Abstract
Considered an antidepressant and anti-anxiety agent, Hypericum perforatum affects multiple neurotransmitters in a non-competitive synergistic manner, and may have nootropic potential. We quantitatively reviewed the pre-clinical literature to examine if there is a cognitive-enhancing effect of H. perforatum in healthy rodents. Additionally, within these studies, we compared the effects observed in intact rodents versus those whose performance has been impaired, mostly through stress manipulations. The meta-analysis incorporated studies that examined the effect of H. perforatum versus placebo on memory indices of task performance. All analyses were based on weighting different studies according to their inverse variance. Thirteen independent studies (published 2000–2014) involving 20 experimental comparisons met our inclusion criteria. The results showed a large positive effect of H. perforatum on cognitive performance for intact, healthy rodents (d = 1.11), though a larger effect emerged for stress-impaired rodents (d = 3.10 for restraint stress). The positive effect on intact rodents was observed in tasks assessing reference memory as well as working memory, and was not moderated by the type of memory or motivation (appetitive versus aversive). Thus, while primarily considered as a medication for depression, H. perforatum shows considerable nootropic potential in rodents.
Hypericum perforatum (H. perforatum), also known as St John’s wort, has been regarded as a medicinal herb for at least two thousand years, being first mentioned in the first century by the Greek herbalist Pedanios Dioskourides1. Traditionally, H. perforatum had been utilized for a variety of medical purposes such as the treatment of burns, wounds, hematomas, inflammations, and muscle pain1,2. It has also been used to reduce fearfulness and melancholy, and is depicted in various religious texts as a “demon chaser”2. This latter psychological effect has recently been rediscovered, with H. perforatum being found to reduce anxiety and depression3,4. Later research showed that it was as effective for reducing symptoms of mild to moderate depression as classic antidepressants such as tricyclic antidepressants (TCA)5 and selective serotonin reuptake inhibitors (SSRIs)6. Today, H. perforatum is an established medicine for mild to moderate depression, registered in many European countries7, and considered to have fewer side effects compared to other antidepressants7,8. In addition to its effect on depression and anxiety, it has been suggested in preclinical studies that H. perforatum also improves some aspects of cognitive functioning, particularly the acquisition and consolidation of memories9,10,11. However, this effect has not always been found to replicate in rodents12,13, while no benefit has been found in humans14,15. The main goals of the current paper are to clarify whether there is a cognitive-enhancing (nootropic) effect of H. perforatum in rodents, and to examine the relationship between the antidepressant and nootropic effects of H. perforatum by evaluating its effects in intact rodents versus those subjected to stress manipulations leading to impaired cognitive performance. We address these research questions by quantitatively reviewing the relevant pre-clinical studies that have examined intact animals. When these studies also examined performance-impaired animals (in a two by two design: impaired/intact × placebo/medication), we assessed the relative benefit of H. perforatum for intact rodents. In particular, we compared the effect for intact animals and those whose performance was impaired, while controlling for the design of the study, the dosage, and the experimental task.
H. perforatum includes at least seven different active ingredients16, among them hypericin and hyperforin are considered the primary constituents7,17. It has been demonstrated that these components have a unique combined pharmacological effect, inhibiting the reuptake of several neurotransmitters in a non-competitive synergistic manner18. Similar to other antidepressants, H. perforatum inhibits the reuptake of monoamine neurotransmitters (5-HT, noradrenaline, and dopamine)18,19,20, which increases the concentration of serotonin and other monoamines in the extracellular space in brain regions such as the hippocampus, thalamus, amygdala, and the prefrontal cortex21. Upregulation of 5-HT in these areas reduces negative affect from impinging into memory processes22,23, while upregulation of dopamine reduces background firing rate of neurons, thus decreasing non-task related activity and improving signal to noise ratio24. These monoaminergic effects are considered quite weak8,16,18; however, they are relatively broad in that not only the expression of 5-HT1A receptors is upregulated as in most SSRIs, but also of 5-HT2 receptors17,18. Another effect of H. perforatum is blocking the binding of GABA3, which results in decreased central nervous system inhibition25. This arousal-related effect is considered to improve the consolidation of memory in the long term storage26. H. perforatum further regulates NMDA receptors18,27 which play a pivotal role in memory and nootropics28. The existence of these neuropharmacological pathways suggests that H. perforatum can enhance the cognitive performance of healthy intact animals, either because of its anti-anxiolytic effects which may alleviate task stress or through its effects on memory and task-related attention.
Methods
A Google Scholar structured search using different keywords was conducted in order to find relevant studies (last search run on March 2016). No limits were applied for language and foreign papers were translated. We used the following search terms: “hypericum”, “animal”, and one of the following: “nootropic”, “cognitive enhancing”, “cognitive enhancers”, “memory enhancing”, or “memory enhancement”. We also included all relevant studies referred to by a previous comprehensive review7.
