Abstract
Aims
Aim of this study was to compare a minimally fluoroscopic radiofrequency catheter ablation with conventional fluoroscopy-guided ablation for supraventricular tachycardias (SVTs) in terms of ionizing radiation exposure for patient and operator and to estimate patients' lifetime attributable risks associated with such exposure.
Methods and results
We performed a prospective, multicentre, randomized controlled trial in six electrophysiology (EP) laboratories in Italy. A total of 262 patients undergoing EP studies for SVT were randomized to perform a minimally fluoroscopic approach (MFA) procedure with the EnSiteTMNavXTM navigation system or a conventional approach (ConvA) procedure. The MFA was associated with a significant reduction in patients' radiation dose (0 mSv, iqr 0–0.08 vs. 8.87 mSv, iqr 3.67–22.01; P < 0.00001), total fluoroscopy time (0 s, iqr 0–12 vs. 859 s, iqr 545–1346; P < 0.00001), and operator radiation dose (1.55 vs. 25.33 µS per procedure; P < 0.001). In the MFA group, X-ray was not used at all in 72% (96/134) of cases. The acute success and complication rates were not different between the two groups (P = ns). The reduction in patients' exposure shows a 96% reduction in the estimated risks of cancer incidence and mortality and an important reduction in estimated years of life lost and years of life affected. Based on economic considerations, the benefits of MFA for patients and professionals are likely to justify its additional costs.
Conclusion
This is the first multicentre randomized trial showing that a MFA in the ablation of SVTs dramatically reduces patients' exposure, risks of cancer incidence and mortality, and years of life affected and lost, keeping safety and efficacy.
Trial registration
clinicaltrials.gov Identifier: NCT01132274.
Keywords: Supraventricular tachycardia, Radiofrequency ablation, Electroanatomical mapping, Radiation exposure
What's new?
The radiation exposure during electrophysiology procedures is non-negligible for both patients and laboratory staff.
The use of a minimally fluoroscopic approach (MFA) with the EnSiteTMNavXTM navigation system is associated with a significant reduction in total fluoroscopy time, patients' exposure, and operator radiation dose without any significant difference in terms of success and complication rates. The reduction in patients' exposure shows a 96% reduction in the estimated risks of cancer incidence and mortality and an important reduction in estimated years of life lost and years of life affected.
The increase in life expectancy and in the period of cancer-free life makes the MFA economically affordable at a rough economical analysis. Thus, faced with such an advantage in terms of cancer prevention, it would be desirable that MFA procedures were increasingly performed, at least in young patients.
Introduction
Electrophysiology (EP) procedures are traditionally performed under fluoroscopic guidance and often involve a non-negligible radiation exposure1–4 for both patients and laboratory staff.
Fluoroscopy is certainly a highly effective way to navigate catheters and to monitor their location; unfortunately, fluoroscopy requires the administration of ionizing radiation, and recent epidemiological evidence shows that even low doses can be harmful and that no completely safe dose exists.4
In the past two decades, non-fluoroscopic three-dimensional (3D) mapping systems have been used in complex arrhythmias ablation to guide the ablation strategy. More recently, non-fluoroscopic 3D mapping systems have been broadly investigated for the complete or near-complete abolishment of radiation exposure during ablation procedures, both in paediatric patients5–12 and in adults,13–21 showing that catheter ablation through a minimally fluoroscopic approach (MFA) is feasible and safe. However, most of the data come from monocentric, non-randomized, feasibility studies. Therefore, it remains unclear whether such an approach results in a clinically significant reduction in exposure to ionizing radiation for both patient and operator.
The multicentre, randomized controlled NO-PARTY trial (www.clinicaltrials.gov identifier NCT01132274) has been designed to compare a minimally fluoroscopic radiofrequency catheter ablation (RFCA) guided by the EnSiteTMNavXTM navigation system with conventional fluoroscopy-guided RFCA for supraventricular tachycardias (SVTs) in terms of ionizing radiation exposure for both patient and operator and to estimate patients' lifetime attributable risks (LAR) associated with such exposure.
Methods
An investigator-initiated, multicentre, randomized controlled trial was performed in six Italian EP laboratories. Randomization of participants occurred between January 2010 and February 2013; follow-up was completed in March 2014. The complete study protocol has been previously described22 (see Supplementary material online). Written informed consent was obtained from all participants. The trial was approved by each institutional review board.
Patients aged 14–50 years undergoing SVTs RFCA were randomized on a 1 to 1 ratio to perform an EP procedure with a MFA or conventional approach (ConvA). In case of a MFA procedure, the EnSiteTMNavXTM system was used as the primary catheter visualization tool (Figure 1) and procedures were performed only by MFA-skilled operators.23–26 Anyway, the use of fluoroscopy was allowed in case the operator would have considered it necessary.
