Abstract
Contexts surround and imbue meaning to events; they are essential for recollecting the past, interpreting the present and anticipating the future. Indeed, the brain’s capacity to contextualize information permits enormous cognitive and behavioural flexibility. Studies of Pavlovian fear conditioning and extinction in rodents and humans suggest that a neural circuit including the hippocampus, amygdala and medial prefrontal cortex is involved in the learning and memory processes that enable context-dependent behaviour. Dysfunction in this network may be involved in several forms of psychopathology, including post-traumatic stress disorder, schizophrenia and substance abuse disorders.
One morning I shot an elephant in my pajamas. How he got in my pajamas, I don’t know. Groucho Marx, Animal Crackers, 1930
Contexts serve a psychological function — they are essential for abstracting situationally informed meaning from the world. Contexts shape and define the perception of sensory traces, memories of episodes past, the content of thought, the meaning of words and the goals of purposive behaviour. Contexts are routinely encoded without awareness1. But what is a context? Here, we take a very broad view of context (BOX 1) and define it as the internal (cognitive and hormonal) and external (environmental and social) backdrop against which psychological processes operate2. It assigns contingencies, spatial locations, necessary conditions and special circumstances to salient cues and memory traces. Context includes perceptions of time, thereby framing the memory of an experience (for recollection, recognition and familiarity) and shaping future expectations of similar experiences (for anticipation, foresight and planning). As such, contexts enable the flexible representation and retrieval of information and have a central role in resolving ambiguity, all of which are necessary for adaptive behaviour. Importantly, contexts can be distinguished from the discrete cues, such as Pavlovian conditional stimuli (CSs) and unconditional stimuli (USs), that they inform3. This very broad view of context that we adopt here might be criticized as being too general, especially by those investigators who study it in a well-controlled experimental environment in animals. However, the concept of context has been extensively used in the human and clinical literature to refer to general cognitive, semantic or ‘emotional’ backgrounds. As the aim of this Review is to highlight the common brain circuitry underlying context processing across different species, we opted for a definition of context that is broader, albeit less discriminating.
Box 1. What is context?
A context is broadly defined as the set of circumstances around an event. In the learning literature, contexts are typically distinguished from cues, such as conditional stimuli, that are arranged as discrete signals for other events (that is, unconditional stimuli). Unlike discrete cues, contexts are typically multisensory, diffuse and continuously present. Although places are clearly contexts, there are many other forms of context that define experience.
Spatial context
Everything we do occurs in a place. Moreover, a large part of the nervous system is devoted to getting us from one place to another. The places, and the configuration of objects and features of those places, define the spatial contexts that surround events.
Temporal context
All of our thoughts and actions occur in a moment in time and are referenced to the time at which they occur. Events are often defined by their temporal properties, such as their frequency, and this information can itself serve as a context.
Interoceptive context
Hormonal and physiological states, such as hunger or stress, also serve as contexts: for example, we particularly note the opening of a wine bottle if we are hungrily anticipating a fine meal at a restaurant. Hunger, then, can influence how we understand the sound of the cork being pulled from a bottle.
Cognitive context
Cognition is at the heart of contextual processing and can itself serve as a context for how information is encoded and retrieved. For instance, an instruction that a red light is likely to be followed by an aversive shock (even if it never is) sets a cognitive context for the outcome that is expected when seeing the light.
Social and cultural contexts
Life unfolds in a social network. The individuals with whom we experience life events, as well as the broader cultural contexts in which those experiences take place, often define our experiences. These social settings strongly influence how we understand the world and ourselves.
Given the essential role of context in emotion and cognition, a major scientific challenge is to understand how the brain processes contextual information. Indeed, an inability to appropriately contextualize information may lead to psychological dysfunction characterized by inaccurate percepts or inappropriate responses that contribute to specific psychopathologies. In the past two decades, considerable research in animals has explored how contexts are encoded in the brain. More recent studies have explored the neural mechanisms by which context modulates memory retrieval induced by ambiguous cues. This research in animals has provided a foundation for studies in humans, which have begun to explore the neuroanatomy of context processing in both healthy subjects and patients with psychiatric disorders. The goal of this Review is to synthesize this work and to propose an integrated circuit model of context processing in the brain. We will focus on the neurocircuitry that mediates the processing of environmental contexts in emotional learning and memory tasks, particularly fear conditioning.
Context and associative learning
Recent years have witnessed an amazing proliferation of research on the neural mechanisms by which context representations are encoded in the brain. This work has largely come from studies of associative learning in both animals and humans4,5. In Pavlovian fear conditioning, for example, an environmental context (a conditioning chamber) may be arranged to signal the delivery of a footshock (a US), which then leads to conditioned responses to the context, such as freezing behaviour in rats.
In order for context learning to occur, animals must first form a representation of the context. Contexts are composed of many stimulus elements that are assembled into configural (also called ‘contextual’) representations (BOX 1). Although contextual representations include the elements that they encompass, they can be distinguished from these elements (FIG. 1). These sorts of contextual representations are learned incidentally (with mere exposure to the context) as a ‘gestalt’ and are acquired very rapidly. After they have been encoded, context representations can themselves come to be associated with other events, such as the occurrence of an aversive footshock. Hence, during a typical context fear-conditioning procedure, animals first encode a representation of the context (when the animal explores the context before a footshock) and then associate that representation with the US. These two learning processes are referred to as context encoding and context conditioning, respectively (FIG. 2). Context encoding is necessary for context conditioning. Indeed, animals that are shocked immediately upon placement in a chamber do not show context conditioning6, which suggests that they must encode a context representation in order for conditioning to occur.
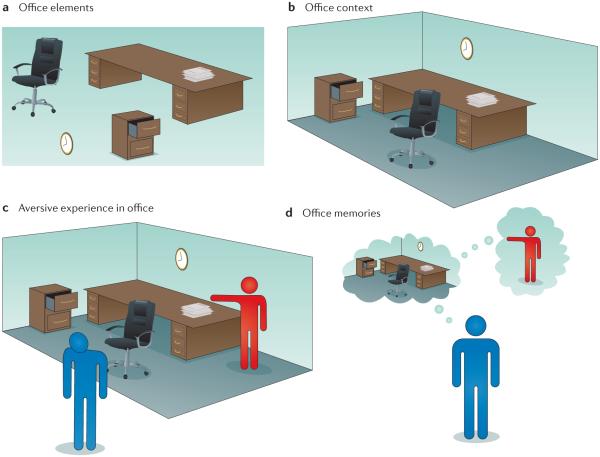
Figure 1. Stimulus elements, context and memory.
Contexts are complex and multimodal representation that are formed by binding constituent elements into a unified representation. This figure illustrates the typical stimulus elements found in an office context and how these elements and their context might set the occasion for an aversive experience (such as getting fired) in that office. a,b | A complete representation of an office space might include not only the unique items found in an office, such as a clock, filing cabinet and desk (part a) but also the unique space in which those items are found (an office at work) and the conjunctive representation of those items in that context, such as the position of the filing cabinet, the clock on the wall next to the desk, and so on (part b). c | After they are encoded, context representations can themselves come to be associated with an event, such as getting fired in the office. d | In this case, memories of the office might come to provoke stress and anxiety by virtue of its association with a disgruntled boss and the circumstances around losing a job — indeed, the aversive memory associated with losing a job might generalize to any office-like setting.
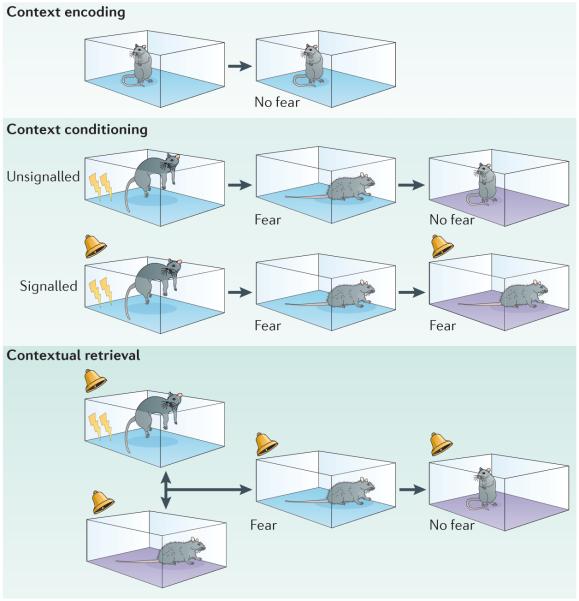
Figure 2. Context encoding, conditioning and retrieval tasks in rodents.