The following eligibility criteria were used: with respect to study type, we included only studies that examined the effect of H. perforatum versus placebo. No language, publication date, or publication status restrictions were imposed. With respect to types of participants, we included only animal studies with healthy intact animals. Thus, we included studies fulfilling the following two criteria: random assignment of animals to groups, and a complete design with at least one healthy, intact, control group treated with placebo and a healthy intact rodent group treated with H. perforatum. With respect to the type of intervention, we included any dosage of H. perforatum administered for any duration, versus placebo but not versus no medication at all. With respect to types of outcome measure, our analysis focused on learning and memory indices of task-performance. Further to this, we focused on response time measures of these indices (which were commonly reported). Studies or conditions involving tasks that focused on anxiolytic effects of H. perforatum, such as time to escape an unknown dangerous place, were excluded (because this examination confounds nootropic and antidepressant/anxiolytic effects). Methods of the analysis and inclusion criteria were specified in advance and documented. Eligibility assessment was performed independently in an unblinded standardized manner by two reviewers. Disagreements between reviewers were resolved by consensus (though no such case emerged).
We used a data extraction protocol based on the Cochrane Consumers and Communication Review Group’s data extraction template. One review author extracted the data from included studies and the second author checked the extracted data. When a paper lacked statistical data (averages, standard errors), we requested data from the authors. In cases of no response, we extracted means and SDs from figures using the ImageJ® software (V. 1.48), following the protocol used in other reviews29,30.
Information was extracted from each included trial on: (1) characteristics of organisms (species, age), and the trial’s inclusion and exclusion criteria; (2) type of intervention (including type, dose, duration, and frequency of H. perforatum administration; versus placebo; and any impairment condition, and (3) type of outcome measure (task name and all indices of task performance). The primary measure was speed of performance. The tasks used in the different studies are shown in Table 1.
Table 1. Preclinical studies examining the effect of Hypericum perforatum on cognitive performance of healthy intact rodents.
| Authors, year | H. perforatum maximal dose and admin. Duration | Rodent age (months) | Task(s)a | No. of healthy animals (max dose) | Impairment method | No. of impaired animals (max dose) |
|---|---|---|---|---|---|---|
| Kumar et al., 200010 | 200 mg/kg × 3 daysc | Adults | Active avoidance response, passive avoidance learningb | 72 | Drug induced amnesia | 48 |
| Klusa et al., 20019 | 50 mg/kg × 8 daysc | Adults | Conditioned avoidance response, passive avoidance learningb | 39 | — | — |
| Misane & Ogren, 200137 | 30 mg/kg × 1 day | Adults | Passive avoidance learning testb | 16 | — | — |
| Kumar et al., 200216 | 200 mg/kg × 3 daysc | Adults | Active avoidance response, passive avoidance learningb | 24 | Electroconvulsive shock induced amnesia | 24 |
| Widy-Tyszkiewicz et al., 200241 | 13 mg/kg × 63 daysc | 6 m | Morris water maze58 | 17 | — | — |
| Beijamini & Andreatini, 200331 | 300 mg/kg × 7 daysc | Adults | Elevated T maze59 b | 48 | — | — |
| Trofimiuk et al., 200539 | 350 mg/kg × 21 days | 2 m | Morris water maze58, Object recognition test60 | 40 | Restraint stress; cortisol-induced stress | 78 |
| Trofimiuk et al., 200612 | 350 mg/kg × 21 days | 2 m | Passive avoidance learning testb | 20 | Restraint stress; cortisol-induced stress | 40 |
| Trofimiuk & Braszko, 200811 | 350 mg/kg × 21 days | 2 m | Morris water maze58 | 20 | Restraint stress; cortisol-induced stress | 41 |
| Prakash et al., 201038 | 200 mg/kg × 21 days | Adults | T maze59 | 16 | Restraint stress | 16 |
| Hasenein & Shahidi, 201136 | 25 mg/kg × 30 daysc | 3–4 m | Passive avoidance learning testb | 14 | Drug induced diabetes | 14 |
| Trofimiuk et al., 201140 | 350 mg/kg × 21 days | 2 m | Barnes maze61 | 32 | Restraint stress; cortisol-induced stress | 64 |
| Asadi et al., 201413 | 350 mg/kg × 7 days | Adults | Passive avoidance learning test62 b | 12 | Restraint stress | 12 |
Note: C – Control group (Intact rodents), H – H. perforatum group (Intact rodents), I –Impaired group.
IH – Impaired and H. perforatum group.
aMean latency or duration of performance was measured, unless otherwise noted.
bDuration of avoiding a dark compartment where rats had previously undergone electric shock. Effect size was inversed.
cMultiple dosages of H. perforatum were administrated (maximal dose is noted).
To examine the effect on different types of memory, we categorized the tasks into those assessing primarily working memory, reference memory (i.e., long term storage of fixed trial properties), and recognition memory. To ascertain whether the effect only emerged for avoidance-related responses we further categorized each task as appetitive or aversive and tested whether effect size may differ under these two categories. This categorization of tasks into memory types and appetitive/aversive motivation appears on Supplementary Table S1. Finally, each paper was reviewed to determine whether animals had access to food and water and were kept in ample temperature and humidity prior to the experiment, and the blinding of data collectors and outcome assessors.
The meta-analysis was based on all relevant tasks within the included papers. In cases where multiple dosages were used in different conditions, we considered the maximal one. However, in order to examine the moderating effect of dosage we conducted a secondary analysis including all dosages levels. In studies with multiple measurements of performance along several days10,16,31, we used the results obtained on the day after first acquisition.