Figure 1.
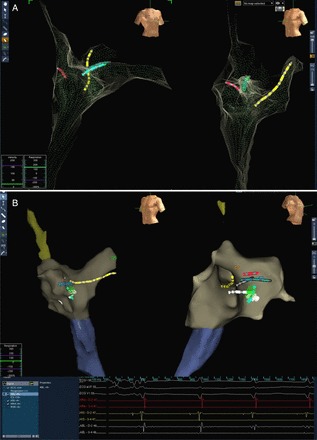
(A) Non-fluoroscopic three-view reconstruction of the right atrium in right anterior oblique (on the left) and in left anterior oblique (on the right) views. The cloud of green points shows the sites reached by the mapping catheters and used for geometry reconstruction. (B) The same geometry showing catheter position inside the atrium: yellow catheter in coronary sinus, blue catheter in Hisian region, and white ablation catheter mapping Koch triangle. The mapping system allows to mark the areas of interest: in this case, we marked where ablation pulses were safely and effectively delivered as shown in the intracavitary electrograms recording box (bottom).
For both groups, fluoroscopic units were optimized at lower-exposure settings; in Supplementary material online, Table S1, characteristics of radiographic/fluoroscopic units are described.
For all patients, total procedural time, time necessary to position catheters, EP study time, and cumulative radiofrequency delivery time were collected.
Ionizing radiation use was calculated as total fluoroscopy time; patient's radiation exposure was measured in terms of dose-area product (DAP), while operator exposure was analysed with a specific kit of radiation dosimeters.
A follow-up outpatient visit was scheduled for each patient at 1, 3, 6, and 12 months to take an updated history, perform physical examination, and obtain a 12-lead electrocardiogram.
Endpoints
The primary endpoint of the study was the reduction in the total radiation dose to the patient. Secondary endpoints were procedural success, reduction in the procedural fluoroscopy time, and reduction in the operator radiation dose. Moreover, an economic assessment has been performed to evaluate the additional costs associated with a MFA approach.
Radiation dose analysis
For each fluoroscopy, the interactions between the X-ray beam and the patient were simulated via Monte Carlo calculation based on the geometry field of the procedure using the validated software PCXMC version 2.0.27 The X-ray spectra were reconstructed from the X-ray tube settings (tube potential, anode angle, filtration) according to Birch and Marshall theory,28 and a modified Cristy and Eckerman mathematical hermaphrodite phantom was used to represent a digital phantom shaped on patient's height, weight, and age. Finally, measurements of DAP were converted into organ doses, and effective doses (EDs) were estimated for each projection using the ICRP 103 weighing factor. Effective dose was also calculated with the accepted formula: mSv = DAP (Gy cm2) × 0.20 to better compare our data with others published.29
The risk of late effects induced by ionizing radiation exposure was assessed in terms of LAR from equivalent organ doses calculated with the Monte Carlo code, according to the Biological Effects of Ionizing Radiation (BEIR) empirical risk models. Years of life lost (YLL) and years of life affected (YLA) were determined with the same risk model.30
Finally, starting from the YLL and YLA, a rough economic analysis has been performed using the Health Technology Assessment.31
The ED to the first operator was assessed from data collected by lithium–fluoride thermoluminescent dosimeters (TLD) positioned on chest. The cumulative dose was measured over consecutive procedures to achieve better accuracy and avoid underestimation due to the small exposure per procedure. Different dosimeter sets were used during ConvA and MFA procedures. Dosimeters were replaced every 2 months.
Statistical analysis
Descriptive statistic has been reported as mean ± standard deviation or median and interquartile Q1–Q3 range (iqr) for skewed distributions in the case of continuous variables and as absolute frequencies and percentages in the case of categorical variables. Between-groups comparison has been performed with the unpaired Student's t-test, the Mann–Whitney U test, or Fisher's exact test. Statistical differences in ED and fluoroscopy time between groups were tested with the independent Mann–Whitney U test (95% confidence level). All tests are two sided, and a P-value of <0.05 has been considered statistically significant. All statistical analyses were performed with the SPSS 21.0 software.
Results
Patients' enrolment in the study is depicted in Figure 2.
Figure 2.
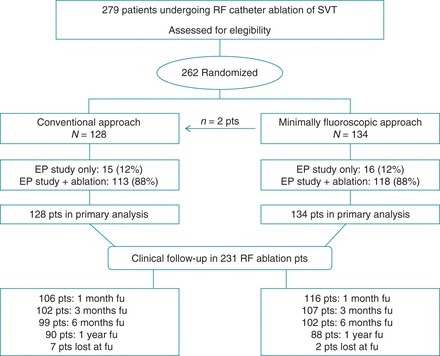
Study flowchart showing enrolment, randomization and follow-up of participants. In two cases, the MFA procedure was shifted to a ConvA one because of first operator change in one case and mapping system failure in the other one. Both these procedures were included in the MFA group as an intention-to-treat analysis.
