Abstract
Aims
To assess the yield of screening for atrial fibrillation (AF) with a hand-held single-lead electrocardiogram (ECG) device during influenza vaccination in primary care in the Netherlands.
Methods and results
We used the MyDiagnostick to screen for AF in persons who participated in influenza vaccination sessions of ten Dutch primary care practices. In case of suspected AF detection by the stick, the recorded 1-min ECG registrations were analysed by a cardiologist. We scrutinized electronic medical files of the general practitioners to obtain information about the cases screened. Multivariable logistic regression analysis was performed to predict the relation between patient characteristics and a new screen-detected diagnosis of AF. In total, 3269 persons were screened for AF during the influenza vaccination sessions of 10 general practitioner practices. As a result, 37 (1.1%) new cases of AF were detected. Prior transient ischeamic attack or stroke (OR 6.05; 95%CI 1.93–19.0), and age (OR 1.09 per year; 95% CI 1.05–1.14) were independent predictors for such newly screen-detected AF. Of the 37 screen-detected AF cases, 2.7% had a CHA2DS2-VASc of 0, 18.9% a score of 1, and 78.4% a score of 2 or more. The majority needed oral anticoagulant therapy.
Conclusions
Screening seems feasible with an easy to use single-lead, hand-held ECG device with automatic AF detection during influenza vaccination in primary care and results in a ‘1-day’ yield of 1.1% new cases of AF.
Trial registration clinicaltrials.gov
Keywords: Atrial fibrillation, Screening, Primary care, Influenza vaccination
What's new.
We have demonstrated that single-lead electrocardiogram screening with an easy to use hand-held device can be blended with influenza vaccination and thus is scalable to a nationwide approach with as a result tens of thousands new cases of atrial fibrillation (AF) that could receive adequate stroke prevention.
The prevalence of newly detected AF cases during influenza vaccination was 1.1%. Below 60 years, no new cases were detected.
Screen-detected cases of AF should in the large majority receive anticoagulation considering their age and co-morbidities.
Introduction
Atrial fibrillation (AF) affects 1–2% of the total population, with prevalences increasing with age.1 If untreated, AF increases the risk of ischaemic stroke, heart failure, and mortality.2 Anticoagulants are very effective and reduce the stroke risk by 60%, and all-cause mortality by 25%.3 Underdiagnosis of AF is, however, common and may at least partly be related to a lack of symptoms, so-called ‘silent AF’.4 In patients admitted with an ischaemic stroke in the presence of AF, the arrhythmia was unknown in one-fourth to almost half of the patients.5,6 Early detection of AF followed by adequate anticoagulation can help prevent ischaemic strokes.1 Older age and co-morbidities such as heart failure, hypertension, diabetes, prior transient ischeamic attack (TIA)/stroke, and vascular disease (CHA2DS2-VASc score) drive the risk of thromboembolism.7 Guidelines recommend to prescribe anticoagulation therapy to AF patients with a CHA2DS2-VASc score of 1 or more (or 2 or more), independent of whether AF is paroxysmal or persistent, screen detected, or diagnosed in patients with symptoms.1,4,8,9
The 2010 European Society of Cardiology (ESC) guidelines recommend screening for AF among those aged 65 years and over in primary care, for instance by pulse palpation during blood pressure measurement, and followed by an electrocardiogram (ECG) in case of irregularity.1 Practice studies in primary care showed that active pulse feeling is infrequently performed nowadays, and there seems to be room for improvement of (early) detection of AF with devices.10 Non-invasive devices such as specialized blood pressure monitors that automatically detect AF (MicroLife RR monitor), and devices that register single-lead ECGs (AliveCor, MyDiagnostick) may be considered good alternatives for AF screening.11,12 The MyDiagnostick is an easy to apply device that registers and automatically analyses a single-lead I rhythm strip after holding the device with both hands for one minute. It signals a red light in case of rhythm irregularity suspicious for AF, and a green light in case of absence of AF. The rhythm strip can be visualized and analysed by linking the device to a computer. A recent validation study showed that the sensitivity and negative predictive value of a green light signal was very good (both 100%) in a cardiology setting with a prevalence of AF of 28%. In a pilot study, this device seemed feasible as a screening tool during influenza vaccination in primary care.12 These results need confirmation in a larger study to quantify the yield of selective ‘mass screening’ during influenza vaccination.
