Abstract
Earlier research has been primarily focused on WC as one of the most promising earth-abundant electrocatalysts for hydrogen evolution reaction (HER), whereas the other compound in this carbide family—W2C—has received far less attention. Our theoretical calculations suggest that such a focus is misplaced and W2C is potentially more HER-active than WC. Nevertheless, the preparation of phase pure and sintering-free W2C nanostructures represents a formidable challenge. Here we develop an improved carburization method and successfully prepare ultrasmall and phase-pure W2C nanoparticles. When evaluated for HER electrocatalysis, W2C nanoparticles exhibit a small onset overpotential of 50 mV, a Tafel slope of 45 mV dec−1 and outstanding long-term cycling stability, which are dramatically improved over all existing WC-based materials. In addition, the integration of W2C nanoparticles with p-type Si nanowires enables highly active and sustainable solar-driven hydrogen production. Our results highlight the great potential of this traditionally non-popular material in HER electrocatalysis.
 Tungsten carbide has yet to live up to its long-believed potential as a replacement for precious metal electrocatalysts. Here, Li and co-workers demonstrate that ditungsten carbide in the form of ultrasmall, phase-pure nanoparticles is a better candidate for the hydrogen evolution reaction.
Tungsten carbide has yet to live up to its long-believed potential as a replacement for precious metal electrocatalysts. Here, Li and co-workers demonstrate that ditungsten carbide in the form of ultrasmall, phase-pure nanoparticles is a better candidate for the hydrogen evolution reaction.
Splitting water molecules to gaseous hydrogen and oxygen using renewable resources such as solar energy represents one of the biggest challenges facing humanity in the modern age1,2,3,4. Among several obstacles to its practical realization at a large scale is the development of active and durable catalyst materials for hydrogen evolution reaction (HER) at the cathode and oxygen evolution reaction (OER) at the anode4,5,6,7. Even though OER remains the bottleneck of water splitting, HER is no less challenging. Platinum group metals are the most active HER electrocatalysts, yet all of them suffer from low abundance and high cost4,6,7. The search for non-precious metal based alternatives has never been more demanding than it is today. Despite the recent exciting advances of many transition metal chalcogenide, nitride or phosphide-based HER electrocatalysts, there are few candidates available at present that can simultaneously meet the activity and stability requirements4,5,6,7.
Early transition metal carbides have attracted wide interest owing to their low cost, high chemical and electrochemical stability, and unique catalytic activities towards a range of important reactions8,9,10,11. Theoretical calculations have demonstrated that the presence of carbon interstitial atoms in the lattice of early transition metals can afford them with higher d-band electronic density of states (DOS) at the Fermi level and hence characteristics resembling those of platinum11,12. In particular, tungsten carbide (WC)-based materials have been long advocated and investigated as potential replacements of platinum for HER electrocatalysis ever since the first report by Trasatti more than half a century ago13. Unfortunately, these materials have yet to be capable of replacing precious metal catalysts due to their poor activities: very few have onset overpotentials within 100 mV (refs 7, 10, 14, 15). The challenges are multifold7,10,15,16. First, these carbides have been conventionally prepared using high-temperature (>700 °C) reduction of metal precursors with gaseous carbon precursors (such as CH4, C2H6 or CO), which usually induces uncontrollable particle sintering, resulting in materials with extremely low surface areas. Second, the introduction of excessive gaseous carbon precursors may also risk extensive coking of catalyst surfaces, seriously deteriorating its catalytic performance. Third and most importantly, there are very few existing methods to selectively engineer different phases of carbides for maximal HER activity. For decades, research on tungsten carbides has been exclusively focused on WC. The other carbide compound—W2C has received far less attention. It is mainly due to the lack of a reliable synthetic method for this carbon-deficient material. Among the few reports available up to date, W2C is usually obtained as a by-product of WC17,18. This is not surprising since the formation of W2C is not thermodynamically favoured below 1,250 °C according to the W–C phase diagram19. Despite its unpopularity among material scientists and electrochemists, our theoretical calculations predict that W2C is a significantly more active HER catalyst than WC with a much less negative Gibbs free energy of hydrogen adsorption (ΔGH) and higher electronic DOS at the Fermi level. As a result, the preparation of nanosized, coking-free and phase-pure W2C particles and assessment of their potential in HER electrocatalysis are a daunting but highly rewarding task. Yet, nobody has been able to accomplish this up to now.
In this study, we advance a solution to this challenge via the carburization reaction between non-volatile, crystalline solid carbon precursor and pre-formed WOx nanoparticles at high temperature and low pressure. Multi-walled carbon nanotubes (MWNTs) are selected as such a solid carbon precursor. Due to their chemical inertness, the diffusion rate of carbon atoms from MWNTs to tungsten lattice is slowed down, which not only leads to suppressed sintering of carbide nanoparticles, but also avoids excessive carbon deposition on their surfaces. In this way, we are able to achieve the selective synthesis of ultrasmall and phase-pure W2C particles with excellent activity and durability for both electrocatalytic and photoelectrochemical hydrogen evolution.
Results
Theoretical calculations
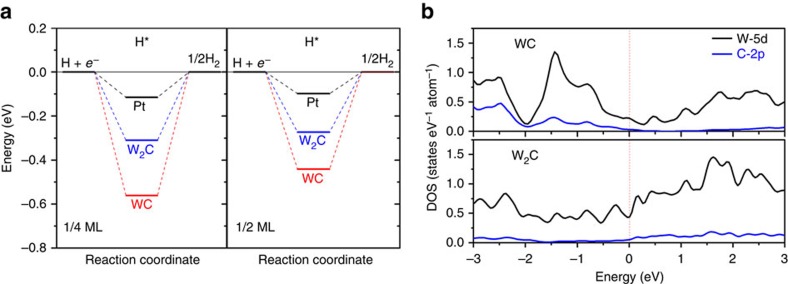
We start with the first-principles density functional theory (DFT) calculations to unveil the greater potential of W2C over WC for HER electrocatalysis. ΔGH has been advocated as a useful descriptor in the selection of proper HER electrocatalysts20,21,22,23,24. According to the Sabatier principle, the optimal electrocatalysts should have ΔGH neither too negative nor too positive (ΔGH ≈0 eV) as we have seen for the case of platinum22,23. Here, we first computed and compared the ΔGH on the (0001) surface of hexagonal WC and W2C, alongside with the (111) surface of cubic Pt as a reference. The three-state diagram of HER free-energy process in Fig. 1a shows that at the low hydrogen adsorption coverage of 1/4 monolayer (MLH), the ΔGH on WC is −0.56 eV, in good agreement with the result reported by Peterson et al. and suggesting that hydrogen intermediates adsorb onto WC surfaces more strongly than ideal25. Under the same condition, the ΔGH on W2C is only −0.31 eV. This value represents a significant improvement over WC, even though it is still slightly more negative than the Pt reference (which has a calculated ΔGH of −0.11 eV in our study, close to the result reported by Nørskov et al.21) and indicates that HER on W2C is still limited by the hydrogen release step. At an increased hydrogen adsorption coverage of 1/2 MLH, ΔGH on WC and W2C shifts positively, and reaches −0.44 and −0.27 eV, respectively (Fig. 1a). It is therefore conclusive that HER is more favoured at high hydrogen coverage for both W2C and WC, nevertheless the former remains far superior to the latter.