Of the 262 participants (110 males, mean age 35.8 ± 10.4 years), 134 (51%) were assigned to MFA group and 128 (49%) to ConvA group. The clinical characteristics of the two groups were comparable (Table 1).
Table 1.
Demographic characteristics
| MFA n = 134 |
ConvA n = 128 |
P | |
|---|---|---|---|
| Female, n (%) | 79 (59) | 73 (57) | ns |
| Age (years) | 36.3 ± 10.4 | 35.4 ± 10.4 | ns |
| BMI | 24.4 ± 4.4 | 23.5 ± 4.4 | ns |
| Previous ablation, n (%) | 10 (8) | 13 (10) | ns |
| EPS, n (%) | 16 (12) | 15 (12) | ns |
| AVNRT, n (%) | 84 (63) | 79 (62) | ns |
| Right AP, n (%) | 10 (8) | 11 (9) | ns |
| Left AP, n (%) | 11 (8) | 14 (11) | ns |
| AFl, n (%) | 10 (8) | 6 (5) | ns |
| AT, n (%) | 3 (2) | 3 (2) | ns |
BMI, body mass index; EPS, electrophysiological study; AVNRT, atrioventricular node re-entry tachycardia; AP, accessory pathway; AFl, atrial flutter; AT, atrial tachycardia.
Procedural features
Of the 262 enrolled patients, 231 (88%) underwent an ablation following the EP study. Supplementary material online, Results section and Table S2, summarizes procedural data.
A 99% acute success rate was achieved in both groups (P = ns) with a complications rate of 1.1% (P = ns). The procedural complications were as follows: one arteriovenous fistula solved by compression in MFA group and two first-degree atrioventricular node blocks spontaneously solved in 48 h in ConvA group. The mean follow-up time was 12 ± 4 months, during which no procedure-related complication occurred. Of the 231 patients who underwent an ablation, the long-term success rate was 97% in MFA group and 94% in ConvA group (P = ns).
Fluoroscopy use and effective dose
Fluoroscopy time and patient ED were significantly lower in the MFA group (0 s, iqr 0–12; 0 mSv, iqr 0–0.08) when compared with ConvA group (859 s, iqr 545–1346; 8.87 mSv, iqr 3.67–22.01; P < 0.00001). Similar results were obtained when the analyses were restricted to the 231 procedures that ended with an ablation.
In the MFA group, X-ray was not used at all throughout the procedure in 72% (96/134) of cases.
Table 2 and Supplementary material online, Results section, describe fluoroscopy data.
Table 2.
Ionizing radiation data
| MFA | ConvA | P | |
|---|---|---|---|
| All patients (n = 262) | |||
| Fluoroscopy time (s) | 0 [0–12] | 859 [545–1346] | <0.00001 |
| DAP (cGy cm2) | 278 [80–791] | 2036 [854–5297] | <0.00001 |
| ED (mSv) | 0 [0–0.08] | 8.87 [3.67–22.01] | <0.00001 |
| Extrapolated ED (mSv) | 0 [0–0] | 3.96 [1.68–10.54] | <0.00001 |
| Fluoro on pelvic area, n (%) | 3/134 (2) | 62/128 (48) | <0.0001 |
Extrapolated ED: ED extrapolated by the formula: mSv = DAP (Gy cm2) × 0.20.
ED, effective dose; DAP, dose-area product.
Lifetime attributable cancer risk
LAR estimates are reported in Table 3 and Supplementary material online, Results section and Tables S3–S8.
Table 3.
Lifetime attributable risks
| LAR | Age | MFA |
ConvA |
||
|---|---|---|---|---|---|
| Man | Woman | Man | Woman | ||
| Mortality | 15 | 4.8 (2.5–8.2) | 6.1 (3.9–9.2) | 136 (82–215) | 186 (131–265) |
| 25 | 4.0 (1.8–7.0) | 4.7 (2.8–7.4) | 105 (59–171) | 138 (94–200) | |
| 35 | 3.7 (1.6–6.7) | 4.2 (2.4–6.7) | 94 (51–156) | 119 (79–175) | |
| 45 | 3.7 (1.5–6.9) | 4.1 (2.3–6.7) | 94 (49–158) | 115 (76–171) | |
| Incidence | 15 | 11.0 (6.0–18.6) | 15.4 (9.9–25.3) | 321 (198–512) | 486 (333–773) |
| 25 | 8.4 (4.3–14.4) | 10.9 (6.9–17.4) | 236 (140–377) | 335 (230–509) | |
| 35 | 7.4 (3.6–12.9) | 8.9 (5.5–14.0) | 201 (117–324) | 267 (183–393) | |
| 45 | 7.3 (3.4–12.8) | 8.2 (5.0–12.8) | 195 (111–315) | 241 (165–350) | |
Lifetime attributable risks of all cancers mortality and incidence, calculated according to BEIR risk models, with 95% confidence intervals from MFA (N = 134) and ConvA procedures (N = 128) in function of age at exposure and sex (number of cases in 100.000).