Every autumn, general practitioners (GPs) in the Netherlands invite eligible community-dwelling persons for a 1-day influenza vaccination session. Dutch indications for influenza vaccination are: (i) age 60 years or over, and (ii) for younger persons, (a history of) diabetes mellitus, COPD, asthma, ischaemic heart disease, or heart failure.13 This setting provides an ideal opportunity for selective screening of a large population of community-dwelling persons who are at increased risk of AF.
We aim to (i) calculate the proportion of newly detected cases, (ii) assess feasibility of large-scale screening with a single-lead ECG device during influenza vaccination, (iii) evaluate the patient characteristics most predictive for a new screen-detected diagnosis of AF, (iv) determine the CHA2DS2-VASc score of novel screen-detected cases and compare these with known cases with AF who received influenza vaccination, and (v) identify enablers and barriers to the implementation of screening with the MyDiagnostick during influenza vaccination.
Methods
Study population
Ten general practices participated, all located in the northern part of the Netherlands, in the vicinity of Groningen. These practices had 49 190 community-dwelling persons enlisted and in the year 2013, 15 032 (30.6%) persons were eligible and invited for the yearly influenza vaccination. Eventually, 9450 (62.9%) showed up to receive the influenza vaccination at the 1-day session. We invited a sample of 3269 persons (34.6% of all participants of the influenza vaccination) to hold the MyDiagnostick. Patients were invited to participate, irrespective of whether the patient was already known with the arrhythmia. Patients were informed that AF mainly affects elderly, that is, those aged ≥65, and research nurses were instructed to selectively screen people aged over 60, including those already known with AF. All participants signed informed consent. The management of newly detected cases of AF was at the discretion of the participating GP.
The study was approved by the medical ethics committee of the Martini Hospital Groningen.
Study procedure
Most general practices in the Netherlands use two rooms for the mass influenza vaccination. Two GP nurses perform the registration in one room, and in the second room three to four healthcare workers (a mix of nurses and GPs) do the immunization. During the year of the study, the 10 participating GP practices performed their influenza vaccination each on another evening. Thus, one and the same research team could visit each practice and blend screening for AF with the immunization programme. Two trained research nurses explained the MyDiagnostick to influenza participants older than 60, and two other nurses took care of the handling of the device by patients, the informed consent, and the registration of the results (green or red signalling). The four research nurses received a training of 30 min on the ins and outs of the MyDiagnostick device, about asking informed consent, and the filling out of the case record form.
Within the logistics of the influenza vaccination every eligible participant received a short lasting instruction on how to hold the device during 1 min and information on the consequences of a green and red signal. Our research team used 10 sticks every evening and was able to screen ∼160 persons per hour. For the purpose of this study, a cardiologist was present during the influenza vaccination session in all 10 locations, and immediately judged the one-channel ECG on the computer with the stick connected to it.
After the screening sessions, the MyDiagnostick rhythm registrations of all 3269 participants were analysed. In case of a red light, the ECG recording was analysed by two cardiologists (R.T. and L.J.G.) for the presence or absence of AF. In case of conflicting interpretation, the two cardiologists discussed the case to come to consensus. The ECG recordings of the green MyDiagnostick results were analysed by one single cardiologist (R.T.). In this article, we refer to a green MyDiagnostick light in combination with no AF on the single-lead ECG registration as a ‘negative MyDiagnostick result’. A red light in combination with confirmation of AF on the ECG strip is a ‘positive MyDiagnostick result’.
Data collection from the electronic medical files of the participating general practitioners
Of all participants, age and gender were registered, and from a random sample of 220 persons with a negative MyDiagnostick result the information on co-morbidities was gathered by scrutinizing the GP's electronic medical files including letters from medical specialists. The same was done in all AF cases with the screening, both new and already known cases.
Data analysis
For comparison between groups, we used the χ2 test or Fisher's exact test for dichotomous variables and Student's t-test for continuous variables. Medical characteristics between sample with no AF at screening (n = 220) and the screen-detected cases (n = 37) were first compared using univariable logistic regression. We included age, male gender, history of stroke or TIA and at least one of remaining CHA2DS2-VASc score co-morbidities. We used only four predictors for both univariable and multivariable logistic regression analyses because we had 37 new cases of screen-detected AF. All analyses were performed with R for windows version 3.1 (The R foundation statistical computing, http://cran.r-project.org).
