Abstract
Regulatory T cells (Tregs), a key mediator in regulating anti-tumor immune suppression, tumor immune escape, metastasis and relapse, are considered an important therapeutic target in immunotherapy of human cancers. In the present investigation, elevated CD19+ CD24+ CD38+ regulatory B cells (Bregs) were observed in PBMCs of invasive carcinoma of breast (IBCa) patients compared with that in patients with fibroadenoma (FIBma) or healthy individuals, and the positive correlation existed between Bregs and CD4+ CD25+ CD127− Tregs (r = 0.316, P = 0.001). We found that PD-L1 expression was higher on Bregs in IBCa patients compared with patients with FIBma or healthy individuals (P < 0.05, respectively), and that a tight correlation exists between CD19+ CD24+ CD38+ PD-L1+ Bregs and CD19+ CD24+ CD38+ Bregs (r = 0.267, P = 0.007), poor TNM phases and up-regulated expression of PD-L1 on Bregs. The pattern of PD-1 expression on CD4+ T cells indicated that high level of PD-1hi expressed on CD4+ CD25+ CD127+ effector T cells (P < 0.001). More importantly, the presence of PD-L1 on Bregs was positively correlated with Tregs (r = 0.299, P = 0.003), but negatively correlated with PD-1hi effector T cells (r = −0.22, P = 0.031). Together, results of the present study indicated that PD-L1 is an important molecule on Bregs, mediated the generation of Tregs in IBCa.
Regulatory B cells (Bregs), a subset of B cells, play a suppressive role in autoimmune diseases, inflammation, and anti-tumor immune response1,2,3,4,5. Based on the expression of various surface molecules, several populations of Bregs have been reported including B10 cells6, CD1dhi CD5+ CD19+ B cells7, CD19+ CD24+ CD38+ B cells2 or CD19+ CD24+ CD27+ B cells8 and so on. Recently, IL-10-producing was recognized as one of the most important characters of functional Bregs6,9,10,11. Increasing evidence showed that, IL-10, as an inhibitory cytokine, suppresses the differentiation and proliferation of Th17, inhibits the secretion of IFN-γ, and reduces the accumulation of NK cells5. In one of our early studies, we also found high percentage and density of CD19+ B cells in the tissues of invasive carcinoma of breast (IBCa) which expressed IL-10 in cytoplasm. We demonstrated that CD19+ B cells from IBCa patients but not that from healthy individuals could induce the expansion of Treg cells in vitro12. The immunosuppressive effects of Bregs have also been shown to be mediated via by promoting Tregs in mice models of autoimmune diseases13, and non-autoimmune diseases including cancers14. Nevertheless, the function and mechanism of Bregs in immune response remain poorly understood.
Programmed death-ligand 1 (PD-L1), as a critical suppressive molecule, constitutively expresses on B lymphocytes, T lymphocytes, dendritic cells and monocytes15,16,17. The expression of PD-L1 is induced by ligation of cell surface receptors and/or stimulation with the TH1-associated cytokine IFNγ15. Its receptor, the programmed death-1 (PD-1), is up-regulated in activated T or B cells17. The PD-1/PD-L1 signaling axis has been shown to be a critical regulator for maintaining peripheral tolerance15.
It is clear that there was an increased number of CD19+ CD24+ CD38+ Bregs in the peripheral blood mononuclear cells (PBMCs) of IBCa patients12, however it is not currently known whether PD-1/PD-L1 expressed on CD19+ CD24+ CD38+ Bregs acts exclusively on Tregs or other components of IBCa. In the present study, in an effort to further understand the role of Bregs in the etiology and pathogenesis of breast cancer, we examined CD4+ T cells, CD19+ B cells and their subsets in PBMCs of breast tumor patients, and investigated the relationship among CD4+ T cells, CD19+ B cells and their subsets in breast tumor.
Results
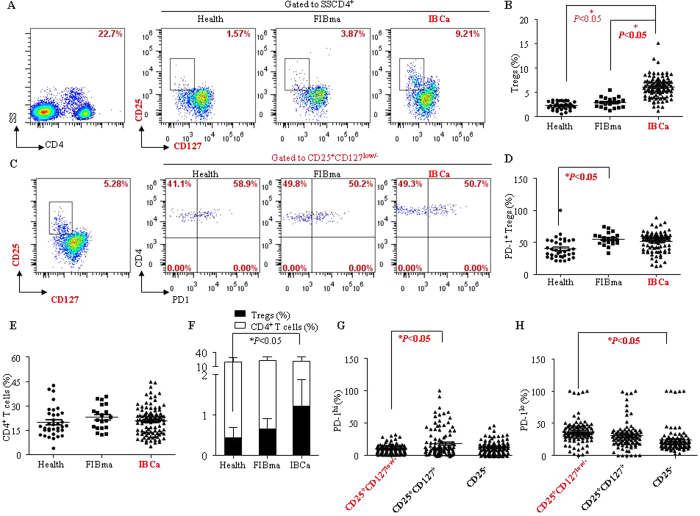
CD4+CD25+CD127low/− Tregs predominated in PBMCs of IBCa patients
CD4+ T lymphocytes, regarded as T helper cells, regulate the immune responses by activating other immune cells or dividing to Tregs to suppress immune reaction. In the present study, we evaluated the percentages of CD4+ T cells and its subsets in the PBMCs of breast tumor patients. The percentages of CD4+ T cells in PBMCs of health individuals (19.90 ± 9.02%), fibroadenoma (FIBma) (22.98 ± 7.51%) and IBCa patients (20.74 ± 8.84%) were comparable (P > 0.05; Fig. 1A,E). However, The percentage of CD4+CD25+CD127low/− T cells was significantly higher in the PBMCs of IBCa patients (6.06 ± 2.17%) compared with that in FIBma patients (2.91 ± 1.06%) and health individuals (2.28 ± 0.79%) (P < 0.05 for both; Fig. 1A,B). Similarly, the ratio of CD4+CD25+CD127low/− Tregs and CD4+ T cells was among the highest in IBCa patients in comparison with health individuals and FIBma patients (P < 0.05; Fig. 1F).
Figure 1. CD4+CD25+CD127low/− T lymphocyte predominated in PBMCs of IBCa patients.