The risks of cancer incidence and mortality from a MFA procedure were reduced by 96% compared with ConvA procedure. As expected, all risks decreased with aging and were always higher for female patients at the same age of intervention.
Furthermore, a dramatic reduction has been calculated in YLL and YLA in MFA group in comparison with ConvA group (Table 4). Undergoing a ConvA EP procedure at 35 years of age results in almost 1 week of ‘life-lost’ and in about 2 weeks of ‘life-affected’ per patient, in contrast with 5 h and half a day, respectively, with the MFA approach.
Table 4.
Years of life lost and years of life affected
| Age | MFA |
ConvA |
|||
|---|---|---|---|---|---|
| Man | Woman | Man | Woman | ||
| YLL | 15 | 0.00088 (0.00038–0.0016) | 0.00112 (0.00065–0.00180) | 0.023 (0.012–0.038) | 0.032 (0.022–0.048) |
| 25 | 0.00061 (0.00026–0.00112) | 0.00079 (0.00046–0.00127) | 0.016 (0.008–0.026) | 0.023 (0.015–0.033) | |
| 35 | 0.00052 (0.00022–0.00095) | 0.00065 (0.00037–0.00105) | 0.013 (0.007–0.022) | 0.018 (0.012–0.027) | |
| 45 | 0.00049 (0.00019–0.00090) | 0.00059 (0.00033–0.00097) | 0.012 (0.006–0.020) | 0.017 (0.011–0.025) | |
| YLA | 15 | 0.0023 (0.0011–0.0042) | 0.0037 (0.0022–0.0065) | 0.063 (0.035–0.106) | 0.113 (0.073–0.192) |
| 25 | 0.0015 (0.0007–0.0027) | 0.0023 (0.0014–0.0039) | 0.042 (0.024–0.068) | 0.071 (0.048–0.112) | |
| 35 | 0.0012 (0.0006–0.0021) | 0.0017 (0.0010–0.0027) | 0.032 (0.018–0.053) | 0.051 (0.035–0.076) | |
| 45 | 0.0011 (0.0005–0.0019) | 0.0014 (0.0008–0.0022) | 0.029 (0.016–0.047) | 0.040 (0.027–0.059) | |
Years of life lost and YLA with 95% confidence intervals from MFA (N = 134) and ConvA procedures (N = 128) in function of age at exposure and sex. Years of life lost are estimated subtracting the effective years of life lived from the life expectancy for every patient who dies from cancer according to the LAR calculation and dividing it by the number of subjects who underwent the procedure. It results in an estimation of YLL per patient. For example, performing a ConvA procedure on a woman aged 15 years means that this woman will live 11.68 days (0.032×365 days/year) less than expected. Translating it into a population of 1000 women aged 15 years, it means that the procedures would account for a total of 32 YLL (0.032×1000 subjects).
Economic considerations
The significant reduction in YLL and YLA observed with the MFA could also be seen as an increase in life expectancy and in the period of life without cancer. Assuming that the period of life with cancer halves optimal quality of life, it can be derived that each patient gains 2 adjusted quality of life weeks, e.g. 0.0348 quality-adjusted life years. Using a range for the cost-effectiveness threshold between 30 000 and 50 000 as suggested in some jurisdictions,32 the intervention would be affordable for an additional net cost ranging between €1151 and €1918. This cost is approximately equivalent to the extra cost deriving from the electroanatomic mapping system.
Ionizing radiation risk for electrophysiology physicians
For EP physicians, the estimated cumulative EDs were calculated taking into account the shielding correction for using the protective apron and the thyroid collar and were obtained from the data collected by a total number of 213 procedures (113 MFA and 100 ConvA groups). As for patients, the ED was significantly lower in MFA group (1.55 µSv per procedure) in comparison with ConvA group (25.33 µSv per procedure; P < 0.001).