There are many learning and memory processes involved in acquiring and expressing information about contexts. Merely exposing animals to a novel context results in a memory of that context, a process called contextual encoding (top panel). If that context is subsequently paired with an aversive stimulus, such as an electric footshock (indicated by the lightning symbol), it will yield an association between the context and shock; this process is called context conditioning (middle panel). Context conditioning occurs with either a signalled shock, in which a conditional stimulus (CS), for example, a sound, is paired with the shock (the unconditional stimulus (US)) or with an unsignalled shock, in which the US is presented without a CS. Contexts can also acquire modulatory properties in which they set the occasion for when a CS and US are paired. For example, in a conditional discrimination task based on context, a CS is paired with the US in one context but not in another context. The context subsequently serves to retrieve the meaning of the CS in each context in which it occurs, a process termed contextual retrieval (bottom panel).
In addition to direct associations with USs, contexts can also signal the relationship between other stimuli and the US. For example, in a context discrimination task, a CS, such as a light, is followed by a footshock (the US) in one context but not in another. In this case, the CS acquires two competing ‘meanings’: one predicting the upcoming US and another predicting that the US will not occur. When animals are then presented with the ambiguous CS, a contextual retrieval process determines which CS–US relationship is in effect, thereby enabling animals to discriminate between the two relationships. As with context conditioning, animals must form representations of the distinct contexts in order to be able to discriminate between them. However, unlike a context conditioning procedure, it is not the direct association between the context and shock that guides performance but the relationship between the context and the events it has hosted that controls behaviour. Once encoded, both context memories and CS–US memories undergo consolidation, although with different time courses7. For the purposes of this discussion, we will use a broad definition of ‘encoding’ that also encompasses the consolidation processes that lead to long-term memories.
Brain systems for encoding context
Animal studies: role of the hippocampus
Considering the essential role of the hippocampus in episodic memory and spatial representation in both animals and humans, neurobiological studies of contextual processing have focused on this brain area. Indeed, many studies now indicate that the hippocampus has a crucial role in tasks involving learning and remembering contexts8. For example, hippocampal lesions in rodents produce deficits in freezing behaviour during exposure to a shock-paired context9–12. These deficits are especially robust when hippocampal lesions are made soon (1 day) after contextual fear conditioning but are minimal when lesions are made at later times (30–100 days after conditioning)11,13,14. This pattern of temporally graded amnesia, in which recent memory is more susceptible to hippocampal damage than remote memory, is typical after hippocampal damage in humans as well15. Interestingly, hippocampal lesions do not necessarily disrupt context fear conditioning when they are made before conditioning12,13,16. This suggests that the stimulus elements that make up a context are represented outside the hippocampus and are sufficient for learning but that these representations are normally (that is, when the hippocampus is intact) superseded by a configural representation of the context in the hippocampus13,17–19. However, it has been shown that animals with hippocampal damage show less context conditioning when the interval between placement in the conditioning chamber and receiving the footshock is reduced. Because the immediate-shock deficit reflects a failure of context encoding, this suggests that rats with hippocampal lesions are not simply learning using elements but are in fact using contextual information, albeit less efficiently3,16.
Deficits in context fear memory following hippocampal damage appear to be due to a deficit in forming and storing the contextual representation itself (that is, in context encoding) rather than due to a deficit in forming the context–US association (that is, in context conditioning). For example, pre-exposing rats to the to-be-conditioned context eliminates the effect of hippocampal lesions on context fear conditioning20. In addition, pharmacological inactivation of the hippocampus during pre-exposure disrupts the context pre-exposure facilitation effect, in which brief pre-exposure to a context facilitates conditioning that normally fails under immediate-shock conditions21. Deficits in context encoding caused by hippocampal lesions have also been demonstrated in appetitive procedures, in which contexts signal the delivery of food22,23. There are reports that the reinstatement of fear after extinction, which is attributed to context–US associations, is disrupted by hippocampal lesions24,25, although this has not been found in appetitive tasks26. Collectively, these studies indicate that the hippocampus is involved in encoding context representations and that context–US associations per se are not disrupted by hippocampal damage. It is now well established that the amygdala is critical for encoding, storing and retrieving direct associations between contexts or cues and aversive stimuli27–30.
The role of the hippocampus in encoding context representations is consistent with the large literature on the role of the hippocampus in spatial representation and navigation. This is hardly surprising given that the immediate environment is a component of the overall contextual representation for any organism, and associating environments (rather than just the specific cues within them) with safety versus danger could be key to an animal’s survival. Neurons in the hippocampus respond vigorously to many types of stimuli, including non-spatial cues, but they are particularly responsive to spatial location. For instance, ‘place cells’ in the hippocampus respond to unique locations in space, such as when an animal explores a novel arena. Place cell firing is also heavily influenced by context31. For instance, in one study, the authors created four distinct contexts by changing olfactory and visual contextual stimuli within the same enclosure and then examined the place field in the hippocampus of rats while they explored each environment32. Individual neurons exhibited a heterogeneous pattern of re-mapping, with some neurons maintaining their positional firing profile across all contexts and other neurons re-mapping their field or dropping their place field altogether in some contexts. This suggests that unique context representations are established in the hippocampus and are represented by population coding of both the spatial properties of the environment and the integration of spatial and non-spatial (that is, contextual) stimuli in the environment33.
In addition to representing exteroceptive contexts, such as the places an animal visits, the hippocampus is involved in the learning and retention of interoceptive contexts. For example, rats with hippocampal lesions have impaired performance in contextual discrimination tasks in which the animal’s deprivation state (hunger versus satiety) signals when a US (a footshock or sucrose) will be delivered34,35. In this case, hippocampal lesions might impair either the encoding of interoceptive contexts or the ability to use those representations to guide memory retrieval. It seems likely that the hippocampus is particularly important for the contextual memory retrieval processes that are required to disambiguate whether a US will be delivered; indeed, animals will have had considerable past experiences of hunger and thirst, and those interoceptive states would certainly not be uniquely related to task performance. Consistent with this perspective, hippocampal neurons have been found to encode the relationship between interoceptive state (hunger versus thirst) and response–outcome relationships in instrumental food-motivated tasks36,37. That is, hippocampal activity encodes what to do in which interoceptive context38.
Animal studies: role of cortical areas
Beyond the hippocampus, there is growing evidence for cortical involvement in encoding context. Lesions in the entorhinal cortex, the primary cortical input to the hippocampus, produce a pattern of deficits that mirrors those of hippocampal damage itself39–41. Damage to other parahippocampal cortices, including the perirhinal and postrhinal cortices, after conditioning also produces impairments in contextual fear conditioning and retention, even when lesions are made as late as 100 days after training41. Pharmacological inactivation of the retrosplenial cortex also impairs retention of long-term context memories42. In addition, the anterior cingulate cortex (ACC) may have a role in the storage of contextual memories, insofar as inactivation of this area impairs the expression of remote context memory; and anterior cingulate neurons exhibit greater activity (as indexed by FOS expression (the protein product of an immediate-early gene that is associated with neural activity)) during the retrieval of remote relative to recent context fear memories43. Medial prefrontal cortical lesions have also been reported to disrupt remote context memories44. Given the time-limited role of the hippocampus in maintaining context memories, as discussed above (but see REF. 45), these data suggest that cortical areas with which the hippocampus is reciprocally connected may be essential for maintaining context representations over time.
Human studies: role of the hippocampus
The development of in vivo functional neuroimaging has revolutionized the study of cognitive processes, including learning and memory, in humans. However, relatively little research has directly examined the neural mechanisms of contextual processing in humans. One limitation has been the inherent difficulty in establishing different ‘contexts’ in the functional neuroimaging environment. That is, the MRI scanner suite, which itself is the context of the neuroimaging experiments it hosts, has posed a challenge for human studies. As a result, studies of contextual representations in humans have primarily manipulated the visual background (for example, alternating colour on background screens or introducing a ‘virtual’ environment through virtual-reality technology). The main concern in this type of manipulation is that most of the key elements of a context (such as the actual location, experimental setting or interoceptive state) remain identical in experimentally manipulated contexts and is therefore ‘invisible’ to standard functional MRI (fMRI) subtraction methodology.
