Abstract
Objective
This study was undertaken to determine the prevalence of filarial infection in the districts of Madhya Pradesh, which were classified as non-endemic by the national program for control of filariasis. These districts showed evidence of clinical symptoms related to filariasis. To confirm the presence of filariasis, night blood surveys and entomological surveys were carried out to detect microfilaria in humans and filarial infection in vectors.
Materials and methods
For microfilaria surveys, thick blood smears were prepared by taking 60 μL of blood between 8.00 and 11.00 pm and examined under a microscope. Vectors Culex quinquefasciatus were dissected for the filarial infection from the affected villages of four non-endemic districts.
Results
Prevalence of microfilaria in the four districts ranged from 3.8% in district Shivpuri to 11.2% in district Bhind (overall mf rate 6.9%, 95% CI = 6.2–7.7). Infection and infectivity rates among vectors were recorded from three districts which varied from 2 to 13% and from 1.3 to 3.6%.
Conclusion
The study confirmed the presence of circulating microfilaria in non-endemic population of four districts, which has significant public health implications. To assess filarial endemicity and hot spots, precise filarial risk mapping using new efficient diagnostic tools is needed to reinforce and extend the strategy in other areas to achieve elimination of lymphatic filariasis.
Keywords: Lymphatic filariasis, Mass drug administration, Filaria elimination, Wuchereria bancrofti
Introduction
Lymphatic filariasis (LF) is one of the major neglected vector borne diseases in the world and the second leading cause of human deformities. This disease is caused by three nematode parasites i.e. Wuchereria bancrofti, Brugia malayi, and B. Timori and expressed in a variety of clinical manifestations, such as hydrocele and chronic lymphodema/elephantiasis of the legs or arms resulting in disability, social stigma, and economic consequences.1 It is commonly prevalent among marginalized people living in rural areas with poor housing and sanitation conditions1 with about 1.4 billion people in 73 countries at risk of disease2 and nearly 120 million persons infected worldwide.3
The World Health Assembly in 1997 passed a resolution to eliminate the disease globally by 2020.4,5 Subsequently, a global program to eliminate lymphatic filariasis (GPELF) was launched in the year 2000.6 The main strategy of GPELF has two folds. First is to identify filarial endemic areas and treat the entire population with antifilarial drug using di-ethylcarbamazine citrate (DEC) plus albendazole, except for children below 2 years, pregnant women and seriously sick persons. This strategy is known as mass drug administration (MDA), is aimed to reduce microfilaria (mf) load in the community and lower the transmission since mf is the essential stage for transmission of infection and disease. Secondly, to provide effective morbidity management to reduce human suffering, the twofold strategy is supported by selective integrated vector management. It is expected that with the MDA coverage of 70–80% continuously for 5–6 annual rounds, transmission may reduce to a very low level, leading to the elimination of the disease.7
India contributes about a third of the global filarial burden,4 with about 600 million people living in 250 districts of 20 different states at risk.8 In India, 99.4% of the cases are caused by W. bancrofti whereas only 0.6% cases are caused by B. malayi [http://nvbdcp.gov.in/fil4.html accessed on 27 November 2015]. Culex quinquefasciatus is the principal vector which coexists with W. bancrofti.9 India launched the National Filarial Control Program (NFCP) in 1955 covering about 40 million populations, mainly in urban areas. The treatment strategy involved selective chemotherapy (DEC 6 mg kg−1 day−1 for 12 days) to individuals tested positive for the parasite through night blood survey.9 Later, the World Health Organization (WHO) recommended that a single dose of DEC is as effective as 12 spaced doses.10 The asymptomatic nature of most parasite carriers and non-suitability of a cumbersome night blood survey for large population, alternative control methods were felt necessary and idea of MDA to the entire population at risk was mooted. The first pilot project of MDA using DEC was launched in India in 1996 and covered 13 districts in 7 states based on mf prevalence data collected between 1958 and 1975,8 much before the launch of GPELF. Later in 1997, the state of Tamil Nadu launched MDA in 31 districts.11 Being a signatory to the World Health Assembly resolution for eliminating the disease by 2020, the Indian health policy of 2002 envisages the elimination of filariasis from the country by 2015.12 Subsequently, in 2004, the National Vector Borne Disease Control Program launched MDA in 202 districts out of 593 based on the filaria map of 1995.13,14