Figure 1. DFT computations on the HER activity and electronic structure of W2C.
(a) Free-energy diagrams for HER on the (0001) surfaces of W2C and WC, as well as Pt (111) surface at both low (1/4 MLH) and high (1/2 MLH) hydrogen adsorption coverage. (b) Calculated partial density of states of W and C atoms in W2C and WC. The red dashed line denotes the position of the Fermi level.
Other than its less negative ΔGH, the advantage of W2C over WC as the HER electrocatalyst is also manifested from the comparison of their electronic structures. Figure 1b illustrates the partial density of states of individual W and C atoms in WC and W2C. WC has long been suggested as platinum-like because of its modified d-band electron structure with a greater density of states (DOS) near the Fermi level upon the incorporation of carbon interstitial atoms11,12. This is again confirmed by our study here showing the considerable W 5d contribution at the Fermi level. Interestingly, we find that electronic DOS of W2C near the Fermi level is even larger than that of WC. The higher W content in W2C enhances its metallicity, and results in a higher carrier density, which would greatly benefit the charge transfer process during electrochemical reactions26. Furthermore, we calculate that the d-band center of W2C (0001) (−1.74 eV) is close to that of Pt (111) (−1.92 eV), but much lower than that of WC (0001) (−0.93 eV; Supplementary Fig. 1). According to the d-band theory, a lower d-band center results in weaker bonding between the catalyst and the adsorbate. This result rationalizes the more favourable ΔGH on W2C than on WC, and suggests that the former is more Pt-like than the latter.
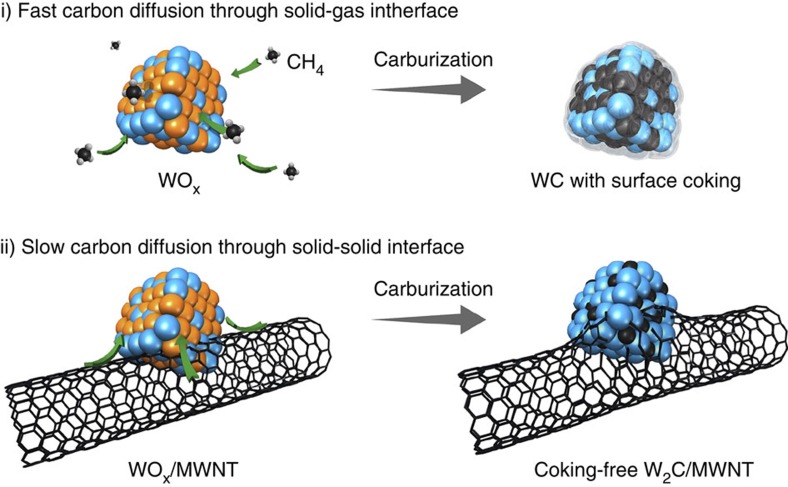
The above DFT calculations have unambiguously established that W2C is a potentially more active HER electrocatalyst than WC because of its more favourable ΔGH and higher electronic DOS. However, the preparation of nanosized W2C is challenging, and so far not successful. The W–C phase diagram suggests that the formation of W2C is not thermodynamically favoured below 1,250 °C (ref. 19). Moreover, the synthesis has to be carried out in a carbon-deficient environment to avoid the undesired formation of WC (see Supplementary Table 1 for the summarized synthetic methods of W2C)27,28,29,30,31. Most conventional approaches (such as those using gaseous carbon precursors) are unable to yield W2C as the main product since the relative amount of carbon precursor to tungsten precursor is often out of control, and the carbon diffusion through the solid-gas interface into the tungsten lattice usually proceeds too fast to allow for the product phase to be carefully engineered (Fig. 2a)10,15. Here, we reason that using a non-volatile, crystalline solid carbon precursor such as carbon nanotubes may present a unique solution to this problem. Under high temperatures, the chemical reactivity of non-volatile solid carbon precursors is considerably lower. They would render the carbon diffusion through the solid-solid interface much slower, opening up the possibility to tailor the product phase via selecting proper reaction conditions (Fig. 2b).
Figure 2. Schematic illustration showing the influence of different carbon precursors.
Gaseous carbon precursor (for example, CH4) and non-volatile solid carbon precursor (for example, CNT) may have different impacts on the chemical composition and microstructure of the carburization product.
Synthesis of W2C nanoparticles
Guided by this design principle, we report here a two-step approach to prepare ultrasmall and phase-pure W2C particles supported on MWNTs (see the Methods section for details). In the first step, ultrasmall WOx nanoparticles were grown on pre-oxidized MWNTs via the controlled hydrolysis of WCl6 in a mixed solvent of ethanol and deionized water (Supplementary Fig. 2). This was mediated through the strong interaction between metal precursors and rich oxygen functionalities on MWNTs32,33,34. Subsequently, the hybrid was subjected to carburization at simultaneously high temperature and low pressure, during which carbon atoms from the MWNT support are properly activated, diffuse into WOx lattices, and selectively reduce them to W2C (Fig. 2b). The optimal conditions to afford small-sized and phase-pure product were found to be 900 °C and 320 Pa.
