Abstract
Nanoparticles do not exist in thermodynamical equilibrium because of high surface free energy, thus they have only kinetic stability. Spontaneous changes can be delayed by designed surface coating. In biomedical applications, superparamagnetic iron oxide nanoparticles (SPIONs) require an optimized coating in order to fulfil the expectation of medicine regulatory agencies and ultimately that of biocompatibility. In this work, we show the high surface reactivity of naked SPIONs due to ≡Fe–OH sites, which can react with H+/OH− to form pH- and ionic strength-dependent charges. We explain the post-coating of naked SPIONs with organic polyacids via multi-site complex bonds formed spontaneously. The excess polyacids can be removed from the medium. The free COOH groups in coating are prone to react with active biomolecules like proteins. Charging and pH- and salt-dependent behaviour of carboxylated SPIONs were characterized quantitatively. The interrelation between the coating quality and colloidal stability measured under biorelevant conditions is discussed. Our coagulation kinetics results allow us to predict colloidal stability both on storage and in use; however, a simpler method would be required to test SPION preparations. Haemocompatibility tests (smears) support our qualification for good and bad SPION manufacturing; the latter ‘promises’ fatal outcome in vivo.
Keywords: magnetic nanoparticles, colloidal stability, surface charging, biocompatibility, haemocompatibility, smears
1. Background
The use of nanomaterial systems has been greatly expanded in the last decade. Working with nanoparticles (NPs) is not easy, and researchers/producers always face a basic problem originating from the inherent propensity of NPs, i.e. they do not exist in thermodynamical equilibrium. As is well known in colloid science [1], the surface to volume ratio of particles starts to increase unquestionably below the micrometre range, along with the increase in surface free energy, which no longer remains negligible in comparison with the total energy of the system. Therefore, the particles in the colloidal and especially the nano (subcolloidal) systems existing over the 1–1000 nm and 1–100 nm size ranges, respectively, keep coarsening: their size increases in time due to the surface energy loss while reaching the state of energy minimum. After their preparation, the nanomaterial systems have only kinetic stability. Outstanding stability like Faraday's gold colloid existing from 1856 [2] can be reached via perfect coating.
In biomedical applications, any NPs or here, superparamagnetic iron oxide NPs (SPIONs) require an optimized coating, via which IONs can see any biological entities (e.g. proteins, cell membranes). The only fabricable part of interaction compartment at the nanobio interface is the coating on the engineered NPs. The NP's core possesses almost all of the most important NP characteristics such as the chemical composition of materials, shape and angle of curvature, porosity and surface crystallinity, heterogeneity, roughness as listed by Nel et al. [3], while the coating shell is responsible for the surface functionalization and hydrophobicity/hydrophilicity that determine the particle behaviour in a given medium. Nel et al. [3] discussed separately the other quantifiable properties of NP interactions, such as effective surface charge (zeta potential), particle aggregation, state of dispersion, stability/biodegradability, dissolution characteristics, hydration and valence of the surface layer, since these are significantly influenced by the characteristics of the suspending media, including the ionic strength, pH, temperature and the presence of large organic molecules (e.g. proteins) or specifically adsorbing molecules or ions (e.g. detergents generally or phosphate ions). The composition and structure of interfacial layer on coated NPs and its changes on the nanoscale also affect their microscale and more macroscale behaviour. Just an example to better see the point is the charge screening effect of dissolved electrolytes. Dispersions of charge carrier NPs are stable like common solutions at low salt content; however, the repulsive forces between NPs decrease with increasing salt concentration and above a certain limit, NPs stick together (nano- to microscale change) and settle down, which is obvious on macroscale. We have worked out a systematic physico-chemical and colloidal characterization protocol of SPION interfaces with reference to the related microscopic and even macroscopic properties of finely divided SPION systems [4]. Viewing the recent literature, our impression is that the measurable physico-chemical properties of SPION interactions, e.g. dissolution, charging, pH and salt tolerance, adsorption of proteins or specific ions and as a result the change in colloidal stability, i.e. nanoparticle aggregation in the desired medium (e.g. blood if intravenous administration as mostly in biomedical applications is planned), are not adequately addressed even in the top articles. Of course, there are prudent reviews, for example, Laurent et al. [5] pointing out that coating is a major determinant of SPION stability in both solution and physiological media. We have also emphasized the importance of colloidal stability and the role of coating (both its quality and quantity) as well as the solution conditions (pH, ionic strength, specific properties of media, etc.) in the particle aggregation [6]. Laurent et al. [5] have stated that more research should be devoted to the stability of SPIONs in colloidal solutions and serum, as well as to the critical physiological factors that might affect the efficiency of outcome (e.g. protein corona).
Although over the last three decades, many first-generation nanomedicines have successfully entered routine clinical use, among them nano-sized colloidal iron-based preparations, there are global discussions recently on the appropriate evolution of regulatory guidance to meet the needs of nano-specific properties. Even the description of nanomaterials is dissimilar at the medicines regulatory agencies in Europe (EMA) and in the USA (FDA) as compared in table 2 of the report of Tinkle et al. [7]. The FDA published guidance drafts for industry to address technical issues related to the use of nanotechnology in different products, the last one of which about the use of nanomaterials in food for animals [8] was published in 2015. The EMA issued reflection papers on different topics like coating [9]; the last one was on the data requirements for intravenous iron-based nano-colloidal products [10]. Based on these we intend to sum up the nano-related problems deriving from the high surface free energy, i.e. non-equilibrium nature, first of all in the production: weak reproducibility, the lack of precise control over nanoparticle manufacturing; and secondly in the products: NPs tend to stick to each other to form larger aggregates (agglomerates as preferred recently), interact with the testing medium or bind to different substances including proteins in the test medium and biological fluids, resulting in an altered biological activity (e.g. biodistribution, efficacy, biocompatibility, toxicity). Since nanomaterials can settle, diffuse and aggregate differentially according to their density, size and surface chemistry, the assessment of their aggregation in the media of in vitro and in vivo systems should be addressed. Proper characterization relevant to non-equilibrium nature of nanomaterials should include measurements of particle size, size distribution and aggregation characteristics under the testing conditions and conditions expected in the use of final product. The surface chemistry (zeta potential/surface charge, surface coating, functionalization and catalytic activity), morphology (shape, surface area, surface topology, crystallinity), solubility and stability should be characterized in the proposed formulations (e.g. solubility in biological fluids, stability on storage) and under the proposed conditions of use (in-use stability, e.g. in blood) [8–10]. It is mentioned [10] that there are no standardized characterization techniques available for accurate measurements of some characteristics such as the particle size, shape, surface area and surface properties critical, for example, to in vivo performance of nano-sized colloidal intravenous iron preparations.