On the basis of reports received from different states, 250 districts were designated as endemic for filariasis by 2006 and the MDA program has been scaled up in these districts. Since in India, district is the administrative unit and a program officer coordinates with the state and national program for the control of vector borne diseases in each district, therefore, each district is designated as an implementing unit. For other 190 districts, mf data was not available and hence these districts were not considered for MDA program. However, recently based on geo-environmental variables, a model was developed by Sabesan et al.15 to create a filariasis transmission risk map of India and 113 districts out of 190 unsurveyed ones were predicted to be at risk. Although this model has a sensitivity of 80% and specificity of 64%, probability mapping revealed that the risk of filarial infection is not uniform throughout the country. Under the national policy, MDA is administered with a target of minimum 85% compliance rate.14 The impact of the program is monitored once a year (about one month prior to the MDA) by screening the population from four sentinels along with an equal number of spot check sites for microfilaraemia in each implementing unit examining their 20 μL of finger prick blood samples from a minimum of 500 persons.14 After eight rounds of MDA, 192 districts reported < 1% mf prevalence which is the recommended threshold for further epidemiological assessment.16
In the state of Madhya Pradesh (MP), MDA has been carried out in 11 districts known for filarial endemicity. In the first four rounds, only DEC was administered17 while albendazole was included in the subsequent rounds. The National Institute for Research in Tribal Health (NIRTH), Jabalpur (Indian Council of Medical Research) had undertaken comprehensive, multidisciplinary research studies on health aspects of tribal people living in MP, which involved extensive field surveys. During such surveys, chronic patients with symptoms related to filariasis were brought in the knowledge of the survey team in some districts where filaria was not previously documented. Since the district program officers confirmed that mf survey had never been carried out in these districts, we carried out the mf survey in four districts of MP along with vector surveillance from May 2013 to April 2015 to ascertain endemicity of filariasis, and inclusion of these areas under MDA.
Materials and methods
Area and population
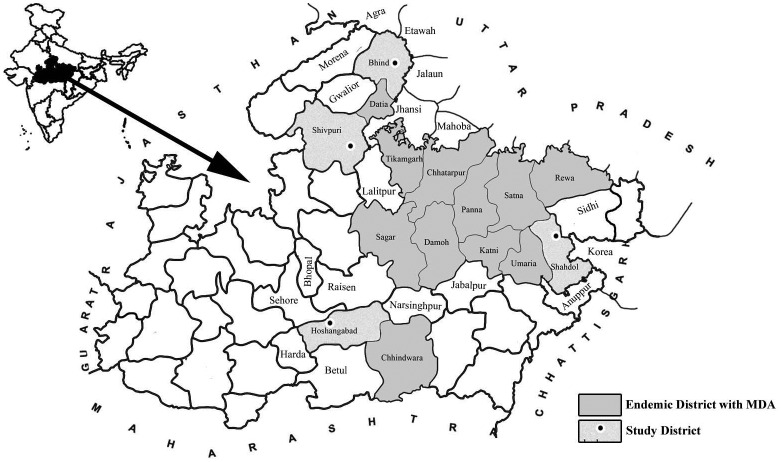
Deformities related to filarial disease were brought in the knowledge of research team working in the villages of the districts Shivpuri, Bhind, Shahdol and Hosangabad, through the practicing medical officers in these areas and by the local residents. The locations of these districts are shown in Fig. 1 and their salient features are:
-
(1)
Shahdol: this district is located at 24°5′ N latitude and 81°38′ E longitude (338 m above mean sea level (MSL)) with an average annual rainfall of 1250 mm. The village Beohari (Population of 24,000) which is the tehsil head quarter was surveyed for mf prevalence. The majority of the population is engaged in agriculture and small-scale business. Paddy and maize are the main agricultural crops.
-
(2)
Shivpuri: this district is located at 24°6′ N latitude and 77°4′ E longitude on the border of district Jhansi of Uttar Pradesh and Datia and Gwalior of MP (521 m above MSL) with an average annual rainfall of 875 mm. The village Pichhore (Population of 18,500) which is the tehsil head quarter and two other villages (Population of 7000) were surveyed. Ethnic tribes along with other socially and economically weaker groups of the community constitute about 30% of the population in these two villages. Mustard is the main agricultural crop of the area.