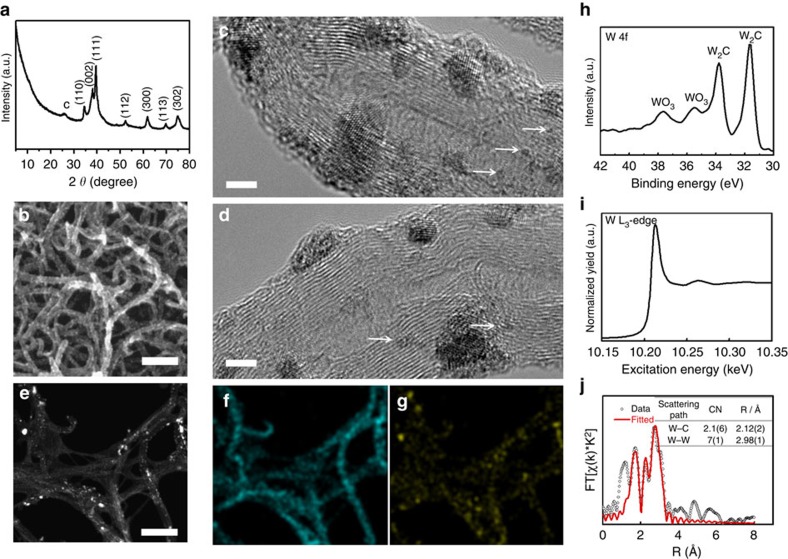
We first examined the chemical composition and microstructure of the final product using X-ray powder diffraction (XRD) and electron microscopy as summarized in Fig. 3a. From X-ray diffraction, except for the weak signal at 25.7o from MWNTs, all the diffraction peaks are assignable to W2C with no sign of the formation of WC or other species. Scanning electron microscopy (SEM) shows that after the high-temperature carburization, MWNTs retain the one-dimensional morphology and are uniformly decorated with small W2C nanoparticles on the surfaces (Fig. 3b). Unlike conventional carbide-based materials, no further aggregation of W2C nanoparticles or their detachment from the support is evidenced here owing to their strong physical immobilization on the conductive scaffold. Fig. 3c,d are the representative high-resolution transmission electron microscopy (TEM) images of the final product. Dark contrast W2C nanoparticles can be readily distinguished from the MWNT background. Most of them have a fine size between 2 and 5 nm with clear lattice fringes. In addition, we also identify some smaller clusters having sizes in the sub-nanometer range such as those marked by arrows. The distribution of W2C nanoparticles on MWNTs over a large area was garnered using high-angle annular dark field (HAADF) scanning transmission electron microscopy (STEM) imaging, and energy dispersive spectroscopy (EDS) elemental mapping under STEM mode. HAADF imaging has a significantly higher sensitivity towards heavy elements35. Bright spots in Fig. 3e reveal the spatial dispersion of W species along MWNTs. Most of them are no larger than a few pixels, thereby attesting to the ultrasmall size of W2C nanoparticles. The EDS signal of W corresponds well with the HAADF image (Fig. 3f). Its high spatial correlation with the C signal (Fig. 3g) also supports the uniform distribution of W2C nanoparticles on MWNTs. The weight percentage of W2C in the composite is analysed to be ∼69 wt% by thermogravimetric analysis (Supplementary Fig. 3).
Figure 3. Microscopic and spectroscopic characterizations of W2C nanoparticles supported on MWNTs.
(a) X-ray diffraction pattern, (b) SEM image and (c,d) TEM images of W2C/MWNT. (e) HAADF image and (f,g) corresponding C and W EDS element mapping of W2C/MWNT. (h) W 4f XPS spectrum, (i) W L3-edge XANES spectrum and (j) Fourier transform EXAFS spectrum and associated fitting curve of W2C/MWNT at the W L3-edge. The inset in j shows the fitting results. Scale bar, 100 nm (b,e); 3 nm (c,d).
To gain insight on the chemical environment and bonding configuration of W2C nanoparticles, multiple spectroscopic characterizations were performed on the carburized product. Its W 4f X-ray photoelectron spectroscopy (XPS) spectrum is featured with two pronounced peaks centered at 31.7 eV and 33.8 eV, typical to tungsten carbides in general (Fig. 3h)36,37. Peaks at the higher binding energy of 35.5 and 37.6 eV are resulted from the inevitable surface oxidation of W2C nanoparticles upon exposure to air36,38. Figure 3i,j depict the W L3-edge XANES spectrum and its corresponding Fourier-transformed extended X-ray absorption fine structure (EXAFS) spectrum of W2C. From the theoretical fitting of the latter, we derive an average W–C bond length of 2.12 Å and W–W bond length of 2.98 Å (see the inserted table in Fig. 3j). These values are more consistent with the crystal structure of hexagonal W2C than WC, and again corroborate the formation of W2C instead of WC. Most importantly, the coordination numbers (CN) of W–C and W–W are fit to be 2.1±0.6 and 7±1, respectively. Both are markedly smaller compared with the expected values for bulk materials (CN=6 for W-C and CN=12 for W–W). This under-coordination can be rationalized by the ultrasmall size and defective nature of the resultant W2C nanoparticles, in good agreement with above TEM results.
The above microscopic and spectroscopic studies establish that using MWNTs as the non-volatile solid carbon precursor, we have accomplished the selective carburization to form ultrasmall and phase-pure W2C nanoparticles. It is worth highlighting that MWNTs play a pivotal role in our control over both the size and phase of the carbide nanoparticles. Among many previous attempts to mitigate particle sintering, Romón–Leshkov and coworkers developed a removable ceramic coating method by encapsulating WOx within SiO2 nanospheres as a hard template to physically prevent their sintering during the high-temperature carburizing15. Here we believe that similar physical confinement can also be in effect via the strong interaction between W precursors and their supporting materials. When firmly immobilized on the conductive MWNT scaffold, the further aggregation or overgrowth of W2C nanoparticles is fully suppressed. On the other hand, the slow carbon diffusion from MWNTs to WOx through the solid–solid interface creates an overall carbon-deficient environment, which is responsible for the favorable formation of W2C over the more common WC phase (Fig. 2b). It is in stark contrast to conventional carbide preparation methods using gaseous carbon precursors (such as CH4, C2H6 or CO), where the carbon diffusion through the solid–gas interface usually proceeds too fast to be controlled (Fig. 2a)10,15,16,18,39. Indeed, our control experiment shows that when CH4 is fed as the gaseous carbon precursor under otherwise identical conditions, the final product is exclusively comprised of WC with a similar size distribution (Supplementary Fig. 4).
