuitable when a radioactive material is used as initial reagent. Microwave technology uses dielectric heating and can produce high-quality IONPs in shorter times, more adequate for this strategy [60–62]. Moreover, microwave technology provides synthetic methods with high reproducibility.
The first example of core-doped iron oxide nanoparticles was described in 2012 by Wong et al. [63]. In this work, the authors described a microwave-driven synthesis of IONPs doped with 64Cu. In this case, the radiolabelling yield was modest (33%) giving a low specific activity [63].
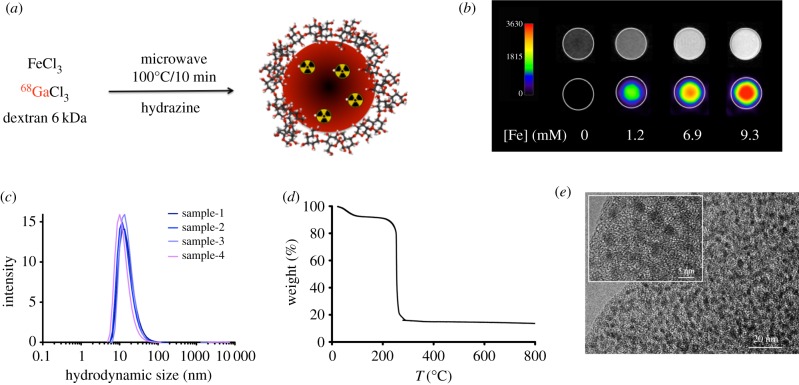
Very recently, our group described the synthesis of IONPs doped with a short half-life radioisotope such as 68Ga with microwave the driving force of the reaction (figure 4a). In 15 min overall time, our protocol retrieved 68Ga core-doped nanoparticles with very large radiolabelling yield (93%) and specific activity (7.6 GBq/mmol Fe). Furthermore, the particles showed large r1 value, which enables their use as T1-weighted (positive) contrast agents for MRI. This was the first example of the combination of IONPs for PET/(T1) MRI (figure 4b). One of the key aspects on this approach is the reproducibility (figure 4c) and the thick organic layer (figure 4d) that guarantees very good colloidal stability and large number of functional groups for further functionalization for such extremely small nanoparticles of 2.5 nm core size (figure 4e).
Figure 4.
(a) Synthesis of 68Ga core-doped IONPs; (b) image phantoms obtained at different iron concentrations by MRI (top row) and PET (bottom row); (c) hydrodynamic size of four different samples of 68Ga core-doped IONPs; (d) thermogravimetric curve of 68Ga core-doped IONPs and (e) TEM images of the sample. Adapted from [64].
2.3. Applications of iron oxide nanoparticles as multimodal imaging probes
2.3.1. Nanotechnology for cancer
In the past two decades, there have been countless discoveries regarding molecular bases of cancer; however, very few of them endure the vigorous clinical trials they must undergo in order to become useful in clinics [65]. Furthermore, there is still an absence of implementations to directly study molecular events in depth. For this reason, attention of oncologists and researchers is drawn to the development of novel approaches and techniques to improve outcomes [66,67]. Nanotechnology, as an interdisciplinary field involving biology, chemistry, engineering, medicine and physics, offers a wide variety of advantages in cancer diagnosis and therapy [34,68–70]. Multimodal therapy, early diagnosis and image-guided surgery or therapy are some examples of the same [66,71].
In this direction, IONPs have been widely used as MRI contrast agents as well as an excellent platform to which radionuclides can be incorporated, finally enabling an engineered molecular probe for dual imaging.
For example, radiolabelled poly(aspartic acid)-coated and PLGA-coated IONPs were used in two different studies for PET/MRI scanner of tumour integrin αvβ3 expression [72,73]. IONPs were coupled to RGD peptide and DOTA chelator prior to labelling with 64Cu. The probe was proved to be tumour-specific by both PET and MRI.
Another example of IONP-based dual probes for PET/MRI is represented by 124I-labelled IONPs. These nanoparticles were coated with serum albumin to secure high colloidal stability in a wide pH range and at high salt concentrations. They were used to track lymph nodes, crucial trademarks for cancer staging.
Likewise, Yang et al. [74] chose to monitor lymph node uptake of 68Ga doped IONPs for T2-weighted MR imaging and PET. Multimodal mannose IONPs containing NOTA chelator were radiolabelled with 68Ga. In vivo imaging showed these complexes could be specifically taken up by macrophages in lymph nodes.
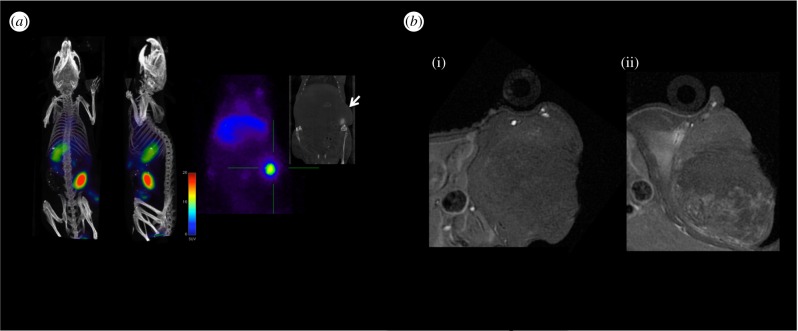
Very recently, Pellico et al. reported the practical synthesis of chelator-free iron oxide-based nanotracers labelled with 68Ga. In more detail, dextran-coated IONPs were core-doped with the radioisotope by a microwave-driven protocol, allowing an efficient and rapid synthesis [64]. These 68Ga core-doped IONPs (68Ga-C-IONP) were conjugated to RGD and assessed in an angiogenesis murine model. In vivo experiments confirm their specific accumulation in the tumour, by both PET and MRI. Figure 5 shows how the extremely high sensitivity of PET nicely matches the high resolution of MRI. Plus the core doping of the nanomaterial easily enables studying the biodistribution by gammacounter with sensitivity unparalleled by any other technique.
Figure 5.
(a) PET/CT imaging of tumour-bearing mice 1 h post injection of 68Ga-C-IONP showing activity in tumour. (b) Axial T1-weighted MRI of tumour area in a murine model previous to injection (i) and 24 h post injection of 68Ga-C-IONP (ii). Adapted from [64].
2.3.2. Nanotechnology for cardiovascular diseases
CVD encompasses all diseases concerning the heart and circulation, including atherosclerosis, coronary heart disease, angina, heart attack, congenital heart disease and stroke [75]. It is the leading cause of death globally and usually stems from vascular dysfunctions. Major advances in treating these diseases have taken place in the past few decades [76], mainly regarding early diagnosis in which new imaging approaches play a crucial role. Molecular imaging allows aiming at specific molecular targets, biological processes and certain cell types, providing a valuable insight into molecular and cellular mechanisms [77]. Nonetheless, molecular imaging requires extremely sensitive and specific agents which should include a signal detection compound and an affinity ligand which directs it to the intended site. As a result, nanoparticles have gained significant interest as agents for cardiovascular imaging and therapy [78]. Nanoconjugates arise as platforms for multiple entity integration, including targeting ligands, therapeutic agents and contrast materials. Different kinds of NPs have been used for imaging and therapy of different CVD.
Ischaemic and infarction lesions have been monitored and treated with several radiolabelled polymeric conjugates in different studies. Polyethylene glycol/phosphatidyl-ethanolamine (PEG-PE). 111In-labelled micelles were successfully used by Lukyanov et al. [79] to passively target infarcted myocardium in rabbit models, taking advantage of enhanced permeability and retention (EPR) effect. Radiolabelled lipoprotein-composed nanoparticles (LDL and HDL) have been used to monitor lipoprotein circulation and uptake in atheromatous lesions [80]. Other radiolabelled NP types such as dextran NPs, dendrimers and polymeric NPs have also been reported as agents for atherosclerosis detection. Their targeting at inflammatory cells has confirmed their virtue as effective agents for intraplaque inflammation detection and may allow targeted drug delivery and release to stabilize plaques before they rupture and originate severe vascular events. Examples of these are the 64Cu-labelled dendrimers used by Seo et al. to target macrophages with LyP-1 cyclic peptide [81]. 64Cu was also chosen by Luehmann et al. [82] to label comb-like polymer NPs targeted towards chemokine receptor 5 (CCR5), which has been reported to be an active participant in late stages of atherosclerosis.
IONPs have been reportedly used in a variety of CVDs. A multifunctional probe composed by dextran-coated cross-linked IONPs labelled with a near-infrared fluorochrome and 18F radionuclide for PET has been studied as a blood pool contrast agent detectable by PET fluorescence molecular tomography and MR imaging [83].