Results
In total, 9450 persons participated in the influenza vaccination (mean age 64.1, SD 16.5, years). The 3269 persons (34.6%) who held the MyDiagnostick were more often men (49.0 vs. 44.9%) and on average 8.6 years older than the 6181 persons who were not screened (Table 1 and Figure 1). It was logistically not feasible to ask all eligible persons to participate because providing written informed consent took some time.
Table 1.
Patient characteristics of attendees of the influenza vaccination, irrespective of AF status
| Persons attending influenza vaccination N = 9450 |
Individuals who did not hold the MyDiagnostick N = 6181 |
Individuals who held the MyDiagnostick N = 3269 |
P-value* | |
|---|---|---|---|---|
| Men (%) | 4375 (46.3) | 2773 (44.9) | 1602 (49.0) | <0.001 |
| Mean age in years (±SD) | 64.1 ± 16.5 | 60.8 ± 18.3 | 69.4 ± 8.9 | <0.001 |
| Age ≥60 years (%) | 6795 (71.9) | 3797 (61.4) | 2998 (91.7) | <0.001 |
| Age ≥65 years (%) | 5306 (56.1) | 2749 (44.5) | 2557 (78.2) | <0.001 |
| Age ≥75 years (%) | 2153 (22.8) | 1325 (21.4) | 828 (26.2) | <0.001 |
*P-value is given for the comparison of individuals who held the MyDiagnostick (N = 3269) and those who did not hold this device (N = 6181).
Figure 1.
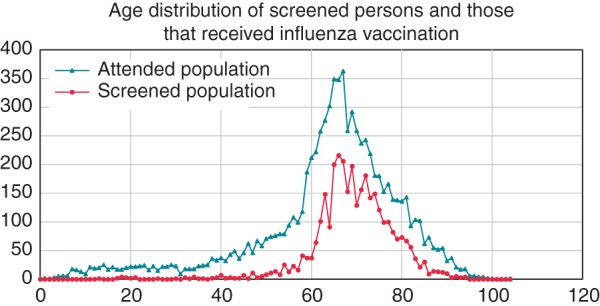
Age distribution of the 9450 persons attending influenza vaccination compared with 3269 screened persons. Attended population: all persons who came for influenza vaccination in 2013. Screened population: all persons who held the MyDiagnostick during this vaccination.
In total, 193 participants (5.9% of the screened population) had a red signalling with the MyDiagnostick. Of them, 121 (3.7% of the screened population) had AF on the single-lead rhythm strip according to the cardiologists (Figure 2). Eighty-four cases were already known with AF, and 37 (1.1% of the screened population) were new screen-detected cases. In 3 of the 193 cases with a red signal, the rhythm strip could not be interpreted by either cardiologist and these were considered as ‘no AF’. In all 3076 cases with a green light, the cardiologist could confirm sinus rhythm.
Figure 2.
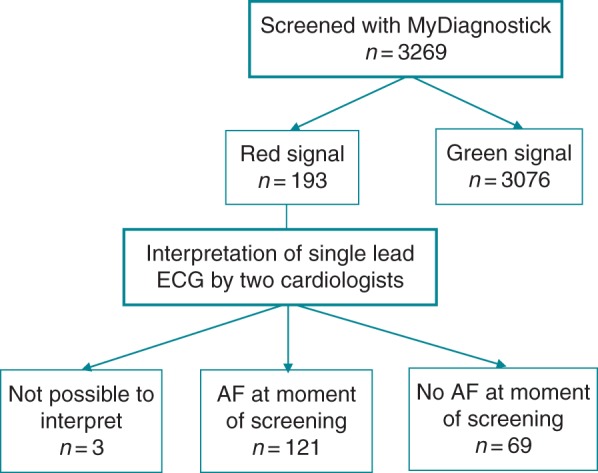
Flow chart of study.