-
(3)
Bhind: this district is located at 26°36′ N latitude and 78°46′ E longitude (190 m above MSL) in the northern part of the state with an average annual rainfall of 700 mm. Five villages (Population of 13,500) situated in the Raun community health centre (CHC) of the district were surveyed. These villages are compact and literacy is very high. Barley, linseed and mustard are the main agricultural crops. The economic condition of the inhabitants of these villages is good with a regular source of income.
-
(4)
Hoshangabad: this district is located at 22°75′ N latitude and 77°7′ E longitude (331 m above MSL) with an average annual rainfall of 1340 mm. Two villages situated in Kesla CHC (Population of 4800) near the Itarsi railway junction and Sohagpur village of Sohagpur CHC (Population of 25,000) located 60 km. from Itarsi town were surveyed. Narmada and Tawa are the two main rivers flowing through the district. In Kesla block, population of selected villages is engaged in agriculture as laborers, whereas businesses and agriculture are the major profession in Sohagpur. Wheat, paddy, and soyabean are the main agricultural crops of this district.
Figure 1.
Map showing location of non MDA district surveyed for microfilaria.
Notes: District shown in light color and with small circle: non MDA study districts. Districts shown in dark color: filaria endemic districts having MDA.
In all the villages under study, the sewage system does not exist and uses of mosquito protection measures are also rare in all these areas.
Sampling
No information was available regarding filarial disease from non endemic districts. Therefore, WHO criteria of examining 300–500 persons from each village were adopted for determining mf prevalence.18 Villages having suspected patients with chronic symptoms related to filariasis were surveyed on the request of the local population. Microfilaria surveys were carried out between 8:00 and 11:00 pm and thick smear was prepared on a glass slide by taking 60 μL of finger prick blood after obtaining written informed consent. The villagers were informed during the day about the team visit in the night for collection of blood samples. Slides were dehaemoglobinised, stained with JSB-I and examined in 100× magnification.19 Microfilaria density (mfd) was calculated as per WHO guidelines.18 List of mf positive persons were provided to the national and state program officials for treatment and possible inclusion of these areas under the MDA program.
For determining infection and infectivity rate in vector species, mosquitoes were collected between 6.00 and 9.00 am from human dwellings in the same villages of these districts by trained technicians using flash light and aspirator. Mosquitoes were identified up to the species level using standard key.20 Specimens of vector species C. quinquefasciatus were dissected manually in normal saline. Each specimen was divided into three parts – head, thorax, and abdomen. Each part was teased separately for the presence of developing filarial larvae which were then classified as microfilaria, larva I, larva II, and larva III (infective stage). The proportion of specimens having any stage of worm development was classified as the infection rate while the proportion of specimens having infective stage larvae were classified as infectivity rate.
Data analysis
Data in excel sheet were analyzed using SPSS-21 software (SPSS Inc., Chicago, USA). The 95% Confidence Interval (CI) for the prevalence of mf was estimated. The significance of difference between genders was tested using the χ2 test. ANOVA was used to assess the difference in mf density among different age groups. The study was approved by the Institutional Ethical Committee and the Scientific Advisory Committee of NIRTH.