Radiolabelled IONPs have also been used for inflammatory cell imaging. Dextran-coated cross-linked IONPs labelled with 64Cu were used by Ueno et al. [84] to quantify myeloid cell infiltration in murine cardiac allografts. The same group previously used 18F-labelled cross-linked IONPs to target macrophages and monocytes in a murine model of aortic aneurysm in order to determine its dimensional stability. Jung et al. [85] carried out a study to assess the capability of HDL conjugates to assess atherosclerotic lesions in murine models by multimodal detection. Three different HDL-coated NPs were used for this purpose. Firstly, HDL-QD conjugates composed by Cd/Se/CdS/ZnS core–shell–shell and coated with HDL for optical imaging were obtained. A second probe, to be used as MRI contrast agent, was obtained using superparamagnetic IONP core and HDL coating (HDL-SPIO). The third probe type was composed of HDL-SPIO radiolabelled with 59Fe, to be used in MRI and to detect radioactivity using a gamma counter. Biodistribution of these conjugates was studied using the different detection techniques. The similarities of HDL-NP conjugates with respect to endogenous HDL allowed quantification of radiolabelled complexes' accumulation in atherosclerotic plaques through MRI, X-ray fluorescence microscopy, confocal fluorescence microscopy and light microscopy.
3. Liposomes for multimodal theranosis
3.1. General properties of liposomes
Liposomes have gained large attention in the nanomedicine field for research and clinical applications [86,87]. They are vesicles consisting of amphiphilic phospholipids (e.g. phosphatidylcholine, phosphatidyl-ethanolamine, phosphatidylserine and phosphatidylglycerol) that form a lipid bilayer enclosing an aqueous core. Hence, they can encapsulate both hydrophobic and hydrophilic molecules that will be arranged in the opportune compartment (figure 6). This is an interesting advantage because biomolecules/drugs of different nature can be encapsulated together or alone in these interesting nanosystems depending on their characteristics. Other advantages of liposomes consist of degradation prevention of the incorporated biomolecule, reduction of drug toxicity with improved efficacy and therapeutic effect, versatility and biocompatibility [86,87].
Figure 6.
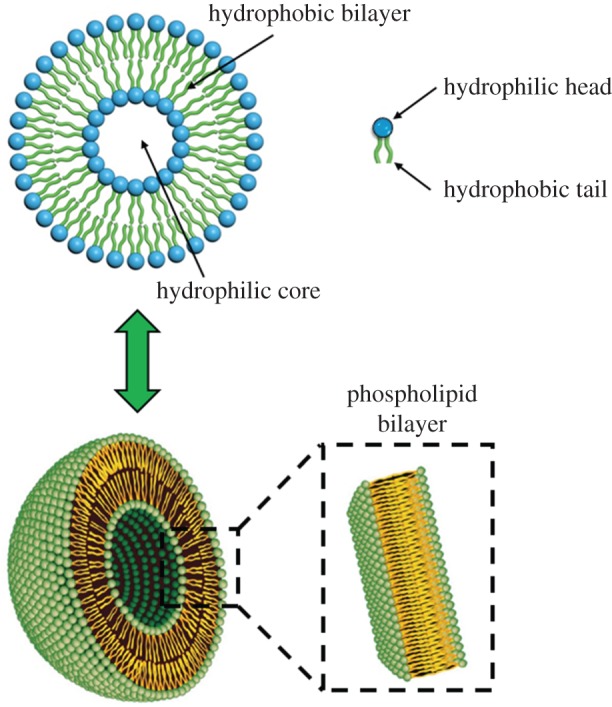
General scheme of liposomes. (Online version in colour.)
Liposome surface can be functionalized to enhance the control of their pharmacokinetics and biodistribution. For example, polyethylene glycol (PEG) can be used to increase the nanosystem lifetime in the blood or targeting molecules can be anchored on the liposome surface promoting their directing to target cells. These allow the nanosystem to recognize the microenvironment and react in a dynamic way mimicking the response of living organisms and permitting the drug release at the selected place by time-controlled mechanism [88].
For all these reasons liposomes have been widely studied, overall as drug delivery systems, as reported in several reviews [86–94]. Moreover, they attracted the attention of pharmaceutical industries as demonstrated by the approval of several liposomal drug formulations for cancer therapies or other diseases (infections, pain, meningitis, hepatitis A, influenza, etc.; table 2). Besides the approved ones, other liposome formulations are undergoing clinical trials, more of them for cancer treatment [86]. Examples of liposome-based formulation that are actually in trial phase III for cancer treatment are represented by ThermoDox (for the release of doxorubucin in breast cancer) [105] and Lipoplatin (for the controlled release of cisplatin in pancreatic, breast, non-small cell lung, head and neck cancers) [106–110].
Table 2.
Commercial liposome-based drug delivery systems.
| product name | encapsulated drug | approved treatment |
|---|---|---|
| Myocet | doxorubicin | metastatic breast cancer [95] |
| Doxil | doxorubicin | Kaposi's sarcoma, ovarian and breast cancer [96–98] |
| Lipodox | doxorubicin | Kaposi's sarcoma, ovarian and breast cancer [99] |
| Marqibo | vincristine sulfate | acute lymphoblastic leukaemia [100] |
| DaunoXome | daunorubicin | blood tumours [101] |
| Epaxal | inactivated hepatitis A virus | hepatitis A [102] |
| DepoDur | morphine sulfate | pain management [103] |
| DepoCyt | cytarabine | neoplastic meningitis and lymphomatous meningitis [104] |
3.2. Methods of liposome preparation
Several methods reporting the preparation of liposomes with numerous variants have been reported [86,87]. On continuation, a description of the most common used methods for the development of these nanosystems will be briefly reviewed.
The easier and older method, described in 1965, is the Bangham method or thin lipid film hydration method [111]. It consists of the formation of a thin film of lipids after evaporation of organic solvents followed by freeze-drying to ensure solvents complete elimination. This film is then rehydrated by aqueous solvents. The molecules that must be incorporated inside the liposome can be solubilized in organic or aqueous medium depending on their nature. To reduce the size of the prepared liposomes (less than or equal to 200 nm) sonication, homogenization or extrusion methods are used, depending on the final desired size.
An alternative method is the reverse phase evaporation technique. Inverted micelles or water-in-oil emulsions containing the molecule of interest in the aqueous phase and lipids composing the organic phase are initially formed [82,87]. The slow elimination of the organic solvent leads to the formation of liposomes. A higher internal aqueous loading with respect to the anterior method is obtained. The excess of solvent can be eliminated by dialysis, centrifugation or gel filtration [112].
Another method is the solvent injection technique. This is based on the injection of phospholipids dissolved in organic solvent (ethanol or ether) into an excess of aqueous solution containing the drug [113,114].
Also other novel methods for the preparation of liposomes such as double emulsion, freeze–thaw, dehydration–rehydration or fast-extrusion have been described [87]. For example, very recently, a technique based on the use of supercritical carbon dioxide has been developed [115].
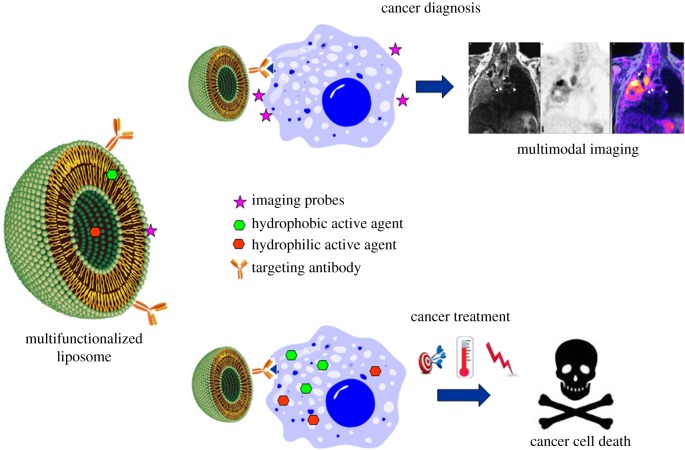
3.3. Multifunctional liposomes
Besides their use as drug delivery systems, recently, the liposomes were considered in order to develop patient personalized therapies, to learn about genetic makeup of the subject and how the specific tumour is evolving [116–118]. By this way prevention, screening and treatment strategies should be more effective and the side effects reduced. Thus, the resistance problems associated with the use of a single therapeutic strategy, causing the failure of the treatment, can be overcome [119].
For this purpose, different strategies could be adopted. Nanosystems could be employed as theranostic agents or to induce multiactive therapies [117]. In the first case, tumour diagnosis and treatment can be obtained by the same formulation. With the second approach the disease can be monitored and fought by the synergistic effect of more than one therapy (figure 7).
Figure 7.
Schematic of multifunctionalized liposome and its application for cancer diagnosis and therapy.