With those caveats aside, an early study used background screen colour to impart conditional discrimination in a fear-conditioning task46. In this design, one tone (CS+) was paired with an aversive stimulus only when the visual background was set to a particular colour (CTX+) but not when the other colour (CTX−) was present. Thus, the background colour provided a context signal that indexed the overall likelihood of receiving the (aversive) US. During training, the auditory cortex produced larger responses to acoustic stimuli in the safe context (CTX−) than in the conditioning context (CTX+), which is consistent with its role in discriminatory fear conditioning. The amygdala was activated by CS+ presentations in the conditioning context, which is consistent with its role in fear conditioning. However, the hippocampal blood-oxygen-level-dependent (BOLD) signal to the CS+ did not depend on the context in which it was presented — this is surprising, given the preponderance of evidence in animals that context information is encoded by the hippocampus and converges with information about the US in the amygdala. The relatively weak visual (as opposed to spatial) context manipulation in this task or the use of discrete cues that signalled threat stimuli may have accounted for the failure to detect context-related hippocampal activation in this study. In support of the possible use of discrete cues, recent human fMRI studies have confirmed the involvement of both the amygdala and the hippocampus in the acquisition of contextual fear conditioning by presenting discrete cues (shapes and faces) and USs without pairing them (which favours context conditioning) in contexts consisting of differently coloured screen backgrounds47 or in more visually complex contexts (such as pictures of ‘real’48 or ‘virtual’49 rooms).
A positron emission tomography (PET) study has revealed differential hippocampal engagement depending on whether USs were signalled or unsignalled50. In this study, subjects experienced epochs in which wrist shocks were either signalled by a visual stimulus (the ‘cue fear’ condition) or were presented independently of the stimulus (the ‘context fear’ condition). In both conditions, the visual stimulus elicited an amygdala response, but only the ‘context fear’ condition increased activation of the hippocampus (and subgenual ACC) in response to the visual cue50. In direct support of this finding, a structural MRI study demonstrated that in healthy participants, hippocampal volumes correlated with contextual fear responses using whole-scanner colour contexts, whereas individual differences in amygdala volumes did not have any influence on the ability to acquire or extinguish contextual fear51.
In another experiment, different visual cues (circles and triangles) were either paired (signalled) or unpaired (unsignalled) with the US, which was given in a particular visual spatial context (for example, one of several scenes of different rooms in a house)49. In this case, the hippocampal BOLD response was most strongly increased in the condition in which the US was unsignalled, whereas the amygdala signal was increased in both the signalled and unsignalled conditions. As has been previously reported, the activity increases in both structures were most pronounced early in acquisition and declined as the conditioning session proceeded49. Consistent with the animal literature, these data suggest that the amygdala has a general role in aversive conditioning and that context conditioning additionally recruits the hippocampus. Interestingly, more sustained contextual responses were noted in the insula, ACC, inferior parietal cortex and lateral orbital cortex — regions that are often implicated in anticipatory anxiety52–55, making it difficult to discern their involvement in contextual encoding from their involvement in regulating the emotional response (that is, anxiety) to such information.
The transient hippocampal response48,49 during contextual conditioning suggests that this structure may be involved in contextual encoding, although it is difficult to parse context encoding and conditioning in these experimental designs. A recent study in which a coloured background (a visual context) was associated with an unsignalled US showed that the precise hippocampal response depended on the acquisition phase; the posterior hippocampus was active during the early phase of acquisition, but a more rostral hippocampal region was active during later phases of acquisition47. The activation of the posterior hippocampus in humans (which is analogous to the dorsal hippocampus in rats) during early acquisition may reflect contextual fear encoding, whereas the engagement of rostral regions during later phases of acquisition may reflect the emotional expression of that fear56,57. It is noteworthy that contextual stimuli are also engaged in the activation of the amygdala, ACC, mPFC, inferior and orbital frontal cortices, insula, ventral putamen and/or parietal cortex47–49, further demonstrating that a broad network is involved in the encoding of context representations in humans.
Brain systems for contextual retrieval
Encountering a particular stimulus may require radically different responses in different situations, and an important function of context processing is to elicit the most appropriate response4 (FIG. 3). For instance, the sound of a loud boat horn at a college football game would be interpreted as a rallying cry for the home team, but the same sound in a harbour would be a warning to make way for another vessel. In the laboratory, contexts can be manipulated to provide crucial information for response selection, and in these cases contextual retrieval processes operate to guide performance. Note that ‘contextual retrieval’ here refers to the memory processes by which the meaning of a cue, such as a CS, is understood with reference to the context in which it is retrieved (which also depends on retrieving a memory of the context itself). When contexts retrieve the meaning of CSs they are typically referred to as occasion setters8,58. For example, in a conditional discrimination task based on context, one context might signal when an auditory CS is followed by food and another context might signal when it is not. In this example, the context sets the occasion for when (and where) the same CS will yield food. Occasion setters themselves do not elicit conditioned behaviour; rather, they serve as modulators that inform when another stimulus will result in a particular outcome. Although occasion setting has been studied largely in animals, it has relevance for humans, as it influences how stimuli are evaluated in many contexts, including different places, social settings, and hormonal and motivational states.
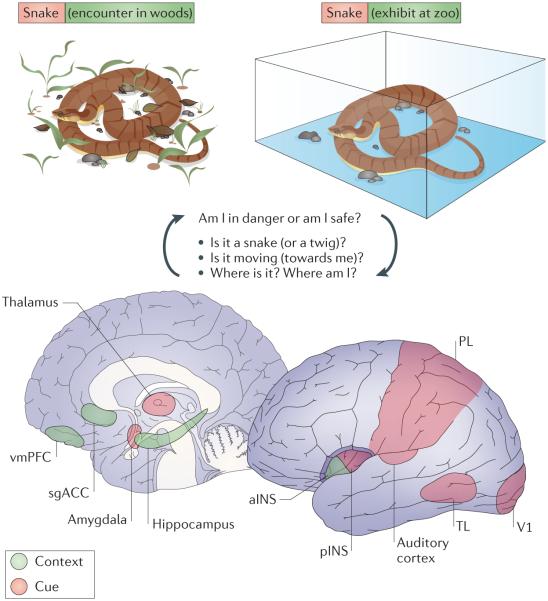
Figure 3. Brain circuits involved in cue and context processing in the human brain.
The brain has separate, parallel systems for processing cues (pink) and processing contexts (green), and the context-processing system is essential for understanding the meaning of cues in a particular context. For example, a poisonous snake has a different meaning when it is encountered in the wild (where it could signal ‘danger’) than when it is seen behind glass in a zoo (where it could mean ‘interesting’). The cue-processing system includes the thalamus, amygdala, sensory cortices (the primary visual cortex (V1) and auditory cortex), posterior insula (pINS) and association areas (in the parietal lobe (PL) and temporal lobe (TL)), whereas as context processing systems involve the ventromedial prefrontal cortex (vmPFC), hippocampus, anterior insula (aINS) and subgenual anterior cingulate cortex (sgACC). There is extensive interaction between these systems insofar as contexts influence the processing of cues and the conjunctions of cues, and contexts are likely to be represented in connections between these networks.
Contextual control of fear behaviour (and, by proxy, contextual retrieval) can be readily examined using extinction and fear renewal procedures in rats4 (FIG. 4). After Pavlovian fear conditioning, a CS will evoke high levels of fear wherever it is encountered, regardless of whether that is in the context in which conditioning occurred, in another, novel context or even in the home environment; that is, it is context-independent. Subsequent repeated presentation of the CS by itself (that is, without the US) yields a loss of fear, as the CS no longer predicts the aversive outcome. This learning process, known as extinction, has obvious clinical relevance in that it is thought to be the core process for therapeutic interventions such as exposure therapy in patients with anxiety disorders. Importantly, extinction training only reduces fear in response to the CS in the place where extinction occurs — that is, extinction is context-dependent. Fear in response to the previously extinguished CS will return when it is presented outside the extinction context, regardless of whether this is in a novel context or in the original conditioning context. This phenomenon, termed renewal, indicates that presentations of the CS during extinction training do not eliminate the CS–US memory that yields learned fear responses but rather that they produce a new, inhibitory CS–no-US memory that competes with the original CS–US memory.