To investigate the phenotype and subsets of CD4+ T cells in patients and healthy individuals, PBMCs were isolated from peripheral blood, incubated with CD4-ECD, CD25-PE-cy7, CD127-FITC or PD-1-PE, and analyzed by flow cytometry. (A,B) The percentage of CD4+CD25+CD127low/− T cells was among the highest in PBMCs of IBCa patients (6.06 ± 2.17%) compared with FIBma patients (2.91 ± 1.06%) and health individuals (2.28 ± 0.79%) (P < 0.05 for all). (C,D) The expression of PD-1 on CD4+CD25+CD127low/− Tregs in PBMCs of health individuals, FIBma and IBCa patients analyzed by flow cytometry. These was significant difference between the PBMCs of FIBma patients and health individuals (P < 0.05). (E) The percentages of CD4+ T cells in PBMCs of health individuals, FIBma and IBCa patients were 19.90 ± 9.02%, 22.98 ± 7.51%, and 20.74 ± 8.84%, respectively. And there no difference was found between health individuals and FIBma patients or health individuals and IBCa patients (P > 0.05). (F) The ratio of CD4+CD25+CD127low/− Tregs and CD4+ T cells was highest in PBMCs of IBCa patients compared with health individuals and FIBma (P < 0.001). (G,H) The expression of PD-1 compartmentalized into two distinct (PD-1lo and PD-1hi) populations. The high level PD-1hi expression was found on CD4+CD25+CD127+ T cells (20.00 ± 22.15%) and CD4+CD25− T cells (13.92 ± 10.15%), compared with CD4+CD25+CD127low/− Tregs (9.57 ± 7.27%). And there was significantly difference between CD4+CD25+CD127low/− Tregs and CD4+CD25+CD127+ effector T cells (P < 0.05). On the other hand, there was high level PD-1lo expression was found on CD4+CD25+CD127low/− Tregs (37.17 ± 19.27%), compared with CD4+CD25+CD127+ effector T cells (31.03 ± 20.84%) or CD4+CD25− T cells (23.87 ± 19.68%) (P > 0.05 and P < 0.05, respectively).
Because PD-1 is an important molecule in immune suppression, we then evaluated the expression of PD-1 on CD4+ T cells and its subsets including CD4+CD25+CD127low/− Tregs in PBMCs. Based on the the expression level of PD-1, each cell population was divided into two distinct subpopulations, PD-1lo and PD-1hi (Fig. S1). Although the expression levels of PD-1 on CD4+CD25+CD127low/− Tregs were significantly different between the PBMCs of FIBma (55.19 ± 9.98%) and healthy individuals (39.95 ± 16.10%)(P < 0.05; Fig. 1C,D), no significant difference between IBCa patients (48.10 ± 18.60%) and healthy individuals (P > 0.05; Fig. 1C,D) were seen. However, the percentage of PD-1hi CD4+CD25+CD127low/− Tregs (9.57 ± 7.27%) were significantly lower than the PD-1hi CD4+CD25+CD127+ T cells (20.00 ± 22.15%) in IBCa (P < 0.05; Fig. 1G). The percentage of PD-1hi CD4+CD25− T cells was 13.92 ± 10.15%, which was not significantly different from PD-1hi CD4+CD25+CD127low/− Tregs or PD-1hi CD4+CD25+CD127+ effector T cells (Fig. 1G). In contrast, there were statistically significantly more PD-1lo CD4+CD25+CD127low/− Tregs (37.17 ± 19.27%) than PD-1lo CD4+CD25+CD127+ effector T cells (31.03 ± 20.84%) and PD-1lo CD4+CD25− T (23.87 ± 19.68%) (P > 0.05 and P < 0.05, respectively) in IBCa (Fig. 1H).
Further analysis of the relationship among the percentages of CD4+ T cells, CD4+ CD25+ CD127low/− Tregs, and CD4+ CD25+ CD127low/− PD-1+ Tregs in IBCa and the histopathological characteristics of IBCa revealed that the CD4+ T cells were higher in patients of 49 years or older (P = 0.047) (Table 1). The percentage of the above cell populations were not significantly different among all other histopathological characters examined including tumor grade, status of metastasis, TNM staging, ER, PR and HER2 status, as well as tumor size (Table 1).
Table 1. Relationship between the proportion of CD4+ T cells and its subset in PBMCs and the clinicopathological parameters of IBCa patients.
| Variables | All cases | T cells | ||
|---|---|---|---|---|
| CD4+ | CD4+CD25+CD127− | CD4+CD25+CD127−PD-1+ | ||
| IDCa | 98 | |||
| Grade | 98 | |||
| G1 | 14 | 24.08 ± 9.13 | 5.54 ± 2.09 | 53.49 ± 13.66 |
| G2 | 63 | 20.20 ± 8.97 | 6.03 ± 2.25 | 45.99 ± 20.93 |