In this paper, we intend to show the inherent instability and high surface reactivity of naked SPIONs. We propose a post-coating strategy and discuss the interrelation between the coating quality and the colloidal stability and how colloidal stability is predictable both on storage and in use with the help of the accurate characterization of interfacial layer. Some examples will be given for good and bad SPION manufacturing.
2. Experimental procedure
Synthetic magnetite NPs (approx. 10 nm primary particle size) were prepared by alkaline hydrolysis of iron(II) and iron(III) salts [11]. Surface charge formation was measured by potentiometric acid–base titration method [11]. The pH-dependent particle aggregation and zeta potential were measured by dynamic light scattering (DLS) laser Doppler electrophoresis (NanoZS) [12]. Haemocompatibility tests such as blood smears were performed in the same way as earlier [4].
3. Results and discussion
3.1. Naked superparamagnetic iron oxide nanoparticles in water: reactive surface sites, pH-dependent charging and colloidal stability
In an aqueous medium, pH and ionic strength-dependent charges develop on amphoteric surface hydroxyls (≡Fe–OH) in the protonation
| 3.1 |
;and deprotonation
| 3.2 |
;reactions taking place spontaneously on the SPION surface under acidic and alkaline conditions, respectively (e.g. [11,13]).
When surface charge development occurs by direct proton transfer from the aqueous phase, the surface charge density (σ0,H) and surface potential (ψ0) can be defined analogously to that of Nernstian surfaces:
| 3.3 |
;and
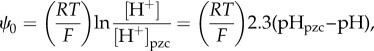 |
3.4 |
;where Γi is the surface excess concentration of species i, R is the gas constant, T is the temperature and F is the Faraday constant; pHpzc is the point of zero charge at which both σ0,H and ψ0 must be equal to zero in the presence of indifferent electrolytes (obey only the electrostatic constraints).
The pH-dependent surface charge density is experimentally accessible from potentiometric acid–base titration of oxide suspensions (e.g. [11,14]). The net proton surface excess amount 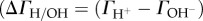 given in mmol m–2) can be directly calculated at each point of titration from the material balance of H+/OH– and the mass of oxide sample titrated and its specific surface area (as, m2 g−1), if no other acid or base consuming reactions take place during titration. The σ0,H (or ΔΓH/OH) versus pH curves, the so-called charge-potential functions, are determined at three concentrations of an indifferent electrolyte, which intersect at a particular pH referred to as the point of zero charge (PZC, where
given in mmol m–2) can be directly calculated at each point of titration from the material balance of H+/OH– and the mass of oxide sample titrated and its specific surface area (as, m2 g−1), if no other acid or base consuming reactions take place during titration. The σ0,H (or ΔΓH/OH) versus pH curves, the so-called charge-potential functions, are determined at three concentrations of an indifferent electrolyte, which intersect at a particular pH referred to as the point of zero charge (PZC, where 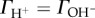 ) characteristic of metal oxides in aqueous medium.
) characteristic of metal oxides in aqueous medium.
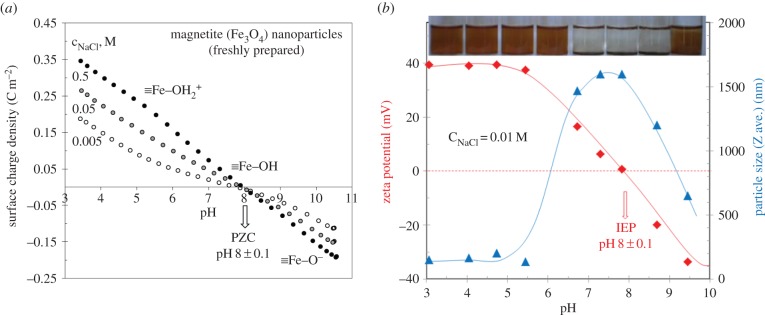
The pH-dependence of the surface charge density (σ0,H) of magnetic iron oxide nanoparticles (MIONs) can be seen in figure 1a. The reversibility of forward and backward titration was excellent (not shown here). Solubility of iron oxides mainly below pH ∼ 4 and above pH ∼ 10 is influenced not only by the pH, temperature and ionic strength of the system, but also by the particle size and crystal defects in the oxide. Magnetite usually dissolves faster than pure Fe(III) oxides due to its Fe(II) content and also because Fe(III) occurs in octahedral and tetrahedral sites. However, the activity of dissolved Fe(III) species remains below approximately 10–5 mol dm−3, between pH ∼ 4 and 10 [13]. The PZC of MION seems to be at pH = 8.0 ± 0.1 and this value falls in the range given in the literature [14]. The surface charge density range (0.19–0.35 C m−2) reached at pH ∼ 3.5 in figure 1a suggests that only approximately 1–2 sites nm–2 on the MION surface are prone to bind protons, which is less than half of the amount of ≡Fe–OH site density (approx. 5 sites nm−2) common for iron oxides [13].