Discussion
Main findings of the study
This is the first prospective multicentre randomized study comparing conventional fluoroscopy-guided procedures with procedures performed using the electroanatomical mapping system as the primary catheter visualization tool in patients undergoing EP study for SVTs. The results confirm safety and efficacy of a MFA in the ablation of a wide range of SVTs. Notably, this randomized study shows that MFA allows a significant reduction in patient's exposure and a decrease in the estimated risks of cancer incidence and mortality and of YLA and YLL. Of course, the choice of MFA also affects the ionizing radiation exposure of the medical staff.
Obviously, MFA involves a greater expense, but the benefits deriving from it are offset in terms of cancer prevention strategy, and, based on our data, the increase in life expectancy makes it economically affordable in most European countries.
Radiological exposure and risks
Medical radiological exposure and risks are topical and emerging issues of last year literature.33–36 Cardiologists are responsible for ∼40% of the entire ED from all medical sources, as a consequence of new capabilities and widespread availability of new imaging techniques that require X-rays.37 Radiation exposure involves patients and operators, resulting in a non-negligible health risk for both.
A cornerstone to enhance radiation safety is optimization, i.e. reducing as much as possible the use of X-rays for a given technique.26 Techniques that allow high-quality imaging with lower radiation exposure should therefore be used when available.
In our study, we showed that the use of one of these techniques (EnSiteTMNavXTM system) is effective in reducing radiation exposure during EP procedures, with an identical success rate and without significantly increasing procedural time.
Considering that X-rays are proven carcinogens (Class 1), the significant increase in the cumulative exposure of patients and population is likely to cause an increased incidence of cancer, with an important yet potentially avoidable public health threat.27 This is the only published study that estimates the lifetime attributable cancer risk of radiation with the Monte Carlo code. The use of MFA reduced LAR by 96%, particularly in the youngest patients and in women. Also YLL and YLA were significantly reduced in the MFA group. The difference between median EDs calculated with the Monte Carlo code and with the international formula31 is probably due to the characteristics of our study population, which is mainly constituted by young and female patients. The advantage of the use of the electroanatomic system was in any case statistically significant.
Reduction in radiation exposure during medical procedures is crucial also for the medical staff. The occupational radiation exposure of cardiac electrophysiologists is two to three times higher than that of diagnostic radiologists.27 Cardiac use of fluoroscopy almost never reaches the threshold for deterministic radiation injury, but it gives an additional lifetime risk of fatal and non-fatal cancers ranging from 1/10 000 to 1/1000 that we should not underestimate. In our study, we confirmed that performing MFA ablation of SVTs significantly reduces professional exposure.
Economic considerations
The Health Technology Assessment evaluates medical technologies under clinical, ethical, organizational, and economic points of view to assess if they are worth being funded.38,39
The MFA clearly produces clinical benefits for both patients and medical staff as it decreases the risk of cancer due to radiation exposure. It may be argued that avoiding patients' risks for unrelated diseases and protecting medical staff in its professional environment deserve a higher priority. From a strictly economic perspective, the crucial issue is whether MFA in ablation is affordable given the constraints in available resources. This study does not provide enough data to conduct a cost-effectiveness analysis, but it gives robust evidence in terms of increase in life expectancy and in period of life free from cancer. This means that MFA procedures might be considered economically affordable in most European countries.
Procedural features
Considering technical and procedural aspects, the 3D electroanatomic system shows advantages not only as an efficient navigation system but also as a mapping system, thus reducing X-ray exposure.
For example, a precise definition of the anatomy of the triangle of Koch and of the His bundle (which is visualized as an area rather than just a point) facilitates atrioventricular node re-entry tachycardia (AVNRT) ablation and avoids the need for continuously monitoring with fluoroscopy the exact position of the node. Furthermore, the 3D electroanatomic system overcomes the difficulties deriving from the disappearance of pre-excitation after the initial radiofrequency pulses, and it makes it easier to accurately re-localize the accessory pathway (AP), thus avoiding time- and radiation-consuming electrophysiological manoeuvres. Moreover, thanks to the possibility of creating an activation map, it permits to eventually localize the gap in case of an incomplete line during atrial flutter ablation. Finally, it allows greater catheter stability in every kind of procedures, as demonstrated by fewer but longer radiofrequency pulses observed in our MFA group.
As a consequence of all these advantages, we believe that specific training in the ‘minimal fluoroscopic approach’ should be part of every EP education programme.
Study limitations
The main limitation of our study is that the relationship between the ED and its lifetime attributable risk was developed according to internationally accepted empirical risk models,29 and it could be criticized that these models are not recommended for estimating risks in individual patients. Nevertheless, it must be emphasized that no epidemiological study exists about low levels of ionizing radiation and we regret that no biomarkers of acute ionizing radiation injury were investigated.