| G3 | 21 | 18.90 ± 7.57 | 6.57 ± 1.99 | 50.60 ± 12.91 |
| P | 0.213 | 0.381 | 0.314 | |
| LN metastasis | ||||
| No | 48 | 21.68 ± 16.69 | 5.90 ± 2.12 | 47.27 ± 17.93 |
| Yes | 50 | 19.84 ± 8.75 | 6.20 ± 2.23 | 48.90 ± 19.37 |
| P | 0.303 | 0.496 | 0.671 | |
| TNM | ||||
| I | 24 | 23.29 ± 8.60 | 5.86 ± 2.45 | 43.44 ± 16.71 |
| II | 41 | 19.61 ± 9.10 | 5.84 ± 1.70 | 51.00 ± 20.57 |
| III | 24 | 20.81 ± 7.58 | 6.45 ± 2.51 | 47.29 ± 15.61 |
| IV | 9 | 18.91 ± 11.22 | 6.46 ± 2.55 | 50.15 ± 21.97 |
| P | 0.388 | 0.650 | 0.462 | |
| ER | ||||
| Negative | 32 | 21.95 ± 9.38 | 6.00 ± 1.77 | 48.91 ± 17.99 |
| Positive | 66 | 20.15 ± 8.58 | 6.08 ± 2.35 | 47.72 ± 19.01 |
| P | 0.348 | 0.856 | 0.771 | |
| PR | ||||
| Negative | 56 | 21.63 ± 9.08 | 6.01 ± 1.85 | 47.06 ± 18.80 |
| Positive | 42 | 19.55 ± 8.48 | 6.12 ± 2.56 | 49.50 ± 18.47 |
| P | 0.252 | 0.793 | 0.529 | |
| HER2 | ||||
| Negative | 62 | 21.12 ± 8.87 | 6.10 ± 2.41 | 49.35 ± 16.97 |
| Positive | 36 | 20.09 ± 8.87 | 5.98 ± 1.70 | 45.93 ± 21.26 |
| P | 0.580 | 0.796 | 0.389 | |
| Age | ||||
| <49 y | 39 | 18.57 ± 8.66 | 6.23 ± 2.21 | 47.79 ± 18.32 |
| ≥49 y | 59 | 22.18 ± 8.74 | 5.94 ± 2.16 | 48.31 ± 18.94 |
| P | 0.047 | 0.510 | 0.894 | |
| Tumor size | ||||
| <3 cm | 59 | 19.96 ± 8.83 | 5.89 ± 2.02 | 46.66 ± 18.56 |
| ≥3 cm | 39 | 21.93 ± 8.85 | 6.31 ± 2.38 | 50.21 ± 18.69 |
| P | 0.283 | 0.346 | 0.361 | |
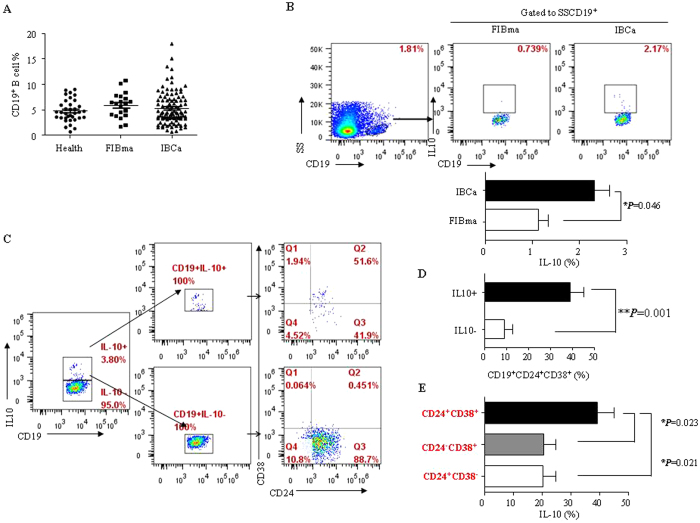
IL-10+ B cells were enriched in the CD19+CD24+CD38+ B cell population
Different from the positive regulation in immune response, the new functions of B cells in antitumor immunity warrant further investigation. CD19+IL-10+ B cells, also known as B10, were regarded as regulatory B cells which play an important role in immune suppression. In present study, we evaluated the percentage of CD19+ B cells in PBMCs of breast tumor patients. No significant difference was found in the percentage of CD19+ B cells in PBMCs among FIBma, IBCa patients and healthy individuals (5.89 ± 2.44%, 5.31 ± 3.23%, and 4.75 ± 2.16%, respectively) (Fig. 2A). However, the level of IL-10 secreting CD19+ B cells was higher in IBCa patients compared with that in FIBma patients (2.31 ± 1.01% vs 1.12 ± 0.41%; P = 0.046) (Fig. 2B).
Figure 2. IL-10+ B cell were enriched with the CD19+CD24+CD38+ B cells population.
(A) The percentages of CD19+ B cells in PBMCs had no different among FIBma patients, IBCa patients and health individuals (5.89 ± 2.44%, 5.31 ± 3.23%, and 4.75 ± 2.16%, respectively). (B) A higher level of IL-10 (2.31 ± 1.01%) in IBCa patients compared with FIBma patients (1.12 ± 0.41%) (P = 0.046). (C,D) In our early report, CD19+CD24+CD38+ B cell was identified as regulatory B cells in breast cancer12. So, to identify the phenotype of CD19+L-10+ B cells in PBMCs of IBCa, stained with CD24 and CD38 and analyzed by flow cytometry. A significantly higher percentage of CD24+CD38+ in the CD19+IL-10+ group (39.18 ± 5.98%) compared to that in the CD19+IL-10− group (8.87 ± 4.08%) (P = 0.001). (E) The higher level of IL-10 secreted by CD19+CD24+CD38+ B lymphocytes (39.18 ± 18.91%) than CD19+CD24−CD38+ B cells (20.72 ± 13.01%) or CD19+CD24+CD38− B cells (20.48 ± 13.46%) (P = 0.023, P = 0.0231).
We have previously demonstrated that CD19+CD24+CD38+ B cells were regulatory B cells in breast cancer12. In the current study, to further determine the phenotype of CD19+IL-10+ B cells in PBMCs of IBCa, isolated CD19+IL-10+ B cells were labeled with anti-CD24 and anti-CD38 antibodies and analyzed by flow cytometry. A significantly higher percentage of CD24+CD38+ in the CD19+IL-10+ group (39.18 ± 5.98%) was seen compared to that in the CD19+IL-10− group (8.87 ± 4.08%) (P = 0.001) (Fig. 2C,D). Consistent with the findings of our previous study12, the level of IL-10 was significantly higher in the CD19+CD24+CD38+ B lymphocytes (39.18 ± 18.91%) than in the CD19+CD24−CD38+ B cells (20.72 ± 13.01%) and CD19+CD24+CD38− B cells (20.48 ± 13.46%) (P = 0.023 and P = 0.0231, respectively) (Fig. 2E).