Examples of multifunctionalized liposome preparation and application to obtain a personalized cancer therapy are reported in the following paragraphs.
3.3.1. Theranostic liposomes
MRI-guided drug delivery is a new approach to obtain a personalized therapy combining cancer diagnosis with therapy accompanied by monitoring of clinical response in real time.
A theranostic liposomal system for lung cancer including both hydrophilic (carboplatin) and hydrophobic (paclitaxel) drugs was designed by Ren et al. [120]. The imaging ability was assured by the presence of a T1-contrast agent (gadodiamide) and a fluorescent molecule (rhodamine). The surface was functionalized with a targeting peptide (c(RGDyK)) specific for receptors overexpressed in many tumour cells. After liposome internalization by endocytosis, its payload was released enhancing diagnostic and therapeutic results. Hence, the cancer cells were firstly detected by MRI and confocal microscopy. Owing to the architecture of the system, it was proved that the tumour signal was enhanced in comparison with commercial contrast agent Omniscan® (figure 8), and liposome biodistribution in vivo imaged via T1-weighted MRI in real time and simultaneous chemotherapeutic effect was shown [120].
Figure 8.
MRI analysis of the tumours after treatment with the developed multifunctionalized liposome compared with Omniscan®. (a) Increase of T1 relaxation rate (R1) over time. (b) R1 was enhanced 11- and 36-fold, respectively, 3.7 h and 4.7 h after injection with respect the commercial formulation Omniscan®. Adapted from [120].
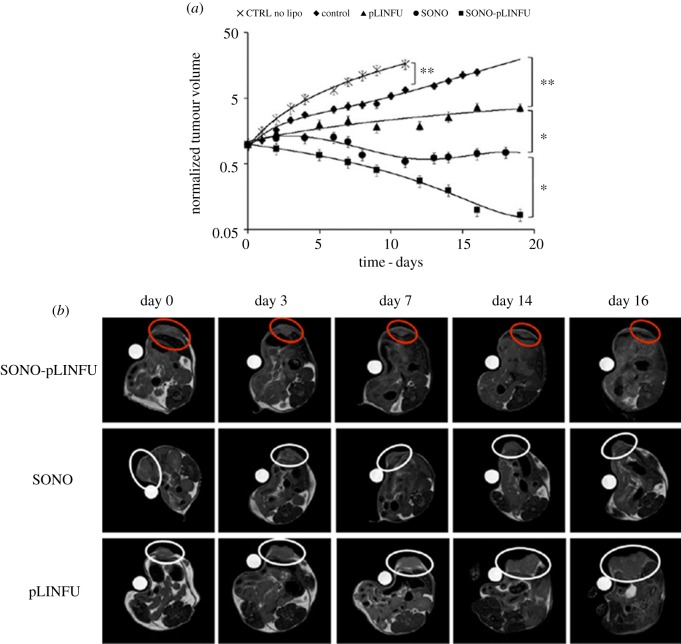
In another work, an MRI-based theranostic liposome was developed for the diagnosis and treatment of breast cancer [121]. A hydrophobic chemotherapeutic drug (doxorubicin) and a hydrophilic contrast agent (gadoteridol) were co-encapsulated inside a liposome. This nanosystem was able to release its content into the tumour cells. Thanks to the presence of the contrast agent it was possible to visualize the doxorubicin release by MRI. The therapeutic efficacy of the drug was improved by two different techniques based on pulsed US. More in detail, the US waves acted at the same time as stimuli useful to trigger the drug release as well as sonoporation stimulus enhancing tumour vascular permeability to the drug. A marked increase of drug concentration in the cancer cells followed by a complete tumour regression was so obtained (figure 9).
Figure 9.
MRI images of (a) tumour progression; (b) US-treated mice. The images were acquired at the time of the first MRI session (day 0) and after 3, 7, 14 and 16 days. ‘Sonoporation stimulus’ (SONO) was applied during the liposome injection, while the ‘release stimulus’ (pLINFU) was applied just after sonoporation. CTRL no LIPO refers to a group of mice that was not injected with liposomes. Adapted from [121].
Theranostic liposomes were also developed using T2-weighted contrast agents such as magnetic nanoparticles (MNPs). The unique property of magnetism of these particles allows an active targeting to the desired cells by using a permanent magnetic field. Carboxymethyldextran-coated magneto liposomes were hence prepared demonstrating their utility in diagnostic/therapeutic efficacy for some cancers such as brain cancer. Doxorubicin and MNPs were loaded into liposomes and released at target cells through pH- and magnetic-dependent mechanisms [122].
3.3.2. Multitherapeutic liposomes
3.3.2.1. Emergent cancer treatments
As previously mentioned, the synergistic effect of more than one therapy can greatly improve the efficacy of the cancer defeat. Beside the conventional methods such as chemotherapy, other novel techniques have been using for the treatment of tumours. Among these, magnetic hyperthermia, photodynamic therapy (PDT) or immunotherapy have been rapidly emerging due to their high therapeutic potential.
Hyperthermia is an emergent and particularly attractive strategy based on heat generation by MNPs on the tumour site presenting fewer side effects compared with chemo- and radiotherapy and that can be used in combination with all conventional therapeutic treatments. The mechanism of hyperthermia relies on selective tumoural cell heating (in the temperature range of 41–46°C) resulting in the activation of natural intracellular and extracellular degradation mechanisms that finally lead to apoptosis with cancer cell death [123,124].
PDT is another modality that in the last decades was surprisingly developed in cancer treatment. This is a clinically approved therapeutic modality based on photo-oxidation of biological materials induced by photosensitizers (PSs) (localized selectively in certain cells or tumoural tissues) activated by a light with appropriate wavelength and in sufficient doses upon irradiation. The activated PS transfers its excited-state energy to surrounding oxygen, resulting in reactive oxygen species, such as singlet oxygen or free radicals, finally causing tumoural cell death with minimal healthy tissue damage [125–127].
Recently, the interest in cancer immunotherapy that stimulates the immune system cells to fight the disease and pursues an opposite strategy with respect to conventional treatments is gaining tremendous interest. This therapeutic strategy does not affect tumoural cells directly, but it activates patient T lymphocytes becoming able to destroy the tumour. Immunotherapy can be obtained by different approaches: cytokines, tumour antigen-targeted monoclonal antibodies, immunological checkpoint inhibitors and therapeutic cancer vaccines. Each of these treatment types has a distinct mechanism of action; however, they all are designed to boost or restore immune function in some manner [107,128–130].
3.3.2.2. Application of liposome as multiactive systems
As clearly stated above, liposomes are very suitable nanocarriers to be employed as multitherapeutic systems.
For example, Pradhan et al. [131] developed thermosensitive magnetic liposomes using MNPs for hyperthermia-triggered drug release. Liposomes based on thermosensitive PEG were prepared encapsulating IONPs and doxorubicin. Folic acid was conjugated on the surface as targeting molecules for their affinity with folate receptors present on cancer cells. The nanosystems were directed to cancer cells for the presence of a permanent magnetic field, and after internalization an alternating magnetic field was applied to induce MNP heating useful for MNPs and drug release as well as for magnetic hyperthermia. Hence, hyperthermia and chemotherapy treatments were simultaneously promoted and a synergistic effect between the two therapies was observed [131]. Similar thermosensitive liposomes have been prepared to combine photothermal therapy (PTT) and PDT [132]. PTT is nowadays attracting attention due to the possibility of controlling the incorporation of light-activated heating nanoparticles into tumours with consequent high heat deposition in the tumour area at low laser intensities, minimizing the damage in the surrounding healthy tissue [133,134].
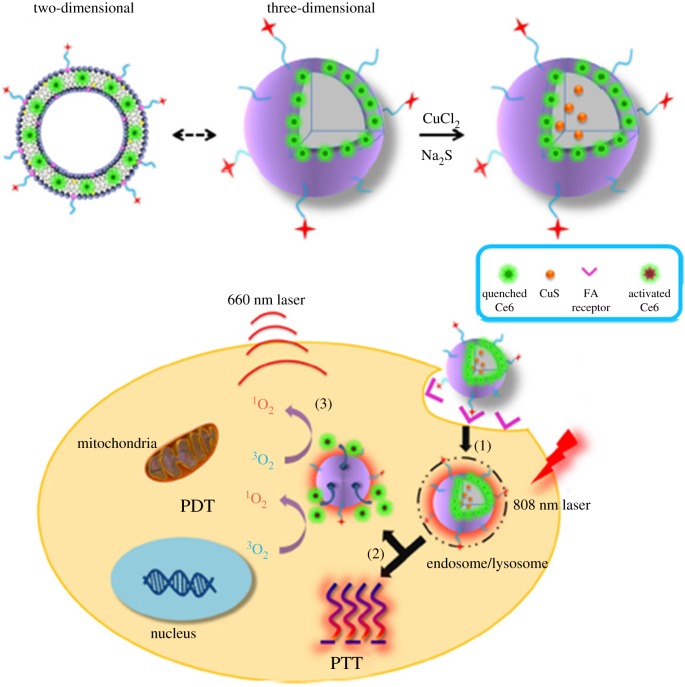
In more detail, a photosensitizer (Ce6) was incorporated in the lipid bilayer and copper sulfide (CuS) was encapsulated into the aqueous compartment of the liposome [132]. After the targeted delivery of the photosensitizer into the cancer cells, CuS was activated by laser irradiation inducing PTT-mediated cell killing and at the same time degradation of the liposome. The released Ce6 performed PDT obtaining a synergistic effect with PTT to kill tumour cells (figure 10).