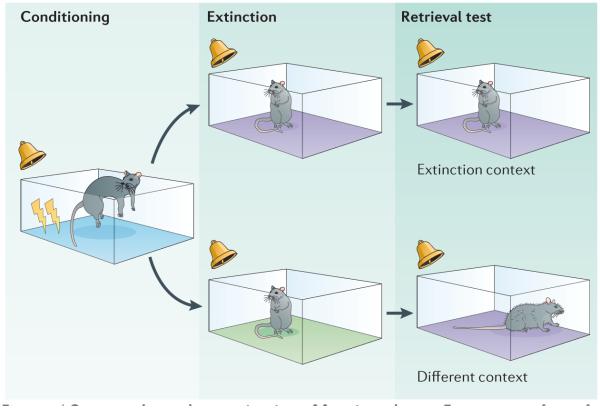
Figure 4. Context-dependent extinction of fear in rodents.
Extinction is a form of learning in which a conditional stimulus (CS) is presented alone after conditioning. Such CS-alone presentations decrease the magnitude and frequency of the learned response, and this loss of responding to the CS is context-specific. As a result, a diminished conditioned response to the CS is expressed only in the context in which the extinction occurred, but the response will return (or ‘renew’) in any other context. The figure shows the typical procedure for studying the context-dependence of extinction in rodents. In this procedure, rats are first conditioned to an auditory CS in one context (blue). Subsequently, extinction training to that CS occurs in another context (either the purple or green context), and rats are then tested in a context that is either the same or different as the one in which extinction took place. In this example, the purple context is the same context as extinction for half of the animals but a different context for the other half of the animals.
In extinction procedures, context serves as an occasion setter that favours retrieval of the ‘safe’ CS–no-US memory in the extinction context, and the ‘fearful’ CS–US memory in any other context. Interestingly, the CS–US memory also becomes more context-dependent after extinction, in that after extinction, fear in response to the CS is highest in the conditioning context and lower in other contexts59. The context-dependence of fear extinction has special clinical relevance, because a major challenge in psychotherapy is to achieve a reduction in fear that generalizes beyond the therapeutic setting60,61. Hence, what follows below is relevant to understanding both the brain systems involved in contextual memory retrieval and the forms of psychopathology that may result from deficits in fear extinction, such as post-traumatic stress disorder (PTSD).
Animal studies: context-dependence of extinction
Studies of the neural basis of extinction have revealed a neural circuit that involves contextual control of fear expression at the level of the amygdala27,62,63. Both the central nucleus of the amygdala and the basolateral complex of the amygdala (BLA) are crucial for the acquisition and expression of conditioned fear 27–30. However, extinction learning also appears to involve plasticity in the amygdala63–65, raising the interesting question of how the expression of fear in response to the CS is retained in a circuit that can effectively suppress its expression in response to the same CS under certain conditions. Recent work indicates that some amygdala neurons fire preferentially in response to CSs during the renewal of fear outside the extinction context, whereas others fire in response to CSs during the suppression of fear in the extinction context66. This suggests that there may be parallel codes for fear and safety in the amygdala — but how are these different codes retrieved to yield appropriate behavioural responses to the CS?
In rodents, neurons in the BLA exhibit robust increases in spike firing in response to the CS after fear conditioning, and these responses are often reduced in magnitude after extinction67–70. In addition, some BLA neurons encode extinction; that is, they increase their firing in response to CS presentation in the extinction context after extinction training66,71,72. Interestingly, CS-elicited activity in the amygdala exhibits context-dependence, in that CS presentations outside the extinction context cause ‘neuronal renewal’ — a return of CS-elicited spike firing in response to the extinguished CS66,71 (FIG. 5).
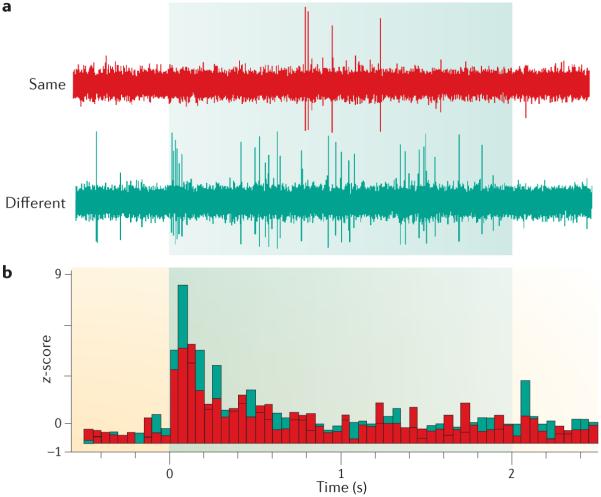
Figure 5. Context-dependence of neuronal activity in the rat amygdala.
The return of fear in response to an extinguished conditional stimulus (CS) is correlated with neuronal firing in response to the CS in the amygdala in rats. a | The traces are high-pass filtered signals recorded from a single multi-unit recording wire implanted in the lateral amygdala; the sweep length is 3 s, which includes 500 ms pre- and post-tone periods and the 2-s tone CS (shaded area). Presentation of the CS occurred in either the extinction context (same) or another context (different). Neuronal responses to the CS were reduced in the extinction context but increased in the different context. b | The histogram shows the average neuronal responses to the extinguished CS in both contexts. Figure is modified, with permission, from REF. 75 © (2007) Cold Spring Harbor Laboratory Press.
The context-dependent expression of fear after extinction (that is, when the meaning of the CS is ambiguous) appears to involve a ‘gating’ of CS–US and CS–no-US associations that are encoded in the amygdala. What brain areas might regulate such gating? Considerable work implicates the hippocampus in regulating the context-dependence of extinction memories. For instance, animal studies have shown that pharmacological inactivation of the hippocampus reduces the renewal of fear in response to an extinguished CS when it is encountered outside the extinction context73–76. This impairment in fear renewal is neither due to a failure to appreciate fear signals per se73 nor is it due to an inability to discriminate the test context from the extinction context77,78. Indeed, hippocampal inactivation in this case appears to specifically impair the retrieval of the CS–context associations that are necessary to support fear renewal. Deficits in contextual memory retrieval after hippocampal damage have not only been reported after fear extinction but also in many other tasks that involve retrieving the meaning of cues in particular contexts, such as occasion setting79–82. Similar to the behavioural renewal of fear, neuronal renewal of CS-elicited activity in the amygdala depends on the hippocampus75. The role of the hippocampus in shaping context-dependent neuronal responses in the amygdala has been supported by computational models83,84.
These data indicate that hippocampus–amygdala interactions are involved in the contextual retrieval of fear memories after extinction. There are at least two anatomical routes by which this interaction might occur (FIG. 6). First, direct projections from the hippocampus to the amygdala terminate on neurons that fire during renewal outside the extinction context66,85. Second, it is also possible that the hippocampus might indirectly influence amygdala neurons through dense projections to the medial prefrontal cortex (mPFC), which in turn projects to both excitatory and inhibitory neurons in the amygdala. This projection has been implicated in anxiety86–88 and fear expression89. Studies in rats have shown that the infralimbic region of the mPFC projects to inhibitory intercalated neurons that limit excitation of the central amygdala induced by activity in upstream BLA neurons90,91. Moreover, the prelimbic region of the mPFC innervates the BLA and may thereby have a role in fear expression92. Hence, hippocampal modulation of mPFC activity might modulate amygdala output to influence the expression of fear in response to an extinguished CS93. Consistent with this possibility, neurons in the prelimbic and infralimbic cortex exhibit reciprocal patterns of FOS expression during the renewal and suppression of fear, respectively94. Ventral hippocampal projections to both the BLA and the mPFC are essential for fear renewal85,95, suggesting that a convergence of both direct and indirect streams of information in the amygdala supports the contextual retrieval of fear memories.
Figure 6. Neural circuit for context-dependent regulation of fear memory.
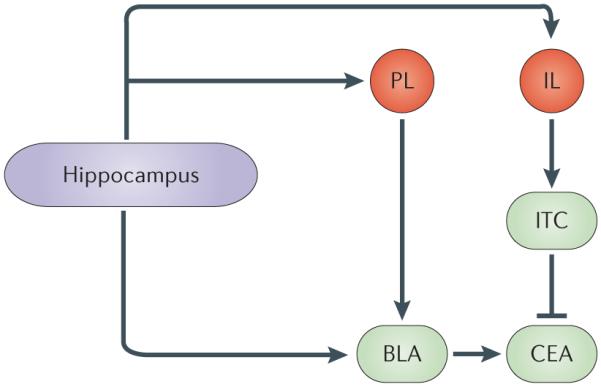
The context-dependence of fear memory involves a neural circuit that includes the hippocampus, the medial prefrontal cortex (specifically, the infralimbic cortex (IL) and the prelimbic cortex (PL)) and the amygdala (specifically, the basolateral amygdala (BLA), central amygdala (CEA) and intercalated (ITC) cells). The hippocampus projects directly to the BLA, and this projection may be crucial for the renewal of fear expression in response to an extinguished conditional stimulus. Indirect projections between the hippocampus and amygdala via the medial prefrontal cortex might also mediate the context-dependent expression of fear in response to an extinguished conditional stimulus. In particular, PL projections to the BLA are involved in fear renewal, whereas IL projections to ITC cells, which in turn inhibit CEA output, are involved in suppressing the expression of fear in response to an extinguished conditional stimulus.