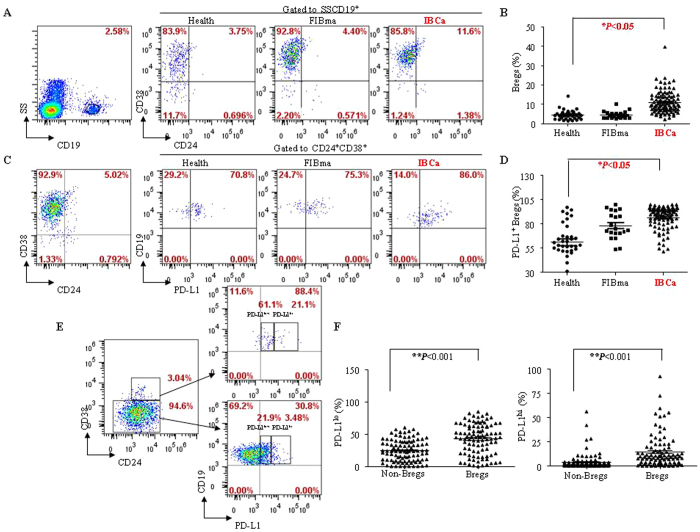
CD19+CD24+CD38+ Bregs and its subsets were expanded in breast cancer patients
Subsequently, we investigated the distribution of CD19+CD24+CD38+ Bregs and various subsets in the PBMCs of breast tumor patients. In comparison with FIBma patients and healthy participants (4.48 ± 1.91% and 4.49 ± 2.48%, respectively), the percentage of CD19+CD24+CD38+ B cells was significantly higher in the PBMCs of IBCa patients with 10.99 ± 5.70% (P < 0.05) (Fig. 3A,B).
Figure 3. CD19+CD24+CD38+ B cell and its subsets are expanded in breast cancer patients.
(A,B) The percentage of CD19+CD24+CD38+ B cells in PBMCs of IBCa patients (10.99 ± 5.70%) was significant higher than in that of FIBma patients (4.48 ± 1.91%) and health individuals (4.49 ± 2.48%) (P < 0.05). (C,D) PD-L1 was detected on CD19+CD24+CD38+ Bregs of breast tumor patients and health individuals. The significant higher CD19+CD24+CD38+PD-L1+ subset (83.74 ± 19.32%) was observed in PBMCs of IBCa patients compared with that in FIBma patients (78.40 ± 13.96%) or health individuals (61.13 ± 20.51%) (P < 0.05). (E,F) The pattern of PD-L1 on CD19+CD24+CD38+ Bregs in PBMCs of IBCa patients was analyzed by cytometry. Wherever PD-L1lo or PD-L1hi expression was significant higher on CD19+CD24+CD38+ Bregs (42.36 ± 23.27% or 14.02 ± 16.99%) than that on Non-Bregs (24.58 ± 16.51% or 3.92 ± 8.18%) in IBCa (P < 0.001 for all).
Similar to the experiments in CD4+ T cells and Tregs, we determined the expression pattern of the negative immune regulator PD-L1 in B cells and CD19+CD24+CD38+ Bregs (Fig. 3C). Significantly higher level of CD19+CD24+CD38+PD-L1+ subset (83.74 ± 19.32%) was observed in the PBMCs of IBCa patients compared with that in FIBma patients (78.40 ± 13.96%) or healthy individuals (61.13 ± 20.51%) (P < 0.05, respectively) (Fig. 3C,D). In addition, both the PD-L1lo and PD-L1hi expression were found to be significantly higher on CD19+CD24+CD38+ Bregs (42.36 ± 23.27% and 14.02 ± 16.99%) compared with non-Bregs (24.58 ± 16.51% and 3.92 ± 8.18%; P < 0.001 for both) (Fig. 3E,F).
We further investigated the relationships among the levels of CD19+ B cells, CD19+ CD24+ CD38+ Bregs, as well as CD19+ CD24+ CD38+ PD-L1+ Bregs in IBCa and the histopathological characteristics of IBCa, and found that the CD19+ B cells were significantly higher in patients with tumors more than 3cm (P = 0.019) and CD19+ CD24+ CD38+ PD-L1+ Bregs were among the highest patient at TNM stage T4 (P = 0.011) (Table 2).
Table 2. Relationship between the proportion of CD19+ B cells and its subset in PBMCs and the clinicopathological parameters of IBCa patients.
| Variables | All cases | B cells | ||
|---|---|---|---|---|
| CD19+ | CD19+CD24+CD38+ | CD19+CD24+CD38+PD-L1+ | ||
| IDCa | 98 | |||
| Grade | ||||
| G1 | 14 | 5.32 ± 3.21 | 9.78 ± 5.41 | 82.88 ± 20.54 |
| G2 | 63 | 5.17 ± 3.15 | 11.83 ± 6.18 | 83.09 ± 21.06 |
| G3 | 21 | 5.26 ± 3.18 | 9.50 ± 3.69 | 86.69 ± 12.63 |
| P | 0.985 | 0.178 | 0.751 | |
| LN metastasis | ||||
| No | 48 | 5.33 ± 3.60 | 11.60 ± 6.84 | 81.30 ± 21.63 |
| Yes | 50 | 5.28 ± 2.85 | 10.40 ± 4.34 | 86.08 ± 16.69 |
| P | 0.931 | 0.303 | 0.223 | |
| TNM | ||||
| I | 24 | 4.74 ± 2.23 | 10.61 ± 5.69 | 87.30 ± 9.37 |
| II | 41 | 5.49 ± 3.92 | 11.07 ± 6.56 | 76.53 ± 26.35 |
| III | 24 | 5.79 ± 3.00 | 11.48 ± 4.67 | 88.30 ± 9.73 |
| IV | 9 | 4.85 ± 2.72 | 10.31 ± 4.66 | 94.88 ± 4.72 |
| P | 0.700 | 0.939 | 0.011 | |
| ER | ||||
| Negative | 32 | 6.36 ± 4.14 | 11.23 ± 6.98 | 82.28 ± 20.35 |
| Positive | 66 | 4.79 ± 2.56 | 10.87 ± 5.03 | 84.44 ± 18.92 |
| P | 0.056 | 0.773 | 0.605 | |
| PR | ||||
| Negative | 56 | 5.39 ± 3.61 | 10.67 ± 6.17 | 82.79 ± 20.31 |
| Positive | 42 | 5.19 ± 2.66 | 11.41 ± 5.06 | 84.99 ± 18.08 |
| P | 0.759 | 0.531 | 0.580 | |
| HER2 | ||||
| Negative | 62 | 5.54 ± 3.17 | 10.69 ± 6.12 | 83.61 ± 17.05 |
| Positive | 36 | 4.90 ± 3.32 | 11.50 ± 4.95 | 83.96 ± 22.97 |
| P | 0.345 | 0.501 | 0.930 | |
| Age | ||||
| <49 y | 39 | 5.30 ± 3.72 | 12.13 ± 6.84 | 82.25 ± 23.48 |
| ≥49 y | 59 | 5.31 ± 2.89 | 10.23 ± 4.73 | 84.72 ± 16.14 |
| P | 0.983 | 0.108 | 0.539 | |
| Tumor size | ||||
| <3 cm | 59 | 4.69 ± 3.03 | 11.09 ± 5.17 | 85.48 ± 18.81 |
| ≥3 cm | 39 | 6.24 ± 3.33 | 10.83 ± 6.50 | 81.21 ± 20.04 |
| P | 0.019 | 0.829 | 0.295 | |