Figure 10.
Schematic of thermosensitive liposome encapsulating Ce6 and CuS and its working mechanism; (1) targeting delivery and cell uptake, (2) 808 nm NIR laser-induced PTT effect and PTT-induced Ce6 release and (3) 660 nm laser-induced PDT effect. Adapted from [132].
PDT was also associated with chemotherapy to obtain more effective combinations against cancer in the clinic. For example, a novel PEGylated liposome system, named DAFODIL incorporating doxorubicin and 5-flurouracil, was developed by Camacho et al. [135]. This nanosystem offered superior therapeutic efficacies compared with free drug administrations and less cytotoxicity. The synergistic effect led to a high reduction (90%) in the tumour growth of murine 4T1 mammary carcinoma in vivo [135]. Example of immunotherapy combined with PDT was reported by Mir et al. [136]. A photo-immuno-conjugate-associating liposome incorporating the photosensitizer benzoporphyrin derivative monoacid A and the FDA-approved Cetuximab antibody for epidermal growth factor receptor (EGFR) was prepared. By means of this engineered nanosytem, the inhibition of EGFR signalling enhanced PDT-mediated ovarian cancer cell death and the overall synergistic and preferential phototoxicity in an ovarian cancer cell model in vitro [136].
In this line, Meraz et al. [137] prepared cationic liposomes containing monophosphoryl lipid A (MPL) and interleukin (IL)-12 to demonstrate that the intratumoural administration produced a regression of breast tumour and a systemic immune response. Cationic liposomes have inherent cytotoxicity and the presence of MPL allows recruiting and activation of immune cells besides the cytokine release supporting tumour regression. To improve the immune response, the IL-12 was added to the MPL liposome. Combined therapy of the liposome incorporating MPL and IL-12 was superior to the activity of any single inhibiting agent and—thanks to the intratumoural administration—tumour growth (4T1 mouse model of breast cancer) was completely blocked. This combined liposomal therapy was able to induce similar reductions in tumour growth in both treated and distal tumours, suggesting a systemic immune response [137].
4. Conclusion
In this review, we discussed recent advances concerning the preparation and application of different multifunctional nanocarriers as molecular imaging-based theranostics. The achieved development in nanomaterial-based combination of therapy and multimodal imaging here reported have shown several unique features that are untenable in traditional medicine. Moreover, the approach of using nanomaterials for specific targeting, molecular imaging and selective therapy has been shown to be both general as well as versatile. This rapidly evolving discipline demonstrates possessing potential to play key roles in every aspect of clinical practice, including early disease detection, diagnosis, staging, personalized treatment, treatment monitoring and follow-up. Those synergistic ‘multiple-in-one’ modalities make personalized and integrated therapy feasible and extremely promising.
Nevertheless, in order to ensure confidence in translating nanomaterials into clinical applications and achieve their definitive utilization, several crucial aspects must be still addressed. Firstly, standard synthetic protocols, measurements and techniques which are necessary for producing and defining a nanoparticle system and for quality control must be unequivocally defined (nanoparticle metrology). Furthermore, nanotoxicity (size-dependent toxicity is one of the most critical issues) and nanomaterial metabolism (the in vivo metabolic pathway of nanomaterials) are still not fully biologically understood.
To this scope, we consider that a much stronger involvement of cross-disciplinary researchers (i.e. biologists, pathologists, chemists, material scientists, physicians and engineers) will undoubtedly address these challenges and finally grant the definitive clinical application of nanomedicine.
Competing interests
We declare we have no competing interests.
Funding
The CNIC is supported by the Spanish Ministry of Economy and Competitiveness (MINECO) and the Pro CNIC Foundation and by Severo Ochoa Center of Excellence (MINECO award SEV-2015-0505). We also thank MINECO for grant MAT2013-47303-P, SAF2016-79593-P and for the research grant no. SAF2014-59118-JIN (‘Proyectos de I+D+i para jóvenes investigadores’, 2014) also co-funded by Fondo Europeo de Desarrollo Regional (FEDER).
References
- 1.Schork NJ. 2015. Personalized medicine: time for one-person trials. Nature 520, 609–611. ( 10.1038/520609a) [DOI] [PubMed] [Google Scholar]
- 2.Wistuba II, Gelovani JG, Jacoby JJ, Davis SE, Herbst RS. 2011. Methodological and practical challenges for personalized cancer therapies. Nat. Rev. Clin. Oncol. 8, 135–141. ( 10.1038/nrclinonc.2011.2) [DOI] [PubMed] [Google Scholar]
- 3.Chan IS, Ginsburg GS. 2011. Personalized medicine: progress and promise. Annu. Rev. Genomics Hum. Genet. 12, 217–244. ( 10.1146/annurev-genom-082410-101446) [DOI] [PubMed] [Google Scholar]
- 4.Kircher MF, Hricak H, Larson SM. 2012. Molecular imaging for personalized cancer care. Mol. Oncol. 6, 182–195. ( 10.1016/j.molonc.2012.02.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willmann JK, van Bruggen N, Dinkelborg LM, Gambhir SS. 2008. Molecular imaging in drug development. Nat. Rev. Drug Discov. 7, 591–607. ( 10.1038/nrd2290) [DOI] [PubMed] [Google Scholar]
- 6.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. 2007. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2, 751–760. ( 10.1038/nnano.2007.387) [DOI] [PubMed] [Google Scholar]
- 7.Moghimi SM, Hunter AC, Murray JC. 2005. Nanomedicine: current status and future prospects. FASEB J. 19, 311–330. ( 10.1096/fj.04-2747rev) [DOI] [PubMed] [Google Scholar]
- 8.Lee DE, Koo H, Sun IC, Ryu JH, Kim K, Kwon IC. 2012. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem. Soc. Rev. 41, 2656–2672. ( 10.1039/c2cs15261d) [DOI] [PubMed] [Google Scholar]
- 9.Huang Y, He S, Cao W, Cai K, Liang XJ. 2012. Biomedical nanomaterials for imaging-guided cancer therapy. Nanoscale 4, 6135–6149. ( 10.1039/c2nr31715j) [DOI] [PubMed] [Google Scholar]
- 10.Lee SY, Jeon SI, Jung S, Chung IJ, Ahn CH. 2014. Targeted multimodal imaging modalities. Adv. Drug Deliv. Rev. 76, 60–78. ( 10.1016/j.addr.2014.07.009) [DOI] [PubMed] [Google Scholar]
- 11.Shin TH, Choi Y, Kim S, Cheon J. 2015. Recent advances in magnetic nanoparticle-based multi-modal imaging. Chem. Soc. Rev. 44, 4501–4516. ( 10.1039/c4cs00345d) [DOI] [PubMed] [Google Scholar]
- 12.Nazir S, Hussain T, Ayub A, Rashid U, MacRobert AJ. 2014. Nanomaterials in combating cancer: therapeutic applications and developments. Nanomedicine 10, 19–34. ( 10.1016/j.nano.2013.07.001) [DOI] [PubMed] [Google Scholar]
- 13.Lee N, Yoo D, Ling D, Cho MH, Hyeon T, Cheon J. 2015. Iron oxide based nanoparticles for multimodal imaging and magnetoresponsive therapy. Chem. Rev. 115, 10 637–10 689. ( 10.1021/acs.chemrev.5b00112) [DOI] [PubMed] [Google Scholar]
