Summary
Lipid recovery and purification from microalgal cells continues to be a significant bottleneck in biodiesel production due to high costs involved and a high energy demand. Therefore, there is a considerable necessity to develop an extraction method which meets the essential requirements of being safe, cost‐effective, robust, efficient, selective, environmentally friendly, feasible for large‐scale production and free of product contamination. The use of wet concentrated algal biomass as a feedstock for oil extraction is especially desirable as it would avoid the requirement for further concentration and/or drying. This would save considerable costs and circumvent at least two lengthy processes during algae‐based oil production. This article provides an overview on recent progress that has been made on the extraction of lipids from wet algal biomass. The biggest contributing factors appear to be the composition of algal cell walls, pre‐treatments of biomass and the use of solvents (e.g. a solvent mixture or solvent‐free lipid extraction). We compare recently developed wet extraction processes for oleaginous microalgae and make recommendations towards future research to improve lipid extraction from wet algal biomass.
Introduction
Microalgal feedstocks have been considered a suitable alternative to traditional oil‐bearing crops as a source of biodiesel production due to simple cultivation systems, reduced pressure on competing arable land and natural resources, and the potential to meet current demands of global biofuel mandates (Singh and Dhar, 2011). The production of biodiesel from microalgae consists of extracting cytosolic lipid bodies that contain large amounts of triacylglycerides (TAG) and can be further refined into biodiesel via transesterification (Chisti, 2007; Hu et al., 2008). Table 1 provides an overview of microalgal strains that have been assessed for TAG production as potential feedstock for biodiesel or other algal oil‐based products. However, due to the presence of thick and robust algal cell walls, releasing the lipids has become a major drawback for manufacturing biodiesel, as techniques that are currently in place are derived from expensive conventional methods for oil‐bearing terrestrial crops. These include concentrating and dewatering the microalgae before undergoing extraction, which not only are energy intensive and ineffective but also toxic due to organic solvents, deeming them unfeasible on the industrial scale (Uduman et al., 2010; de Boer et al., 2012; Lee et al., 2012; Passell et al., 2013; Torres et al., 2013). To mitigate this, lipid extraction from wet algal biomass has been proposed as an ideal solution, disrupting the algal cells in solution and eliminating the costs and impacts that are associated above (shown in Fig. 1). In this paper, we review recent progress made towards developing wet lipid extraction techniques and their constraints.
Table 1.
Examples of microalgal strains being evaluated for TAG production
| Strain | Lipid content (% of DCW) | Lipid productivity (mg/L/d) | Reference |
|---|---|---|---|
| Chaetoceros calcitrans CS 178 | 40 | 18 | Rodolfi et al. (2009) |
| Chaetoceros muelleri F&M‐M43 | 34 | 22 | Rodolfi et al. (2009) |
| Chlorella protothecoides | 43–46 | 1881–1840 | Cheng et al. (2009) |
| Chlorella protothecoides | 55 | 932 | Xu et al. (2006) |
| Chlorella sorokiniana IAM‐212 | 19 | 45 | Rodolfi et al. (2009) |
| Chlorella vulgaris | 21 | 254 | Liang et al. (2009) |
| Chlorella vulgaris | 5.1 | 180 | Gouveia and Oliveira (2009) |
| Chlorella zofingiensis | 51 | 354 | Liu et al. (2011) |
| Chlorococcum sp. UMACC 112 | 19 | 54 | Rodolfi et al. (2009) |
| Dunaliella tertiolecta | 17 | 120 | Gouveia and Oliveira (2009) |
| Isochrysis sp. F&M‐M28 | 22 | 38 | Rodolfi et al. (2009) |
| Monodus subterraneus UTEX 151 | 16 | 30 | Rodolfi et al. (2009) |
| Nannochloropsis oculata | 23–30 | 84–142 | Chiu et al. (2009) |
| Nannochloropsis sp. | 35–48 | 385–413 | Pal et al. (2011) |
| Nannochloropsis sp. | 29 | 90 | Gouveia and Oliveira (2009) |
| Neochloris oleoabundans | 29 | 90 | Gouveia and Oliveira (2009) |
| Pavlova lutheri CS 182 | 36 | 50 | Rodolfi et al. (2009) |
| Scenedesmus obliquus | 39 | 79 | Ho et al. (2010) |
| Scenedesmus obliquus | 18 | 90 | Gouveia and Oliveira (2009) |
| Scenedesmus quadricauda | 18 | 35 | Rodolfi et al. (2009) |
| Spirulina maxima | 4 | 210 | Gouveia and Oliveira (2009) |
| Tetraselmis sp. F&M‐M34 | 15 | 43 | Rodolfi et al. (2009) |
DCW, dry cell weight.