We find that carburization conditions also have a large influence on the phase and size of the final product. When the carburization temperature is raised from 900 °C to 1,000–1,100 °C, WC phase emerges and grows in percentage based on X-ray diffraction analysis as the result of the accelerated carbon diffusion (Supplementary Fig. 5)14,28. When the temperature is maintained at 900 °C, annealing in vacuum (5 Pa) or atmospheric pressure results in predominantly metallic W or WO2 phase, respectively, with drastically different morphologies (Supplementary Fig. 6). Moreover, cubic or hexagonal WN can be prepared when the carrier gas is switched from N2 to NH3 (Supplementary Fig. 7).
Electrocatalytic HER performance of W2C
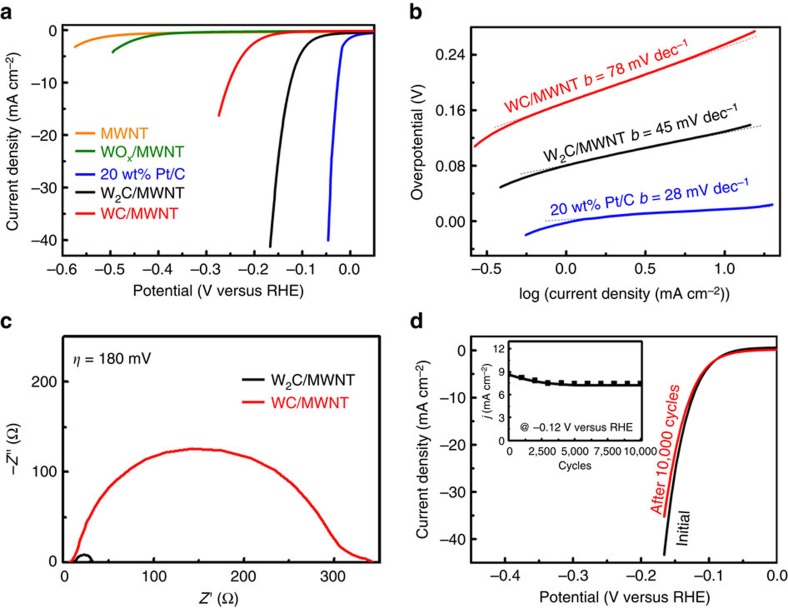
The electrocatalytic performance of W2C nanoparticles for HER was assessed by using a standard three-electrode setup in 0.5 M H2SO4 (see details in Methods section). HER polarization curves of different electrocatalysts were collected and compared alongside with the commercial 20 wt% Pt/C benchmark. As shown in Fig. 4a, MWNTs alone or WOx/MWNT from the first step has negligible HER activity. By contrast, W2C/MWNT exhibits an impressive HER activity with a small onset overpotential of ∼50 mV and achieving a geometric current density of 10 mA cm−2 at η=123 mV versus reversible hydrogen electrode (RHE). Such an activity excels most previous carbide-based (WC and Mo2C) HER electrocatalysts to our best knowledge, and is among the best non-precious metal based electrocatalysts (Supplementary Table 2)7,10,15,28,40. Tafel analysis yields a small slope of 45 mV dec−1 for W2C/MWNT (Fig. 4b), again representing the smallest value ever reported, and indicative of possible Volmer–Heyrovsky pathway with the electrochemical desorption reaction as the rate determining step41. Its fast HER reaction kinetics is also reflected by a small charge transfer resistance of 20 Ω at η=180 mV from electrochemical impedance spectroscopy analysis (Fig. 4c). Interestingly, we note that WC/MWNT prepared using CH4 as the feed gas in our experiment has much inferior performance (Supplementary Fig. 4). It delivers j=10 mA cm−2 at η=250 mV; its charge transfer resistance at η=180 mV is 15 times larger than W2C/MWNT—a performance consistent with the best WC-based HER electrocatalysts (such as the one reported by Romón–Leshkov using the removable ceramic coating method15). Given the largely similar size distribution of WC and W2C nanoparticles here, we believe that the striking difference in their HER activities clearly underscores the advantageous electronic structure of W2C over WC as elucidated by the above DFT calculations.
Figure 4. Electrochemical measurements of W2C/MWNT for HER in 0.5 M H2SO4.
(a) Polarization curve of W2C/MWNT in comparison with 20 wt% Pt/C, WC/MWNT, WOx/MWNT or MWNTs alone. (b) Tafel plot of W2C/MWNT in comparison with 20 wt% Pt/C and WC/MWNT. (c) Electrochemical impedance spectrum of W2C/MWNT at η=180 mV in comparison with WC/MWNT. (d) Polarization curve of W2C/MWNT before and after 10,000 potential cycles. The inset shows the change of HER cathodic current density at −0.12 V versus RHE with respect to the number of potential cycles.
Meanwhile, it is worth emphasizing that the ultrasmall size of W2C is also an indispensable structural factor to achieve its superior HER activity. Since electrocatalysis is a surface-limiting process, a smaller particle size results in larger surface areas and exposes more catalytically active sites for HER. To estimate its electrochemical surface area (ECSA), W2C is electrochemically oxidized in an anodic sweep (Supplementary Fig. 8). From the charge integrated over the oxidation envelope, we estimate ∼60% of W2C is electrochemically accessible. This value is very impressive and consistent with the ultrasmall size of W2C particles. By contrast, non-structured W2C is significantly less active. For example, W2C thin film prepared from the magnetron sputtering only has a mediocre HER activity with j=1 mA cm−2 achieved at η∼200 mV (ref. 30).
HER activities of a range of control samples are summarized in the Supplementary Information. We have systematically explored the influence of starting precursor ratio (Supplementary Fig. 9), carburization temperature (Supplementary Fig. 5), pressure (Supplementary Fig. 6), and carrier gas (Supplementary Fig. 4 and 7), and concluded that the present conditions yield the most active HER electrocatalyst.
Furthermore, W2C/MWNT has satisfactory long-term stability. To demonstrate this, the catalyst was continuously cycled up to 10,000 cycles. Its polarization curve was periodically collected every 1,000 cycles, and cathodic current density at η=120 mV extracted and plotted against the cycle number as depicted in the insert of Fig. 4d. We can see that despite a slight initial activity loss, the cathodic current density levels off after 4,000 cycles, and remains constant at ∼7.4 mA cm−2. When the initial and final polarization curves are compared in Fig. 4d, this activity loss translates to <10 mV increment in overpotential. The outstanding cycling stability of W2C/MWNT attests to its great structural and electrochemical robustness. Postmortem X-ray diffraction and XPS analysis of W2C/MWNT after HER reveal that its crystallinity is retained while its surface becomes slightly more oxidized (Supplementary Fig. 10).