- 14.Sharma P, Allison JP. 2015. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 161, 205–214. ( 10.1016/j.cell.2015.03.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castellano JM, Bueno H, Fuster V. 2015. The cardiovascular polypill: clinical data and ongoing studies. Int. J. Cardiol. 201, S8–S14. ( 10.1016/s0167-5273(15)31027-5) [DOI] [PubMed] [Google Scholar]
- 16.Lonn E, Bosch J, Teo KK, Pais P, Xavier D, Yusuf S. 2010. The polypill in the prevention of cardiovascular diseases: key concepts, current status, challenges, and future directions. Circulation 122, 2078–2088. ( 10.1161/CIRCULATIONAHA.109.873232) [DOI] [PubMed] [Google Scholar]
- 17.Hu C-MJ, Aryal S, Zhang L. 2010. Nanoparticle-assisted combination therapies for effective cancer treatment. Ther. Deliv. 1, 323–334. ( 10.4155/tde.10.13) [DOI] [PubMed] [Google Scholar]
- 18.Talelli M, Aires A, Marciello M. 2016. Protein-modified magnetic nanoparticles for biomedical applications. Curr. Org. Chem. 20, 1252–1261. ( 10.2174/1385272819666150810221009) [DOI] [Google Scholar]
- 19.Marciello M, Connord V, Veintemillas-Verdaguer S, Vergés MA, Carrey J, Respaud M, Serna CJ, Morales MP. 2013. Large scale production of biocompatible magnetite nanocrystals with high saturation magnetization values through green aqueous synthesis. J. Mat. Chem. B 1, 5995–6004. ( 10.1039/c3tb20949k) [DOI] [PubMed] [Google Scholar]
- 20.Etheridge ML, Campbell SA, Erdman AG, Haynes CL, Wolf SM, McCullough J. 2013. The big picture on nanomedicine: the state of investigational and approved nanomedicine products. Nanomedicine 9, 1–14. ( 10.1016/j.nano.2012.05.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kranz LM, et al. 2016. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534, 396–401. ( 10.1038/nature18300) [DOI] [PubMed] [Google Scholar]
- 22.Yang X, et al. 2011. CRGD-functionalized, DOX-conjugated, and 64Cu-labeled superparamagnetic iron oxide nanoparticles for targeted anticancer drug delivery and PET/MR imaging. Biomaterials 32, 4151–4160. ( 10.1016/j.biomaterials.2011.02.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouziotis P, Psimadas D, Tsotakos T, Stamopoulos D, Tsoukalas C. 2012. Radiolabeled iron oxide nanoparticles as dual-modality SPECT/MRI and PET/MRI agents. Curr. Top. Med. Chem. 12, 1–9. ( 10.2174/1568026611212230007) [DOI] [PubMed] [Google Scholar]
- 24.Schlachter EK, et al. 2011. Metabolic pathway and distribution of superparamagnetic iron oxide nanoparticles: in vivo study. Int. J. Nanomed. 6, 1793–1800. ( 10.2147/IJN.S23638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang XD, et al. 2011. Size-dependent in vivo toxicity of PEG-coated gold nanoparticles. Int. J. Nanomed. 6, 2071–2081. ( 10.2147/IJN.S21657) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong H, Chen F, Cai W. 2013. Pharmacokinetic issues of imaging with nanoparticles: focusing on carbon nanotubes and quantum dots. Mol. Imaging Biol. 15, 507–520. ( 10.1007/s11307-013-0648-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glaus C, Rossin R, Welch MJ, Bao G. 2010. In vivo evaluation of 64Cu-labeled magnetic nanoparticles as a dual-modality PET/MR imaging agent. Bioconjug. Chem. 21, 715–722. ( 10.1021/bc900511j) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai W, Chen K, Li Z-B, Gambhir SS, Chen X. 2007. Dual-function probe for PET and near-infrared fluorescence imaging of tumor vasculature. J. Nucl. Med. 48, 1862–1870. ( 10.2967/jnumed.107.043216) [DOI] [PubMed] [Google Scholar]
- 29.Zhang R, Lu W, Wen X, Huang M, Zhou M, Liang D, Li C. 2011. Annexin A5-conjugated polymeric micelles for dual SPECT and optical detection of apoptosis. J. Nucl. Med. 52, 958–964. ( 10.2967/jnumed.110.083220) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi J-S, et al. 2008. A hybrid nanoparticle probe for dual-modality positron emission tomography and magnetic resonance imaging. Angew. Chem. Int. Ed. 47, 6259–6262. ( 10.1002/anie.200801369) [DOI] [PubMed] [Google Scholar]
- 31.Däpp S, Müller C, Garayoa E, Bläuenstein P, Maes V, Brans L, Tourwé DA, Schibli R. 2012. PEGylation, increasing specific activity and multiple dosing as strategies to improve the risk-benefit profile of targeted radionuclide therapy with 177Lu-DOTA-bombesin analogues. EJNMMI Res. 2, 24 ( 10.1186/2191-219X-2-24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Däpp S, Garayoa EG, Maes V, Brans L, Tourwé DA, Müller C, Schibli R. 2011. PEGylation of 99mTc-labeled bombesin analogues improves their pharmacokinetic properties. Nucl. Med. Biol. 38, 997–1009. ( 10.1016/j.nucmedbio.2011.02.014) [DOI] [PubMed] [Google Scholar]
- 33.de Barros A, Tsourkas A, Saboury B, Cardoso V, Alavi A. 2012. Emerging role of radiolabeled nanoparticles as an effective diagnostic technique. EJNMMI Res. 2, 39 ( 10.1186/2191-219X-2-39) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong H, Zhang Y, Sun J, Cai W. 2009. Molecular imaging and therapy of cancer with radiolabeled nanoparticles. Nano Today 4, 399–413. ( 10.1016/j.nantod.2009.07.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thorek DLJ, Chen AK, Czupryna J, Tsourkas A. 2006. Superparamagnetic iron oxide nanoparticle probes for molecular imaging. Ann. Biomed. Eng. 34, 23–38. ( 10.1007/s10439-005-9002-7) [DOI] [PubMed] [Google Scholar]
- 36.Beyer T, et al. 2000. A combined PET/CT scanner for clinical oncology. J. Nucl. Med. 41, 1369–1380. [PubMed] [Google Scholar]
- 37.Lee N, Hyeon T. 2012. Designed synthesis of uniformly sized iron oxide nanoparticles for efficient magnetic resonance imaging contrast agents. Chem. Soc. Rev. 41, 2575–2589. ( 10.1039/C1CS15248C) [DOI] [PubMed] [Google Scholar]
- 38.Zhao Z, et al. 2013. Octapod iron oxide nanoparticles as high-performance T2 contrast agents for magnetic resonance imaging. Nat. Commun. 4, 2266 ( 10.1038/ncomms3266) [DOI] [PubMed] [Google Scholar]
- 39.Xiao N, Gu W, Wang H, Deng Y, Shi X, Ye L. 2014. T1–T2 dual-modal MRI of brain gliomas using PEGylated Gd-doped iron oxide nanoparticles. J. Colloid Interface Sci. 417, 159–165. ( 10.1016/j.jcis.2013.11.020) [DOI] [PubMed] [Google Scholar]
- 40.Lin C, Cai S, Feng J. 2012. Positive contrast imaging of SPIO nanoparticles. J. Nanomater. 2012, 1–9. ( 10.1155/2012/734842) [DOI] [Google Scholar]
- 41.Zahraei M, et al. 2016. Versatile theranostics agents designed by coating ferrite nanoparticles with biocompatible polymers. Nanotechnology 27, 25 5702–255 714. ( 10.1088/0957-4484/27/25/255702) [DOI] [PubMed] [Google Scholar]
- 42.Bhavesh R, Lechuga-Vieco AV, Ruiz-Cabello J, Herranz F. 2015. T1-MRI fluorescent iron oxide nanoparticles by microwave assisted synthesis. Nanomaterials 5, 1880–1890. ( 10.3390/nano5041880) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Z, et al. 2013. Engineered iron-oxide-based nanoparticles as enhanced T1 contrast agents for efficient tumor imaging. ACS Nano 7, 3287–3296. ( 10.1021/nn305991e) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valk PE, Delbeke D, Bailey DL, Townsend DW, Maisey MN. 2010. Positron emission tomography: clinical practice. London, UK: Springer-Verlag ( 10.1007/1-84628-187-3) [DOI] [Google Scholar]