Figure 1.
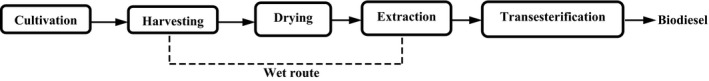
A pipeline model for the production of biodiesel from microalgae. Microalgae are cultivated by providing light, nutrients (especially nitrogen and phosphate), carbon dioxide and water in an open or closed bioreactor. Once there is concentrated biomass, it is harvested and dewatered by sedimentation, flocculation, filtration, centrifugation, flotation or electrophoresis techniques. Lipid extraction is performed after cell disruption. A ‘wet route’ would eliminate the drying process while lowering the production costs. The final step for biodiesel production is algal oil transesterification.
Recent progress in lipid extraction from wet algal biomass
By definition, lipid extraction from wet algal biomass consists of disrupting/damaging the algal cell walls in the solution in which the microalgae were cultivated in. These techniques can be grouped into organic solvent‐based and solvent‐free approaches. Considering solvent‐based lipid extraction techniques on wet biomass, water plays a barrier role between the intracellular lipids and non‐ploar organic solvents. Increasing the polarity of the solvent can remove this obstacle to a great extent. Safety and environmental issues are the main limitations of organic solvent‐based techniques, so a new direction of research focuses on developing organic solvent‐free approaches which are not only safe and environmental friendly but also minimizing the need to separate contaminants from the extracted lipid product. The most desirable solvent‐free techniques are the ones which can be implemented on a diverse range of algal strains with low energy consumption and negligible initial set‐up costs for infrastructure. As discussed below, promising approaches come from microalgal cell rupturing techniques that free up lipid bodies which subsequently need to be recovered. Although still immature, solvent‐free extraction is seen to be a promising technique for the industrial production of primary extracted lipids. Similar to the vegetable industry, the feasibility of secondary extraction of remaining cellular lipids from partially defatted algae by using organic solvents needs to be assessed.
Pre‐treatments of biomass
For microalgae lipid extraction, the cells have to be disrupted properly, irrespective if an organic solvent is to be used or not. Pre‐treatments can be applied on the biomass to enhance lipid recovery efficiency through breaking or weakening the microalgal cell walls which in turn facilitates easier cellular lipid extraction (Cooney et al., 2009; Yoo et al., 2012). There are different methods which can be applied as pre‐treatments; including mechanical, chemical, physical and biological approaches. Some examples are pressing, bead milling, electroporation, homogenization and osmotic shock (mechanical), cell lysing with acid/base (chemical), lyophilization, sonication, microwave, thermal (physical) and enzymatic polysaccharide and/or protein degradation (biological) (Kita et al., 2010; Mercer and Armenta, 2011; Kim et al., 2013). In mechanical pre‐treatments (compared with chemical and biological pre‐treatments), the risk of degradation or degeneration of the target compound is considered reduced (Greenwell et al., 2009; Kim et al., 2013). Although almost all of these pre‐treatments are used in conjunction with solvent techniques for the recovery of extracted lipids, less solvent can be used compared with untreated biomass (Mercer and Armenta, 2011).