PEC hydrogen generation on W2C decorated Si nanowires
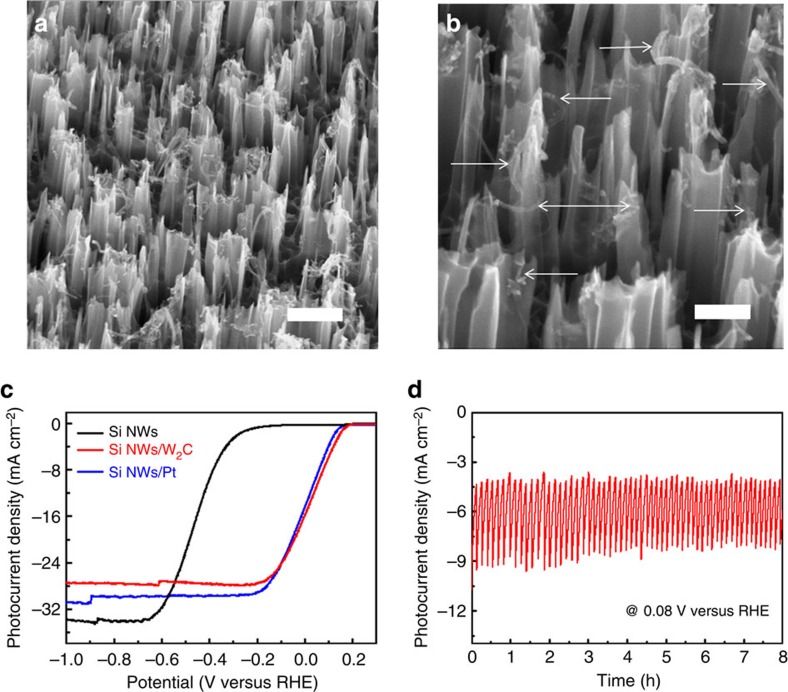
We integrated W2C/MWNT with p-type Si nanowires and studied their performance for photoelectrochemical (PEC) hydrogen evolution. P-type Si nanowires having an average length of 500 nm were prepared from the Ag-assisted etching of Si wafer (see details in Methods)42,43. W2C/MWNT was directly dropcast onto these nanowires. Figure 5a shows a tilted SEM image of the photocathode. These vertically aligned Si nanowires have adequate open space among them, and are uniformly infiltrated with W2C/MWNT catalysts over a large area. Close examination reveals that many W2C/MWNT fibers coil around individual Si nanowires or bridge neighbouring Si nanowires (marked by arrows in Fig. 5b). Even though Si nanowires may not be in direct physical contact with W2C nanoparticles, they are electrically connected through MWNTs.
Figure 5. Integration of W2C/MWNT with p-type Si nanowires for PEC hydrogen generation in 0.5 M H2SO4.
(a,b) SEM images of the photocathode loaded with W2C/MWNT catalyst as marked by arrows. Scale bar, 400 nm (a) and 200 nm (b). (c) Polarization curve of Si nanowire photocathode loaded with W2C/MWNT under one sun illumination in comparison with the same photocathode loaded with 20 wt% Pt/C or Si nanowire photocathode alone. (d) Amperometric i−t curve at a constant potential of 0.08 V versus RHE under one sun illumination.
The PEC polarization curves of Si nanowire photocathodes with or without catalyst under 1 sun illumination are presented and compared in Fig. 5c. Si nanowires alone have a poor PEC activity with no appreciable photocurrent density to the positive of −0.2 V versus RHE. After integration of Si nanowires with W2C/MWNT, their onset potential improves significantly to 0.2 V, and a photocurrent density of 16 mA cm−2 is reached at 0 V. Both metrics are well comparable to the photocathode loaded with 20 wt% Pt/C, and superior to previous p-type Si nanowire electrodes integrated with other earth-abundant electrocatalysts, such as Mo3S4, Ni–Mo alloy or Mo2C (Supplementary Table 3)44,45,46,47. Note that the slightly lower saturation photocurrent density of catalyst-loaded Si nanowires (∼30 mA cm−2) compared with bare Si nanowires (∼34 mA cm−2) probably suggests that a fraction of the incoming light is blocked by the catalyst powder. Moreover, the operation durability of Si/W2C/MWNT was investigated by galvanostatic (i∼t) experiment at 0.08 V up to 8 h (Fig. 5d). Even though the photocurrent density fluctuates due to the periodic accumulation and release of H2 bubbles from the photocathode surface, there is no sign of PEC activity decay over the course of experiment. All these results would not be possible if it was not for the great HER activity and durability of our W2C/MWNT catalyst. The physical integration of Si nanowires and W2C/MWNT is also strong enough to survive the vigorous bubbling on the electrode surface.
Discussion
In conclusion, built on our improved understanding of the pitfalls associated with conventional preparation methods and the great potential of W2C predicted by theoretical calculations, we demonstrated the selective synthesis of ultrasmall and phase-pure W2C particles using non-volatile crystalline solid carbon precursor (that is, MWNTs), and achieved efficient and sustainable electrocatalytic and photoelectrochemical hydrogen evolution. The slow carbon diffusion from MWNT support to WOx nanoparticles through the solid–solid interface created an overall carbon-deficient environment, and led to the favorable formation of W2C over the more common WC. It also prevented the extensive coking and sintering of the resultant carbide nanoparticles. As a result, W2C/MWNT exhibited impressive electrocatalytic HER performance, featuring a small onset overpotential of ∼50 mV, a cathodic current density of 10 mA cm−2 at η=123 mV, and long-term cycling stability. W2C/MWNT is far superior to all existing WC-based HER electrocatalysts. In addition, W2C/MWNT could be readily integrated with p-type Si nanowires to enable solar-driven hydrogen production, and achieve highly competitive performance to that of the photocathode decorated with Pt. We also expect our ultrasmall W2C nanoparticles to be more active than WC towards a range of other catalytic or electrocatalytic reactions.