Figure 1.
The pH-dependent surface charge development on SPIONs at different ionic strengths (a). Zeta potential (calculated by Smoluchowski equation) proportional to the particle charge at shear plane and aggregation of SPIONs (DLS sizes reflecting the given kinetic state measured 2 min after sonication) as a function of pH and a photo of pH-adjusted series taken an hour after sonication (b). (The points of surface charge density versus pH curves (a) were calculated from the material balance of H+/OH− in the course of equilibrium acid–base titration considering the specific surface area of SPIONs of 105 m2 g−1.) (Online version in colour.)
The charge state of amphoteric iron oxides changes characteristically with pH and electrolyte concentration. Electrokinetic measurements provide independent information on the electric double layer of charged particles. The measured electrophoretic mobility can be converted to electrokinetic (zeta) potential on the well-known theoretical basis with several assumptions and limitations [15]. In principle, the Hückel and Smoluchowski approaches can be applied to convert the mobility values to electrokinetic (zeta) potential. While the sign of these data is the same as that of the surface charge of a particle in the absence of specific ions (e.g. phosphate), its magnitude is only somewhat proportional to it, since the electrokinetic mobility is a function of the charge density at the shear plane. As Nel et al. [3] described, the effective surface charge manifests in zeta potential. While surface potential (ψ0) of oxides depends only on the pH, but not on the salt concentration, zeta potential changes significantly with the ionic strength, and it becomes zero at high enough salt concentration typically at and above 0.15 M [15]. The sign of the electrophoretic mobility measured in the metal oxide dispersions reverses at a characteristic pH, where these amphoteric particles do not hold excess charge. It can be identified as the pH of the isoelectric point (IEP) (figure 1b). Around the IEP, the colliding particles stick together and the large aggregates settle down in the absence of electrostatic stabilization as seen in the photo of pH-adjusted series of MION dispersions (figure 1b, top). Aggregation processes in dilute systems can be characterized by particle size determination. DLS can provide reliable size data even when the system is undergoing aggregation. The pH-dependence of the hydrodynamic size (Z average) calculated from the cumulant analysis of correlation functions is shown in figure 1b. A significant increase in average particle size over the range of pH ∼ 7–9 even at low salt concentration is obvious, proving a pronounced aggregation near the pH of PZC, where the electrostatic repulsion between particles is negligible and particles generally undergo fast coagulation, in accordance with the basic colloid literature (e.g. [15]). All measured points of figure 1b are suitable only for comparing a given kinetic state of coagulating systems.
To sum up, the surface of naked SPIONs in aqueous medium is covered by reactive ≡Fe–OH groups (approx. 5 sites nm−2) prone to react with H+/OH− and form variable charges (up to 2 charges nm−2) depending both on the pH and salt concentration. Naked SPIONs are electrostatically stabilized: the particles either aggregate or disperse depending on the structure of the local electric field formed on their surface. Aggregation takes place at pH near to PZC or IEP (pH ∼ 8 for freshly prepared magnetite) even at low ionic strength as a result of weak electrostatic field, and at all pH, if salt concentration is high enough to screen the surface charges formed in protonation/deprotonation reactions. From the colloidal stability point of view, the physiological conditions in body fluids like blood having pH ∼ 7.4 and 0.15 M salt are not favourable: naked SPION particles aggregate strongly, which is out of the question in biomedical applications. In the latter, the fact that iron is a Fenton-active metal [16] is not negligible at all. The naked surface of SPIONs and the iron ions dissolved even in trace amount can catalyse the oxidation of biomolecules via enhancing reactive oxygen species generation.
3.2. Polyelectrolyte-coated (carboxylated) superparamagnetic iron oxide nanoparticles in biorelevant media
The prevention of SPIONs' aggregation in biological milieu at certain pH, salt and protein concentration in general is an absolute requirement, if biomedical applications are planned [8,9]. Therefore, SPIONs have to be accurately coated either during or after their synthesis. The literature distinguishes in situ coating, post-synthesis adsorption or post-synthesis grafting [5]. In the latter, organic molecules with functional groups are anchored to the SPION surface, forming brush-like chains. Coatings largely influence not only the colloidal stability, but also the functionality and biological fate of SPIONs. One can distinguish several diverse functions of coating [6], namely (i) colloidal stabilization under physiological conditions (protecting against aggregation at biological pHs and salty medium), (ii) inhibiting corrosion and oxidation of magnetic core (passivation reducing the iron leakage), (iii) hindering non-specific protein adsorption in biological milieu, (iv) providing reactive groups for grafting drugs and targeting molecules, and (v) control of nano-biointerfacial interactions (bio/haemocompatibility, reticuloendothelial system uptake, blood circulation time, IONP internalization efficiency, toxicity, targeting efficiency, in vivo fate, etc.) as discussed in detail [3,5,17,18]. These largely coincide with the general issues suggested by EMA [9] to consider during the development of nanomedicine products with covalent or non-covalent coating.