Solvent‐based techniques
Use of solvent mixtures
As mentioned before, solvents still play a main role in both extraction and recovery of microalgal lipids. Suitable solvents should be chosen as per the target compound polarity. The main microalgal lipid material for biodiesel production are TAGs, which are non‐polar and hence, more soluble in non‐polar organic solvents (Levine et al., 2010; Chen et al., 2012). However, a successful extraction solvent is one which can fully penetrate the biomass, making physical contact with the targeted lipid material and subsequently dissolve it completely (Mercer and Armenta, 2011). The lipid extraction/recovery can be enhanced through increasing the polarity of the solvent by mixing the polar and non‐polar solvents. This is due to the ability of the polar solvents to release the lipids from their protein–lipid complexes which facilitates their dissolving in the non‐polar solvent (Ryckebosch et al., 2012). This effect is even higher when the lipid extraction/recovery is to be performed on wet biomass, as the polar solvent can penetrate the water layer and make the lipids available for non‐polar solvent solvation (Yoo et al., 2012). We recently reported that not only the total lipid recovery can be increased through utilizing a solvent mixture but also the total FAME recovery can be increased when using a mixture of polar and non‐polar solvents (Ghasemi Naghdi et al., 2014). Utilizing a solvent mixture of hexane and ethanol at a 3:1 ratio, respectively, through a Soxhlet system, when applied on Tetraselmis sp. significantly enhanced FAME recovery by 50%.
Microwave‐assisted extraction
Microwave‐assisted extractions (MAE) were first established in the mid 1980s as a means to obtain lipids and pesticides from seeds, foods, feeds and soil (Ganzler et al., 1986). When applied to microalgal cultures, microwave technology has been proven to be not only relatively safe, rapid and economical but also reduced the cost associated with dewatering and extracting of dry algal biomass (Lee et al., 2010). Typically, the contact between a dielectric or polar material (e.g. water) and a rapidly oscillating electric field (produced by microwaves) generates heat due to frictional forces arising from inter‐ and intra‐molecular movements. As heat is produced, water vapour begins to form within the cell, eventually rupturing it, leading to an electroporation effect which further opens up the cell membrane and releasing the intracellular contents (Amarni and Kadi, 2010). Šoštarič et al. (2012) previously reported that microwaves, when used in conjunction with other mechanical extraction methods such as sonication, have yielded higher levels of lipid in Chlorella vulgaris, while Refaat et al. (2008) indicated that microwave irradiation can also assist in the transesterification process post extraction by substituting conventional heating. When implementing MAE, considerations regarding the time, temperature, dielectric properties of the process mixture, the solid–liquid ratio, and the type and concentration of the solvent should be taken into account (Eskilsson and Björklund, 2000). For example, Balasubramanian et al. (2011) demonstrated that higher temperatures at longer times resulted in higher oil extraction efficiency when compared with standard methods such as the Soxhlet extraction; however, the presence of TAGs is highest at elevated temperatures accompanied by shorter times. However, if the target compounds and/or the solvent(s) are non‐polar or volatile, the efficiency of MAE can be hindered dramatically (Wang and Weller, 2006). Currently, MAE is evaluated to be a cost‐effective method for wet lipid extraction from microalgae based on short reaction times and the extraction of high‐quality lipids; however, when scaling up commercially, maintenance cost is viewed as a limiting factor.