Results
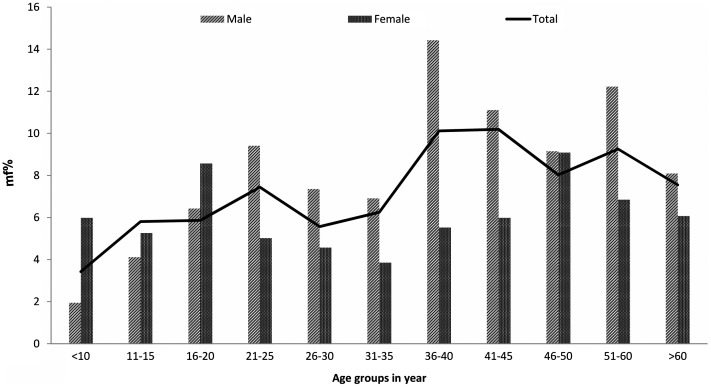
In all, 5042 persons were examined from 12 villages in four districts. Among them, 346 (6.9%, 95% CI = 6.2–7.7) persons were found having mf. In one village of Shivpuri district, mf was not found while the mf rate in remaining 11 villages, varied from 0.3% (95% CI = 0–1.6) in Pichhore village of Shivpuri district to 16.9% (95% CI = 13.6–20.7), in the Mehdwan village of Bhind district (Table 1). Overall district wise, mf rate ranged from 3.8% (95% CI = 2.7–4.9) in district Shivpuri to 11.2% (95% CI = 9.7–12.6) in district Bhind, and variation in the mf rate was significant (χ2 = 68.5.0, p < 0.001). While the sex-wise mf rate was significantly higher in males (7.6%) than in females (6.0%) (χ2 = 4.8, p < 0.05); however, in the district Bhind, mf rate was higher among females than males though the difference was not significant (p > 0.05). Prevalence of mf was lowest in children below 10 years of age and increased with the advancement of age and showed maximum occurrence in the age group of 36–45 years (Fig. 2). The age group-wise variation in mf rate was also statistically significant (χ2 trend = 19.09, p < 0.001).
Table 1.
Microfilaria prevalence in study areas not known for filariasis
| Districts | Village | M | Pos (mf %) | F | Pos (mf %) | Total (mf %) | OR (CI) |
|---|---|---|---|---|---|---|---|
| Shahadol | Beohari | 309 | 26(8.4) | 228 | 5(2.2) | 31(5.8) | 4.1*(1.5–13.8) |
| Bhind | Chandaveli | 178 | 21(11.8) | 177 | 24(13.6) | 45(12.7) | 0.9 (0.4–1.7) |
| Mehdwan | 240 | 37(15.4) | 221 | 41(18.6) | 78(16.9) | 0.8 (0.5–1.3) | |
| Gauri | 205 | 25(12.2) | 186 | 17(9.1) | 42(10.7) | 1.3 (0.6–2.7) | |
| Jaitpura | 114 | 7(6.1) | 60 | 10(16.7) | 17(9.8) | 0.3**(0.1–1.0) | |
| Mangarh | 239 | 13(5.4) | 173 | 5(2.9) | 18(4.4) | 1.9 (0.6–7.3 | |
| Total | 976 | 103(10.6) | 817 | 97(11.9) | 200(11.2) | 0.9 (0.7–1.2) | |
| Hosangabad | Sohagpur | 315 | 13(4.1) | 265 | 6(2.3) | 19(3.3) | 1.8 (0.6–5.8) |
| Ghatli | 193 | 10(5.2) | 196 | 8(4.1) | 18(4.6) | 1.3 (0.5–3.7) | |
| Sankheda | 214 | 24(11.2) | 129 | 9(7.0) | 33(9.6) | 1.7 (0.7–4.0) | |
| Total | 722 | 47(6.5) | 590 | 23(3.9) | 70(5.3) | 1.7*(1.0–3.0) | |
| Shivpuri | Khod | 499 | 35(7.0) | 338 | 9(2.7) | 44(5.3) | 2.8*(1.3–6.6) |
| Pichhore | 187 | 1(0.5) | 167 | 0(0.0) | 1(0.3) | NA | |
| Ganesh Kheda | 112 | 0(0.0) | 97 | 0(0.0) | 0(0.0) | NA | |
| Total | 798 | 36(4.5) | 602 | 9(1.5) | 45(3.2) | 3.1*(1.4–7.0) | |
| G. total | 2805 | 212(7.6) | 2237 | 134(6.0) | 346(6.9) | 1.3*(1.1–1.7) |
Notes: M = male, F = female, pos – positive, OR – odd ratio between male and female (reference), NA = not applicable.
p < 0.05 significantly more in male.
p < 0.05 significantly more in female.
Figure 2.
Age group wise microfilaria rate in non MDA study areas.
Notes: Light bar and dark represent mf% in male and female, respectively. The line represent overall age group wise mf %.