Although it is known that NCL (Nanotechnology Characterization Laboratory) has developed several protocols that rigorously characterize nanoparticle physico-chemical properties, as well as in vitro and in vivo characteristics [7], none of them has been standardized yet. There are serious methodological problems; there is no single technique available that measures for example the particle size, shape, surface area and surface properties accurately [10]. As we see, not the technique itself, but the conditions under which the measurements are or should be conducted are the main source of problems. The colloidal stability of SPIONs has to be satisfactory under physiological conditions, i.e. at biological pHs, in salty medium, in the presence of proteins and also in cell culture media. The measurements, if any, are realized under arbitrary conditions using buffers, which contain specific ions (e.g. in the most frequently used phosphate-buffered saline, phosphate ions form strong surface complexes on iron oxide, so ligand exchange process is probable) or cell culture media with proteins forming corona immediately on SPIONs. At a conference held in Vancouver recently, Jerome Lewis [19], one of the invited speakers, talked about the difficulties during approval of SPION products. He emphasized the colloidal stability expectations during storage and even after freezing of the products, which are intended to be approved. He said ‘Colloids hate buffers’, warning of the presence of specific ions having chemical affinity to the SPION surface. This means that the original feature of a SPION product may be masked because of ligand exchange between the specific ions of buffer and the molecules of original coating (replacement of, for example, carboxylate with phosphate compounds is probable chemically). Testing aggregation (DLS sizing, filtration test, visual observation of sedimentation) under arbitrary conditions is quite usual [18]. Coagulation kinetics experiment is a correct method to test the colloidal stability of SPIONs, i.e. their salt tolerance, and so their resistance against aggregation in physiological environment can be predicted [20]. We have suggested a straightforward route of physico-chemical (iron dissolution) and colloidal (pH-dependent charging and particle size, salt tolerance from coagulation kinetics) measurements to assess eligibility of SPIONs for in vitro tests [4]. Citrated SPIONs are the best example for enhanced dissolution of iron core due to the reductive and complex forming ability of citric acid as we demonstrated previously [4] together with its inadequate salt tolerance, its biomedical use is still favoured in the literature [6] regardless of the fact that the dissolved iron ions may cause oxidative stress [16] besides the danger of particle aggregation in vivo.
We have worked out a spontaneous one-step process to form carboxylated shell on SPIONs by post-coating of purified synthesis products, in which optimized magnetic cores were combined with different commercial polyelectrolytes (e.g. poly(acrylic acid), PAA [12,21], poly(acrylic acid-co-maleic acid), PAM [22] and designed polymer molecules [23]). The carboxylated shells are bound to SPION surface via multipoint inner- and outer-sphere surface complexes and hydrogen bonds proved by X-ray photoelectron spectroscopy (XPS) and attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectroscopy [4,21,22]. We recall here only one of our previous plots [24] summarizing the gradual reversal of zeta potential with increasing amounts of organic polyacids added to the purified dispersions of SPIONs (figure 2). The inset photo shows the macroscopic feature of a series with increasing polyacid concentration an hour after its addition. The starting point is a colloidally stable system of naked SPIONs having positive surface and particle charges under the given conditions. In the presence of trace amount of each polyacid, the negatively charged macroions neutralize the positively charged surface sites at pH below the PZC of MIONs and so promote the coagulation of SPIONs by reducing the positive charge density to a certain extent, and creating negatively charged patches on oxide surface (patch-wise charge heterogeneity). The gradual reversal of particle charge is obvious from the successive shift of zeta potential values to the negative region with increasing polyacid loading. Relatively small amount of polyanionic compounds (less than 0.15 mmol COOH g−1) can entirely cover the positive charges of particles (approx. 0.05 mmol g−1) neutralizing and destabilizing them (seen as the settling systems on the photo insert in figure 2). Further increase in polyacid loading reverses the sign of particle charge then overcharges them reaching zeta potential values of −40 to −45 mV and inducing particles to disperse, i.e. a significant increase in colloidal stability occurs due to the combined steric and electrostatic repulsion.
Figure 2.
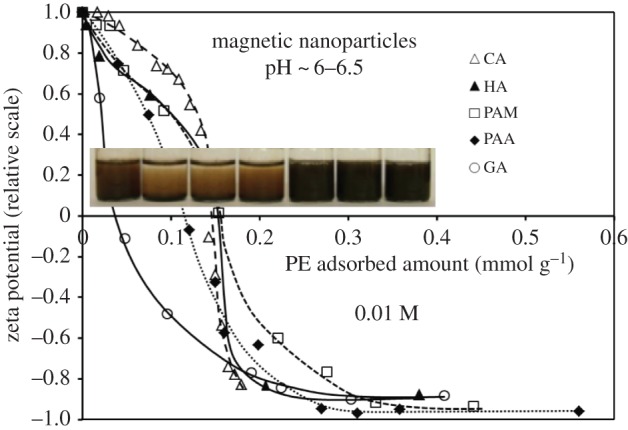
Change in the electrokinetic potential of SPIONs with increasing surface coverage of polyacids CA, HA, PAM, PAA and GA, measured at pH ∼ 6–6.5 and 0.01 M NaCl. (CA, citric acid; HA, humic acid; PAA, poly(acrylic acid); PAM, poly(acrylic-co-maleic acid); the amount of the macromolecular polyacids (HA, PAA and PAM) was related to the moles of their carboxylic groups. The error in the relative values of zeta potentials is ±0.1 mV.) Reproduced with permission from [24] under CC-BY. The inset photo shows the colloidal stability of a series of SPION dispersions with increasing HA concentration. (Online version in colour.)