Collectively, these data suggest that a network of structures, including the hippocampus, PFC and amygdala, is involved in contextual regulation of associative fear learning and retrieval. Indeed, human studies have confirmed the engagement of the amygdala96–100, of an area that includes the subgenual ACC, ventromedial PFC (vmPFC) and orbitofrontal cortex47,97,99, of the dorsal ACC and rostral mPFC47,96–99,101, and of the hippocampus100 in extinction of fear memory. It should be noted that amygdala involvement is not consistently reported in human studies102 and may only appear during the early phase of extinction learning99,100 (but see REF. 96), and that activation of the hippocampus is not commonly detected during extinction learning per se (see REF. 103 for a review). Interestingly, it has been reported that vmPFC activation is specific to extinction learning — that is, it was engaged during fear extinction but not fear acquisition47,96,97, linking it to contextual encoding of the extinction memory.
Human studies: contextual control of fear
The neural model derived from animal studies that implicates the hippocampus and mPFC in the retrieval of extinction memory has recently been explored in human studies. The vmPFC (including the subgenual anterior cingulate region) has been associated with the retrieval of extinction memory99, although it remains unclear whether it is engaged in a context-dependent manner. Contextual discriminations can be used in fMRI experiments to test the neural response to an extinguished CS in either the extinction context (in which fear in response to the CS is suppressed) or in the conditioning context (in which fear in response to the CS renews). In these designs, human subjects acquire CS–US pairings in a conditioning context, and these pairings are subsequently extinguished in another context, leading to robust contextual discrimination54,96.
In one recent study, the vmPFC and anterior hippocampus (extending into the entorhinal cortex) were activated during the retrieval of fear extinction, whereas the striatum, temporal cortex, posterior hippocampus (and the amygdala at a slightly more liberal threshold) were activated during the renewal of fear54. Furthermore, CS+-evoked left anterior hippocampal activation during retrieval of extinction memory was positively correlated with the corresponding vmPFC activation; that is, individuals with strong extinction-related hippocampal activation also had strong extinction-related vmPFC activation. Interestingly, this hippocampal–ventromedial pre-frontal cortical relationship was specific to extinction as the correlation was smaller (statistically non-significant) in the conditioning context54. This suggests that the hippocampus may support the retrieval of extinction, as it contains contextual information supporting retrieval of that memory. This is consistent with the suggestion that hippocampal–prefrontal interactions are involved in the contextual retrieval of fear memory after extinction93–95,104.
Another study using a contextual discrimination design also showed that both the vmPFC and hippocampus are engaged during the retrieval of extinction memory96. Moreover, signal increases in these areas correlated with the behavioural expression of extinction memory, and vmPFC activation was correlated with hippocampal activation96. In other words, individuals showing the greatest suppression of conditional responding also had greater vmPFC and hippocampal activation. Importantly, the vmPFC was hyperactive in response to the CS+ (relative to the CS−) during extinction learning, particularly in the late stages, but was hypoactive in response to the CS+ (relative to the CS–) during fear acquisition. Together, these studies suggest that both the vmPFC and hippocampus are involved in the retrieval of extinction memories, although it is not clear to what extent they are involved in the context-dependent expression of these memories.
The paucity of pharmacological ‘inactivation’ and lesion studies in humans on contextual retrieval limits causal inference of the critical role of the hippocampus and vmPFC in these processes. Nonetheless, there is some evidence that amnesic patients fail to show reinstatement of extinguished fear after presentations of the aversive US alone outside the conditioning context despite being able to acquire the original fear association105–107. This suggests that humans with hippocampal damage have deficits in context encoding or conditioning, which is consistent with findings from many animal studies8,25. However, there are no human lesion studies to date examining whether the vmPFC is necessary for extinction learning, extinction retrieval or contextual retrieval, although structural MRI data suggest a role for this area in these functions108. More definitive mechanistic studies are needed to determine whether damage to the hippocampus and vmPFC is sufficient and/or necessary for causing deficits in the retrieval of contextual fear extinction memory in humans.
Contextual processing in psychopathology
Considering the central role of context in the flexible representation and retrieval of information and in resolving ambiguity regarding the meaning of stimuli, deficits in contextual processing often lead to inflexible, rigid and inappropriate behavioural responses. In humans, these can in turn lead to various symptoms — from paranoid beliefs or intrusive thoughts to compulsive behaviours — that are seen in multiple psychiatric disorders, including schizophrenia, PTSD, depression and drug addiction. Among these disorders, PTSD may be most representative of context processing pathology, given that the core features of PTSD involve intrusive thoughts, memories and perceptions (flashbacks) that are experienced outside the current context — as if the person was re-experiencing the traumatic event. Deficits in the extinction of fear memory have been hypothesized to contribute to PTSD and have been identified as therapeutic targets for extinction-based behavioural therapies109–111. These deficits may reflect a loss of contextual control of extinction, causing extinguished fear to inappropriately renew in any context.
However, few studies have directly and specifically implicated impaired contextual processing in PTSD pathophysiology112. In one study in which participants underwent a fear-conditioning–extinction protocol, patients with PTSD exhibited a robust conditioned fear response (an increase in skin conductance) to the previously extinguished CS, indicating impaired retention of extinction113. This was associated with impaired activation of the hippocampus and vmPFC and exaggerated dorsal ACC responses during extinction recall compared with control subjects113. In another experiment, subjects were shown an image of an indoor scene (that is, an office containing desk and a lamp) in which illumination of the lamp (the CS) was paired with an aversive event (the US). During extinction, the subjects were shown a different indoor scene (that is, a library containing a shelf and a lamp), and illumination of the lamp was not followed by the US. In this task, contextual information (the room) provides information about whether the lamp will be followed by the US. Compared with controls, patients with PTSD exhibited lower activity in the vmPFC in response to the contextual stimuli during both late extinction training and extinction recall113,114. This suggests that patients with PTSD may not be able to use contexts to limit fear responses to a CS that no longer yields an aversive outcome or to learn new relationships that define when formerly dangerous cues are safe112. This, in turn, may give rise to maladaptive behavioural responses to both trauma-relevant cues and other salient stimuli, including inappropriate fear responses (for example, exaggerated startle) to cues that resemble the trauma event (for example, a seemingly innocuous loud noise). These considerations raise the possibility that contextual processing deficits are at the core of PTSD pathophysiology, although further testing of this hypothesis is required.
Deficits in contextual processing have also been implicated in responses to drugs of abuse115–119 and in the enhancement of the incentive properties of amphetamines120. The role of abnormal contextual processing in the development of substance abuse in humans has received little attention relative to many studies that have examined responses to drugs and drug-associated cues121,122. However, it is well documented in animal models that context plays a crucial part in the propensity of animals to self-administer drugs and in modulating the expression of drug-induced neuroplasticity and drug tolerance116. Given the important role of the setting in determining the quantity and even the type of drug that is consumed, it stands to reason that dysregulation in contextual processing would influence drug-taking behaviour. For instance, a loss of context-specific tolerance so that tolerance is experienced in any context might cause drug use to escalate in any context in which it has previously been taken. Similarly, the expression of drug sensitization, which is normally limited to the context of drug-taking, can be generalized if contextual processing is altered.
Abnormal contextual processing has also been associated with schizophrenia, insofar as individuals with this disorder have deficits in the so-called AX-continuous performance test, in which one cue (the letter A) sets the occasion for when subjects should respond (for example, by pressing a response key to another cue (the letter X)123,124. Interestingly, fMRI studies have implicated the dorsolateral PFC, a key working memory region, in this task123,124, but there is little evidence for hippocampal involvement, which may not be surprising given that discrete cues, rather than contexts, inform behavioural responses in this task. Nonetheless, a substantial body of literature implicates mPFC and hippocampus abnormalities in schizophrenia125, suggesting that dysfunction in contextual processing may account for some of the symptoms associated with this disorder. In addition, deficits in eliciting appropriate emotional responses or suppressing inappropriate ones (as the case may be in paranoid ideation) might reflect a failure to modulate thoughts and actions in response to environmental stimuli based on contextual information; examination of mPFC–hippocampal neurocircuitry using context-focused designs could yield important new information regarding the pathophysiology of schizophrenia.