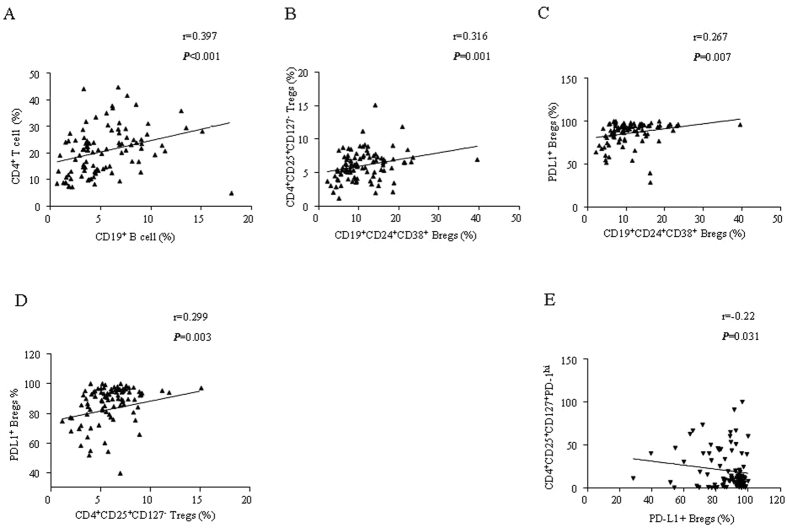
The relationship between CD4+ T cell subsets and CD19+ B cell subsets in IBCa
In our previous studies, we have shown that B cells from IBCa could induce Tregs in vitro12. In the present study, we further examined the correlation between CD4+ T cell subsets and CD19+ B cell subsets in PBMCs of IBCa patients. The results showed a positive correlation between the percentages of CD4+ T cells and CD19+ B cells (r = 0.397, P < 0.001), CD4+CD25+CD127low/− Tregs and CD19+CD24+CD38+ Bregs (r = 0.316, P = 0.001), PD-L1+ Bregs and Bregs (r = 0.267, P = 0.007), PD-L1+ Bregs and CD4+CD25+CD127low/− Tregs (r = 0.299, P = 0.003) (Fig. 4A–D). In contrast, PD-L1+ Bregs and PD-1hi CD4+ CD25+ CD127+ effector T cells were inversely correlated with each other (r = -0.220, P = 0.031; Fig. 4E).
Figure 4. The correlation between CD4+ T cell subsets and CD19+ B cell subsets in IBCa.
(A) The positive correlation was found between the percentages of CD4+ T cells and CD19+ B cells (r = 0.397, P < 0.001). (B) The positive correlation was found between CD4+CD25+CD127low/− Tregs and CD19+CD24+CD38+ Bregs (r = 0.316, P = 0.001). (C) The positive correlation was found between PD-L1+ Bregs and Bregs (r = 0.267, P = 0.007). (D) The positive correlation was found between PD-L1+ Bregs and CD4+CD25+CD127low/− Tregs (r = 0.299, P = 0.003). (E) There was significantly negative correlation between PD-L1+ Bregs vs PD-1hi CD4+ CD25+ CD127+ effector T cells (r = −0.220, P = 0.031).
Discussion
Although the overall survival rate has been improved considerably over the past few decades with the advancement of treatment modalities, breast cancer is still the leading causes of cancer mortality in women18. The immunosuppression and immune escape are regarded as predicting factors for poor prognosis of solid cancers. Regulatory T cells (Tregs), defined as CD4+CD25+Foxp3+ or CD4+CD25+CD127low/−, mediate peripheral tolerance and prevent autoimmunity in healthy individuals. It has been recognized that Tregs serve as a key mediator that regulates and maintains the balance of immune response by suppressing the expansion of effector T cells19. Therefore, Tregs play a beneficial role in treatment of autoimmunity diseases. In contrast, there is usually an increased number of Tregs in the peripheral blood or tumor sites in patients who have cancer, including head and neck squamous cell carcinoma, breast cancer, lung cancer, colorectal cancer, pancreatic cancer, ovarian cancer and melanoma20,21,22. Numerous reports showed Tregs were associated with inhibition of immune response against cancer, tumor immune escape and metastasis19. Higher number of Tregs has been associated with higher risk of relapse and shorter relapse-free survival. A recent investigation suggested that Tregs may be an important pathological factor predicting a response to hormone therapy or chemotherapy in breast cancer patients and can be potential therapeutic target for breast cancer23. However, the mechanisms that regulate Tregs activity is only beginning to be elucidated.
We have shown in the present study and our previous report12 that there was a significantly elevated number of CD4+ CD25+ Foxp3+ or CD4+ CD25+ CD127− Tregs in the PBMCs of IBCa patients compared with that in patients with breast benign tumor or healthy women. We revealed that it was the ratio of CD4+ T cells to CD4+ CD25+ CD127− Treg instead of their number changes that governs their suppressive activity. On the other hand, CD19+ B cells from IBCa was recently shown to promote the expansiveness of Tregs in a T and B cell co-culture system, and an elevated number of CD19+ CD24+ CD38+ B cells, identified as Bregs, were found in IBCa12. The majority of studies on Bregs have focused on their suppression in the ranges of autoimmune, allergic conditions and cancer in both mice and man. The immune regulatory functions of Tregs have been described, yet little is known about the regulatory role B cells toward on Tregs responses against tumor. In the present investigation, significantly high percentage of CD19+ CD24+ CD38+ Bregs was seen in the PMBCs of IBCa patients compared with that in benign tumor patients or healthy individuals. A positive correlation between CD19+ CD24+ CD38+ Bregs and CD4+ CD25+ CD127− Tregs was also observed.