We acknowledge that we did not perform an analysis about the dose for allied professionals involved in the procedures and that we did not estimate the lifetime risks for operators and allied professionals. These matters should be properly evaluated by a specifically designed trial.
Furthermore, both ConvA and MFA procedures were performed differently in various EP labs (i.e. numbers of diagnostic catheters, strategy in ablating left APs, etc.), but in the study protocol, we intentionally decided to allow the operators working as routinary as possible to have a better snapshot of MFA in everyday live EP labs.
Finally, a more exhaustive cost-effectiveness analysis would be desirable. The economic observations reported suggest a first point of reference about the additional costs for which the undiscounted gain benefits for patients might be considered affordable in European countries, although crucial elements that may largely affect the overall value for money are missing.
Conclusion
The NO-PARTY study is the first multicentre randomized controlled trial confirming that MFA in SVTs ablation results in a clinically significant reduction in exposure to ionizing radiation for both patient and operator. Notably, the reduction in patients' exposure achieves a dramatic reduction in the estimated risks of cancer incidence and mortality and in YLA and YLL. The increase in life expectancy and in the period of cancer-free life makes the MFA economically affordable at a rough economical analysis. Thus, faced with such an advantage in terms of cancer prevention, it would be desirable that MFA procedures were increasingly performed, at least in young patients.
Supplementary material
Funding
The NO-PARTY was an independent study. St. Jude Medical, Italy, provided a research grant conditioned to the e-CRF creation and the dosimeters management, provided technical support, and supervised the implementation of the study, but it had no access to the database and did not participate in the analysis of the results or in writing of the manuscript. Funding to pay the Open Access publication charges for this article was provided by St. Jude Medical.
Supplementary Material
Acknowledgements
The authors thank Engineers: Roberta Annibali, Federico Borselli, Michele Ciani, Pasquale De Juliis, Silvia Gilardone, Flavia Giovannetti, Stefano Indiani, Claudia Licciardello, Fabio Lissa, and Anna Tranquilli (St. Jude Medical, Italy) for their expert technical support. We also thank Dr Viviana Biagioli for editorial assistance. Finally, we wish to thank Irene Colangelo, Antonio Mininno, Rosanna Poggi, and Enrico Romano.
Conflict of interest: C.T. has served as member of the advisory board of Biosense-Webster and has been consultant for St. Jude Medical. A.N. has received compensation for belonging to the speakers’ bureau for St. Jude Medical, Boston Scientific, Medtronic, and Biosense-Webster and has received a research grant from St. Jude Medical. A.N. is consultant for Biosense-Webster. L.D.B. is consultant for Hansen Medical, Biosense-Webster, and St. Jude Medical. L.D.B. has received speaker honoraria from Biotronik, EPI-EP, and Atricure. G.F. has received compensation from St. Jude and Medtronic. Other authors declare no relationships with industry.
References
- 1. Picano E, Vaño E. The radiation issue in cardiology: the time for action is now. Cardiovasc Ultrasound 2011;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vaño E, Gonzales L, Guibelalde E, Fernandez JM, Ten JI. Radiation exposure to medical staff in interventional and cardiac radiology. Br J Radiol 1998;71:793–808. [DOI] [PubMed] [Google Scholar]
- 3. Linet MS, Kim KP, Miller DL, Kleinerman RA, Simon S, Berrington de Gonzalez A. Historical review of occupational exposures and cancer risks in medical radiation workers. Radiat Res 2010;174:793–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation; Nuclear and Radiation Studies Board, Division on Earth and Life Studies, National Research Council of the National Academies. Health Risks From Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. Washington, DC: The National Academies Press; 2006. [PubMed] [Google Scholar]
- 5. Papagiannis J, Tsoutsinos A, Kirvassilis G, Sofianidou I, Koussi T, Laskari C et al. Nonfluoroscopic catheter navigation for radiofrequency catheter ablation of supraventricular tachycardia in children. Pacing Clin Electrophysiol 2006;29:971–8. [DOI] [PubMed] [Google Scholar]