Means and standard deviations (or standard errors) were obtained from each study to estimate the pooled effect sizes (Cohen’s ds) of the treatment effect on intact and impaired rodents. Confidence intervals for each study’s effect size, and pooled effect sizes and their confidence intervals were calculated using the common practice of weighting different studies according to their inverse variance32,33. If within a study, multiple tasks were conducted on different animals, n sizes were based on the total number of organisms taking part in the study, such that each study received weight according to the number of organisms tested. Additionally, in order to test for publication bias we plotted the effect size of each experiment by its sample size. The symmetry of such ‘funnel plots’ was assessed to see if the effect decreased with increasing sample size34,35.
To examine moderating effects we conducted Weighted Least Squares (WLS) regressions, which included the duration of H. perforatum administration (number of consecutive days), dosage level, type of memory predominantly assessed, and motivation (appetitive versus aversive) as predictors. In this latter examination we needed to delve into the properties of individual tasks within each study because some have used multiple task types. Therefore, each observation was further weighted according to the number of tasks within a study (the weight score was divided by the number of tasks). In light of the small number of studies, each of the four moderators (duration, dosage, memory type, and motivation) was individually entered into a separate regression model as a predictor.
Results
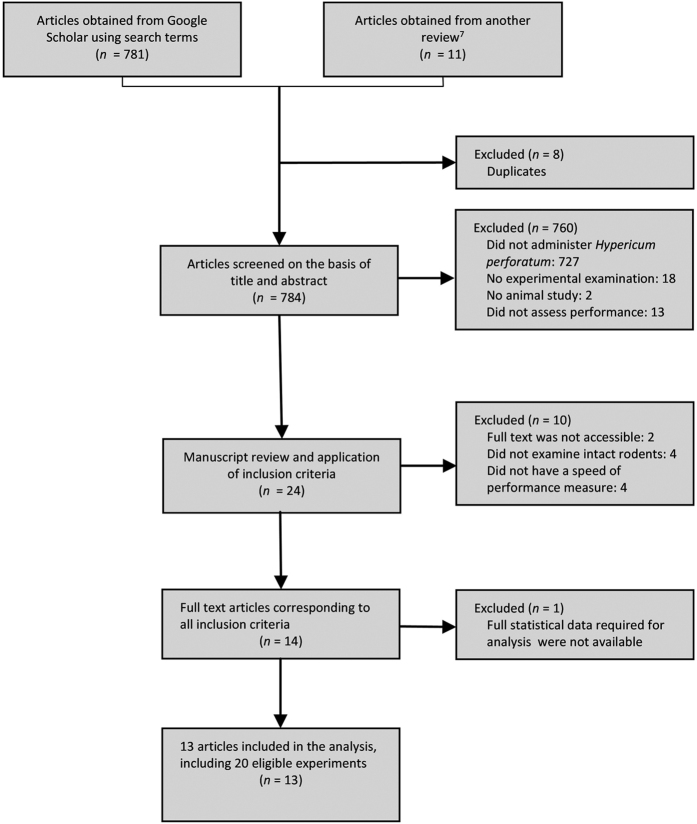
The literature search provided a total of 792 citations, of which 13 studies met the criteria and were included in the review. Figure 1 provides a complete flow diagram of this process. The 13 reviewed studies (each published as a separate paper between the years 2000 and 2014)9,10,11,12,13,16,31,36,37,38,39,40,41 involved 20 experimental comparisons. Tested animals had access to food and water and were kept in ample temperature and humidity prior to the experiment. All studies, except for two, used rats (Prakash et al., 2010 38 used mice and Klusa et al., 2011 9 used both rats and mice). The number of animals in each experimental condition varied between 6 and 16 (M = 8.3, SD = 2.8). No information on blinding of experimenters and data evaluators was available. Table 1 includes the detailed characteristics of each study.
Figure 1. Flow diagram of literature search (based on Moher et al.) 57.
Overall, for non-impaired organisms H. perforatum was administered to 289 healthy intact rodents (maximal dosages groups: n = 170) and placebo to 200 healthy intact rodents. Additionally, nine studies (involving 13 experiments) also compared the intact animals - as a control group - to a group in which cognitive performance had been impaired. Across experiments, the predominant means of impairment was by restraint-induced stress (k = 6); others were cortisol induced stress (k = 4), scopolamine, sodium nitrite, and electroconvulsive shock induced amnesia (k = 2), and diabetes (k = 1). With the exception of two studies which administrated H. perforatum acutely (in 1 day), others maintained daily H. perforatum administration sub-chronically (4 days) to chronically (from 21 to even 64 days) before measuring performance. Daily dosages varied greatly, between 1 to 350 mg/kg.