Methods
Materials synthesis
To prepare W2C/MWNT, MWNTs were first oxidized by modified Hummers method following a previous report48. In brief, 1 g of MWNTs were oxidized by 200 mg of NaNO3 and 1 g of KMnO4 in 23 ml of concentrated H2SO4 at 40 °C for 30 min. Subsequently, 16 mg of pre-oxidized MWNTs and 128 mg of anhydrous WCl6 were dispersed in 40 ml of ethanol and 4 ml of water. The temperature of the solution was then raised to and maintained at 80 °C overnight in an oil bath under vigorous magnetic stirring. After cooling back to room temperature, the solid product was collected by centrifugation, repeatedly washed with absolute ethanol and water, and lyophilized. To carry out the carburization reaction, the as-prepared solid powder was placed in the center of a tube furnace. The system was evacuated to 320 Pa at a continuous Ar flow rate of 100 sccm. The specimen was heated to 900 °C at a ramping rate of 10 °C min−1, and maintained at this temperature for 1 h.
Structure characterizations
Powder X-ray diffraction was performed on a PANalytical X-ray diffractometer at a scan rate of 0.05° s−1. XPS were collected on an Ultra-DLD XPS Spectrometer. Scanning electron microscopy (s.e.m.) images were taken on a Supera 55 Zeiss scanning electron microscope. TEM was done with a FEI F20 microscope, and the HAADF-STEM and STEM-EDS mapping were carried out on a FEI Titan G2 80–200 microscope. Both of them were operated at an acceleration voltage of 200 kV. W L3 edge XANES spectra were collected on the VESPERS (Very Sensitive Elemental and Structural Probe Employing Radiation from a Synchrotron) beamline at the Canadian Light Source. The energy scan was achieved by using a double crystal Si(111) monochromator. The emitted X-ray fluorescence spectrum was recorded by a 4-element Vortex silicon drift detector, which is positioned 50 mm away from the sample at 45 degree angle to the sample and 90 degrees to the incoming beam within the horizontal plane. Subsequently, the XANES spectrum was background-subtracted using low-order polynomials and normalized based on its post-absorption edge intensity. EXAFS data fitting was performed using WinXAS software in conjunction with scattering paths generated by FEFF8 (refs 49, 50). Absorption edge-shift (ΔE0) values were correlated between the two scattering paths, and all other parameters were allowed to vary freely. Both W–C and W–W bond lengths were obtained from a series of tungsten carbide structures and compared to those obtained experimentally from the fitted EXAFS spectra.
Electrochemical measurements
To prepare the catalyst ink, 1 mg of active materials, 0.5 mg of Ketjenblack carbon and 5 μl of Nafion solution (5 wt%) were dispersed in a mixture of 250 μl of H2O and ethanol (ν/ν, 1/1) with the assistance of ultrasonication for at least 30 min. Then 10 μl of the ink was dropcast onto a glassy carbon electrode of 3 mm in diameter (catalyst loading ∼0.56 mg cm−2). Electrochemical measurements were performed in a standard three-electrode set-up in 0.5 M H2SO4 solution, using a saturated calomel electrode (SCE), the catalyst loaded glassy carbon electrode, and a graphite rod as the reference, working and counter electrode, respectively. Before each measurement, the SCE reference electrode was carefully calibrated against a commercial RHE from ASL Japan. Linear sweep voltammetry (LSV) was carried out at a scan rate of 10 mV s−1. Electrochemical impedance measurements were carried out at different overpotentials in the frequency range of 100,000–0.05 Hz.
Fabrication of photocathode and PEC measurements
Si nanowire arrays were fabricated via the metal assisted chemical etching of Si wafers. In brief, a (100)-oriented Si wafer (p-type, 0.2–0.8 Ω cm−1) was first degreased by ultrasonication in ethanol and acetone sequentially, each for 15 min. It was then treated in a boiling piranha solution (H2O2:H2SO4=1:4 v/v) for 30 min. Thus cleaned Si wafer was etched in an aqueous etchant containing 20 mM AgNO3 and 5 M HF for 15 min, subsequently treated in 5% HNO3 solution for 30 min to remove surface Ag residue. Resultant Si nanowire arrays were rinsed with a copious amount of water, and finally dried at 80 °C for further use. To integrate Si nanowire arrays with W2C nanoparticles, Si nanowires were etched in 0.5 M HF for 15 s and were blow-dried under N2 purge. Ten microlitre of 4 mg ml−1 W2C/MWNT in ethanol dispersion was uniformly spin-coated onto a 15 mm × 15 mm Si nanowire electrode at 2,500 r.p.m., and naturally dried in air. Ohmic contact to the backside of electrode was made by scratching with Ga–In eutectic and affixing a Cu wire to it. PEC experiments were carried out in a custom-built square cell with a circular 0.38 cm2 aperture, which was sealed by the working electrode. Light was provided by a 300 W Xe arc lamp from Newport Corporation fitted with an AM 1.5 G filtre. Its power density was adjusted to 100 mW cm−2, and calibrated with an optical power meter equipped with a thermopile detector. LSV curves were collected at a scan rate of 10 mV s−1.
Theoretical calculations
DFT calculations were performed using the plane-wave technique with exchange-correlation interactions modeled by the revised Perdew–Burke–Ernzerhof generalized gradient approximation functional51, as implemented in the Vienna ab Initio Simulation package52,53. The ion–electron interaction was treated within the projector-augmented plane wave pseudopotentials54,55. A plane-wave cutoff energy of 420 eV was used in all computations. The electronic structure calculations were employed with a Fermi-level smearing of 0.1 eV for all surface calculations and 0.01 eV for gas-phase species. The Brillouin zone was sampled with 12 × 12 × 12 for bulk calculations and 6 × 6 × 1 k-points for surfaces calculations. The convergence of energy and forces were set to be 1 × 10−5 eV and 0.02 eV Å−1, respectively. For all surfaces, a 2 × 2 supercell was used and a vacuum region of 15 Å was set along the z direction to avoid the interaction between periodic images (Supplementary Fig. 11). The Pt (111) surface was modeled with four atomic layers where only the top layer and adsorbate were allowed to fully relax. The WC(0001) and W2C (0001) planes were, respectively, constructed with ten and nine atomic layers respectively where the top two layers and adsorbate were allowed to fully relax.