Average mfd among mf positive persons was 26.5 ± 72.2 per 60 μL of blood. The mfd value was relatively high in Bhind (37.3 ± 92.1) followed by Hoshangabad (10.3 ± 14.4), Shivpuri (9.3 ± 9.8) and Shahdol districts (8.4 ± 15). In Bhind district, two persons were having exceptionally high mfd value (830 and 828 mf per 60 μL of blood). Overall, mfd was more in females 30.6 ± 80.9 than in males (23.4 ± 66.0) though not significant statistically. Age group-wise analysis revealed that mfd value was lowest in young children of less than 10 years and highest in the 16–20 years age group. This difference in mfd value among different age groups was also not significant (F = 1.1, p > 0.05).
Two hundred thirty-six specimens of C. quinquefasciatus were dissected from three districts. Twenty vector specimens were having developing filarial larvae (infection rate – 8.5%) and six specimens had infective larvae (infectivity rate – 2.5%). Area wise infection and infectivity rate were higher in Bhind district (Table 2). Infection was found in thorax and abdomen region of the vector specimen but parasite was not found in head region. In all, 21 specimens of infective larvae were found in six vector specimens, ranging from 1 to 8 larvae in thorax and abdomen. In five vector specimens, mf was found only in the abdomen region. LI and LII were seen only in the thorax region.
Table 2.
Infection and infectivity rate of vector C. quinquefasciatus
| Districts | No. dissected | Infection positive (%) | Larva III positive (%) |
|---|---|---|---|
| Shivpuri | 76 | 5(7.1) | 1(1.3) |
| Shahdol | 50 | 1(2) | 1(2) |
| Bhind | 111 | 14(13) | 4(3.6) |
| Hosangabad | ND | ND | ND |
| Total | 236 | 20(8.5) | 6(2.5) |
Discussion
This study confirmed the presence of mf above the threshold level (1%) in all non-MDA districts surveyed, which was then reported to concerned state authorities and treatment was started immediately along with field surveys in the four districts. The chronic symptoms of hydrocele and lymphodema prevalent in these districts are certainly due to the filarial infection by W. bancrofti. High mf rate has also been reported earlier from non-MDA districts of MP and Orissa, which were considered filaria-free.21,22 While filariasis in general is a problem prevalent in the eastern half of the country,13 the areas under study are located in central India and mf prevalence is comparable with the pre intervention status of highly endemic district in the state of MP.23,24 Moreover, the distribution of mf cases is highly heterogeneous even within a specific area as recorded in Bhind district. Infection in both sexes and in all the age groups also revealed that filarial transmission was established many years ago, but detection in these sites might have escaped as symptoms remained unseen and unreported due to hesitation of people in revealing the deformities in comprehension of being discarded by the society or being the subject of mocking.
It is noteworthy that all the four districts are not only geographically located far away from each other but also differ in rainfall, agriculture, altitude, and demographic variables in terms of literacy, occupation, and socioeconomic conditions. These variables are known to be associated with the occurrence and transmission of filariasis.25–27 The presence of filariasis in diverse situations revealed that infection and disease may have high potential of adaptation. Presence of filariasis particularly in village Khod of Shivpuri and Bhind district are prime examples. Large proportions of population in Khod village consist of tribal people and other marginalized communities who lives in extreme poverty and mf status in this village is comparable with the mf prevalence in tribal community of Panna district.28,29 In contrast, the people of Bhind district belong to the higher caste group and are an economically rich community. However, despite high literacy numbers and good financial conditions, these people still sought treatment of hydrocele from untrained and unqualified medical practitioners (quacks).
High infection and the infectivity rate among vector C. quinquefasciatus in the study villages further confirm the filarial endemicity in non MDA districts which suggest that the local climatic conditions are conducive for maintaining high vector longevity (more than 10–12 days) and development of parasite (from mf to infective stage larvae) in the vector.30 Stagnant water in open drainage system blocked with garbage and/or cess pits around human dwellings are the ideal source of vector C. quinquefasciatus breeding and maintaining high vector density as recorded in an earlier study.31
For successful elimination of LF, accurate mapping of areas to effectively target MDA is essential particularly for the bordering villages of filarial endemic districts and areas where population migration is very high. This is particularly important in an Indian context since India bears about 40% of the global LF burden with approximately 48 million people infected.13 Under the GPELF, 13 out of 73 countries have completed MDA and have started post MDA surveillance. Since India leads in providing 71% of total global treatment1 and consequently, after initiation of MDA mf prevalence reduced from 1.24% in 2004 to 0.4% in 2014 and 203 districts are showing mf prevalence less than 1%. Further 49 endemic districts have also qualified for stoppage of MDA. (www.nvbdcp.gov.in/filariasis.html accessed on 3 August 2015). Despite an impressive gain in decreasing LF burden in the known endemic areas, the present survey highlights the huge hidden burden of disease because of lack of evidence-based mapping. As per the filarial risk map prepared on the basis of geo environmental factors for these unsurveyed districts, no district was predicted as at risk of filariasis.15 However, evidence-based detection of filariasis in the study districts hitherto classified as filaria risk-free, suggest limitation of the model.