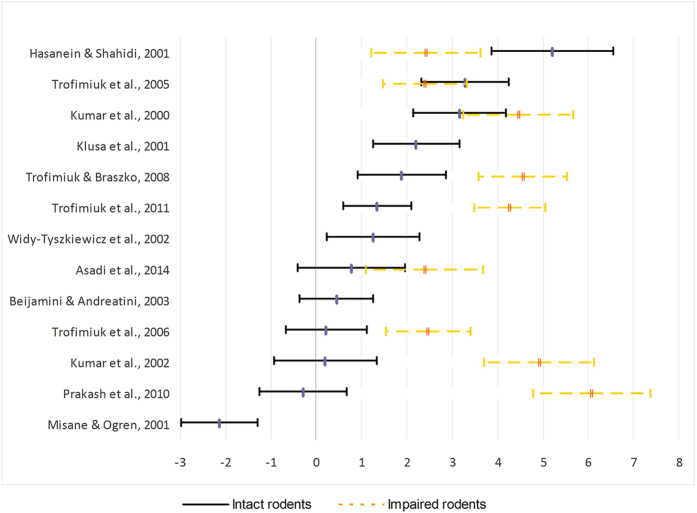
Figure 2 illustrates the effect size and 95% confidence interval for each of the 13 studies. Across studies, there was a large positive effect of H. perforatum on intact rodents’ performance (mean d = 1.11) which was statistically significant (95% CI: 0.84 – 1.37), indicating that administration considerably enhanced memory processes among healthy, intact, rodents. For the impaired rodents the effect was larger (mean d = 3.70, 95% CI: 3.35 – 4.04). This was true both for rodents whose performance was impaired by restraint stress (mean d = 3.10, 95% CI: 2.68 – 3.52) or by other means (mean d = 4.04, 95% CI: 3.62 – 4.46). The differences between the effect for intact versus impaired rodents were statistically significant (intact vs. restraint stress: z = 6.56, p < 0.001; intact vs. other means of impairment: z = 8.25, p < 0.001). Still, there was large variance in the relative influence on intact and impaired rodents. In five out of nine studies, the administration of H. perforatum led to significantly higher performance levels for impaired rodents; in three studies, there was no significant difference; while in one study intact rodents benefited significantly more than impaired ones.
Figure 2. Effect sizes (Cohen’s d’s) and 95% confidence intervals for the difference between the effect of Hypericum perforatum and placebo on cognitive performance of intact and impaired rodents.
In addition to the effect size estimation, heterogeneity was assessed using the common measurements of Q and I2 42. Cochran’s Q test yielded significant heterogeneity for both intact (Q = 154, p < 0.001) and impaired (Q = 46, p < 0.001) rodent designs. The I2 index also showed large heterogeneity for the intact (92%) and impaired (83%) experimental comparisons. While not proving the existence of moderators driving heterogeneity, these results strongly invite further investigations of the variability across studies43.
As noted above, one major difference between experiments involved the type of memory assessed. The majority of the reviewed studies (k = 11) examined reference memory (i.e., recalling trial fixed features) and because of this the overall effect size reflects differences in reference memory to a greater extent than other types of memory. Hence, we also separately analyzed the tasks assessing each memory type. In studies of reference memory we found an effect size of d = 1.06 (95% CI: 0.76 – 1.35) for intact rodents, and d = 3.31 (95% CI: 2.88 – 3.73) for the impaired ones. In studies assessing primarily working memory there was also a large effect size for healthy intact rodents, d = 1.54 (95% CI: 0.95 – 2.14), and a larger effect size for impaired rodents, d = 4.38 (95% CI: 3.77 – 4.99) (one study focused on recognition memory with the results being similar, intact d = 1.75; impaired d = 2.75).
Weighted Least Squares (WLS) regression analyses were conducted to assess possible moderators within the intact healthy rodents. First, we compared tasks focusing on reference versus working memory (and excluding the single study examining recognition memory): the results show no significant moderating effect (β = 0.12, p = 0.62). Secondly, an examination of appetitive versus aversive motivation similarly showed no moderating effect (β = 0.07, p = 0.78). Finally, there was no moderating effect of duration (β = 0.26, p = 0.27) or dosage (β = 0.13, p = 0.59). For the impaired rodents also, neither memory type, motivation, duration, nor dosage were related to the effect size (p’s > 0.38).
An alternative analysis was conducted taking into consideration not only the maximal dosage, but all dosages (specifically, each dosage group was examined separately and weighted based on the number of different dosage conditions in each study, as indicated above). The effect sizes were similar to those reported above for maximal dosages, both for the intact rodents, d = 1.09 (95% CI: 0.83 – 1.35); and for the impaired ones, d = 3.58 (95% CI: 3.23 – 3.93). WLS regression analyses, analogous to those mentioned above, also did not yield any significant moderator.
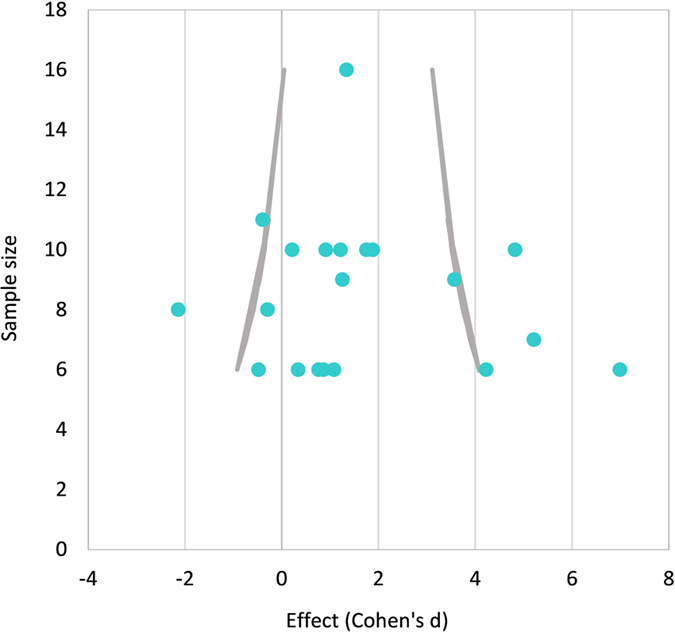
Finally, we tested whether the effect size is inflated due to publication bias (i.e., small sample studies with large effect sizes), using the method proposed by Egger34, which examines the relation between the studies effect size and their sample size. With no publication bias, studies with the largest n are plotted near the average effect size, and smaller studies are spread evenly on both sides, creating a roughly funnel-shaped distribution. The funnel plot, based on all tasks involving intact rodents, is shown in Fig. 3. While the funnel plot is roughly symmetric, reflecting little relation between sample size and effect size, there were four outliers with small sample sizes and extreme results (|z| > 3; 3 positive, 1 negative). Excluding these four cases, a similar size effect remains (d = 1.06, 95% CI: 0.78 – 1.35), emphasizing the validity of the main effect for intact rodents.