- 6. Smith G, Clark JM. Elimination of fluoroscopy use in a pediatric electrophysiology laboratory utilizing three-dimensional mapping. Pacing Clin Electrophysiol 2007;30:510–8. [DOI] [PubMed] [Google Scholar]
- 7. Miyake CY, Mah DY, Atallah J. Nonfluroscopic imaging systems reduce radiation exposure in children undergoing ablation of supraventricular tachycardia. Heart Rhythm 2011;8:519–25. [DOI] [PubMed] [Google Scholar]
- 8. Papagiannis J, Avramidis D, Alexopoulos C, Kirvassilis G. Radiofrequency ablation of accessory pathways in children and congenital heart disease patients: impact of a nonfluoroscopic navigation system. Pacing Clin Electrophysiol 2011;34:1288–96. [DOI] [PubMed] [Google Scholar]
- 9. Kwong W, Neilson AL, Chiu CC, Gross GJ, Hamilton RM, Soucie L et al. The effect of NavX on fluoroscopy times in pediatric catheter ablation. J Interv Card Electrophysiol 2012;33:123–6. [DOI] [PubMed] [Google Scholar]
- 10. Casella M, Dello Russo A, Fassini G, Andreini D, De Iuliis P, Mushtaq S et al. Manifold benefits of choosing a minimally fluoroscopic catheter ablation approach. World J Cardiol 2013;5:8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Scaglione M, Ebrille E, Caponi D, Blandino A, Di Donna P, Siboldi A et al. Single center experience of fluoroless AVNRT ablation guided by electroanatomic reconstruction in children and adolescents. Pacing Clin Electrophysiol 2013;36:1460–7. [DOI] [PubMed] [Google Scholar]
- 12. Mah DY, Miyake CY, Sherwin ED, Walsh A, Anderson MJ, Western K et al. The use of an integrated electroanatomic mapping system and intracardiac echocardiography to reduce radiation exposure in children and young adults undergoing ablation of supraventricular tachycardia. Europace 2014;16:277–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Earley MJ, Showkathali R, Alzetani M, Kistler PM, Gupta D, Abrams DJ et al. Radiofrequency abaltion of arrhythmias guided by non-fluoroscopic catheter location: a prospective randomized trial. Eur Heart J 2006;27:1223–9. [DOI] [PubMed] [Google Scholar]
- 14. Tuzcu V. A nonfluoroscopic approach for electrophysiology and catheter ablation procedures using a three-dimensional navigation system. Pacing Clin Electrophysiol 2007;30:519–25. [DOI] [PubMed] [Google Scholar]
- 15. Alvarez M, Tercedor L, Almansa I, Ros N, Galdeano RS, Burillo F et al. Safety and feasibility of catheter ablation for atrioventricular nodal re-entrant tachycardia without fluoroscopic guidance. Heart Rhythm 2009;6:1714–20. [DOI] [PubMed] [Google Scholar]
- 16. Alvarez M, Tercedor L, Herrera N, Muñoz L, Galdeano RS, Valverde F et al. Cavotricuspid isthmus catheter ablation without the use of fluoroscopy as a first-line treatment. J Cardiovasc Electrophysiol 2011;22:656–62. [DOI] [PubMed] [Google Scholar]
- 17. Casella M, Pelargonio G, Dello Russo A, Riva S, Bartoletti S, Santangeli P et al. “Near-zero” fluoroscopic exposure in supraventricular arrhythmia ablation using the EnSite NavXTM mapping system: personal experience and review of the literature. J Interv Card Electrophysiol 2011;31:109–18. [DOI] [PubMed] [Google Scholar]
- 18. Fernandez-Gomez JM, Moriña-Vazquez P, Del Rio Morales E, Venegas-Gamero J, Barba-Pichardo R, Herrera Carranza M. Exclusion of fluoroscopy use in catheter ablation procedures: six years of experience at a single center. J Cardiovasc Electrophysiol 2014;25:638–44. [DOI] [PubMed] [Google Scholar]
- 19. Schoene K, Rolf S, Schloma D, John S, Arya A, Dinov B et al. Ablation of typical atrial flutter using a non-fluoroscopic catheter tracking system vs. conventional fluoroscopy–results from a prospective randomized study. Europace 2015;17:1117–21. [DOI] [PubMed] [Google Scholar]
- 20. Christoph M, Wunderlich C, Moebius S, Forkmann M, Sitzy J, Salmas J et al. Fluoroscopy integrated 3D mapping significantly reduces radiation exposure during ablation for a wide spectrum of cardiac arrhythmias. Europace 2015;17:928–37. [DOI] [PubMed] [Google Scholar]
- 21. Stabile G, Scaglione M, del Greco M, De Ponti R, Bongiorni MG, Zoppo F et al. Reduced fluoroscopy exposure during ablation of atrial fibrillation using a novel electroanatomical navigation system: a multicentre experience. Europace 2012;14:60–5. [DOI] [PubMed] [Google Scholar]
- 22. Casella M, Dello Russo A, Pelargonio G, Bongiorni MG, Del Greco M, Piacenti M et al. Rationale and design of the NO-PARTY trial: near-zero fluoroscopic exposure during catheter ablation of supraventricular arrhythmias in young patients. Cardiol Young 2012;22:539–46. [DOI] [PubMed] [Google Scholar]