- 45.Fani M, André JP, Maecke H. 2008. 68 Ga-PET: a powerful generator-based alternative to cyclotron-based PET radiopharmaceuticals. Contrast Media Mol. Imaging 3, 53–60. ( 10.1002/cmmi.232) [DOI] [PubMed] [Google Scholar]
- 46.Velikyan I. 2015. 68Ga-based radiopharmaceuticals: production and application relationship. Molecules (Basel, Switzerland) 20, 12 913–12 943. ( 10.3390/molecules200712913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Breeman WAP, de Blois E, Sze Chan H, Konijnenberg M, Kwekkeboom DJ, Krenning EP. 2011. 68Ga-labeled DOTA-peptides and 68Ga labeled radiopharmaceuticals for positron emission tomography: current status of research, clinical applications, and future perspectives. Semin. Nucl. Med. 41, 314–321. ( 10.1053/j.semnuclmed.2011.02.001) [DOI] [PubMed] [Google Scholar]
- 48.Poeppel TD, Binse I, Petersenn S, Lahner H, Schott M, Antoch G, Brandau W, Bockisch A, Boy C. 2013. Differential uptake of 68Ga-DOTATOC and 68Ga-DOTATATE in PET/CT of gastroenteropancreatic neuroendocrine tumors. In Recent Results Cancer Res. 194, 353–371. ( 10.1007/978-3-642-27994-2_18) [DOI] [PubMed] [Google Scholar]
- 49.Kulkarni HR, Baum RP. 2014. Patient selection for personalized peptide receptor radionuclide therapy using Ga-68 somatostatin receptor PET/CT. PET Clin. 9, 83–90. ( 10.1016/j.cpet.2013.08.015) [DOI] [PubMed] [Google Scholar]
- 50.Poeppel TD, Binse I, Petersenn S, Lahner H, Schott M, Antoch G, Brandau W, Bockisch A, Boy C. 2011. 68Ga-DOTATOC versus 68Ga-DOTATATE PET/CT in functional imaging of neuroendocrine tumors. J. Nucl. Med. 52, 1864–1870. ( 10.2967/jnumed.111.091165) [DOI] [PubMed] [Google Scholar]
- 51.Rotello V. 2004. Nanoparticles. Berlin, Germany: Springer Science+Business Media. [Google Scholar]
- 52.Biener J, Wittstock A, Baumann TF, Weissmüller J, Bäumer M, Hamza AV. 2009. Surface chemistry in nanoscale materials. Materials 2, 2404–2428. ( 10.3390/ma2042404) [DOI] [Google Scholar]
- 53.Chen F, et al. 2013. Chelator-free synthesis of a dual-modality PET/MRI agent. Angew. Chem. Int. Ed. 52, 13 319–13 323. ( 10.1002/anie.201306306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morin G, Wang Y, Ona-Nguema G, Juillot F, Calas G, Menguy N, Aubry E, Bargar JR, Brown GE. 2009. EXAFS and HRTEM evidence for As(III)-containing surface precipitates on nanocrystalline magnetite: implications for As sequestration. Langmuir 25, 9119–9128. ( 10.1021/la900655v) [DOI] [PubMed] [Google Scholar]
- 55.Chakravarty R, Shukla R, Ram R, Tyagi AK, Dash A, Venkatesh M. 2011. Development of a nano-zirconia based 68Ge/68Ga generator for biomedical applications. Nucl. Med. Biol. 38, 575–583. ( 10.1016/j.nucmedbio.2010.10.007) [DOI] [PubMed] [Google Scholar]
- 56.de Blois E, Sze Chan H, Naidoo C, Prince D, Krenning EP, Breeman WAP. 2011. Characteristics of SnO2-based 68Ge/68Ga generator and aspects of radiolabelling DOTA-peptides. Appl. Radiat. Isot. 69, 308–315. ( 10.1016/j.apradiso.2010.11.015) [DOI] [PubMed] [Google Scholar]
- 57.Chakravarty R, Valdovinos HF, Chen F, Lewis CM, Ellison PA, Luo H, Meyerand ME, Nickles RJ, Cai W. 2014. Intrinsically germanium-69-labeled iron oxide nanoparticles: synthesis and in-vivo dual-modality PET/MR imaging. Adv. Mater. 26, 5119–5123. ( 10.1002/adma.201401372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim DK, Zhang Y, Voit W, Rao KV, Muhammed M. 2001. Synthesis and characterization of surfactant-coated superparamagnetic monodispersed iron oxide nanoparticles. J. Magn. Magn. Mater. 225, 30–36. ( 10.1016/S0304-8853(00)01224-5) [DOI] [Google Scholar]
- 59.Sun S, Zeng H. 2002. Size-controlled synthesis of magnetite nanoparticles. J. Am. Chem. Soc. 124, 8204–8205. ( 10.1021/ja026501x) [DOI] [PubMed] [Google Scholar]
- 60.Liao X, Zhu J, Zhong W, Chen HY. 2001. Synthesis of amorphous Fe2O3 nanoparticles by microwave irradiation. Mater. Lett. 50, 341–346. ( 10.1016/S0167-577X(01)00251-8) [DOI] [Google Scholar]
- 61.Wang W-W, Zhu Y-J, Ruan M-L. 2007. Microwave-assisted synthesis and magnetic property of magnetite and hematite nanoparticles. J. Nanoparticle Res. 9, 419–426. ( 10.1007/s11051-005-9051-8) [DOI] [Google Scholar]
- 62.Pellico J, Lechuga-Vieco AV, Benito M, García-Segura JM, Fuster V, Ruiz-Cabello J, Herranz F. 2015. Microwave-driven synthesis of bisphosphonate nanoparticles allows in vivo visualisation of atherosclerotic plaque. RSC Adv. 5, 1661–1665. ( 10.1039/C4RA13824D) [DOI] [Google Scholar]
- 63.Wong RM, Gilbert DA, Liu K, Louie AY. 2012. Rapid size-controlled synthesis of dextran-coated, 64Cu-doped iron oxide nanoparticles. ACS Nano 6, 3461–3467. ( 10.1021/nn300494k) [DOI] [PubMed] [Google Scholar]
- 64.Pellico J, et al. 2016. Fast synthesis and bioconjugation of 68Ga core-doped extremely small iron oxide nanoparticles for PET/MR imaging. Contrast Media Mol. Imaging 11, 203–210. ( 10.1002/cmmi.1681) [DOI] [PubMed] [Google Scholar]
- 65.Xing Y, Zhao J, Conti PS, Chen K. 2014. Radiolabeled nanoparticles for multimodality tumor imaging. Theranostics 4, 290–306. ( 10.7150/thno.7341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prasad PN. 2012. Introduction to nanomedicine and nanobioengineering. New York, NY: Wiley. [Google Scholar]
- 67.Enrique M-A, Mariana O-R, Mirshojaei SF, Ahmadi A. 2015. Multifunctional radiolabeled nanoparticles: strategies and novel classification of radiopharmaceuticals for cancer treatment. J. Drug Target 23, 191–201. ( 10.3109/1061186X.2014.988216) [DOI] [PubMed] [Google Scholar]
- 68.Cai W, Chen X. 2007. Nanoplatforms for targeted molecular imaging in living subjects. Small 3, 1840–1854. ( 10.1002/smll.200700351) [DOI] [PubMed] [Google Scholar]
- 69.Rudolph C, Gleich B, Flemmer AW. 2010. Cancer nanotechnology. Methods Mol. Biol. (Clifton NJ) 624, 267–280. ( 10.1007/978-1-60761-609-2) [DOI] [PubMed] [Google Scholar]
- 70.Cuenca AG, Jiang H, Hochwald SN, Delano M, Cance WG, Grobmyer SR. 2006. Emerging implications of nanotechnology on cancer diagnostics and therapeutics. Cancer 107, 459–466. ( 10.1002/cncr.22035) [DOI] [PubMed] [Google Scholar]
- 71.Chakravarty R, Hong H, Cai W. 2014. Positron emission tomography image-guided drug delivery: current status and future perspectives. Mol. Pharm. 11, 3777–3797. ( 10.1021/mp500173s) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee H-Y, Li Z, Chen K, Hsu AR, Xu C, Xie J, Sun S, Chen X. 2008. PET/MRI dual-modality tumor imaging using arginine-glycine-aspartic (RGD)–conjugated radiolabeled iron oxide nanoparticles. J. Nucl. Med. 49, 1371–1379. ( 10.2967/jnumed.108.051243) [DOI] [PubMed] [Google Scholar]
- 73.Aryal S, Key J, Stigliano C, Landis MD, Lee DY, Decuzzi P. 2014. Positron emitting magnetic nanoconstructs for PET/MR imaging. Small 10, 2688–2696. ( 10.1002/smll.201303933) [DOI] [PubMed] [Google Scholar]
- 74.Yang BY, Moon S-H, Seelam SR, Jeon MJ, Lee Y-S, Lee DS, Chung J-K, Kim YI, Jeong JM. 2015. Development of a multimodal imaging probe by encapsulating iron oxide nanoparticles with functionalized amphiphiles for lymph node imaging. Nanomedicine 10, 1899–1910. ( 10.2217/nnm.15.41) [DOI] [PubMed] [Google Scholar]
- 75.British Heart Foundation. Cardiovascular disease. See https://www.bhf.org.uk/heart-health/conditions/cardiovascular-disease.