Figure 3. Funnel plot of experimental comparisons on intact rodents (maximal dosages only, different tasks shown separately).
Margins are at Z = 3 (99.7%).
Discussion
A meta-analytic summary of preclinical studies revealed that though primarily considered as a medication for depression, H. perforatum seems to have large nootropic effects. As might be expected, across studies the effect was more pronounced in rodents with impaired cognitive performance, either due to stress or amnestic agents. However, particularly for the stress-impaired rodents there was large heterogeneity between studies and only in about a third of the studies was the effect significantly higher for the impaired compared to the intact rodents. Classic antidepressant drugs, such as SSRIs and SNRIs, are not usually considered to possess nootropic traits, unless administered to depressed or anxious populations, in whom they can reduce cognitive biases and restore normal cognitive performance44,45. Our results suggest that at least for H. perforatum, this assumption should be re-evaluated.
Interestingly, the nootropic effect was observed in tasks involving different characteristics: first, the effect was observed both in tasks assessing reference memory as well as in tasks assessing primarily working memory. Secondly, H. perforatum enhanced performance both in tasks involving appetitive and aversive stimuli. This implies that the effect of H. perforatum on performance is not mediated solely by enhanced avoidance responses due to its effect on the mood system37, but rather that it modulates broader learning and memory processes.
Our view is that two basic aspects contribute to this nootropic effect for healthy intact rodents: First, there is extensive documentation that performance-related stress may impinge upon the achievements of intact organisms as well, thus reducing it may have positive effects5,46. In addition to its effect on a variety of neurotransmitters noted above, H. perforatum also has a long term influence on the expression of genes involved in the regulation of the hypothalamic-pituitary-adrenal (HPA) axis, which leads to reduced plasma adrenocorticotropic hormone and corticosterone level46 and inhibits stress-induced increases in gene transcription in the hippocampus and the pituitary46. An additional neuroendocrine interaction is via suppression of interleukin (IL-6) release, which inhibits substance-P mediated stress responses47. These diverse stress-related mechanisms are consistent with our finding that the nootropic effect was larger in stress-induced rodents. Secondly, H. perforatum may have additional nootropic effects that should be further investigated. In part, these processes may be specific to spatial tasks: H. perforatum uniquely upregulates 5-HT2 receptors, an effect which is thought to contribute to the enhancement of spatial memory41,48. Alternatively, it may have a general effect on memory performance, via its dopaminergic activity27, or due to its GABAergic traits modulating working memory49, or combined GABA and glutamate neuromechanisms regulating NMDA receptors18.
Limitations of the current study include the heterogeneity of the tasks performed, and the overall small number of studies. As well, as with any meta-analysis one should consider the likelihood that studies with null results were not published, thus inflating the effect size found. Our analysis using Egger’s method34 suggests marginal inflation of effect size in the present investigation, though it does not completely rule out the possibility of a drawer effect. Still, it should be stressed that in the majority of the reviewed studies the underlying assumption was an interaction effect, such that there would be a positive effect of H. perforatum on performance for the stress-impaired rodents and not for the intact ones. Nevertheless, a considerable and unexpected positive effect emerged for the intact control group.
In light of these findings it is interesting to consider two human studies examining the effect of H. perforatum administration on cognition14,15. The first study involved 12 healthy volunteers, who underwent three within-subject conditions of a single acute dose (Placebo, H. perforatum 900 mg or H. perforatum 1800 mg). No significant effect of H. perforatum was obtained in tasks assessing several cognitive functions (working memory, delayed recall, and reaction time). Furthermore, the highest dose (1800 mg) even impaired performance in some of the tasks14. The second study was also based on a small sample of 12 healthy volunteers, and examined the effect of 14 days of H. perforatum administration (standard antidepressant dosage containing 900 μg hypericin). No effects were found in cognitive tasks and physiological measures of autonomic nervous system activity15.