We further investigated the function and relationship of PD-L1/PD-1 between Tregs and Bregs. PD-L1 as a ligand of PD-1 is constitutively expressed on B lymophocytes, macrophages and dendritic cells (DCs)15. Meanwhile, PD-1 is also inducible on activated T-cell subsets24. The PD-1/PD-L1 interaction exerts inhibitory effects that limit effector cells response, prevent the triggering of immune-mediated tissue damage, and regulate the balance between T cell activation and tolerance25. A number of studies have indicated that PD-1/PD-L1 pathway is a crucial modulator of host immune responses in regulation of autoimmunity, tumor immunity, transplantation immunity, and allergy26,27,28. The majority of studies have focused on PD-L1 expressed on DCs or macrophages that suppress effector CD4+ T cells26,29. In the present study, we analyzed the expression of PD-L1 on CD19+ CD24+ CD38+ Bregs in breast tumor patients with and healthy individuals. We confirmed that PD-L1 was higher on CD19+CD24+CD38+ Bregs in IBCa patients compared with patients with benign tumor or healthy individuals. Though the percentages of CD19+ B cells, CD19+CD24+CD38+ B cells, and CD19+ CD24+ CD38+ PD-L1+ B cells were not significantly different in terms of tumor grades, lymph node metastasis, ER, PR, and HER2 status, a tight correlation was seen between upregulated expression of PD-L1 on CD19+CD24+CD38+ Bregs and higher TNM phases of IBCa. More importantly, a tight correlation exists between CD19+ CD24+ CD38+ Bregs and PD-L1 expression in CD19+ CD24+ CD38+ Bregs, and PD-L1 maybe an important molecule in CD19+ CD24+ CD38+ Bregs. We further found that both PD-L1hi and PD-L1lo was significantly higher on CD19+ CD24+ CD38+ Bregs compared with that on non-Bregs (CD19+ CD24− CD38+ B cells). We therefore speculate that high PD-L1 expression may contribute to the immunosuppressive role of CD19+ CD24+ CD38+ Bregs.
When the expression pattern of PD-1 on CD4+ CD25+ CD127− Tregs as well as CD4+ non-Tregs was examined, we found that PD-1hi was mainly expressed on non-Tregs, and the expression level of PD-1lo was not significantly different between Tregs and non-Tregs. The presence of PD-L1 on CD19+ CD24+ CD38+ Bregs was found to be positively correlated with CD4+ CD25+ CD127− Tregs, and inversely associated with PD-1hi non-Tregs. As elegantly described by Adnan et al.30, PD-L1hi B cells could suppress inflammation in EAE model through limiting the expansion of CD4+ CXCR5+ PD-1+ follicular helper T cells (TFH-cell). This, in conjunction with our recent finding that that CD19+ B cells from IBCa could promoted Tregs in a T and B cells co-culture system12, led us to hypothesize that PD-L1 expressed on Bregs might inhibit the proliferation of PD-1hi non-Tregs, and promote the expansion of Tregs to suppress immune response.
As negative function of B cells was demonstrated, B-cell depletion by anti-CD20 antibody was used to augment immunotherapy in multiple solid tumor models31. Further investigations confirmed that the absence of B lymphocytes reduced the number and function of Tregs and enhanced the anti-tumor response in a murine tumor model31. However, as important immune cells, depletion of B lymphocytes would lead to deficient adaptive immune responses. Furthermore, published studies have showed that the depletion of B lymphocytes by anti-CD20 antibody would enrich CD20low/− Bregs and promote cancer escape32. So, Bregs maybe a useful target cells in tumor immunotherapy base on Breg-mediated immunosuppression. And the precise phenotype of Bregs need identified and further investigated in cancer. Here, we have shown the level of PD-L1 was higher on CD19+CD24+CD38+ Bregs in IBCa, and the percentage of PD-L1+CD19+CD24+CD38+ Bregs was positively correlated with Tregs, but negatively correlated with PD-1hi non-Tregs. These observations suggested that PD-L1 maybe another important molecule on Bregs, and a critical molecule mediated the promotion of Tregs in advanced breast cancer. CD19+ CD24+ CD38+ PD-L1+ Bregs may serve as a target in immunotherapy.
Materials and Methods
Reagents
The following monoclonal antibodies and reagents were used in this study: anti-human CD19-ECD (clone: J3-119) (Beckman Coulter Company, Marseille, France) and anti-human CD4-ECD (clone: SFCI12T411) were purchased from Beckman (USA); anti-human CD24-PE-cy7 (clone: eBioSN3), anti-human CD38-APC (clone: HIT2), and anti-human CD25-PE-cy7 (clone: BC96) were from eBiosciencs (San Jose, CA, USA); anti-human CD127-FITC (clone: A019D5) and anti-human PD-1-PE (clone: PD1.3.1.3) were from Miltenyi Biotec (Germany); PDL1-PE (clone: 29E.2A3) was obtained from BioLegend (USA). Fixation/Permeabilization Kit (with GolgiStop protein transport inhibitor containing monensin) was purchased from BD Company (BD Biosciences, USA). The Isotype control antibodies including FITC Mouse IgG1 (clone: MOPC-21), PE-cy7 Mouse IgG1 (clone: MOPC-21), APC Mouse IgG1 (clone: MOPC-21), PE Mouse IgG2b (clone: MPC-11) were purchased from Biolegend (USA). ECD Mouse IgG1 (clone: A07797) was purchased from Beckman (USA). The following antibodies for compensation controls: CD4-FITC (clone: VIT4, Miltenyi Biotec, Germany), CD4-PE (clone: VIT4, Miltenyi Biotec, Germany), CD4-ECD (clone: SFCI12T411, Bechman, USA), CD4-APC (clone: RPA-T4, eBiosciencs, USA), CD4-PE-cy7 (clone: RPA-T4, eBiosciencs, USA). The following antibodies for Fluorescence Minus One (FMO): CD3-FITC (clone: BW264/56, Miltenyi Biotec, Germany), PD-L1-PE (clone: PD1.3.1.3), CD19-ECD (clone: J3-119), CD4-APC (clone: RPA-T4), CD14-PE 770 (clone: TUK4, Miltenyi Biotec, Germany).
Lipopolysaccharide, PMA and calcium-ionomycin were obtained from Sigma (St Louis, USA).