Detection of microfilaria is the essential part of mapping and monitoring in a LF elimination program. It is important to mention that in 2002 when the MDA strategy was formulated, under NFCP, 206 filaria control units and 199 filaria clinics were functional. These units detected microfilaria by taking blood samples in night blood surveys in the respective districts. This mf detection technique is older and less sensitive as persons with low microfilaria count and amicrofilaraemic condition escaped detection. In addition, there are various operational constraints in conducting night blood surveys. For example, staff members that visit villages during the night for mf survey face non co-operation from the community and people are also reluctant to get themselves examined because they do not feel sick. Moreover, in rural areas, electricity is not often available and blood slides made in inadequate light are of poor quality. Finally, the terrain in the villages is not smooth and public transport system is non-existent. Under these circumstances, night blood surveys are often carried out before 7.00 pm which might lead to false results. Hence, the need of the hour is to adopt the use of more efficient tools for mapping of the filariasis and correct evaluation of the intervention impact, i.e. using antigen-based ICT which has been introduced in the late 1990s32 to overcome the difficulties of night blood survey. Recently with the use of these tools, nine African countries previously considered to be endemic, were found free of LF or with extremely low LF prevalence and then later declared non endemic.1,33 Unfortunately, because of the high cost of these tests, they are used only for Transmission Assessment Survey14,18 and not for routine surveys or periodic evaluation and monitoring in India. However, surveillance by ICT has some limitations too. Its instability is a major challenge for routine use. Under ambient temperature, its shelf life is only 3 months.34 Recently new efficient antigen based – filaria strip test (FST) has been evaluated in many countries. It detects 21–132% more cases as compared to ICT.34,35 This test is much cheaper than the ICT card. Thus, looking at the finding of this study, all difficult and remote areas should be surveyed for mf prevalence using FST on a priority basis so that hidden filarial foci may be covered under elimination.
Finally, in central India, people have immense faith in spiritual leaders and therefore such leaders should be involved for community motivation to visit health facilities if filariasis like symptoms occurs. School teachers can also be of great help in the mass awareness campaign.36 Additionally, mandatory notification of each case of filaria with compulsory reporting from private and other organized government sectors will be useful for the national LF elimination program in identifying hotspots. India has sufficient expertise and technical knowhow in disease eradication of smallpox in the past and of poliomyelitis recently.37 By mobilising all resources and using new tools along with MDA, LF can also be eliminated from the country.
Conflict of interest
There is no conflict of interest by any author.
Contributors
GC was involved in designing the study, data collection and manuscript preparation. LSK and NKC were involved in data collection and analysis and NS was involved in designing the study and manuscript preparation.
Acknowledgments
The authors gratefully acknowledge Dr Altaf L. Lal, Former Health Attache, U.S. Embassy, New Delhi for critically reviewing the manuscript. The authors also thank Shri D.S. Thakur, Shri S.R. Mishra and Shri B.S. Patel for their support in data collection. The help received from local staff of Health department in the collection of data in the field is also thankfully acknowledged.