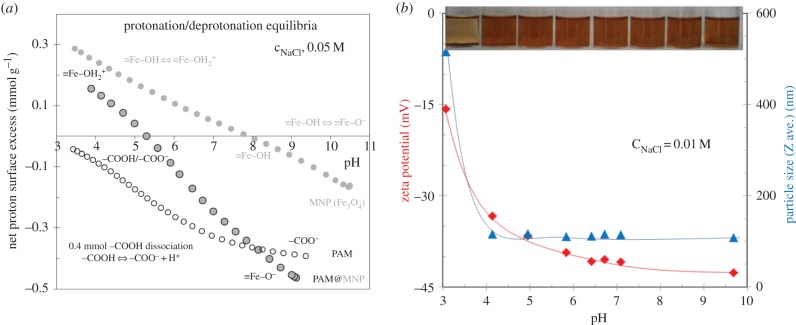
The question is how the shortcomings of naked SPIONs have changed. Thus, we investigated the change in surface charge state by measuring the pH-dependent particle charge and colloidal stability of SPIONs completely covered with polyacids. The pH-dependence of the net surface proton excess of a coated SPION sample at a given salt concentration can be seen in figure 3a (middle curve). The reversibility of forward and backward titration was acceptable (not shown here). For comparison, the titration results of the naked SPION and the coating agent are also shown. The protonation/deprotonation of the interfacial active sites (≡Fe–OH for the MION core and free –COOH for the coating layer) can be quantitatively characterized by acid–base titration. The positive values of calculated net proton surface excess indicate proton accumulation, while their lack in the interfacial layer results in negative excess values (e.g. [11]). Both the sign and the quantitative amount of charges formed on colloids dispersed and dissolved in aqueous medium, i.e. SPION nanoparticles and PAM polyacid, respectively, can be determined. At pHs below approximately 5, the protonation of ≡Fe–OH sites is dominant at the interface of PAM-coated SPIONs; surprisingly, ≡Fe–OH sites are still available in spite of full polyelectrolyte coating. Above pH approximately 5, the negative net proton excess values indicate the dissociation (deprotonation) of –COOH in coating layer, which becomes more and more obvious with increasing pH. The deprotonation of ≡Fe–OH sites also contributes to the increase in negative excess values above pH ∼ 8. For the sake of clarity of our interpretation, the net proton excess versus pH curve of a PAM sample containing 0.4 mmol –COOH is also plotted in figure 3a. We assume that this –COOH amount remains titratable in the PAM coating layer, as it is very close to the high-affinity limit (approx. 0.3 mmol COOH g−1) of PAM adsorption on IO nanoparticles [22], which cannot be removed by exhaustive washing. While acid–base titration proved the presence of both positive and negative charges at the interface of PAM-coated SPIONs over the pH range studied here, we could measure only negative values of electrokinetic (zeta) potential in 0.01 M NaCl solution (figure 3b). Most of the zeta potential values were below −30 mV enough for electrostatic repulsion between colliding particles, i.e. their stabilization. The latter is in good harmony with the DLS results measured under the same conditions, namely hydrodynamic particle sizes are small (approx. 100 nm) above pH ∼ 4 independently of pH. The excellent colloidal stability of these systems is confirmed by the inset photo (figure 3b) showing the macroscopic feature of a series with increasing pH some hours after their preparation. These PAM-coated SPIONs similar to our other carboxylated SPION products meet the pH requirement of biomedical application, i.e. they must be well dispersed in the environment of physiological pH (approx. 7), where IO particles themselves would be fully aggregated because their point of zero charge is at pH = 8.0 ± 0.1 as shown in figure 1. Regarding the colloidal stability on storage, it is predictable that our carboxylated SPION preparations can be stored for a long time preferably in a refrigerator, because they are not sensitive to change in pH. We have non-sterilized carboxylated SPION samples stored at 4°C for years without the sign of destabilization.
Figure 3.
The pH-dependent interfacial equilibria of a carboxylated SPION sample (PAM@MNP) in comparison with that of naked MNP and its coating agent (PAM containing 0.4 mmol COOH). The net proton surface excess related to the unit mass of SPIONs (calculated from the material balance of H+/OH− in the course of equilibrium acid–base titration) is proportional to the amount of surface charges developed in the interfacial layer (a). Zeta potential proportional to the particle charge at shear plane and aggregation of PAM-coated SPION sample (DLS sizes reflect the given kinetic state measured 2 min after sonication) as a function of pH and a photo of pH-adjusted series taken an hour after sonication (b). (Online version in colour.)
If we think of biomedical application, we have to pay attention not only to the pH but also to the salt content of biofluids like 0.15 M NaCl in physiological salt solutions. The colloidal stability especially in electrostatically stabilized systems depends definitely on the salt concentration [15]. Therefore, the resistance of different SPION preparations against electrolytes has to be tested. In colloid science, coagulation kinetics measurements can quantitatively characterize the stability of colloidal dispersions. The size evolution of aggregates can be followed in time by DLS. An example is shown in figure 4a. The stability ratio (W) can be calculated from the initial slopes of kinetic curves as their ratio belonging to the rapid and slow coagulation at each salt concentration. Stability plots recalled from our previous paper [22] can be seen in figure 4b, from which the critical coagulation concentration (CCC) can be determined as the intersection of the fitted straight lines belonging to the slow and fast coagulation regimes. The CCC of the pure MION sol is approximately 1 mM NaCl at pH ∼ 6.5; however, it increases with increasing amount of coating agents (PAA and PAM in the example) reaching noteworthy values (approx. 500 mM at the highest coverage) far above the 150 mM physiological salt content.
Figure 4.