- 76.Sanz J, Fayad ZA. 2008. Imaging of atherosclerotic cardiovascular disease. Nature 451, 953–957. ( 10.1038/nature06803) [DOI] [PubMed] [Google Scholar]
- 77.Jaffer FA, Libby P, Weissleder R. 2007. Molecular imaging of cardiovascular disease. Circulation 116, 1052–1061. ( 10.1161/CIRCULATIONAHA.106.647164) [DOI] [PubMed] [Google Scholar]
- 78.Stendahl JC, Sinusas AJ. 2015. Nanoparticles for cardiovascular imaging and therapeutic delivery, part 2: radiolabeled probes. J. Nucl. Med. 56, 1637–1641. ( 10.2967/jnumed.115.164145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lukyanov AN, Hartner WC, Torchilin VP. 2004. Increased accumulation of PEG–PE micelles in the area of experimental myocardial infarction in rabbits. J. Control Release 94, 187–193. ( 10.1016/j.jconrel.2003.10.008) [DOI] [PubMed] [Google Scholar]
- 80.Majmudar MD, et al. 2013. Polymeric nanoparticle PET/MR imaging allows macrophage detection in atherosclerotic plaques. Circ. Res. 112, 755–761. ( 10.1161/CIRCRESAHA.111.300576) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seo JW, Baek H, Mahakian LM, Kusunose J, Hamzah J, Ruoslahti E, Ferrara KW. 2014. 64Cu-labeled LyP-1-dendrimer for PET-CT imaging of atherosclerotic plaque. Bioconjug. Chem. 25, 231–239. ( 10.1021/bc400347s) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luehmann HP, Pressly ED, Detering L, Wang C, Pierce R, Woodard PK, Gropler RJ, Hawker CJ, Liu Y. 2014. PET/CT imaging of chemokine receptor CCR5 in vascular injury model using targeted nanoparticle. J. Nucl. Med. 55, 629–634. ( 10.2967/jnumed.113.132001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nahrendorf M, Keliher E, Marinelli B, Leuschner F, Robbins CS, Gerszten RE, Pittet MJ, Swirski FK, Weissleder R. 2011. Detection of macrophages in aortic aneurysms by nanoparticle positron emission tomography–computed tomography. Arterioscler. Thromb. Vasc. Biol. 31, 750–757. ( 10.1161/ATVBAHA.110.221499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ueno T, et al. 2013. Nanoparticle PET-CT detects rejection and immunomodulation in cardiac allografts. Circulation 6, 568–573. ( 10.1161/CIRCIMAGING.113.000481) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jung C, et al. 2014. Intraperitoneal injection improves the uptake of nanoparticle-labeled high-density lipoprotein to atherosclerotic plaques compared with intravenous injection: a multimodal imaging study in ApoE knockout mice. Circulation 7, 303–311. ( 10.1161/CIRCIMAGING.113.000607) [DOI] [PubMed] [Google Scholar]
- 86.Pattni BS, Chupin VV, Torchilin VP. 2015. New developments in liposomal drug delivery. Chem. Rev. 115, 10 938–10 966. ( 10.1021/acs.chemrev.5b00046) [DOI] [PubMed] [Google Scholar]
- 87.Nogueira E, Gomes AC, Preto A, Cavaco-Paulo A. 2015. Design of liposomal formulations for cell targeting. Colloid Surf. B 136, 514–526. ( 10.1016/j.colsurfb.2015.09.034) [DOI] [PubMed] [Google Scholar]
- 88.Mura S, Nicolas J, Couvreur P. 2013. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 12, 991–1003. ( 10.1038/NMAT3776) [DOI] [PubMed] [Google Scholar]
- 89.Tila D, Ghasemi S, Yazdani-Arazi SN, Ghanbarzadeh S. 2015. Functional liposomes in the cancer-targeted drug delivery. J. Biomater. Appl. 30, 3–16. ( 10.1177/0885328215578111) [DOI] [PubMed] [Google Scholar]
- 90.Eloy JO, Claro de Souza M, Petrilli R, Barcellos JPA, Lee RJ, Marchetti JM. 2014. Liposomes as carriers of hydrophilic small molecule drugs: strategies to enhance encapsulation and delivery. Colloid Surf. B 123, 345–363. ( 10.1016/j.colsurfb.2014.09.029) [DOI] [PubMed] [Google Scholar]
- 91.Bochicchio S, Dalmoro A, Barba AA, Grassi G, Lamberti G. 2014. Liposomes as siRNA delivery vectors. Curr. Drug Metab. 15, 882–892. ( 10.2174/1389200216666150206124913) [DOI] [PubMed] [Google Scholar]
- 92.Mallick S, Choi JS. 2014. Liposomes: versatile and biocompatible nanovesicles for efficient biomolecules delivery. J. Nanosci. Nanotechnol. 14, 755–765. ( 10.1166/jnn.2014.9080) [DOI] [PubMed] [Google Scholar]
- 93.Du AW, Stenzel MH. 2014. Drug carriers for the delivery of therapeutic peptides. Biomacromolecules 15, 1097–1114. ( 10.1021/bm500169p) [DOI] [PubMed] [Google Scholar]
- 94.Sharma NK, Kumar V. 2014. Liposomes as triggerable carrier for intracellular drug delivery. Drug Deliv. Lett. 4, 12–20. ( 10.2174/2210303103999131211110908) [DOI] [Google Scholar]
- 95.Gardikis K, Tsimplouli C, Dimas K, Micha-Screttas M, Demetzos C. 2010. New chimeric advanced drug delivery nano systems (chi-aDDnSs) as doxorubicin carriers. Int. J. Pharm. 402, 231–237. ( 10.1016/j.ijpharm.2010.10.007) [DOI] [PubMed] [Google Scholar]
- 96.Barenholz Y. 2012. Doxil®–the first FDA-approved nano-drug: lessons learned. J. Control Release 160, 117–134. ( 10.1016/j.jconrel.2012.03.020) [DOI] [PubMed] [Google Scholar]
- 97.Park JW. 2002. Liposome-based drug delivery in breast cancer treatment. Breast Cancer Res. 4, 95–99. ( 10.1186/bcr432) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Andreopoulou E, Gaiotti D, Kim E, Downey A, Mirchandani D, Hamilton A, Jacobs A, Curtin J, Muggia F. 2007. Pegylated liposomal doxorubicin HCL (PLD; Caelyx/Doxil): experience with long-term maintenance in responding patients with recurrent epithelial ovarian cancer. Ann. Oncol. 18, 716–721. ( 10.1093/annonc/mdl484) [DOI] [PubMed] [Google Scholar]
- 99.Chou HH, et al. 2006. Pegylated liposomal doxorubicin (Lipo-Dox) for platinum-resistant or refractory epithelial ovarian carcinoma: a Taiwanese gynecologic oncology group study with long-term follow-up. Gynecol. Oncol. 101, 423–428. ( 10.1016/j.ygyno.2005.10.027) [DOI] [PubMed] [Google Scholar]
- 100.Silverman JA, Deitcher SR. 2013. Marqibo® (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother. Pharmacol. 71, 555–564. ( 10.1007/s00280-012-2042-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rivera E. 2003. Liposomal anthracyclines in metastatic breast cancer: clinical update. Oncologist 8(Suppl. 2), 3–9. ( 10.1634/theoncologist.8-suppl_2-3) [DOI] [PubMed] [Google Scholar]
- 102.D'Acremont V, Herzog C, Genton B. 2006. Immunogenicity and safety of a virosomal hepatitis A vaccine (Epaxal) in the elderly. J. Travel Med.. 13, 78–83. ( 10.1111/j.1708-8305.2006.00001.x) [DOI] [PubMed] [Google Scholar]
- 103.Patil SD, Burgess DJ. 2005. Liposomes, design and manufacturing. In Injectable dispersed systems: formulation, processing and performance (drugs and the pharmaceutical sciences series) (ed. Burgess DJ.), pp. 249–303. New York, NY: Marcel Dekker; (ISBN 9780849336997) [Google Scholar]
- 104. Drugs.com homepage on the Internet. DepoCyt® (cytarabine liposome injection) prescribing information. See http://www.drugs.com/pro/depocyt.html. (accessed 18 August 2016).