What seems to differentiate the former study from most of the animal studies in our review is that it had only a single administration of H. perforatum. For SSRIs and H. perforatum as well, the neurochemical effects are expected to build up and reach significance only after a couple of weeks50,51. In addition, it is worth noting that both human studies used very large dosages, about the same as those recommended for individuals suffering from moderate to severe depression52. Potentially, given the U shape effect found for some of the behavioral outcomes of H. perforatum53, smaller dosages may be more effective. Finally, the tasks used in the human literature markedly differed from those used in the rodent literature, being less based on learning and more on basic working memory capacity54,55. Because of the presumed attention-focusing effects of H. perforatum, the effects in human may be more pronounced in complex learning or interference tasks56. Still, despite these potential differences, the gap between the nootropic effects that we observe in rodent studies and the absence of the effect in human studies remains unclear.
Additional Information
How to cite this article: Ben-Eliezer, D. and Yechiam, E. Hypericum perforatum as a cognitive enhancer in rodents: A meta-analysis. Sci. Rep. 6, 35700; doi: 10.1038/srep35700 (2016).
Supplementary Material
Acknowledgments
We acknowledge our debt of gratitude for the efforts and results of the research groups who have conducted the studies reviewed in this paper. This work was supported in part by the Max Wertheimer Minerva Center for Cognitive Studies and by the I-CORE program of the Planning and Budgeting Committee and the Israel Science Foundation (Grant no. 1821/12).
Footnotes
Author Contributions E.Y. and D.B.-E. designed the study. D.B.-E. collected and analyzed the data with E.Y.’s guidance. D.B.-E. and E.Y. jointly wrote the manuscript.
References
- Istikoglou C. I., Mavreas V. & Geroulanos G. History and therapeutic properties of Hypericum Perforatum from antiquity until today. Psychiatriki. 21, 332–338 (2010). [PubMed] [Google Scholar]
- Moffat B. Archaeological Sources for the History of Herbal Medicine Practice: The case study of St John’s wort with valerian at Soutra medieval hospital. In Francia S., Stobart A. (eds) Critical Approaches to the History of Western Herbal Medicine: From Classical Antiquity to the Early Modern Period. Bloomsbury, London, UK, pp. 253–270 (2014). [Google Scholar]
- Chatterjee S. S., Bhattacharya S. K., Wonnemann M., Singer A. & Müller W. E. Hyperforin as a possible antidepressant component of Hypericum extracts. Life Sci. 63, 499–510 (1998). [DOI] [PubMed] [Google Scholar]
- Kessler R. C. et al. The use of complementary and alternative therapies to treat anxiety and depression in the United States. Am. J. Psychiat. 158, 289–294 (2001). [DOI] [PubMed] [Google Scholar]
- Kim H. L., Streltzer J. & Goebert D. St. John’s wort for depression: a meta-analysis of well-defined clinical trials. J. Nerv. Ment. Dis. 187, 532–538 (1999). [DOI] [PubMed] [Google Scholar]
- Brenner R., Azbel V., Madhusoodanan S. & Pawlowska M. Comparison of an extract of Hypericum (LI 160) and sertraline in the treatment of depression: a double-blind, randomized pilot study. Clin. Ther. 22, 411–419 (2000). [DOI] [PubMed] [Google Scholar]
- Committee on Herbal Medicinal Products (HMPC). Assessment Report on Hypericum Perforatum L., Herba. European Medicines Agency: London, UK (2009). [Google Scholar]
- Barnes J., Anderson L. A. & Phillipson J. D. St John’s wort (Hypericum perforatum L.): A review of its chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 53, 583–600 (2001). [DOI] [PubMed] [Google Scholar]
- Klusa V., Germane S., Nöldner M. & Chatterjee S. S. Hypericum extract and hyperforin: memory-enhancing properties in rodents. Pharmacopsychiatry 34, 61–69 (2001). [DOI] [PubMed] [Google Scholar]
- Kumar V., Singh P. N., Muruganandam A. V. & Bhattacharya S. K. Effect of Indian Hypericum perforatum Linn on animal models of cognitive dysfunction. J. Ethnopharmacol. 72, 119–128 (2000). [DOI] [PubMed] [Google Scholar]
- Trofimiuk E. & Braszko J. J. Alleviation by Hypericum perforatum of the stress-induced impairment of spatial working memory in rats. N-S Arch. Pharmacol. 376, 463–471 (2008). [DOI] [PubMed] [Google Scholar]
- Trofimiuk E., Walesiuk A. & Braszko J. J. St John’s wort (Hypericum perforatum) counteracts deleterious effects of the chronic restraint stress on recall in rats. Acta. Neurobiol. Exp. 66, 129–138 (2006). [DOI] [PubMed] [Google Scholar]
- Asadi P., Roshanaee K. & Mohajerani H. R. Effect of Hypericum perforatum extract on consolidation of passive avoidance learning of acutely restrained male wistar rats. New Cell Mol. Biotechnol. J. 4, 51–56 (2014). [Google Scholar]
- Ellis K. A., Stough C., Vitetta L., Heinrich K. & Nathan P. J. An investigation into the acute nootropic effects of Hypericum perforatum L. (St. John’s Wort) in healthy human volunteers. Behav. Pharmacol. 12, 173–182 (2001). [DOI] [PubMed] [Google Scholar]