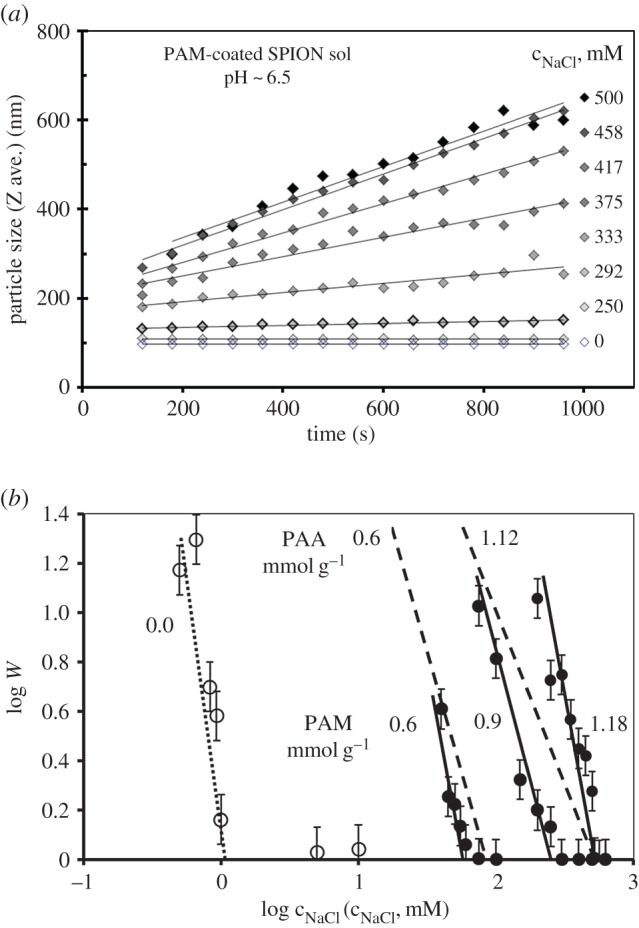
Coagulation kinetics measured by DLS: the size evolution of aggregates in PAM-coated SPION sols at pH ∼6.5 and different NaCl concentrations, at 25 ± 0.1°C (a). Stability plots to determine the values of the critical coagulation concentration of naked (open symbols) and PAA (0.6, 1.12 mmol g−1 dashed lines) and PAM (0.6, 0.9 and 1.18 mmol g−1 solid lines) coated (filled symbols) SPIONs dispersed in aqueous media at pH ∼ 6.5 (b). Reproduced with permission from [22]. Copyright © 2012 American Chemical Society.
Although the coagulation kinetics results predict well the in-use stability of SPION product, a simpler method would be required to test SPION preparations for colloidal stability in biorelevant media. In a recent paper [25], the hydrodynamic diameters (DLS size) of different coated SPIONs were measured at pH 7.3 in various NaCl concentrations from 0 to 0.30 M NaCl. The authors evidenced the good colloidal stabilization of SPION products from the very marginal size variation even at high NaCl concentrations without concerning the kinetic condition of the DLS sizing (i.e. the time elapsed between salt addition and actual size measurements). The latter would be appropriate, but an accurate protocol should be worked out.
The magnetic cores were mostly the same in each product and the carboxylated shells were also similar regarding their hydrophobicity, size and surface charge [4]; and so a very similar behaviour should be expected at the nanobio interface as well, since these are the key parameters according to the conclusions of in vivo experiments [3]. The multiple bindings of polycarboxylated macromolecules on SPIONs via surface complexation of ≡Fe–OH sites by carboxylates are spontaneous. The excess polyelectrolytes can be easily removed by washing exhaustively without the loss of colloidal stability [22]. The formed polyelectrolyte layer hinders SPION dissolution, provides combined electrostatic and steric (electrosteric) stabilization. In addition, free carboxylates still exist in the shell for anchoring bioactive molecules via both covalent and non-covalent strategies, where peptide bonds form through carbodiimide activation and ionic coupling of positively charged bio-entities, respectively. The pH- and ionic strength-dependent dissociation of carboxylated shell and the IEP of complex molecules such as antibodies or proteins [26] need to be correlated for ionic coupling.
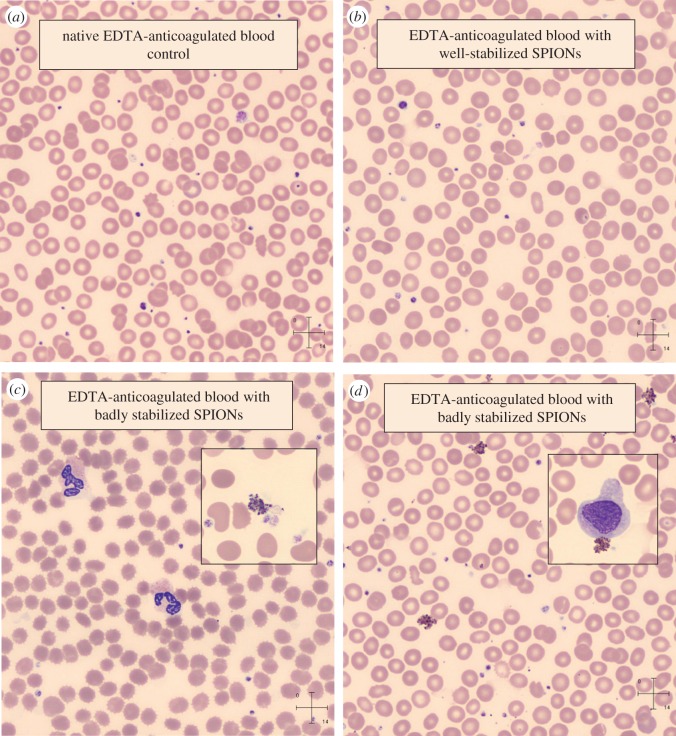
Finally, if we think of biomedical applications like magnetic resonance imaging contrast enhancement, intravenous administration of SPION product is envisioned, so haemocompatibility test should be mandatory. We have introduced some blood tests such as blood sedimentation and peripheral blood smears [4,22,23]. Here, we intend to show only some astonishing results of peripheral blood smear experiments, which give definitely trustworthy information on haemocompatibility of the given SPION preparation. Figure 5 shows results from the whole blood samples of a healthy volunteer collected in EDTA anticoagulant-coated vacutainer tubes mixed with magnetic fluids at 5 : 1 volume ratio. The smears of control and a blood containing well-stabilized SPION do not reveal signs of coagulation (figure 5a,b). Well-dispersed SPIONs are not visible at the resolution of the image of smear tests unless coagulation occurs. The small dots in the enlarged fields are non-aggregated thrombocytes. In the presence of badly stabilized SPIONs in whole blood samples, however, we found strong aggregation of thrombocytes, monocytes and NPs as well (see in the magnified insets of figure 5c,d). These aggregations can be fatal in vivo. In addition, an obvious sign of serious osmotic stress on red blood cells (RBCs) appeared in one of the smears (figure 5c), since the shape of RBCs strongly deformed, they crenated in the hypertonic solution even at 5 : 1 volume ratio. The osmotic stress was caused probably by large excess of additives (surfactant, PEG, etc.) in the given SPION preparation.