Conclusions and future directions
The studies reviewed above indicate that convergent evidence from animal and human studies implicates the hippocampus and mPFC in the processing of spatial contextual information. The experimental manipulations used in animal studies allow firmer inferences regarding the causal relationships and the specific roles of the brain regions involved. However, the differences in the complexity of behaviour and of different kinds of context in humans versus animals, not to mention the difficulty in delineating homologies between rat and human cortical regions, clearly require further studies of the neurocircuitry underlying context processing in humans in vivo.
Nevertheless, converging findings from animal and human studies suggest a key, although not exclusive, role for the hippocampus in spatial context encoding, and of hippocampal and prefrontal cortical regions (mainly of the medial wall of the frontal cortex, including the medial prefrontal regions and the ACC, in humans and of the infralimbic and prelimbic cortices in rats) in spatial context retrieval. The pattern of functional connectivity between the hippocampus, mPFC and amygdala in rats and humans is consistent with a role of the hippocampus in encoding context memories and suggests that hippocampal–mPFC–amygdala circuits mediate contextual retrieval of fear memories after extinction.
A better understanding of the neural circuits involved in context processing in normal subjects is needed to identify the abnormalities in this circuitry that may accompany various psychiatric disorders. Indeed, fascinating early findings have advanced our understanding of PTSD, and novel avenues of future research should include studies of context processing in schizophrenia and substance abuse disorders. Without question, a comprehensive view of the brain circuits that mediate contextual processing and modulation will greatly enrich the future understanding of flexible, adaptive responses to environmental stimuli, and of pathophysiological processes that interfere with this flexibility.
Acknowledgements
Work from the authors’ laboratories described in this paper is supported by grants from the US National Institutes of Health to S.M. (R01MH065961), K.L.P. (R01MH086517 and R01MH071698) and I.L. (R24MH075999).
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- 1.Barrett L, Kensinger E. Context is routinely encoded during emotion perception. Psychol. Sci. 2010;21:599. doi: 10.1177/0956797610363547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spear N. Retrieval of memory in animals. Psychol. Rev. 1973;80:194. [Google Scholar]
- 3.Fanselow MS. From contextual fear to a dynamic view of memory systems. Trends Cogn. Sci. 2010;14:15. doi: 10.1016/j.tics.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouton M. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol. Bull. 1993;114:99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- 5.Bouton M. Context, ambiguity, and classical conditioning. Curr. Direct. Psychol. Sci. 1994;3:53. [Google Scholar]
- 6.Fanselow M. Factors governing one-trial contextual conditioning. Anim. Learn. Behav. 1990;18:270. [Google Scholar]
- 7.Debiec J, LeDoux JE, Nader K. Cellular and systems reconsolidation in the hippocampus. Neuron. 2002;36:538. doi: 10.1016/s0896-6273(02)01001-2. [DOI] [PubMed] [Google Scholar]
- 8.Holland P, Bouton M. Hippocampus and context in classical conditioning. Curr. Opin. Neurobiol. 1999;9:202. doi: 10.1016/s0959-4388(99)80027-0. [DOI] [PubMed] [Google Scholar]
- 9.Phillips R, LeDoux J. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 1992;106:285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 10.Selden N, Everitt B, Jarrard L, Robbins T. Complementary roles for the amygdala and hippocampus in aversive conditioning to explicit and contextual cues. Neuroscience. 1991;42:350. doi: 10.1016/0306-4522(91)90379-3. [DOI] [PubMed] [Google Scholar]
- 11.Kim J, Fanselow M. Modality-specific retrograde amnesia of fear. Science. 1992;256:677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 12.Frankland P, Cestari V, Filipkowski R, McDonald R, Silva A. The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. Behav. Neurosci. 1998;112:874. doi: 10.1037//0735-7044.112.4.863. [DOI] [PubMed] [Google Scholar]
- 13.Maren S, Aharonov G, Fanselow M. S. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav. Brain Res. 1997;88:274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- 14.Anagnostaras SG, Maren S, Fanselow MS. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subjects examination. J. Neurosci. 1999;19:1114. doi: 10.1523/JNEUROSCI.19-03-01106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bayley P, Gold J, Hopkins R, Squire L. The neuroanatomy of remote memory. Neuron. 2005;46:810. doi: 10.1016/j.neuron.2005.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiltgen B, Sanders M, Anagnostaras S, Sage J, Fanselow M. Context fear learning in the absence of the hippocampus. J. Neurosci. 2006;26:5491. doi: 10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maren S. Neurobiology of Pavlovian fear conditioning. Annu. Rev. Neurosci. 2001;24:931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- 18.Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behav. Brain Res. 2000;110:81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- 19.Rudy JW. Context representations, context functions, and the parahippocampal–hippocampal system. Learn. Mem. 2009;16:585. doi: 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young S, Bohenek D, Fanselow M. NMDA processes mediate anterograde amnesia of contextual fear conditioning induced by hippocampal damage: immunization against amnesia by context preexposure. Behav. Neurosci. 1994;108:29. doi: 10.1037//0735-7044.108.1.19. [DOI] [PubMed] [Google Scholar]
- 21.Matus-Amat P, Higgins E, Barrientos R, Rudy J. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. J. Neurosci. 2004;24:2439. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Good M, Honey R. Conditioning and contextual retrieval in hippocampal rats. Behav. Neurosci. 1991;105:509. doi: 10.1037//0735-7044.105.4.499. [DOI] [PubMed] [Google Scholar]
- 23.Butterly D, Petroccione M, Smith D. Hippocampal context processing is critical for interference free recall of odor memories in rats. Hippocampus. 2011;22:913. doi: 10.1002/hipo.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson A, Brooks D, Bouton M. The role of the rat hippocampal system in several effects of context in extinction. Behav. Neurosci. 1995;109:836. doi: 10.1037//0735-7044.109.5.828. [DOI] [PubMed] [Google Scholar]
- 25.Frohardt R, Guarraci F, Bouton M. The effects of neurotoxic hippocampal lesions on two effects of context after fear extinction. Behav. Neurosci. 2000;114:240. doi: 10.1037//0735-7044.114.2.227. [DOI] [PubMed] [Google Scholar]
- 26.Fox G, Holland P. Neurotoxic hippocampal lesions fail to impair reinstatement of an appetitively conditioned response. Behav. Neurosci. 1998;112:260. [PubMed] [Google Scholar]
- 27.Maren S, Quirk G. J. Neuronal signalling of fear memory. Nature Rev. Neurosci. 2004;5:852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 28.Fanselow M, Poulos A. The neuroscience of mammalian associative learning. Annu. Rev. Psychol. 2005;56:234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- 29.LeDoux JE. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 30.Davis M, Whalen P. The amygdala: vigilance and emotion. Mol. Psychiatry. 2001;6:34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 31.Smith D, Mizumori S. Hippocampal place cells, context, and episodic memory. Hippocampus. 2006;16:729. doi: 10.1002/hipo.20208. [DOI] [PubMed] [Google Scholar]
- 32.Anderson M, Jeffery K. Heterogeneous modulation of place cell firing by changes in context. J. Neurosci. 2003;23:8835. doi: 10.1523/JNEUROSCI.23-26-08827.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayman R, Chakraborty S, Anderson M, Jeffery K. Context-specific acquisition of location discrimination by hippocampal place cells. Eur. J. Neurosci. 2003;18:2834. doi: 10.1111/j.1460-9568.2003.03035.x. [DOI] [PubMed] [Google Scholar]
- 34.Davidson T, Jarrard L. A role for hippocampus in the utilization of hunger signals. Behav. Neural Biol. 1993;59:171. doi: 10.1016/0163-1047(93)90925-8. [DOI] [PubMed] [Google Scholar]
- 35.Davidson T, et al. Hippocampal lesions impair retention of discriminative responding based on energy state cues. Behav. Neurosci. 2010;124:105. doi: 10.1037/a0018402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kennedy PJ, Shapiro ML. Motivational states activate distinct hippocampal representations to guide goal-directed behaviors. Proc. Natl Acad. Sci. USA. 2009;106:10810. doi: 10.1073/pnas.0903259106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kennedy PJ, Shapiro ML. Retrieving memories via internal context requires the hippocampus. J. Neurosci. 2004;24:6985. doi: 10.1523/JNEUROSCI.1388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Komorowski R, Manns J, Eichenbaum H. Robust conjunctive item-place coding by hippocampal neurons parallels learning what happens where. J. Neurosci. 2009;29:9929. doi: 10.1523/JNEUROSCI.1378-09.2009. This paper reveals that in rats, hippocampal neurons exhibit context-dependent firing patterns that represent odour–reward associations in specific places. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maren S, Fanselow MS. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J. Neurosci. 1995;15:7564. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maren S, Fanselow MS. Electrolytic lesions of the fimbria/fornix, dorsal hippocampus, or entorhinal cortex produce anterograde deficits in contextual fear conditioning in rats. Neurobiol. Learn. Mem. 1997;67:149. doi: 10.1006/nlme.1996.3752. [DOI] [PubMed] [Google Scholar]