The 37 new screen-detected cases of AF were older, had more co-morbidities such as hypertension (64.9% vs. 43.2%), stroke (18.9% vs. 2.7%), TIA (8.1 vs. 0.5%), and COPD (10.8% vs. 3.2%) than a random sample of 220 participants of the influenza vaccination, but without AF (Table 2). The unadjusted odds ratios of a new screen-detected diagnosis of AF were 9.78 (95% CI 3.38–28.33) for a history of stroke or TIA, 0.65 (95% CI 0.32–1.31) for male gender, 1.09 per year for older age (95% CI 1.05–1.14), and 1.83 (95% CI 0.85–3.98) for a history of either diabetes mellitus, hypertension, heart failure, or vascular disease. Multivariable logistic regression showed that age (OR 1.09 per year; 95% CI 1.05–1.14) and a history of ischaemic stroke or TIA (OR 6.05; 95%CI 1.93–19.0) were independent predictors of a screen-detected diagnosis of AF (Table 3).
Table 2.
Baseline characteristics of individuals who held the MyDiagnostick divided in new screen-detected cases of AF, patients already known with AF, and a random sample of patients with no AF
| Newly detected AF with screening N = 37 |
Already known with AF and a red light with screening N = 84 |
Sample of patients with no AFa N = 220 |
|
|---|---|---|---|
| Men (%) | 21 (56.8) | 49 (58.3) | 101 (45.9) |
| Mean age (SD) | 75.9 (8.6) | 75.6 (8.3) | 65.9 (12.4) |
| Medical history | |||
| Hypertension (%) | 24 (64.9) | 55 (65.5) | 95 (43.2) |
| Diabetes (%) | 9 (24.3) | 23 (27.4) | 52 (23.6) |
| Heart failure (%) | 2 (5.4) | 18 (21.4) | 2 (0.9) |
| Stroke (%)b | 7 (18.9) | 9 (10.7) | 6 (2.7) |
| TIA (%) | 3 (8.1) | 10 (11.9) | 1 (0.5) |
| VTE (%)c | 2 (5.4) | 7 (14.3) | 10 (4.5) |
| Peripheral arterial disease (%) | 3 (8.1) | 2 (2.4) | 7 (3.2) |
| Prior myocardial infarct (%) | 2 (5.4) | 10 (14.3) | 7 (3.2) |
| Valvular repair (%) | 0 (0) | 6 (7.1) | 1 (0.5) |
| CABG/PCI (%) | 2 (5.4) | 14 (16.7) | 19 (8.6) |
| COPD (%) | 4 (10.8) | 12 (17.1) | 17 (7.7) |
| Renal disease (%) | 3 (8.1) | 11 (15.7) | 8 (3.6) |
| Pacemaker (%) | 0 (0.0) | 4 (4.8) | 2 (0.9) |
| Vitamin K antagonists (%) | 2 (5.4)d | 70 (83.3) | 5 (2.3)d |
| NOACs (%) | 0 (0.0) | 7 (8.3) | 0 (0.0) |
| ASA (%) | 11 (29.7) | 7 (8.3) | 46 (20.9) |
| ACE inhibitors (%) | 10 (27.0) | 31 (36.9) | 55 (25.0) |
| Beta-blockers (%) | 8 (21.6) | 59 (70.2) | 58 (26.4) |
| Calcium channel blockers (%) | 13 (35.1) | 21 (25.0) | 27 (12.3) |
aSample of 220 persons unknown with AF and also sinus rhythm on the MyDiagnostick single-lead ECG with screening during influenza vaccination.
bStroke is defined as ischaemic stroke or cryptogenic stroke not being an haemorrhagic infarction.
cVTE = venous thromboembolism, including history of pulmonary embolism and/or deep vein thrombosis.
dIndications for VKA in two cases was (1) a history of deep vein thrombosis and lung embolus, and a history of ischaemic stroke. The indications in five cases with a negative MyDiagnostick result were (1) a history of more than one deep vein thrombosis or lung embolus, (2) heart valve replacement, and (3) secondary prevention after ischaemic stroke.
NOAC, non-vitamin K antagonist oral anticoagulant.
Table 3.