- Siepmann M., Krause S., Joraschky P., Mück-Weymann M. & Kirch W. The effects of St John’s wort extract on heart rate variability, cognitive function and quantitative EEG: A comparison with amitriptyline and placebo in healthy men. Brit. J. Clin. Pharmacol. 54, 277–282 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Khanna V. K., Seth P. K., Singh P. N. & Bhattacharya S. K. Brain neurotransmitter receptor binding and nootropic studies on Indian Hypericum perforatum Linn. Phytother. Res. 16, 210–216 (2002). [DOI] [PubMed] [Google Scholar]
- Greeson J. M., Sanford B. & Monti D. A., St. John’s wort (Hypericum perforatum): A review of the current pharmacological, toxicological, and clinical literature. Psychopharmacology 153, 402–414 (2001). [DOI] [PubMed] [Google Scholar]
- Nathan P. J. Hypericum perforatum (St John’s Wort): A non-selective reuptake inhibitor? A review of the recent advances in its pharmacology. J. Psychopharmacol. 15, 47–54 (2001). [DOI] [PubMed] [Google Scholar]
- Butterweck V., Böckers T., Korte B., Wittkowski W. & Winterhoff H. Long-term effects of St. John’s wort and hypericin on monoamine levels in rat hypothalamus and hippocampus. Brain Res. 930, 21–29 (2002). [DOI] [PubMed] [Google Scholar]
- Bongiorno P. & LoGiudice P. Hypericum for depression. Nat. Med. J. 2, 3–9 (2010). [Google Scholar]
- Bellani M., Dusi N., Yeh P. H., Soares J. C. & Brambilla P. The effects of antidepressants on human brain as detected by imaging studies. Focus on major depression. Prog. Neuro. Psychoph. 35, 1544–1552 (2011). [DOI] [PubMed] [Google Scholar]
- Bauer M., Heinz A. & Whybrow P. C. Thyroid hormones, serotonin and mood: Of synergy and significance in the adult brain. Mol. Psychiatry 7, 140–156 (2002). [DOI] [PubMed] [Google Scholar]
- Ruhé H. G., Mason N. S. & Schene A. H. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: A meta-analysis of monoamine depletion studies. Mol. Psychiatry 12, 331–359 (2007). [DOI] [PubMed] [Google Scholar]
- Volkow N. D., Wang G. J., Fowler J. S. & Ding Y. S. Imaging the effects of methylphenidate on brain dopamine: New model on its therapeutic actions for attention-deficit/hyperactivity disorder. Biol. Psychiatry 57, 1410–1415 (2005). [DOI] [PubMed] [Google Scholar]
- Baureithel K. H., Büter K. B., Engesser A., Burkard W. & Schaffner W. Inhibition of benzodiazepine binding in vitro by amentoflavone, a constituent of various species of Hypericum. Pharm. Acta Helv. 72, 153–157 (1997). [DOI] [PubMed] [Google Scholar]
- Farkas L. & Crowe S. F. The role of the benzodiazepine–GABA system in the memory processes of the day-old chick. Pharmacol. Biochem. Be. 65, 223–231 (2000). [DOI] [PubMed] [Google Scholar]
- Mondadori C. The pharmacology of the nootropics; new insights and new questions. Behav. Brain Res. 59, 1–9 (1993). [DOI] [PubMed] [Google Scholar]
- Levin E. D., McClernon F. J. & Rezvani A. H. Nicotinic effects on cognitive function: Behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology 184, 523–539 (2006). [DOI] [PubMed] [Google Scholar]
- Wever K. E. et al. Ischemic preconditioning in the animal kidney, a systematic review and meta-analysis. Plos One 7, e32296 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilfoyle M. R., Helmy A., Duane D. & Hutchinson P. J. Regional scalp block for postcraniotomy analgesia: a systematic review and meta-analysis. Anesth. Analg. 116, 1093–1102 (2013). [DOI] [PubMed] [Google Scholar]
- Beijamini V. & Andreatini R. Effects of Hypericum perforatum and paroxetine on rat performance in the elevated T-maze. Pharmacol. Res. 48, 199–207 (2003). [DOI] [PubMed] [Google Scholar]
- Borenstein M., Hedges L., Higgins J. & Rothstein H. Introduction to Meta-Analysis. Wiley: Chichester, UK (2009). [Google Scholar]
- Glass G. V., McGaw B. & Smith M. L. Meta-Analysis in Social Research. (Sage: Beverly Hills, CA, 1981). [Google Scholar]
- Egger M. Bias in meta-analysis detected by a simple, graphic test. Brit. Med. J. 315, 629 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne J. A. et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Brit. Med. J. 343, d4002 (2011). [DOI] [PubMed] [Google Scholar]
- Hasanein P. & Shahidi S. Effects of Hypericum perforatum extract on diabetes‐induced learning and memory impairment in rats. Phytother. Res. 25, 544–549 (2011). [DOI] [PubMed] [Google Scholar]
- Misane I. & Ogren S. O. Effects of Hypericum perforatum (St. John’s wort) on passive avoidance in the rat: evaluation of potential neurochemical mechanisms underlying its antidepressant activity. Pharmacopsychiatry 34, 89–97 (2001). [DOI] [PubMed] [Google Scholar]