- 23. Ventura R, Rostock T, Klemm HU, Lutomsky B, Demir C, Weiss C et al. Catheter ablation of common-type atrial flutter guided by three-dimensional right atrial geometry reconstruction and catheter tracking use cutaneous patches: a randomized prospective study. J Cardiovasc Electrophysiol 2004;15:1157–61. [DOI] [PubMed] [Google Scholar]
- 24. Krum D, Goel A, Hauck J, Schweitzer J, Hare J, Attari M et al. Catheter location, tracking, cardiac chamber geometry creation, and ablation using cutaneous patches. J Interv Cardiac Electrophysiol 2005;12:17–22. [DOI] [PubMed] [Google Scholar]
- 25. Rotter M, Takahashi Y, Sanders P, Haissaguerre M, Li-Fern Hsu PJ, Sacher F et al. Reduction of fluoroscopy exposure and procedure duration during ablation of atrial fibrillation using a novel anatomical navigation system. Eur Heart J 2005;26:1415–21. [DOI] [PubMed] [Google Scholar]
- 26. Estner HL, Deisenhofer I, Luik A, Ndrepepa G, von Bary C, Zrenner B et al. Electrical isolation of pulmonary veins in patients with atrial fibrillation: reduction of fluoroscopy exposure and procedure duration by the use of a non-fluoroscopic navigation system (NavX®). Europace 2006;8:583–7. [DOI] [PubMed] [Google Scholar]
- 27. Birch R, Marshall M. Computation of bremsstrahlung x-ray spectra and comparison with spectra measured with a Ge(Li) detector. Phys Med Biol 1979;24:505–17. [DOI] [PubMed] [Google Scholar]
- 28. International Commission on Radiological Protection. ICRP Publication 103, the 2007 Recommendation of the International Commission on Radiological Protection. Ann ICRP 2007;37:60–318. [DOI] [PubMed] [Google Scholar]
- 29. Heidbuchel H, Wittkampf FHM, Vano E, Ernst S, Schilling R, Picano E et al. Practical ways to reduce radiation dose for patients and staff during device implantations and electrophysiological procedures. Europace 2014;16:946–64. [DOI] [PubMed] [Google Scholar]
- 30. Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2014 Sub (1973–2012) - Linked To County Attributes - Total U.S., 1969–2013 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2015, based on the November 2014 submission.
- 31. International Network of Agencies for Health Technology Assessment (INAHTA). http://www.inahta.org/HTA.
- 32. Eichler HG, Kong SX, Gerth WC, Mavros P, Jönsson B. Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Value Health 2004;7:518–28. [DOI] [PubMed] [Google Scholar]
- 33. Fazel R, Gerber T, Balter S, Brenner DJ, Carr JJ, Cerqueira MD et al. on behalf of the American Heart Association Council on Quality of Care and Outcomes Research, Council on Clinical Cardiology, and Council on Cardiovascular Radiology and Intervention. Approaches to enhancing radiation safety in cardiovascular imaging. A scientific statement from the American Heart Association. Circulation 2014;130:1730–48. [DOI] [PubMed] [Google Scholar]
- 34. Picano E, Vaño E, Rehani MM, Cuocolo A, Mont L, Bodi V et al. The appropriate and justified use of medical radiation in cardiovascular imaging: a position document of the ESC Associations of Cardiovascular Imaging, Percutaneous Cardiovascular Interventions and Electrophysiology. Eur Heart J 2014;35:665–72. [DOI] [PubMed] [Google Scholar]
- 35. Natarajan MK, Paul N, Mercuri M, Waller EJ, Leipsic J, Traboulsi M et al. Canadian Cardiovascular Society Position Statement on radiation exposure from cardiac imaging and interventional procedures. Canadian J Cardiol 2013;29:1361–8. [DOI] [PubMed] [Google Scholar]
- 36. Estner HL, Bongiorni MG, Chen J, Dagres N, Hernandez-Madrid A, Blomström-Lundqvist C. Use of fluoroscopy in clinical electrophysiology in Europe: results of the European Heart Rhythm Association Survey. Europace 2015;17:1149–52. [DOI] [PubMed] [Google Scholar]
- 37. National Council on Radiation Protection & Measurements. Ionizing Radiation Exposure of the Population of the United States. NCRP Report No.160 Bethesda, MD: National Council on Radiation Protection & Measurements; March2009. [Google Scholar]
- 38. Henshall C, Mardhani-Bayne L, Fronsdal KB, Klemp M. Interactionsbetween health technology assessment, coverage, and regulatory processes: emerging issues, goals, and opportunities. Int J Technol Assess 2011;27:253–60. [DOI] [PubMed] [Google Scholar]
- 39. Facey K. Health Technology Assessment (HTA) glossary INAHTA–International Network of Agencies for Health Technology Assessment; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