- 41.Burwell RD, Bucci DJ, Sanborn MR, Jutras MJ. Perirhinal and postrhinal contributions to remote memory for context. J. Neurosci. 2004;24:11028. doi: 10.1523/JNEUROSCI.3781-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corcoran K, et al. NMDA receptors in retrosplenial cortex are necessary for retrieval of recent and remote context fear memory. J. Neurosci. 2011;31:11659. doi: 10.1523/JNEUROSCI.2107-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frankland P, Bontempi B, Talton L, Kaczmarek L, Silva A. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- 44.Quinn J, Ma Q, Tinsley M, Koch C, Fanselow M. Inverse temporal contributions of the dorsal hippocampus and medial prefrontal cortex to the expression of long-term fear memories. Learn. Mem. 2008;15:372. doi: 10.1101/lm.813608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sutherland R, Lehmann H. Alternative conceptions of memory consolidation and the role of the hippocampus at the systems level in rodents. Curr. Opin. Neurobiol. 2011;21:451. doi: 10.1016/j.conb.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 46.Armony J, Dolan R. Modulation of auditory neural responses by a visual context in human fear conditioning. Neuroreport. 2001;12:3411. doi: 10.1097/00001756-200110290-00051. [DOI] [PubMed] [Google Scholar]
- 47.Lang S, et al. Context conditioning and extinction in humans: differential contribution of the hippocampus, amygdala and prefrontal cortex. Eur. J. Neurosci. 2009;29:832. doi: 10.1111/j.1460-9568.2009.06624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alvarez R, Biggs A, Chen G, Pine D, Grillon C. Contextual fear conditioning in humans: cortical– hippocampal and amygdala contributions. J. Neurosci. 2008;28:6219. doi: 10.1523/JNEUROSCI.1246-08.2008. An influential study substantiating the role of amygdala–hippocampal brain circuits in representing context during fear conditioning in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marschner A, Kalisch R, Vervliet B, Vansteenwegen D, Büchel C. Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. J. Neurosci. 2008;28:9036. doi: 10.1523/JNEUROSCI.1651-08.2008. This paper differentiates amygdala and hippocampal activity during cued and context fear conditioning in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hasler G, et al. Cerebral blood flow in immediate and sustained anxiety. J. Neurosci. 2007;27:6319. doi: 10.1523/JNEUROSCI.5369-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pohlack S, et al. Hippocampal but not amygdalar volume affects contextual fear conditioning in humans. Hum. Brain Mapp. 2012;33:488. doi: 10.1002/hbm.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ploghaus A, et al. Dissociating pain from its anticipation in the human brain. Science. 1999;284:1981. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- 53.Wager T, et al. Placebo-induced changes in fMRI in the anticipation and experience of pain. Science. 2004;303:1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 54.Kalisch R, et al. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J. Neurosci. 2006;26:9511. doi: 10.1523/JNEUROSCI.2021-06.2006. This study is among the first to report that the ventromedial prefrontal–hippocampal network is involved in context-dependent recall of human extinction memory. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nitschke J, Sarinopoulos I, Mackiewicz K, Schaefer H, Davidson R. Functional neuroanatomy of aversion and its anticipation. Neuroimage. 2006;29:116. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 56.Bannerman D, et al. Ventral hippocampal lesions affect anxiety but not spatial learning. Behav. Brain Res. 2003;139:213. doi: 10.1016/s0166-4328(02)00268-1. [DOI] [PubMed] [Google Scholar]
- 57.Fanselow MS, Dong H-W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bouton M, Nelson J, Schmajuk N, Holland P. In: Occasion Setting: Associative Learning And Cognition In Animals. Schmajuk N, Holland P, editors. American Psychological Association; 1998. pp. 69–112. [Google Scholar]
- 59.Harris J, Jones M, Bailey G, Westbrook R. Contextual control over conditioned responding in an extinction paradigm. J. Exp. Psychol. Anim. Behav. Process. 2000;26:185. doi: 10.1037//0097-7403.26.2.174. [DOI] [PubMed] [Google Scholar]
- 60.Bouton M. Context and ambiguity in the extinction of emotional learning: implications for exposure therapy. Behav. Res. Ther. 1988;26:149. doi: 10.1016/0005-7967(88)90113-1. [DOI] [PubMed] [Google Scholar]
- 61.Bouton M. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol. Psychiatry. 2002;52:986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- 62.Ehrlich I, et al. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 63.Quirk G, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Falls W, Miserendino M, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J. Neurosci. 1992;12:863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herry C, et al. Neuronal circuits of fear extinction. Eur. J. Neurosci. 2010;31:612. doi: 10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- 66.Herry C, et al. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:606. doi: 10.1038/nature07166. A key paper revealing different populations of amygdala neurons that respond either during the expression of extinction or during the renewal of fear; these populations received different patterns of hippocampal and prefrontal input. [DOI] [PubMed] [Google Scholar]
- 67.Repa JC, et al. Two different lateral amygdala cell populations contribute to the initiation and storage of memory. Nature Neurosci. 2001;4:731. doi: 10.1038/89512. [DOI] [PubMed] [Google Scholar]
- 68.Maren S, Poremba A, Gabriel M. Basolateral amygdaloid multi-unit neuronal correlates of discriminative avoidance learning in rabbits. Brain Res. 1991;549:316. doi: 10.1016/0006-8993(91)90473-9. [DOI] [PubMed] [Google Scholar]
- 69.Maren S. Auditory fear conditioning increases CS-elicited spike firing in lateral amygdala neurons even after extensive overtraining. Eur. J. Neurosci. 2000;12:4054. doi: 10.1046/j.1460-9568.2000.00281.x. [DOI] [PubMed] [Google Scholar]
- 70.Quirk G, Repa C, LeDoux J. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- 71.Hobin JA, Goosens KA, Maren S. Context-dependent neuronal activity in the lateral amygdala represents fear memories after extinction. J. Neurosci. 2003;23:8416. doi: 10.1523/JNEUROSCI.23-23-08410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tye K, Cone J, Schairer W, Janak P. Amygdala neural encoding of the absence of reward during extinction. J. Neurosci. 2010;30:125. doi: 10.1523/JNEUROSCI.4240-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Corcoran KA, Maren S. Hippocampal inactivation disrupts contextual retrieval of fear memory after extinction. J. Neurosci. 2001;21:1726. doi: 10.1523/JNEUROSCI.21-05-01720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hobin JA, Ji J, Maren S. Ventral hippocampal muscimol disrupts context-specific fear memory retrieval after extinction in rats. Hippocampus. 2006;16:182. doi: 10.1002/hipo.20144. [DOI] [PubMed] [Google Scholar]
- 75.Maren S, Hobin JA. Hippocampal regulation of context-dependent neuronal activity in the lateral amygdala. Learn. Mem. 2007;14:324. doi: 10.1101/lm.477007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zelikowsky M, Pham DL, Fanselow MS. Temporal factors control hippocampal contributions to fear renewal after extinction. Hippocampus. 2011;22:1106. doi: 10.1002/hipo.20954. This paper reveals that the hippocampus has a critical role in the context-dependent retrieval of extinction memories but that animals that underwent extinction without a hippocampus exhibit contextual control under some conditions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Holt W, Maren S. Muscimol inactivation of the dorsal hippocampus impairs contextual retrieval of fear memory. J. Neurosci. 1999;19:9062. doi: 10.1523/JNEUROSCI.19-20-09054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang S, Teixeira CL, Wheeler A, Frankland P. The precision of remote context memories does not require the hippocampus. Nature Neurosci. 2009;12:255. doi: 10.1038/nn.2263. [DOI] [PubMed] [Google Scholar]