Multivariable logistic regression analysis relating participants’ characteristics to screen-detected AF
| Univariable analysis |
Multivariable analysis |
|||
|---|---|---|---|---|
| OR for screen-detected AF | 95% Confidence interval | OR for screen-detected AF | 95% Confidence interval | |
| Age per year | 1.09 | 1.05–1.14 | 1.09 | 1.05–1.14 |
| Male gender | 0.65 | 0.32–1.31 | 0.56 | 0.25–1.23 |
| History of stroke/TIA | 9.78 | 3.38–28.33 | 6.05 | 1.93–18.98 |
| History of one or more remaining co-morbidities of the CHA2DS2-VASc scorea | 1.83 | 0.85–3.98 | 0.91 | 0.38–2.18 |
aDiabetes mellitus, hypertension, heart failure, and/or vascular disease (coronary heart disease, CABG/PCI, myocardial infarction, and/or peripheral arterial disease).
The prevalence of screen-detected AF cases increased with age, from 0% in those aged <60 years to 4.9% in those aged 85 years and over (Table 4).
Table 4.
Cases with a red signal with the MyDiagnostick divided in those with no AF and with AF on the rhythm strip, categorized per age category and divided by the number of cases screened
| Age | Red light but no AF/screened casesa | % | Red light but already known AF/screened casesb | % | Red light and new screen-detected AF/screened cases c | % |
|---|---|---|---|---|---|---|
| <60 | 5/271 | 1.8 | 3/271 | 1.1 | 0/271 | 0 |
| 60–64 | 10/441 | 2.3 | 4/441 | 0.9 | 4/441 | 0.9 |
| 65–74 | 29/1729d | 1.7 | 31/1729 | 1.8 | 14/1729 | 0.8 |
| 75–84 | 24/725e | 3.3 | 37/725 | 5.1 | 14/725 | 1.9 |
| >85 | 4/103 | 3.9 | 9/103 | 8.7 | 5/103 | 4.9 |
aRed light with MyDiagnostick suggesting irregular heart rhythm and possibly AF, but without AF after interpretation of the single-lead ECG registration of 1 min by the cardiologist, per number screened individuals per age category.
bCases with AF at moment of screening that was already known per number of screened individuals per age category.
cScreen-detected AF cases per number of screened individuals per age category.
dFrom 29 cases, 2 were previously diagnosed with paroxysmal AF but showed no AF at moment of screening.
eFrom 24 cases, 7 were previously diagnosed with paroxysmal AF but showed no AF at moment of screening.
Among screen-detected AF patients, 2.7% had a CHA2DS2-VASc of 0, 18.9% a score of 1, and 78.4% a score of 2 or more. The distribution was similar to cases already known with AF (Table 5).
Table 5.
CHA2DS2-VASca scores for individuals with AF, screen-detected cases vs. previously known AF cases
| Screen-detected AF N = 37 |
Already known AF N = 84 |
P-value | |
|---|---|---|---|
| Mean CHA2DS2-VASc score | 3.4 (1.9) | 3.6 (1.7) | 0.49 |
| CHA2DS2-VASc score 0 | 1 (2.7) | 1 (1.2) | 0.55 |
| CHA2DS2-VASc score 1 | 7 (18.9) | 10 (11.9) | 0.31 |
| CHA2DS2-VASc score ≥2 | 29 (78.4) | 73 (86.9) | 0.23 |
aCHA2DS2-VASc score is a clinical decision rule used to predict the risk of stroke in patients with AF. A higher score indicates a greater risk of stroke. Scores range from 0 to 9, categories include heart failure (1), hypertension (1), age ≥65 years (1), age ≥75 years (1), diabetes mellitus (1), prior ischaemic stroke and/or TIA and/or arterial thromboembolism (2), vascular diseaseb (1), and female gender (1).
bVascular includes coronary heart disease, CABG/PCI, myocardial infarction, and/or peripheral arterial disease.
Of the 193 individuals with a red signal, 72 (37.3%) had no AF on the single-lead I ECG analysed by the two cardiologists. Thirty-four (47.2%) had premature atrial or ventricular complexes, 25 (34.7%) had sinus arrhythmia, and 10 (13.9%) had irregularity caused by artefacts. Three cases had un-interpretable results (0.1% of all cases who held the MyDiagnostick); one because of artefacts, one because of a pacemaker rhythm, and one because of the cardiologists thought that it could be either extra systoles or atrial flutter. With a 12-lead ECG 1 day later, two had sinus rhythm, and one sinus arrhythmia. One of these three cases was known with paroxysmal AF and had DDDR pacemaker for bradycardia, and the pacemaker was active at the time he held the MyDiagnostick and not during the 12-lead ECG.