Patients and peripheral blood cells collection
A total of 153 participants including 98 IBCa, 20 FIBma, and 35 healthy individuals were enrolled in this study. The FIBma patients aged 17–58 years, with a mean of 39.8 years. The healthy individuals aged 25–64 years, with a mean of 45.7 years. The age range of the IBCa patients was from 31 to 90 years, with a mean of 54.2 years. None of the patients with IBCa had received any kind of chemical or radiation therapy before surgery. The characteristics of the IBCa patients were presented in Table 1. 14 out of 98 (14.3%) IBCa were graded as well-differentiated, 63/98 (64.3%) were moderately-differentiated and the rest (21/98, 21.4%) were poorly-differentiated. Carcinoma specimens were classified as stage T1 (24/98, 24.5%), T2 (41/98, 41.8%), T3 (24/98, 24.5%) and T4 (9/98, 9.2%). In addition, local lymph node metastasis occurred in 50 cases of 98 cases (51.0%). Peripheral blood cells were collected from IBCa and FIBma patients, and healthy individuals for flow cytometry analysis of cell surface markers. Written informed consent was obtained from each individual participant. The study protocol was performed in accordance with the guidelines outlined in the Declaration of Helsinki and was approved by the Ethics Committee of Soochow University.
Peripheral immune cell isolation
Peripheral blood mononuclear cells (PBMCs) were isolated from blood sample via a ficoll density gradient (Amersham Biosciences, Sweden) centrifugation at 1800 rpm for 20 min at 4 °C. PBMCs were washed with FACS buffer and centrifuged at 1800 rpm for 5min prior to labeling with antibodies for flow cytometry analysis.
Surface staining and flow cytometry
Following isolation, the PBMCs were immediately labelled with the specific fluorochrome-conjugated antibodies including CD4-ECD, CD25-PE-cy7, CD127-FITC and PD-1-PE to identify the surface molecules of Tregs for 30 min at 4 °C. Labeled cells were re-suspended in 0.5 mL cell staining buffer, and analyzed using a flow cytometer (CytomicsTM FC500, Beckman Coulter, USA). The data was analyzed using FlowJo software (version 7.6.2, Ashland, OR, USA). Isotype controls were included for each staining. FMO controls were included for assessing background fluorescence. Meanwhile, CD4-FITC, CD4-PE, CD4-ECD and CD4-PE-cy7 were used for compensation. The compensation and FMO controls were performed as shown in Supplementary Figures 2 and 3.
The surface molecules of Breg were analyzed similarly. In brief, PBMCs were incubated with the specific fluorochrome-conjugated antibodies including CD19-ECD, CD38-APC, CD24-PE-cy7 and PD-L1-PE for 30 min at 4 °C. Labeled cells were re-suspended in 0.5 mL cell staining buffer, and analyzed using flow cytometer with FlowJo software (version 7.6.2, Ashland, OR, USA). Isotype controls were used for each staining, and FMO controls were included to assess background fluorescence. Meanwhile, CD4-PE, CD4-ECD, CD4-APC, and CD4-PE-cy7 were used for compensation.
Intracellular staining of IL-10 and flow cytometry analysis
Intracellular IL-10 staining and analysis by flow cytometry was performed as previously described12. Briefly, PBMCs cells were cultured in the RMPI 1640 media containing 10% fetal calf serum, 200 μg/mL penicillin, 200 μg/mL streptomycin and stimulated with LPS (1 μg/mL) for 72 hour, then stimulated with PMA (50 ng/mL) and ionomycin (750 ng/mL) for 6 hour. Four hours prior to the termination, cells were blocked with GolgiStop (BD Biosciences, USA). Bregs were labeled as described above using CD19-ECD, CD24-Pecy7 and CD38-APC. For intracellular staining, cells were fixed with Fixation/Permeabilization solution and incubated with anti-human IL-10-FITC antibody for 30 min in the dark. Labeled cells were re-suspended in 0.5 mL cell staining buffer, and analyzed with flow cytometry. Isotype controls and FMO controls were included for all experiments. Meanwhile, CD4-FITC, CD4-PE, CD4-ECD, and CD4-APC, were used for compensation.
Statistical analysis
All of the data were presented as mean ± standard error of the mean and analyzed using ANOVA-test or Student t test. ANOVA-test and Student-Newman-Keuls (SNK) for post hoc test were used to compare the mean of the percentage of CD4+ T cells, CD19+ B cells and their subsets in patients, healthy individuals. Correlation was determined by the Pearson correlation. The P < 0.05 and P < 0.01 were considered as statistically significant and very significant, respectively. All analyses were done using SPSS 17.0 software (USA).
Additional Information
How to cite this article: Guan, H. et al. PD-L1 is a critical mediator of regulatory B cells and T cells in invasive breast cancer. Sci. Rep. 6, 35651; doi: 10.1038/srep35651 (2016).
Supplementary Material
Acknowledgments
This work was supported in part by the National Natural Science Foundation of China (81372343); the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). We thank Mr. Yu Chen from Clinical Immunology Institute for analysis of flow data and review of the manuscript.
Footnotes
Author Contributions F.X., H.G., Z.M.W., Y.W. and X.Z. conceived and designed the study and helped to draft the manuscript. Y.S. and Y.W. performed the statistical analysis. Y.W., J.L., Z.Y.W., Q.W., Y.L. and J.Z. performed the data collection and Flow cytometry analysis. All authors reviewed the manuscript.