Figure 5.
Pictures of blood smears prepared from EDTA-anticoagulated blood without and with well-stabilized (a,b) and badly stabilized (c,d) SPION preparations (insets: thrombocytes, WBCs, crenated RBCs (c) and monocyte (d) and aggregated SPIONs on both). (Staining: May-Grünwald-Giemsa method by automated slide preparation system (Sysmex SP4000i); analysis: CellaVisionTM DM96. Sample description: well-stabilized (b), purified PAM-coated SPION (details in [22]); badly stabilized (c,d): (c) PEGylated oleic acid double-layer-coated SPION with high excess PEG (poly(ethylene glycol) of Mw = 1000 Da) as prepared (details in [27]) and (d) magnetic clusters of PAM-coated SPION linked by PEI (polyethylenimine) as obtained (details in [28]).) (Online version in colour.)
4. Conclusion
Nanoparticle dispersions are non-equilibrium systems, in general; this fact enhances the importance of coating, since the quality of coating governs particle–particle interactions (colloidal stability of systems) and interactions of NPs with different biological entities like proteins, cell membranes, etc. The detailed knowledge regarding the carboxylated coatings on SPIONs allowed us to predict their colloidal behaviour in biorelevant milieu on storage and in use as well.
Magnetic (e.g. VSM, SQUID), structural (e.g. XRD, SAXS), thermal (e.g. TG, DSC), spectroscopic (e.g. XPS, ATR-FTIR), even colloidal such as size (e.g. TEM, magnetic, hydrodynamic), effective charge (e.g. zeta potential), etc., characterization of NP cores and also their coating shell is widely accepted in scientific communities. These mostly cover the determining properties, except for hydrophilicity relevant to the interaction with biological entities at bionano interfaces and to colloidal stability both on storage and in use critical in approval procedures. Thinking of real situations, the latter are rightful expectations and would be the most important in the characterization of SPION products, if we focus indeed on the biomedical application. Unfortunately, direct testing of NP aggregation under harsh conditions (e.g. in saline medium) has not become widespread in the scientific community, although the long-term colloidal stability testing such as sedimentation and freezing is crucial in the approval procedure of FDA considering that storage stability and proper dosage of SPION products are essential in their use.
As is clearly stated both in EMA and FDA documents that colloidal stability of SPION products is crucial during storage and in use, we suggest testing it under conditions approximating to storage and in use; however, development of a simple protocol would be a reasonable expectation of the scientific community. We want to note finally that haemocompatibility of SPION product tests should be mandatory, if intravenous administration is envisioned. It would be worth introducing smear testing as a simple routine method available in most clinical diagnostic laboratories.
Acknowledgement
The authors are grateful for cooperation with Laboratory of Magnetic Fluids, CFATR, Timisoara, Romania. E.T. is especially grateful to Ladislau Vékás for initiating SPION research in Szeged, Hungary.
Authors' contributions
M.S. helped draft the manuscript and participated in data analysis; E.I., I.Y.T. and D.N. carried out the experimental work and evaluated the measured data; T.S. participated in the design of the study and drafted the manuscript; E.T. designed and coordinated the study and wrote the manuscript. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
E.T.'s researches were supported by OTKA (NK 84014) and RADIOMAG (COST action TD1402) grants.
References
- 1.Buzágh A. 1937. Colloid systems: a survey of the phenomena of modern colloid physics and chemistry. London, UK: The Technical Press Ltd. [Google Scholar]
- 2.2016. ‘Michael Faraday's gold colloids | The Royal Institution: Science Lives Here’. See www.rigb.org (retrieved 24 June 2016).
- 3.Nel AE, Mädler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, Klaessig F, Castranova V, Thompson M. 2009. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 8, 543–557. ( 10.1038/nmat2442) [DOI] [PubMed] [Google Scholar]
- 4.Szekeres M, Tóth I, Illés E, Hajdú A, Zupkó I, Farkas K, Oszlánczi G, Tiszlavicz L, Tombácz E. 2013. Chemical and colloidal stability of carboxylated core-shell magnetite nanoparticles designed for biomedical applications. Int. J. Mol. Sci. 14, 14 550–14 574. ( 10.3390/ijms140714550) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laurent S, Saei AA, Behzadi S, Panahifar A, Mahmoudi M. 2014. Super-paramagnetic iron oxide nanoparticles for delivery of therapeutic agents: opportunities and challenges. Expert Opin. Drug Deliv. 11, 1449–1470. ( 10.1517/17425247.2014.924501) [DOI] [PubMed] [Google Scholar]
- 6.Tombácz E, Turcu R, Socoliuc V, Vékás L. 2015. Magnetic iron oxide nanoparticles: recent trends in design and synthesis of magnetoresponsive nanosystems. Biochem. Biophys. Res. Commun. 468, 442–453. ( 10.1016/j.bbrc.2015.08.030) [DOI] [PubMed] [Google Scholar]
- 7.Tinkle S, McNeil SE, Mühlebach S, Bawa R, Borchard G, Barenholz YC, Tamarkin L, Desai N. 2014. Nanomedicines: addressing the scientific and regulatory gap. Ann. NY Acad. Sci. 1313, 35–56. ( 10.1111/nyas.12403) [DOI] [PubMed] [Google Scholar]
- 8.Guidance for Industry Use of Nanomaterials in Food for Animals, August 2015. http://www.fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/UCM401508.pdf (retrieved 24 June 2016).
- 9.2013. Reflection paper on surface coatings: general issues for consideration regarding parenteral administration of coated nanomedicine products, 22 May 2013 EMA/325027/2013. See http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/08/WC500147874.pdf (retrieved 24 June 2016).