- 105.Yarmolenko PS, Zhao Y, Landon C, Spasojevic I, Yuan F, Needham D, Viglianti BL, Dewhirst MW. 2010. Comparative effects of thermosensitive doxorubicin-containing liposomes and hyperthermia in human and murine tumours. Int. J. Hyperthermia 26, 485–498. ( 10.3109/02656731003789284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Farhat FS, et al. 2011. A phase II study of lipoplatin (liposomal cisplatin)/vinorelbine combination in HER-2/neu-negative metastatic breast cancer. Clin. Breast Cancer 11, 384–389. ( 10.1016/j.clbc.2011.08.005) [DOI] [PubMed] [Google Scholar]
- 107.Casagrande N, Celegato M, Borghese C, Mongiat M, Colombatti A, Aldinucci D. 2014. Preclinical activity of the liposomal cisplatin lipoplatin in ovarian cancer. Clin. Cancer Res. 20, 5496–5506. ( 10.1158/1078-0432.CCR-14-0713) [DOI] [PubMed] [Google Scholar]
- 108.Mylonakis N, Athanasiou A, Ziras N, Angel J, Rapti A, Lampaki S, Politis N, Karanikas C, Kosmas C. 2010. Phase II study of liposomal cisplatin (Lipoplatin) plus gemcitabine versus cisplatin plus gemcitabine as first line treatment in inoperable (stage IIIB/IV) nonsmall cell lung cancer. Lung Cancer 68, 240–247. ( 10.1016/j.lungcan.2009.06.017) [DOI] [PubMed] [Google Scholar]
- 109.Ravaioli A, et al. 2009. Lipoplatin monotherapy: a phase II trial of second-line treatment of metastatic non-small-cell lung cancer. J. Chemother. 21, 86–90. ( 10.1179/joc.2009.21.1.86) [DOI] [PubMed] [Google Scholar]
- 110.Stathopoulos GP, Boulikas T. 2012. Lipoplatin formulation review article. J. Drug Deliv. 2012, 581 363–581 373. ( 10.1155/2012/581363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bangham A, Gier J, Greville G. 1967. Osmotic properties and water permeability of phospholipid liquid crystals. Chem. Phys. Lipids 1, 225–246. ( 10.1016/0009-3084(67)90030-8) [DOI] [Google Scholar]
- 112.Szoka F Jr, Papahadjopoulos D. 1978. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc. Natl Acad. Sci. USA 75, 4194–4198. ( 10.1073/pnas.75.9.4194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Batzri S, Korn ED. 1973. Single bilayer liposomes prepared without sonication. Biochim. Biophys. Acta 298, 1015–1019. ( 10.1016/0005-2736(73)90408-2) [DOI] [PubMed] [Google Scholar]
- 114.Laouini A, Jaafar-Maalej C, Limayem-Blouza I, Sfar S, Charcosset C, Fessi H. 2012. Preparation, characterization and applications of liposomes: state of the art. J. Colloid Sci. Biotechnol. 1, 147–168. ( 10.1166/jcsb.2012.1020) [DOI] [Google Scholar]
- 115.Lesoin L, Crampon C, Boutin O, Badens E. 2011. Development of a continuous dense gas process for the production of liposomes. J. Supercrit. Fluids 60, 51–62. ( 10.1016/j.supflu.2011.04.018) [DOI] [Google Scholar]
- 116.Petersen AL, Hansen AE, Gabizon A, Andresen TL. 2012. Liposome imaging agents in personalized medicine. Adv. Drug Deliv. Rev. 64, 1417–1435. ( 10.1016/j.addr.2012.09.003) [DOI] [PubMed] [Google Scholar]
- 117.Perche F, Torchilin VP. 2013. Recent trends in multifunctional liposomal nanocarriers for enhanced tumor targeting. J. Drug Deliv. 2013, 1–32. ( 10.1155/2013/705265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tagami T, Foltz WD, Ernsting MJ, Lee CM, Tannock IF, May JP, Li S-D. 2011. MRI monitoring of intratumoral drug delivery and prediction of the therapeutic effect with a multifunctional thermosensitive liposome. Biomaterials 32, 6570–6578. ( 10.1016/j.biomaterials.2011.05.029) [DOI] [PubMed] [Google Scholar]
- 119.De Palma M, Hanahan D. 2012. The biology of personalized cancer medicine: facing individual complexities underlying hallmark capabilities. Mol. Oncol. 6, 111–127. ( 10.1016/j.molonc.2012.01.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ren L, Chen S, Li H, Zhang Z, Zhong J, Liu M, Zhou X. 2016. MRI-guided liposomes for targeted tandem chemotherapy and therapeutic response prediction. Acta Biomater. 35, 260–268. ( 10.1016/j.actbio.2016.02.011) [DOI] [PubMed] [Google Scholar]
- 121.Rizzitelli S, Giustetto P, Faletto D, Castelli DD, Aime S, Terreno E. 2016. The release of Doxorubicin from liposomes monitored by MRI and triggered by a combination of US stimuli led to a complete tumor regression in a breast cancer mouse model. J. Control Release 230, 57–63. ( 10.1016/j.jconrel.2016.03.040) [DOI] [PubMed] [Google Scholar]
- 122.Guo H, Chen W, Sun X, Liu Y-N, Li J, Wang J. 2015. Theranostic magnetoliposomes coated by carboxymethyl dextran with controlled release by low-frequency alternating magnetic field. Carbohydr. Polym. 118, 209–217. ( 10.1016/j.carbpol.2014.10.076) [DOI] [PubMed] [Google Scholar]
- 123.Hilger I, Kaiser WA. 2012. Iron oxide-based nanostructures for MRI and magnetic hyperthermia. Nanomedicine 7, 1443–1459. ( 10.2217/nnm.12.112) [DOI] [PubMed] [Google Scholar]
- 124.Thiesen B, Jordan A. 2008. Clinical applications of magnetic nanoparticles for hyperthermia. Int. J. Hypertherm. 24, 467–474. ( 10.1080/02656730802104757) [DOI] [PubMed] [Google Scholar]
- 125.Dolmans DE, Fukumura D, Jain RK. 2003. Photodynamic therapy for cancer. Nat. Rev. Cancer 3, 380–387. ( 10.1038/nrc1071) [DOI] [PubMed] [Google Scholar]
- 126.Ethirajan M, Chen Y, Joshi P, Pandey RK. 2011. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 40, 340–362. ( 10.1039/b915149b) [DOI] [PubMed] [Google Scholar]
- 127.Lim CK, et al. 2013. Nanophotosensitizers toward advanced photodynamic therapy of cancer. Cancer Lett. 334, 176–187. ( 10.1016/j.canlet.2012.09.012) [DOI] [PubMed] [Google Scholar]
- 128.Mellman I, Couks G, Dranoff G. 2011. Cancer immunotherapy comes of age. Nature 480, 480–489. ( 10.1038/nature10673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Galon J, et al. 2006. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313, 1960–1964. ( 10.1126/science.1129139) [DOI] [PubMed] [Google Scholar]
- 130.Dunn GP, Koebel CM, Schreiber RD. 2006. Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol. 6, 836–848. ( 10.1038/nri1961) [DOI] [PubMed] [Google Scholar]
- 131.Pradhan P, Giri J, Rieken F, Koch C, Mykhaylyk O, Doblinger M, Baneriee R, Bahadur D, Plank C. 2010. Targeted temperature sensitive liposomes for thermo-chemotherapy. J. Control Release 42, 108–121. ( 10.1016/j.jconrel.2009.10.002) [DOI] [PubMed] [Google Scholar]
- 132.Tan X, Pang X, Lei M, Ma M, Guo F, Wang J, Yu M, Tan F, Li N. 2016. An efficient dual-loaded multifunctional nanocarrier for combined photothermal and photodynamic therapy based on copper sulphide and chlorin e6. Int. J. Pharm. 503, 220–228. ( 10.1016/j.ijpharm.2016.03.019) [DOI] [PubMed] [Google Scholar]
- 133.Jain PK, Huang X, El-Sayed IH, El-Sayed MA. 2008. Noble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc. Chem. Res. 41, 1578–1586. ( 10.1021/ar7002804) [DOI] [PubMed] [Google Scholar]
- 134.Jaque D, Martínez Maestro L, del Rosal B, Haro-Gonzalez P, Benayas A, Plaza JL, Martin Rodríguez E, García Solé J. 2014. Nanoparticles for photothermal therapies. Nanoscale 6, 9494–9530. ( 10.1039/c4nr00708e) [DOI] [PubMed] [Google Scholar]
- 135.Camacho KM, et al. 2016. DAFODIL: a novel liposome-encapsulated synergistic combination of doxorubicin and 5FU for low dose chemotherapy. J. Control Release 229, 154–162. ( 10.1016/j.jconrel.2016.03.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mir Y, Elrington SA, Hasan T. 2013. A new nanoconstruct for epidermal growth factor receptor-targeted photo-immunotherapy of ovarian cancer. Nanomed. Nanotechnol. 9, 1114–1122. ( 10.1016/j.nano.2013.02.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Meraz IM, Savage DJ, Segura-Ibarra V, Li J, Rhudy J, Gu J, Serda RE. 2014. Adjuvant cationic liposomes presenting MPL and IL-12 induce cell death, suppress tumor growth, and alter the cellular phenotype of tumors in a murine model of breast cancer. Mol. Pharm. 11, 3484–3491. ( 10.1021/mp5002697) [DOI] [PMC free article] [PubMed] [Google Scholar]