References
- 1.Ramaiah KD, Ottesen EA. Progress of 13 years of the global programme to eliminate lymphatic filariasis on reducing the burden of filarial disease. PLoS Negl Trop Dis. 2014;8(11):e3319. 10.1371/journal.pntd.0003319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Global program to eliminate lymphatic filariasis: progress report for 2012. Wkly Epidemiol Rec. 2013;88:389–399. [PubMed] [Google Scholar]
- 3.World Health Organization Morbidity management and disability prevention in lymphatic filariasis. New Delhi: WHO (SEARO) 2013; SEA-CD-268. [Google Scholar]
- 4.Molyneux DH, Zagaria N. Lymphatic filariasis elimination: progress in global programme development. Ann Trop Med Parasitol. 2002;96(8):15–40. 10.1179/000349802125002374 [DOI] [PubMed] [Google Scholar]
- 5.Otteson EA. Lymphatic filariasis. Treatment control and elimination. Adv Parasito. 2006;61:395–441. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization Lymphatic filariasis. Progress of disability prevention activities. Wkly Epidemiol Rec. 2004;79:417–424. [PubMed] [Google Scholar]
- 7.Otteson EA, Duke BO, Karam M, Bihbehani K. Strategies and tools for the control/elimination of lymphatic filariasis. Bull World Health Organ. 1997;75:491–503. [PMC free article] [PubMed] [Google Scholar]
- 8.Sabesan S, Vanamail P, Raju KHK, Raju P. Lymphtic filariasis in India: epidemiology and control measures. J Postgrad Med. 2010;56:232–238. 10.4103/0022-3859.68650 [DOI] [PubMed] [Google Scholar]
- 9.Das PK, Pani SP, Krishnamoorthy K. Prospects of elimination of lymphatic filariasis in India. ICMR Bull. 2002;32:41–54. [Google Scholar]
- 10.World Health Organization Lymphatic filariasis: fourth report of the WHO expert committee on filariasis. Tech Rep Ser. 1984;702:3–112. [PubMed] [Google Scholar]
- 11.Sabesan S, Ravi R, Das PK. Elimination of lymphatic filariasis in India. Lancet Infect Dis. 2005;5(1):4–5. 10.1016/S1473-3099(04)01232-0 [DOI] [PubMed] [Google Scholar]
- 12.National Health Policy New Delhi: Ministry of Health and Family Welfare, Government of India; 2002. p. 1–39. [Google Scholar]
- 13.Sabesan S, Palaniyandi M, Das PK, Michael E. Mapping of lymphatic filariasis in India. Ann Trop Med Parasitol. 2000;94:591–606. [DOI] [PubMed] [Google Scholar]
- 14.Government of India Guide lines on elimination of lymphatic filariasis in India. Delhi: Directorate of National Vector Borne Disease Control Program; 2009. [Google Scholar]
- 15.Sabesan S, Raju KHK, Subramanian S, Srivastava PK, Jambulingam P. Lymphatic filariasis transmission risk map of India, based on a geo-environmental risk model. Vector Borne Zoonotic Dis. 2013;13(9):657–665. 10.1089/vbz.2012.1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subramanian S, Perumal V, Adinarayanan S, Kaliannagounder K, Rangachari R, Purushottam J. Epidemiological assessment of eight round of mass drug administration for lymphatic filariasis in India: implication for monitoring and evaluation. PLoS Negl Trop Dis. 2012;6(11):e1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lahariya CK, Mishra AC. Strengthening of mass drug administration implementation is required to eliminate lymphatic filariasis from India: an evaluation study. J Vector Borne Dis. 2007;45:313–320. [PubMed] [Google Scholar]
- 18.World Health Organisation Global programme to eliminate lymphatic filariasis. Monitoring and epidemiological assessment of mass drug administration. Geneva: WHO/HTM/NTD/PCT/2011.4; 2011. [Google Scholar]
- 19.Ramaiah KD, Vanamail P, Yuvaraj J, Das PK. Effect of annual mass administration of diethylcarbamazine and albendazole on bancroftian filariasis in five villages in south India. Trans R Soc Trop Med Hyg. 2011;105:431–437. 10.1016/j.trstmh.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 20.Baraud PJ. Fauna of British India including Ceylon and Burma diptera, culicidae, culicinae. New Delhi: Today and Tomorrow Printers &; 1934. [Google Scholar]