- 10.2015. Reflection paper on the data requirements for intravenous iron-based nano-colloidal products developed with reference to an innovator medicinal product 26 March 2015 EMA/CHMP/SWP/620008/2012. See http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/03/WC500184922.pdf (retrieved 24 June 2016).
- 11.Tombácz E, Illés E, Majzik A, Hajdú A, Rideg N, Szekeres M. 2007. Ageing in the inorganic nanoworld: example of magnetite nanoparticles in aqueous medium. Croat. Chem. Acta 80, 503–515. [Google Scholar]
- 12.Tombácz E, Tóth IY, Nesztor D, Illés E, Hajdú A, Szekeres M, Vékás L. 2013. Adsorption of organic acids on magnetite nanoparticles, pH-dependent colloidal stability and salt tolerance. Colloids Surf. A 435, 91–96. ( 10.1016/j.colsurfa.2013.01.023) [DOI] [Google Scholar]
- 13.Cornell RM, Schwertmann U. 1996. The iron oxides. Weinheim, Germany: VCH. [Google Scholar]
- 14.Kosmulski M. 2001. Chemical properties of material surfaces. New York, NY: Marcel Dekker. [Google Scholar]
- 15.Hunter RJ. 1989. Foundations of colloid science, vol. 1 Oxford, UK: Clarendon Press. [Google Scholar]
- 16.Lemire JA, Harrison JJ, Turner RJ. 2013. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 11, 371–384. ( 10.1038/nrmicro3028) [DOI] [PubMed] [Google Scholar]
- 17.Colombo M, Carregal-Romero S, Casula MF, Gutiérrez L, Morales MP, Böhm IB, Heverhagen JT, Prosperi D, Parak WJ. 2012. Biological applications of magnetic nanoparticles. Chem. Soc. Rev. 41, 4306 ( 10.1039/c2cs15337h) [DOI] [PubMed] [Google Scholar]
- 18.Amstad E, Textor M, Reimhult E. 2011. Stabilization and functionalization of iron oxide nanoparticles for biomedical applications. Nanoscale 3, 2819 ( 10.1039/c1nr10173k) [DOI] [PubMed] [Google Scholar]
- 19.Lewis JM. 2016. Superparamagnetic iron oxides, from the lab to approval. In 11th Int. Conf. on the Scientific and Clinical Applications of Magnetic Carriers, Vancouver, Canada, 31 May–4 June 2016, Book of abstract: Invited Talk#6. Vancouver, Canada: University of British Columbia.
- 20.Forge D, Laurent S, Gossuin Y, Roch A, Vander Elst L, Muller RN. 2011. An original route to stabilize and functionalize magnetite nanoparticles for theranosis applications. J. Magn. Magn. Mater. 323, 410–415. ( 10.1016/j.jmmm.2010.09.031) [DOI] [Google Scholar]
- 21.Hajdú A, Szekeres M, Tóth IY, Bauer RA, Mihály J, Zupkó I, Tombácz E. 2012. Enhanced stability of polyacrylate-coated magnetite nanoparticles in biorelevant media. Colloids Surf. B 94, 242–249. ( 10.1016/j.colsurfb.2012.01.042) [DOI] [PubMed] [Google Scholar]
- 22.Tóth IY, Illés E, Bauer RA, Nesztor D, Szekeres M, Zupkó I, Tombácz E. 2012. Designed polyelectrolyte shell on magnetite nanocore for dilution-resistant biocompatible magnetic fluids. Langmuir 28, 16 638–16 646. ( 10.1021/la302660p) [DOI] [PubMed] [Google Scholar]
- 23.Illés E, Tombácz E, Szekeres M, Tóth IY, Szabó Á, Iván B. 2015. Novel carboxylated PEG-coating on magnetite nanoparticles designed for biomedical applications. J. Magn. Magn. Mater. 380, 132–139. ( 10.1016/j.jmmm.2014.10.146) [DOI] [Google Scholar]
- 24.Tombácz E, Szekeres M, Hajdú A, Tóth IY, Bauer RA, Nesztor D, Illés E, Zupkó I, Vékás L. 2014. Colloidal stability of carboxylated iron oxide nanomagnets for biomedical use. Periodica Polytechn. Chem. Eng. 58, 3–10. ( 10.3311/PPch.7285) [DOI] [Google Scholar]
- 25.Lam T, Avti P, Pouliot P, Maafi F, Tardif J-C, Rhéaume É, Lesage F, Kakkar A. 2016. Fabricating water dispersible superparamagnetic iron oxide nanoparticles for biomedical applications through ligand exchange and direct conjugation. Nanomaterials 6, 100 ( 10.3390/nano6060100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedrich RP, Zaloga J, Schreiber E, Tóth IY, Tombácz E, Lyer S, Alexiou C. 2016. Tissue plasminogen activator binding to superparamagnetic iron oxide nanoparticle—covalent versus adsorptive approach. Nanoscale Res. Lett. 11, 297 ( 10.1186/s11671-016-1521-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Illés E, Szekeres M, Kupcsik E, Tóth IY, Farkas K, Jedlovszky-Hajdú A, Tombácz E. 2014. PEGylation of surfacted magnetite core-shell nanoparticles for biomedical application. Colloids Surf. A Physicochem. Eng. Aspects 460, 429–440. ( 10.1016/j.colsurfa.2014.01.043) [DOI] [Google Scholar]
- 28.Nesztor D, Bali K, Tóth IY, Szekeres M, Tombácz E. 2015. Controlled clustering of carboxylated SPIONs through polyethylenimine. J. Magn. Magn. Mater. 380, 144–149. ( 10.1016/j.jmmm.2014.10.091) [DOI] [Google Scholar]