Discussion
By screening community-dwelling persons during influenza vaccination in primary care AF was detected in 3.7% (2.6% already known cases of AF, and 1.1% new cases). The screen-detected cases of AF had a similar CHA2DS2-VASc score as those already known with AF, and the large majority would need anticoagulation. Age (OR 1.09 per year, 95%CI 1.05–1.14) and a history of TIA or stroke (OR 6.05 95%CI 1.93–18.98) were independent predictors for screen-detected AF.
In a previous study in the UK, community-dwelling person aged 65 years or over from primary care were investigated by systematically taking the pulse followed by a 12-lead ECG if irregular. With this method, 1.6% new cases were detected during 1 year, while 1.0% a year was detected with care as usual.10 A systematic review including 16 screening studies in persons aged 65 years or over from the community, primary care, or cardiology outpatients clinics showed that screening with pulse palpation resulted in 1.4% new screen-detected cases of AF.14 This is in line with our results achieved by a single screening session during influenza vaccination.
Guidelines consider screen-detected AF cases to be at increased risk for stroke and eligible for stroke prevention based on the CHA2DS2-VASc score.15 In a substudy of the AFFIRM study, including 481 asymptomatic and 3576 symptomatic AF patients, the absence of symptoms (silent AF) did not result in a significant difference in mortality after correction for baseline differences (adjusted hazard ratio 1.07, 95% CI 0.79–1.46) or major events (death, disabling stroke, major central nervous system haemorrhage, or cardiac arrest) (adjusted HR 1.14, 95% CI 0.87–1.50) compared with AF with symptoms.4 Another study compared the prognosis of 148 asymptomatic and 952 symptomatic AF patients during a mean follow-up of 10 years. After adjustment for differences in baseline characteristics a borderline-significant increased risk for ischaemic stroke was seen in asymptomatic patients (hazard ratio 1.8, 95% CI 1.0–3.8) compared with symptomatic AF, whereas asymptomatic patients were more often treated with anticoagulants (40 vs. 21%).16 The fact that in the present study 27% of patients with screen-detected AF already had a history of TIA or stroke underlines the importance of detecting silent AF.
Two previous studies validated the accuracy of the light signal of the MyDiagnostick against an immediately followed 12-lead ECG as the reference test. In a case–control design with an AF prevalence of 28 and 53%, respectively, the negative predictive values were 100 and 93%, and the sensitivities 100 and 94%, respectively.12 In a screening setting with a low prevalence of (unknown) AF, a green light signal with the MyDiagnostick will result in a very small number of missing cases (low false negatives). A red signal, however, should be followed by an adequate interpretation of electrocardiographic data, either the 1-min lead I registration recorded by the MyDiagnostick or a 12-lead ECG taken immediately after holding the device. Current guidelines on AF recommend diagnosing AF with either a 12-lead ECG, or a single-lead ECG lasting for 30 s or more.1,9 Ambulatory ECG monitors that record two leads of an ECG showed high sensitivity to detect a variety of cardiac arrhythmias,17 and such devices are widely used nowadays for detecting AF in high-risk patients (e.g. after ischaemic stroke).18 Interpretation of a lead-I ECG by an experienced physician has a high correlation with a 12-lead ECG, with a sensitivity and specificity of 95–96 and 90–95%, respectively.19
Feasibility
We could not detect a single novel case of AF among 271 persons aged <60 years, which underlines that screening of community-dwelling persons should focus on older individuals, i.e. aged 60 or 65 years or over.
Screening during influenza vaccination is a single time-point screening, and thus, paroxysmal AF may be missed. The Stroke Stop study has demonstrated that repeated measurements during 2 weeks in patients aged 75–76 years increases the yield of screening compared with single time-point screening.20 However, this approach requires an extensive and expensive programme. Our study blended single-point screening with the existing programme of influenza vaccination. This is feasible at limited costs and generating a considerable yield. It may therefore have a large impact on general health of those aged over 60 years.
Screening with the MyDiagnostick is easily performed; it takes only 1 min and can be done without supervision. In our study, 160 persons per hour were screened with 10 MyDiagnosticks by four research nurses. In general, the participants of influenza vaccination were very willing to participate. The main barriers are the need for more personnel and the informed consent procedure.