- 21.Chand G, Barde PV, Singh N. New foci of filariasis in Madhya Pradesh. Trans R Soc Trop Med Hyg. 2013;107:462–464. 10.1093/trstmh/trt040 [DOI] [PubMed] [Google Scholar]
- 22.Foo PK, Alessandro T, Aprajit M, Joanne Y, Lakshmi K, Daniel K, et al. . High prevalence of Wucheraria bancrofti infection as detected by immunochromatographic card testing in five districts of Orissa, India, previously considered to be non endemic. Trans R Soc Trop Med Hyg. 2011;105(2):109–114. 10.1016/j.trstmh.2010.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chand G, Tiwary RS, Joshi B. Prevalence of Wuchereria bancrofti infection in rural population of district Panna, Madhya Pradesh – an entomological and parasitological observation. Ind J Prev Soc Med. 1994;25:70–76. [Google Scholar]
- 24.Das D, Kumar S, Sahoo PK, Dash AP. A survey of bancroftian filariasis for microfilariae & circulating antigenaemia in two villages of Madhya Pradesh. Ind J Med Res. 2005;121:771–775. [PubMed] [Google Scholar]
- 25.Brooker S, Michael E. The potential of geographical information system and remote sensing in the epidemiology and control of human helminth infection. Adv Parasitol. 2000;47:246–288. [DOI] [PubMed] [Google Scholar]
- 26.Sherchand JB, Obsomer V, Das TG, Hommel M. Mapping of lymphatic filariasis in Nepal. Filaria J. 2003. doi: 10.1186/1475-2883-2-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galvez Tan JZ. The elimination of lymphatic filariasis: a strategy for poverty alleviation and sustainable development – perspective from the Philippines. Filaria J. 2003. doi: 10.1186/1475-2883-2-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chand G, Pandey GD, Tiwary RS. Prevalence of Wucheraria bancrofti infection among tribals of Panna district of Madhya Pradesh. J Commun Dis. 1996;28:304–307. [PubMed] [Google Scholar]
- 29.Singh N, Shukla MM, Chand G, Barde PV, Singh MP. Vector-borne diseases in central India, with reference to malaria, filaria, dengue and chikungunya. WHO South-East Asia J Public Health. 2014;3(1):28–35. [DOI] [PubMed] [Google Scholar]
- 30.Rajagopalan PK, Kazmi SJ, Mani TR. Some aspects of transmission of Wucheraria bancrofti and ecology of Culex pipiens fatigns in Pondicherry. Ind J Med Res. 1977;66:200–215. [PubMed] [Google Scholar]
- 31.Menon PKB, Rajagopalan PK. Relative importance of different types of breeding habitats in contributing to the population of Culex pipiens fatigans in Pondicherry. Ind J Med Res. 1980;71:725–733. [PubMed] [Google Scholar]
- 32.Bal MS, Beuria MK, Mandal NN, Das MK. Antigenemia in young children living in Wuchereria bancrofti-endemic areas of Orissa, India. Trans R Soc Trop Med Hyg. 2009;103(3):262–265. 10.1016/j.trstmh.2008.08.006 [DOI] [PubMed] [Google Scholar]
- 33.Rebollo MP, Bockarie MJ. Shrinking the lymphatic filariasis map: update on diagnostic tools for mapping and transmission monitoring. Parasitology. 2014;141:1912–1917. 10.1017/S0031182014001231 [DOI] [PubMed] [Google Scholar]
- 34.Weil GJ, Curtis KC, Fakoli L, Fischer K, Gankpala L, Lammie PJ, et al. . Laboratory and field evaluation of a new rapid test for detecting Wucheraria bancrofti antigen in human blood. Am J Trop Med Hyg. 2013;89(1):11–15. 10.4269/ajtmh.13-0089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yahatugoda TC, Supali T, Rao RU, Djuardi YA, Stefani D, Pical F, et al. . A comparison of two tests for filarial antigenemia in areas in Sri Lanka and Indonesia with low level persistence of lymphatic filariasis following mass drug administration. Parasit Vectors. 2015;8:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bagcchi S. India tackles lymphatic filariasis. Lancet Infect Dis. 2015. doi: 10.1016/S1473-3099(15)70116-7 10.1016/S1473-3099(15)70116-7 [DOI] [PubMed] [Google Scholar]
- 37.John TJ, Vashishtha VM. Eradicating poliomyelitis: India’s journey from hyperendemic to polio-free status. Ind J Med Res. 2013;137(5):881–894. [PMC free article] [PubMed] [Google Scholar]