The free energy of adsorbed H (ΔGH) on different surfaces is expressed as:
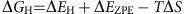 |
where ΔEH is the hydrogen adsorption energy, ΔEZPE and ΔS are the zero point energy difference and the entropy difference between the adsorbed state and the gas phase, respectively, and T is the system temperature (298.15 K, in this work). For each system, its ΔEH was directly determined from the DFT total energies, and its EZPE was computed by summing vibrational frequencies over all normal modes ν (EZPE=1/2Σħν). After exploring possible H adsorption sites, we reported the lowest energy binding location as shown in Supplementary Fig. 11. The free energy of proton and electron (H++e−) at standard conditions was taken as 1/2GH2.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
How to cite this article: Gong, Q. et al. Ultrasmall and phase-pure W2C nanoparticles for efficient electrocatalytic and photoelectrochemical hydrogen evolution. Nat. Commun. 7, 13216 doi: 10.1038/ncomms13216 (2016).
Supplementary Material
Supplementary Figures 1 - 11 and Supplementary Tables 1 - 3
Acknowledgments
We acknowledge supports from the National Natural Science Foundation of China (51472173, 51522208, 51222202 and 21522305), the National Natural Science Foundation of Jiangsu Province (BK20140302, SBK2015010320, BK20150045), the National ‘973' Program of China (2014CB932500), the Priority Academic Program Development of Jiangsu Higher Education Institutions and Collaborative Innovation Center of Suzhou Nano Science and Technology. P.Z. thanks the financial support from NSERC Canada. The CLS is supported by the Canadian Funding agencies CFI, NSERC, NRC, CIHR, the University of Saskatchewan, the Government of Saskatchewan, and Western Economic Diversification Canada. This work also partly made use of the resources of the Center of Electron Microscopy of Zhejiang University.
Footnotes
Author contributions Y.G.L. conceived the project and designed the experiments. Q.G. conducted material synthesis and electrochemical measurements. Y.W. and Y.F.L. carried out theoretical calculations. Q.H. and C.J. performed STEM studies. J.Z, R.F., P.N.D. and P.Z. performed XANES and EXAFS analysis. F.C. conducted PEC measurements. N.H. performed XPS analysis. Q.G., Y.W., Y.F.L. and Y.G.L. co-wrote the paper. All authors discussed the results and commented on the manuscript.
References
- Turner J. A. Sustainable hydrogen production. Science 305, 972–974 (2004). [DOI] [PubMed] [Google Scholar]
- Lewis N. S. & Nocera D. G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl Acad. Sci. USA 103, 15729–15735 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocera D. G. The artificial leaf. Acc. Chem. Res. 45, 767–776 (2012). [DOI] [PubMed] [Google Scholar]
- Walter M. G. et al. Solar water splitting cells. Chem. Rev. 110, 6446–6473 (2010). [DOI] [PubMed] [Google Scholar]
- Morales-Guio C. G., Stern L.-A. & Hu X. Nanostructured hydrotreating catalysts for electrochemical hydrogen evolution. Chem. Soc. Rev. 43, 6555–6569 (2014). [DOI] [PubMed] [Google Scholar]
- Faber M. S. & Jin S. Earth-abundant inorganic electrocatalysts and their nanostructures for energy conversion applications. Energy Environ. Sci. 7, 3519–3542 (2014). [Google Scholar]
- Zeng M. & Li Y. Recent advances in heterogeneous electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A 3, 14942–14962 (2015). [Google Scholar]
- Levy R. & Boudart M. Platinum-like behavior of tungsten carbide in surface catalysis. science 181, 547–549 (1973). [DOI] [PubMed] [Google Scholar]
- Oyama S. T. Introduction to the Chemistry of Transition Metal Carbides and Nitrides 1–27Springer (1996). [Google Scholar]
- Chen W. F., Muckerman J. T. & Fujita E. Recent developments in transition metal carbides and nitrides as hydrogen evolution electrocatalysts. Chem. Commun. 49, 8896–8909 (2013). [DOI] [PubMed] [Google Scholar]
- Chen J. G. Carbide and nitride overlayers on early transition metal surfaces: preparation, characterization, and reactivities. Chem. Rev. 96, 1477–1498 (1996). [DOI] [PubMed] [Google Scholar]
- Kitchin J. R., Nørskov J. K., Barteau M. A. & Chen J. G. Trends in the chemical properties of early transition metal carbide surfaces: a density functional study. Catal. Today 105, 66–73 (2005). [Google Scholar]
- Bianchi G., Mazza F. & Trasatti S. Aynodic behavior and passivity of some interstitial compounds of niobium, tantalum, titanium, and tungsten. Z. Phys. Chem. 226, 40–58 (1964). [Google Scholar]
- Chen W. F. et al. Tungsten carbide-nitride on graphene nanoplatelets as a durable hydrogen evolution electrocatalyst. ChemSusChem 7, 2414–2418 (2014). [DOI] [PubMed] [Google Scholar]
- Hunt S. T., Nimmanwudipong T. & Roman-Leshkov Y. Engineering non-sintered, metal-terminated tungsten carbide nanoparticles for catalysis. Angew. Chem. Int. Ed. 53, 5131–5136 (2014). [DOI] [PubMed] [Google Scholar]
- Kimmel Y. C., Esposito D. V., Birkmire R. W. & Chen J. G. Effect of surface carbon on the hydrogen evolution reactivity of tungsten carbide (WC) and Pt-modified WC electrocatalysts. Int. J. Hydrogen Energy 37, 3019–3024 (2012). [Google Scholar]
- Shen P. K., Yin S., Li Z. & Chen C. Preparation and performance of nanosized tungsten carbides for electrocatalysis. Electrochim. Acta 55, 7969–7974 (2010). [Google Scholar]
- Nikiforov A. et al. WC as a non-platinum hydrogen evolution electrocatalyst for high temperature PEM water electrolysers. Int. J. Hydrogen Energy 37, 18591–18597 (2012). [Google Scholar]
- Kurlov A. S. & Gusev A. I. Tungsten carbides and W-C phase diagram. Inorg. Mater 42, 121–127 (2006). [Google Scholar]
- Hinnemann B. et al. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 127, 5308–5309 (2005). [DOI] [PubMed] [Google Scholar]
- Nørskov J. K. et al. Trends in the Exchange Current for Hydrogen Evolution. J. Electrochem. Soc. 152, J23–J26 (2005). [Google Scholar]