- Prakash D. J., Arulkumar S. & Sabesan M. Effect of nanoHypericum (Hypericum perforatum gold nanoparticles) treatment on restraint stressinduced behavioral and biochemical alteration in male albino mice. Pharmacognosy. Res. 2, 330 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trofimiuk E., Walesiuk A. & Braszko J. J. St John’s wort (Hypericum perforatum) diminishes cognitive impairment caused by the chronic restraint stress in rats. Pharmacol. Res. 51, 239–246 (2005). [DOI] [PubMed] [Google Scholar]
- Trofimiuk E., Holownia A. & Braszko J. J. St. John’s wort may relieve negative effects of stress on spatial working memory by changing synaptic plasticity. N-S Arch. Pharmacol. 383, 415–422 (2011). [DOI] [PubMed] [Google Scholar]
- Widy-Tyszkiewicz E., Piechal A., Joniec I. & Blecharz-Klin K. Long term administration of Hypericum perforatum improves spatial learning and memory in the water maze. Biol. Pharm. Bull. 25, 1289–1294 (2002). [DOI] [PubMed] [Google Scholar]
- Huedo-Medina T. B., Sánchez-Meca J., Marín-Martínez F. & Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Methods 11, 193 (2006). [DOI] [PubMed] [Google Scholar]
- Hedges L. V. & Olkin I. Statistical Methods for Meta-Analysis. Academic press, New York, NY, 2014. Scheibehenne B, Greifeneder R, Todd PM. Can there ever be too many options? A meta-analytic review of choice overload. J. Consum. Res. 37, 409–425 (2010). [Google Scholar]
- Mogg K., Baldwin D. S., Brodrick P. & Bradley B. P. Effect of short-term SSRI treatment on cognitive bias in generalised anxiety disorder. Psychopharmacology 176, 466–470 (2004). [DOI] [PubMed] [Google Scholar]
- Greer T. L., Sunderajan P., Grannemann B. D., Kurian B. T. & Trivedi M. H. Does duloxetine improve cognitive function independently of its antidepressant effect in patients with major depressive disorder and subjective reports of cognitive dysfunction? Depress. Res. Treat. 2014, 627863 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterweck V., Winterhoff H. & Herkenham M. St John’s wort, hypericin, and imipramine: a comparative analysis of mRNA levels in brain areas involved in HPA axis control following short-term and long-term administration in normal and stressed rats. Mol. Psychiatry 6, 547–564 (2001). [DOI] [PubMed] [Google Scholar]
- Thiele B., Brink I. & Ploch M. Modulation of cytokine expression by Hypericum extract. J Geriatr. Psychiatry Neurol. 7, S60–S62 (1994). [DOI] [PubMed] [Google Scholar]
- Khalifa A. E. Hypericum perforatum as a nootropic drug: enhancement of retrieval memory of a passive avoidance conditioning paradigm in mice. J. Ehnopharmacol. 76, 49–57 (2001). [DOI] [PubMed] [Google Scholar]
- Levin E. D., Weber E. & Icenogle L. Baclofen interactions with nicotine in rats: effects on memory. Pharmacol. Biochem. Be. 79, 343–348 (2004). [DOI] [PubMed] [Google Scholar]
- Biringer E., Rongve A. & Lund A. A review of modern antidepressants’ effects on neurocognitive function. Curr. Psychiat. Rev. 5, 164–174 (2009). [Google Scholar]
- Loubinoux I. et al. Modulation of behavior and cortical motor activity in healthy subjects by a chronic administration of a serotonin enhancer. Neuroimage 27, 299–313 (2005). [DOI] [PubMed] [Google Scholar]
- Szegedi A., Kohnen R., Dienel A. & Kieser M. Acute treatment of moderate to severe depression with Hypericum extract WS 5570 (St John’s wort): Randomised controlled double blind non-inferiority trial versus paroxetine. Brit. Med. J. 330, 503–508 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterweck V., Petereit F., Winterhoff H. & Nahrstedt A. Solubilized hypericin and pseudohypericin from Hypericum perforatum exert antidepressant activity in the forced swimming test. Planta Medica 64, 291–294 (1998). [DOI] [PubMed] [Google Scholar]
- Luck S. J. & Vogel E. K. The capacity of visual working memory for features and conjunctions. Nature 390, 279–281 (1997). [DOI] [PubMed] [Google Scholar]
- Vogel E. K. & Machizawa M. G. Neural activity predicts individual differences in visual working memory capacity. Nature 428, 748–751 (2004). [DOI] [PubMed] [Google Scholar]
- Bush G., Shin L. M., Holmes J., Rosen B. R. & Vogt B. A. The Multi-Source Interference Task: Validation study with fMRI in individual subjects. Mol. Psychiatry 8, 60–70 (2003). [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J. & Altman D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Brit. Med. J. 339, b2535 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. G. M. Spatial localization does not require the presence of local cues. Learn. Motiv. 12, 239–260 (1981). [Google Scholar]
- Olton D. S. Mazes, maps, and memory. Am. Psychol. 34, 583–596 (1979). [DOI] [PubMed] [Google Scholar]
- Ennaceur A. & Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain. Res. 31, 47–59 (1988). [DOI] [PubMed] [Google Scholar]
- Barnes C. A. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol. 93, 74–104 (1979). [DOI] [PubMed] [Google Scholar]
- Lorenzini C. A., Bucherelli C. & Giachetti A. Passive and active avoidance behavior in the light-dark box test. Physiol. Behav. 32, 687–689 (1984). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.