- 79.Crombag H, Bossert J, Koya E, Shaham Y. Context-induced relapse to drug seeking: a review. Phil. Trans. R. Soc. B. 2008;363:3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Holland P, Lamoureux J, Han J, Gallagher M. Hippocampal lesions interfere with Pavlovian negative occasion setting. Hippocampus. 1999;9:157. doi: 10.1002/(SICI)1098-1063(1999)9:2<143::AID-HIPO6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 81.Maren S, Holt W. The hippocampus and contextual memory retrieval in Pavlovian conditioning. Behav. Brain Res. 2000;110:108. doi: 10.1016/s0166-4328(99)00188-6. [DOI] [PubMed] [Google Scholar]
- 82.Yoon T, Graham L, Kim J. Hippocampal lesion effects on occasion setting by contextual and discrete stimuli. Neurobiol. Learn. Mem. 2011;95:184. doi: 10.1016/j.nlm.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vlachos I, Herry C, Lüthi A, Aertsen A, Kumar A. Context-dependent encoding of fear and extinction memories in a large-scale network model of the basal amygdala. PLoS Comp. Biol. 2011;7:e1001104. doi: 10.1371/journal.pcbi.1001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Krasne FB, Fanselow MS, Zelikowsky M. Design of a neurally plausible model of fear learning. Front. Behav. Neurosci. 2011;5:41. doi: 10.3389/fnbeh.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Knapska E, et al. Functional anatomy of neural circuits regulating fear and extinction. Proc. Natl Acad. Sci. USA. 2012;109:17098. doi: 10.1073/pnas.1202087109. This study uses a novel transgenic rat to localize convergent hippocampal and prefrontal projections onto amygdala neurons that are differentially active during the expression of extinction or during the renewal of fear. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gordon JA. Oscillations and hippocampal-prefrontal synchrony. Curr. Opin. Neurobiol. 2011;21:491. doi: 10.1016/j.conb.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Adhikari A, Topiwala MA, Gordon JA. Single units in the medial prefrontal cortex with anxiety-related firing patterns are preferentially influenced by ventral hippocampal activity. Neuron. 2011;71:910. doi: 10.1016/j.neuron.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal–prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:767. doi: 10.1038/nature08855. An important paper linking aberrant neuronal synchronization of hippocampal–prefrontal circuits to an animal model of schizophrenia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E, Quirk GJ. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron. 2012;76:812. doi: 10.1016/j.neuron.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Quirk GJ, Likhtik E, Pelletier JG, Paré D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J. Neurosci. 2003;23:8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Paré D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Corcoran K, Quirk G. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J. Neurosci. 2007;27:844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maren S. Seeking a spotless mind: extinction, deconsolidation, and erasure of fear memory. Neuron. 2011;70:845. doi: 10.1016/j.neuron.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Knapska E, Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn. Mem. 2009;16:493. doi: 10.1101/lm.1463909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Orsini CA, Kim JH, Knapska E, Maren S. Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. J. Neurosci. 2011;31:17277. doi: 10.1523/JNEUROSCI.4095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Milad M, et al. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol. Psychiatry. 2007;62:454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 97.Gottfried J, Dolan R. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nature Neurosci. 2004;7:1153. doi: 10.1038/nn1314. [DOI] [PubMed] [Google Scholar]
- 98.LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:945. doi: 10.1016/s0896-6273(00)80475-4. This is the first study to implicate the vmPFC in fear extinction learning in humans. [DOI] [PubMed] [Google Scholar]
- 99.Phelps E, Delgado M, Nearing K, LeDoux J. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 100.Knight D, Cheng D, Smith C, Stein E, Helmstetter F. Neural substrates mediating human delay and trace fear conditioning. J. Neurosci. 2004;24:228. doi: 10.1523/JNEUROSCI.0433-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Veit R, et al. Brain circuits involved in emotional learning in antisocial behavior and social phobia in humans. Neurosci. Lett. 2002;328:236. doi: 10.1016/s0304-3940(02)00519-0. [DOI] [PubMed] [Google Scholar]
- 102.Molchan S, Sunderland T, McIntosh A, Herscovitch P, Schreurs BA. Functional anatomical study of associative learning in humans. Proc. Natl Acad. Sci. USA. 1994;91:8126. doi: 10.1073/pnas.91.17.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sehlmeyer C, et al. Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS ONE. 2009;4:e5865. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sotres-Bayon F, Quirk G. Prefrontal control of fear: more than just extinction. Curr. Opin. Neurobiol. 2010;20:235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Labar KS, Phelps EA. Reinstatement of conditioned fear in humans is context dependent and impaired in amnesia. Behav. Neurosci. 2005;119:686. doi: 10.1037/0735-7044.119.3.677. [DOI] [PubMed] [Google Scholar]
- 106.Bechara A, et al. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- 107.LaBar K, LeDoux J, Spencer D, Phelps E. Impaired fear conditioning following unilateral temporal lobectomy in humans. J. Neurosci. 1995;15:6855. doi: 10.1523/JNEUROSCI.15-10-06846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Milad M, et al. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc. Natl Acad. Sci. USA. 2005;102:10711. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ressler K, Mayberg H. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nature Neurosci. 2007;10:1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rauch S, Shin L, Phelps E. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research - past, present, and future. Biol. Psychiatry. 2006;60:382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 111.Milad M, Quirk G. Fear extinction as a model for translational neuroscience: ten years of progress. Annu. Rev. Psychol. 2012;63:151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liberzon I, Sripada C. The functional neuroanatomy of PTSD: a critical review. Prog. Brain Res. 2007;167:169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- 113.Milad M, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol. Psychiatry. 2009;66:1082. doi: 10.1016/j.biopsych.2009.06.026. This is the first functional neuroimaging study to implicate the vmPFC and hippocampus in contextual retrieval deficits in patients with PTSD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rougemont-Bücking A, et al. Altered processing of contextual information during fear extinction in PTSD: an fMRI study. CNS Neurosci. Ther. 2010;17:236. doi: 10.1111/j.1755-5949.2010.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.MacKillop J, Lisman S. Effects of a context shift and multiple context extinction on reactivity to alcohol cues. Exp. Clin. Psychopharmacol. 2008;16:331. doi: 10.1037/a0012686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Badiani A, Belin D, Epstein D, Calu D, Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nature Rev. Neurosci. 2011;12:700. doi: 10.1038/nrn3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Badiani A, Anagnostaras S, Robinson T. The development of sensitization to the psychomotor stimulant effects of amphetamine is enhanced in a novel environment. Psychopharmacol. 1995;117:452. doi: 10.1007/BF02246217. [DOI] [PubMed] [Google Scholar]
- 118.Anagnostaras S, Robinson T. Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behav. Neurosci. 1996;110:1414. doi: 10.1037//0735-7044.110.6.1397. [DOI] [PubMed] [Google Scholar]
- 119.Siegel S. Morphine analgesic tolerance - situation specificity supports a pavlovian conditioning model. Science. 1976;193:325. doi: 10.1126/science.935870. [DOI] [PubMed] [Google Scholar]
- 120.Childs E, de Wit H. Contextual conditioning enhances the psychostimulant and incentive properties of d-amphetamine in humans. Addict. Biol. 2011 Nov 29; doi: 10.1111/j.1369-1600.2011.00416.x. doi:10.1111/j.1369-1600.2011.00416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wilson S, Sayette M, Fiez J. Prefrontal responses to drug cues: a neurocognitive analysis. Nature Neurosci. 2004;7:214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Carter B, Tiffany S. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:340. [PubMed] [Google Scholar]
- 123.Barch D, Carter C, MacDonald A, Braver T, Cohen J. Context-processing deficits in schizophrenia: diagnostic specificity, 4-week course, and relationships to clinical symptoms. J. Abnorm. Psychol. 2003;112:143. [PubMed] [Google Scholar]
- 124.Cohen J, Barch D, Carter C, Servan-Schreiber D. Context-processing deficits in schizophrenia: converging evidence from three theoretically motivated cognitive tasks. J. Abnorm. Psychol. 1999;108:133. doi: 10.1037//0021-843x.108.1.120. [DOI] [PubMed] [Google Scholar]
- 125.Taylor S, et al. Meta-analysis of functional neuroimaging studies of emotion perception and experience in schizophrenia. Biol. Psychiatry. 2012;71:145. doi: 10.1016/j.biopsych.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]