- Greeley J. et al. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat. Mater. 5, 909–913 (2006). [DOI] [PubMed] [Google Scholar]
- Greeley J. & Nørskov J. K. Large-scale, density functional theory-based screening of alloys for hydrogen evolution. Surf. Sci. 601, 1590–1598 (2007). [Google Scholar]
- Mavrikakis M. Computational methods: A search engine for catalysts. Nat. Mater. 5, 847–848 (2006). [DOI] [PubMed] [Google Scholar]
- Michalsky R., Zhang Y.-J. & Peterson A. A. Trends in the hydrogen evolution activity of metal carbide catalysts. ACS Catal. 4, 1274–1278 (2014). [Google Scholar]
- Xie J. et al. Atomically-thin molybdenum nitride nanosheets with exposed active surface sites for efficient hydrogen evolution. Chem. Sci 5, 4615–4620 (2014). [Google Scholar]
- Weidman M. C., Esposito D. V., Hsu Y.-C. & Chen J. G. Comparison of electrochemical stability of transition metal carbides (WC, W2C, Mo2C) over a wide pH range. J. Power Sources 202, 11–17 (2012). [Google Scholar]
- Ham D. J., Ganesan R. & Lee J. S. Tungsten carbide microsphere as an electrode for cathodic hydrogen evolution from water. Int. J. Hydrogen Energy 33, 6865–6872 (2008). [Google Scholar]
- Liang C. et al. The synthesis of nanostructured W2C on ultrahigh surface area carbon materials via carbothermal hydrogen reduction. Nanotechnology 14, 955 (2003). [Google Scholar]
- Esposito D. V., Hunt S. T., Kimmel Y. C. & Chen J. G. A new class of electrocatalysts for hydrogen production from water electrolysis: metal monolayers supported on low-cost transition metal carbides. J. Am. Chem. Soc. 134, 3025–3033 (2012). [DOI] [PubMed] [Google Scholar]
- Meng F. et al. Biomass-derived high-performance tungsten-based electrocatalysts on graphene for hydrogen evolution. J. Mater. Chem. A 3, 18572–18577 (2015). [Google Scholar]
- Wang H. & Dai H. Strongly coupled inorganic–nano-carbon hybrid materials for energy storage. Chem. Soc. Rev. 42, 3088–3113 (2013). [DOI] [PubMed] [Google Scholar]
- Li Y. et al. Advanced zinc-air batteries based on high-performance hybrid electrocatalysts. Nat. Commun. 4, 1805 (2013). [DOI] [PubMed] [Google Scholar]
- Liang Y. et al. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 10, 780–786 (2011). [DOI] [PubMed] [Google Scholar]
- Li Y. et al. An oxygen reduction electrocatalyst based on carbon nanotube-graphene complexes. Nat. Nanotechnol. 7, 394–400 (2012). [DOI] [PubMed] [Google Scholar]
- Krasovskii P. V. et al. XPS study of surface chemistry of tungsten carbides nanopowders produced through DC thermal plasma/hydrogen annealing process. Appl. Surf. Sci. 339, 46–54 (2015). [Google Scholar]
- Aizawa T., Hishita S., Tanaka T. & Otani S. Surface reconstruction of W2C (0001). J. Phys. Condens. Matter 23, 305007 (2011). [DOI] [PubMed] [Google Scholar]
- Tang C., Wang D., Wu Z. & Duan B. Tungsten carbide hollow microspheres as electrocatalyst and platinum support for hydrogen evolution reaction. Int. J. Hydrogen Energy 40, 3229–3237 (2015). [Google Scholar]
- Zheng H., Huang J., Wang W. & Ma C. Preparation of nano-crystalline tungsten carbide thin film electrode and its electrocatalytic activity for hydrogen evolution. Electrochem. Commun. 7, 1045–1049 (2005). [Google Scholar]
- Vrubel H. & Hu X. Molybdenum boride and carbide catalyze hydrogen evolution in both acidic and basic solutions. Angew. Chemie 124, 12875–12878 (2012). [DOI] [PubMed] [Google Scholar]
- Li Y. et al. MoS2 nanoparticles grown on graphene: an advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 133, 7296–7299 (2011). [DOI] [PubMed] [Google Scholar]
- Huang Z. et al. Ni12P5 Nanoparticles as an efficient catalyst for hydrogen generation via electrolysis and photoelectrolysis. ACS nano 8, 8121–8129 (2014). [DOI] [PubMed] [Google Scholar]
- Hu Y. et al. Metal-catalyzed electroless etching of silicon in aerated HF/H2O vapor for facile fabrication of silicon nanostructures. Nano Lett. 14, 4212–4219 (2014). [DOI] [PubMed] [Google Scholar]
- Hou Y. et al. Bioinspired molecular co-catalysts bonded to a silicon photocathode for solar hydrogen evolution. Nat. Mater. 10, 434–438 (2011). [DOI] [PubMed] [Google Scholar]
- Warren E. L. et al. Hydrogen-evolution characteristics of Ni–Mo-coated, radial junction, n+ p-silicon microwire array photocathodes. Energy Environ. Sci. 5, 9653–9661 (2012). [Google Scholar]
- Seger B. et al. Hydrogen production using a molybdenum sulfide catalyst on a titanium-protected n+p-silicon photocathode. Angew. Chem. Int. Ed. 51, 9128–9131 (2012). [DOI] [PubMed] [Google Scholar]
- Morales-Guio C. G. et al. Solar hydrogen production by amorphous silicon photocathodes coated with a magnetron sputter deposited Mo2C catalyst. J Am Chem Soc. 137, 7035–7038 (2015). [DOI] [PubMed] [Google Scholar]
- Ye H. et al. Iron-based sodium-ion full batteries. J. Mater. Chem. A 4, 1754–1761 (2016). [Google Scholar]
- Ressler T. WinXAS: a program for X-ray absorption spectroscopy data analysis under MS-Windows. J. Synchrotron. Rad. 5, 118–122 (1998). [DOI] [PubMed] [Google Scholar]
- Ankudinov A., Ravel B., Rehr J. & Conradson S. Real-space multiple-scattering calculation and interpretation of x-ray-absorption near-edge structure. Phys. Rev. B 58, 7565 (1998). [Google Scholar]
- Hammer B., Hansen L. B. & Nørskov J. K. Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals. Phys. Rev. B 59, 7413 (1999). [Google Scholar]
- Kresse G. & Hafner J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558 (1993). [DOI] [PubMed] [Google Scholar]
- Kresse G. & Furthmüller J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996). [DOI] [PubMed] [Google Scholar]
- Blöchl P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953 (1994). [DOI] [PubMed] [Google Scholar]
- Kresse G. & Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758 (1999). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1 - 11 and Supplementary Tables 1 - 3
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.