In the present study, silent AF was present in 1.3% of all patients ≥65 years (derived from Table 4; 33/2.557 patients). Seventeen per cent of the in total 17 million inhabitants in the Netherlands is aged at least 65 years, and influenza vaccination rate in this age category is around 80%.13 Blending screening with a handheld device with such vaccination could potentially result in screening of 2.3 million people (0.80×0.17×17 million) with as a result up to 30 000 (1.3%) new cases of AF that could receive adequate stroke prevention. Therefore, such a screening approach is scalable to make a significant nationwide impact on stroke reduction.
A previous study described that screening of community-dwelling people aged over 65 years with 12-lead electrocardiography was cost effective with a participating rate of 50%.8 Our approach, to screen for AF with an easy to use handheld device during an existing influenza vaccination programme, is potentially even more cost effective to reduce ischaemic strokes.
For the purpose of the study, the cardiologist was present on the location of screening and immediately judged the ECG from the stick. When implemented on a large scale, this may not always be possible or desirable. In that case, the MyDiagnostick ECG rhythm strips can be sent to a cardiologist to confirm the presence of AF. In our study, in only 3 cases (0.1%), the rhythm strip was not adequately interpretable.
Limitations
We did not screen all participants of the influenza vaccination, but selectively aimed at those aged 60 years or over. This selection was applied because under the age of 60 years AF is very uncommon. Secondly, we missed some eligible persons because of time commitment for informed consent, and this could have resulted in more selectively inclusion of more healthy and literate patients. We considered it unlikely that this had substantial impact on our point estimate of screen-detected AF, the more because when such a screening is institutionalized a selection towards more healthy and literate persons would also occur.
We had information of a random sample of 220 persons with a negative MyDiagnostick result. We decided to only assess a random selection of all persons with a negative lightning result for practical and logistic reasons. Higher age in the screened population than in the controls may have resulted in bias towards detecting age as an independent factor.
The lead I registrations of the MyDiagnostick were interpreted by the cardiologists while having knowledge of the lightning results. Cardiologists were not blinded to the red/green signalling. Importantly, our aim was the yield of screening, not evaluation of the accuracy of the MyDiagnostick.
Conclusions
Screening with a single-lead ECG device during influenza vaccination in primary care resulted in 1.1% new cases of AF and is a feasible option for large-scale screening.
Declaration of Helsinki and Ethical approval: This study complies with the Declaration of Helsinki. The study was approved by the medical ethics committee of the Martini Hospital Groningen. All participants were informed about the study and signed informed consent. Neither ABS nor Boehringer Ingelheim BV was involved in study design and data collection.
Authors’ contribution
F.K. designed the project and did the data collection and statistical analysis, and drafted the first version of the paper. R.G.T. initiated the project, co-designed the project, analysed single-lead ECG registrations, and revised the paper. L.J.G. analysed single-lead ECG registrations, and revised the paper. F.H.R. co-designed data collection and analysis plan and revised the paper. M.H. co-designed data collection and analysis plan and revised the paper. A.W.H. revised the paper. F.K. is guarantor of the study.
Funding
Funding to pay the Open Access publication charges for this article was provided by Robert Tieleman.
Conflict of interest: All authors have completed the ICMJE uniform disclosure form and declare: no support from any organization for the submitted work; R.G.T. is co-inventor of the MyDiagnostick and receives royalties from Applied Biomedical Systems (ABS) BV. None of the other authors have a financial relationship with any organization that might have an interest in the submitted work in 36 months prior to this study. There are no other relationships or activities that could appear to have influenced the submitted work.
Acknowledgements
We thank all GPs that participated in this study. We thank the nursing students of the Hanzehogeschool Groningen for their participation during screening. The MyDiagnosticks were for the duration of the study delivered on loan basis by Boehringer Inghelheim BV, Comeniusstraat 6, 1718 MS, Alkmaar, The Netherlands.