References
- Rosser E. C. & Mauri C. Regulatory B cells in experimental mouse models of arthritis. Methods in molecular biology 1190, 183–194, doi: 10.1007/978-1-4939-1161-5_13 (2014). [DOI] [PubMed] [Google Scholar]
- Blair P. A. et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity 32, 129–140, doi: 10.1016/j.immuni.2009.11.009 (2010). [DOI] [PubMed] [Google Scholar]
- Yanaba K. et al. IL-10-producing regulatory B10 cells inhibit intestinal injury in a mouse model. The American journal of pathology 178, 735–743, doi: 10.1016/j.ajpath.2010.10.022 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olkhanud P. B. et al. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4(+) T cells to T-regulatory cells. Cancer research 71, 3505–3515, doi: 10.1158/0008-5472.CAN-10-4316 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding T., Yan F., Cao S. & Ren X. Regulatory B cell: New member of immunosuppressive cell club. Human immunology 76, 615–621, doi: 10.1016/j.humimm.2015.09.006 (2015). [DOI] [PubMed] [Google Scholar]
- Kalampokis I., Yoshizaki A. & Tedder T. F. IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis research & therapy 15 Suppl 1, S1, doi: 10.1186/ar3907 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanaba K. et al. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 28, 639–650, doi: 10.1016/j.immuni.2008.03.017 (2008). [DOI] [PubMed] [Google Scholar]
- de Masson A. et al. CD24(hi)CD27(+) and plasmablast-like regulatory B cells in human chronic graft-versus-host disease. Blood 125, 1830–1839, doi: 10.1182/blood-2014-09-599159 (2015). [DOI] [PubMed] [Google Scholar]
- Liu Y. et al. Role of IL-10-producing regulatory B cells in control of cerebral malaria in Plasmodium berghei infected mice. European journal of immunology 43, 2907–2918, doi: 10.1002/eji.201343512 (2013). [DOI] [PubMed] [Google Scholar]
- Das A. et al. IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. Journal of immunology 189, 3925–3935, doi: 10.4049/jimmunol.1103139 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita T., Horikawa M., Iwata Y. & Tedder T. F. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. Journal of immunology 185, 2240–2252, doi: 10.4049/jimmunol.1001307 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan H. et al. PD-L1 mediated the differentiation of tumor-infiltrating CD19 B lymphocytes and T cells in Invasive breast cancer. Oncoimmunology 5, e1075112, doi: 10.1080/2162402X.2015.1075112 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A., Basu S., Williams C. B., Salzman N. H. & Dittel B. N. A novel IL-10-independent regulatory role for B cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. Journal of immunology 188, 3188–3198, doi: 10.4049/jimmunol.1103354 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M., Wang Z. & Han X. B Cells with Regulatory Function in Animal Models of Autoimmune and Non-Autoimmune Diseases. Open J Immunol 5, 9–17, doi: 10.4236/oji.2015.51002 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarnath S. et al. The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Science translational medicine 3, 111ra120, doi: 10.1126/scitranslmed.3003130 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi T. et al. Host APCs augment in vivo expansion of donor natural regulatory T cells via B7H1/B7.1 in allogeneic recipients. Journal of immunology 186, 2739–2749, doi: 10.4049/jimmunol.1002939 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. Z. et al. PD-1 expression defines two distinct T-cell sub-populations in follicular lymphoma that differentially impact patient survival. Blood cancer journal 5, e281, doi: 10.1038/bcj.2015.1 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer. Journal international du cancer 136, E359–E386, doi: 10.1002/ijc.29210 (2015). [DOI] [PubMed] [Google Scholar]
- Elpek K. G., Lacelle C., Singh N. P., Yolcu E. S. & Shirwan H. CD4+CD25+ T Regulatory Cells Dominate Multiple Immune Evasion Mechanisms in Early but Not Late Phases of Tumor Development in a B Cell Lymphoma Model. The Journal of Immunology 178, 6840–6848, doi: 10.4049/jimmunol.178.11.6840 (2007). [DOI] [PubMed] [Google Scholar]
- Martin-Orozco N. et al. Melanoma cells express ICOS ligand to promote the activation and expansion of T-regulatory cells. Cancer research 70, 9581–9590, doi: 10.1158/0008-5472.CAN-10-1379 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthay A. How do regulatory T cells work? Scandinavian journal of immunology 70, 326–336, doi: 10.1111/j.1365-3083.2009.02308.x (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakamatsu E., Mathis D. & Benoist C. Convergent and divergent effects of costimulatory molecules in conventional and regulatory CD4+ T cells. Proceedings of the National Academy of Sciences of the United States of America 110, 1023–1028, doi: 10.1073/pnas.1220688110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su M., Huang C. X. & Dai A. P. Immune Checkpoint Inhibitors: Therapeutic Tools for Breast Cancer. Asian Pacific journal of cancer prevention: APJCP 17, 905–910 (2016). [DOI] [PubMed] [Google Scholar]
- Liu M. F., Weng C. T. & Weng M. Y. Variable increased expression of program death-1 and program death-1 ligands on peripheral mononuclear cells is not impaired in patients with systemic lupus erythematosus. Journal of biomedicine & biotechnology 2009, 406136, doi: 10.1155/2009/406136 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel K. M. et al. B7-H1 expression on non-B and non-T cells promotes distinct effects on T- and B-cell responses in autoimmune arthritis. European journal of immunology 40, 3117–3127, doi: 10.1002/eji.201040690 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. et al. Upregulation of programmed death-1 on T cells and programmed death ligand-1 on monocytes in septic shock patients. Critical Care 15, R70, doi: 10.1186/cc10059 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt A. Q. & Mills K. H. Immunosuppressive networks and checkpoints controlling antitumor immunity and their blockade in the development of cancer immunotherapeutics and vaccines. Oncogene 33, 4623–4631, doi: 10.1038/onc.2013.432 (2014). [DOI] [PubMed] [Google Scholar]
- Salem M. L. & El-Badawy A. Programmed death-1/programmed death-L1 signaling pathway and its blockade in hepatitis C virus immunotherapy. World journal of hepatology 7, 2449–2458, doi: 10.4254/wjh.v7.i23.2449 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll D. M. The blockade of immune check points in cancer immunotherapy. Nature reviews. Cancer 12, 252–264, doi: 10.1038/nrc3239 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. R. et al. PD-L1hi B cells are critical regulators of humoral immunity. Nature communications 6, 5997, doi: 10.1038/ncomms6997 (2015). [DOI] [PubMed] [Google Scholar]
- Kim S. et al. B-cell depletion using an anti-CD20 antibody augments antitumor immune responses and immunotherapy in nonhematopoetic murine tumor models. Journal of immunotherapy 31, 446–457, doi: 10.1097/CJI.0b013e31816d1d6a (2008). [DOI] [PubMed] [Google Scholar]
- Bodogai M. et al. Anti-CD20 antibody promotes cancer escape via enrichment of tumor-evoked regulatory B cells expressing low levels of CD20 and CD137L. Cancer research 73, 2127–2138, doi: 10.1158/0008-5472.CAN-12-4184 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.