References
- 1. Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S et al. . Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace 2010;12:1360–420. [DOI] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98:946–52. [DOI] [PubMed] [Google Scholar]
- 3. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857–67. [DOI] [PubMed] [Google Scholar]
- 4. Flaker GC, Belew K, Beckman K, Vidaillet H, Kron J, Safford R et al. . Asymptomatic atrial fibrillation: demographic features and prognostic information from the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Am Heart J 2005;149:657–63. [DOI] [PubMed] [Google Scholar]
- 5. Pisters R, van Oostenbrugge RJ, Knottnerus IL, de Vos CB, Boreas A, Lodder J et al. . The likelihood of decreasing strokes in atrial fibrillation patients by strict application of guidelines. Europace 2010;12:779–84. [DOI] [PubMed] [Google Scholar]
- 6. Friberg L, Rosenqvist M, Lindgren A, Terent A, Norrving B, Asplund K. High prevalence of atrial fibrillation among patients with ischemic stroke. Stroke; A Journal of Cerebral Circulation 2014;45:2599–605. [DOI] [PubMed] [Google Scholar]
- 7. Boriani G, Botto GL, Padeletti L, Santini M, Capucci A, Gulizia M et al. . Improving stroke risk stratification using the CHADS2 and CHA2DS2-VASc risk scores in patients with paroxysmal atrial fibrillation by continuous arrhythmia burden monitoring. Stroke; A Journal of Cerebral Circulation 2011;42:1768–70. [DOI] [PubMed] [Google Scholar]
- 8. Fitzmaurice DA, McCahon D, Baker J, Murray ET, Jowett S, Sandhar H et al. . Is screening for AF worthwhile? Stroke risk in a screened population from the SAFE study. Fam Pract 2014;31:298–302. [DOI] [PubMed] [Google Scholar]
- 9. NHG-Standaard Atriumfibrilleren. Utrecht: Nederlands Huisartsen Genootschap; 2013. [Google Scholar]
- 10. Fitzmaurice DA, Hobbs FD, Jowett S, Mant J, Murray ET, Holder R et al. . Screening versus routine practice in detection of atrial fibrillation in patients aged 65 or over: cluster randomised controlled trial. BMJ (Clinical Research ed) 2007;335:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wiesel J, Fitzig L, Herschman Y, Messineo FC. Detection of atrial fibrillation using a modified microlife blood pressure monitor. Am J Hypertens 2009;22:848–52. [DOI] [PubMed] [Google Scholar]
- 12. Tieleman RG, Plantinga Y, Rinkes D, Bartels GL, Posma JL, Cator R et al. . Validation and clinical use of a novel diagnostic device for screening of atrial fibrillation. Europace 2014;16:1291–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tacken M, Mulder J, Van den Hoogen H, Donkers J, Tiersma W, Verheij R. Monitoring Griepvaccinatiecampagne 2006. Utrecht: LINH-NIVEL; 2007. [Google Scholar]
- 14. Lowres N, Neubeck L, Redfern J, Freedman SB. Screening to identify unknown atrial fibrillation. A systematic review. Thromb Haemost 2013;110:213–22. [DOI] [PubMed] [Google Scholar]
- 15. Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH et al. . 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Europace 2012;14:1385–413. [DOI] [PubMed] [Google Scholar]
- 16. Potpara TS, Polovina MM, Marinkovic JM, Lip GY. A comparison of clinical characteristics and long-term prognosis in asymptomatic and symptomatic patients with first-diagnosed atrial fibrillation: the Belgrade Atrial Fibrillation Study. Int J Cardiol 2013;168:4744–9. [DOI] [PubMed] [Google Scholar]
- 17. Zimetbaum P, Goldman A. Ambulatory arrhythmia monitoring: choosing the right device. Circulation 2010;122:1629–36. [DOI] [PubMed] [Google Scholar]
- 18. Adams HP Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A et al. . Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Circulation 2007;115:e478–534. [DOI] [PubMed] [Google Scholar]
- 19. Kearley K, Selwood M, Van den Bruel A, Thompson M, Mant D, Hobbs FR et al. . Triage tests for identifying atrial fibrillation in primary care: a diagnostic accuracy study comparing single-lead ECG and modified BP monitors. BMJ Open 2014;4:e004565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Svennberg E, Engdahl J, Al-Khalili F, Friberg L, Frykman V, Rosenqvist M. Mass screening for untreated atrial fibrillation: The STROKESTOP study. Circulation 2015;131:2176–84. [DOI] [PubMed] [Google Scholar]