Ultrasound‐assisted extraction
Ultrasound‐assisted extraction (UAE) eliminates the issues associated with conventional mechanical disruption, and is advantageous due to low set‐up costs, fast operational time and high purity of the final product. In the presence of liquid cultures, UAE can rupture the cells via cavitation which produces microbubbles around the cell as a result of an ultrasonic wave. The eventual collapse of these bubbles emits a shockwave which shatters the cell wall, hence releasing the intracellular contents (Suslick and Flannigan, 2008; Harun et al., 2010). Metherel et al. (2009) showed that an increase in amplitude of the exposure time can result in higher lipid recoveries, with further enhancement using a mixture of polar and non‐polar solvents, while Vinatoru et al. (1997) demonstrated that UAE can not only reduce extraction time but also facilitate the absorption of cell contents into the solvent through mass transfer and penetration of the solvent into the cell. UAE extraction using ethanol as the only solvent may improve environmental safety. In addition, UAE can also be advantageous to MAE as it can be conducted under low temperatures, reducing thermal denaturation of essential biomolecules (Ranjith Kumar et al., 2015). However, if the microalgal cultures are subjected to prolonged exposure to sonication, this can lead to the generation of free radicals that can deteriorate the quality of the lipids through oxidation (Chemat et al., 2004). Nevertheless, oxidation can be limited by utilizing non‐polar organic solvents which are not susceptible to peroxide formation, such as hexane. Metherel et al. (2009) reported that the ideal ratio of extraction solvents for UAE in flaxseed is 2:1 chloroform:methanol and 3:2 hexane:isopropanol, reducing lipid oxidation and resulting in higher yields.
Hydrothermal liquefaction
Hydrothermal liquefaction (HTL) is a thermochemical conversion technique that processes the whole microalgal biomass by applying medium to subcritical temperature (below 374°C) and high pressure (10–25 MPa) (Garcia Alba et al., 2011; Barreiro et al., 2013). Under hydrothermal conditions, fatty acids and hydrogenated compounds (‘biocrude’) are produced from lipids (Biller et al., 2011). As substantial equipment and running costs are associated with this technology, economical feasibility and scalability need to be considered. While these still need to be demonstrated, HTL reportedly achieved an energy recovery from biomass to fuel up to 80% (Toor et al., 2011). As HTL can be used on wet algal biomass with a water content as high as 80–95%, it was also claimed to require less than 5% of the energy costs otherwise needed for complete drying (Garcia Alba et al., 2011). Toor et al. (2013) reported a biocrude yield (lipid conversion to free fatty acids) around 34–38% for Spirulina platensis after HTL at 310°C and 115 bar and 34–46% for Nannochloropsis salina at 350°C and 175 bar. A biocrude yield of 27% and 47% from HTL of Scenedesmus (350°C) and Chlorella (300°C), respectively, was reported by Biller et al. (2012). These conversion rates are very low when compared with other feedstocks, e.g. for soybean where, with the right catalyst, more than 90% of the lipids can be converted to fatty acids. The use of catalysts to improve oil yield in HTL treatment was studied by Ross et al. (2010). They evaluated the influences of temperature and catalyst type (alkali, potassium hydroxide and sodium carbonate and the organic acids, acetic acid and formic acid) on the production and nature of biocrude produced by Chlorella and Spirulina after HTL. Their results show that biocrude yield is higher in the presence of organic acids and at higher temperatures. Due to the high amounts of nitrogen in chlorophyll and proteins in algal cells, the process may lead to high NOx emissions, one of the biggest bottlenecks for this process to be a real alternative to biofuel production (Barreiro et al., 2013). Garcia Alba et al. (2011) found that hydrothermal treatment of Desmodesmus sp. produces up to 6% of nitrogen content in the oil yield, while Toor et al. (2013) reported a nitrogen content of 4–6% in oil extracted from Chlorella and Spirulina. Scalability, safety, and equipment costs and maintenance appear to be issues that deserve further investigation.
Osmotic shock
By osmotic shock, algae cells burst, liberating their contents due to an abrupt lowering of osmotic pressure (Mercer and Armenta, 2011). Lipid extraction after osmotic shock has been studied during recent years (Lee et al., 2010; Prabakaran and Ravindran, 2011; Yoo et al., 2012). Yoo et al. (2012) evaluated lipid recovery from wet biomass of Chlamydomonas reinhardtii by osmotic shock along with both polar and non‐polar organic solvents. Their results suggest that osmotic shock could increase lipid recovery approximately two times. Despite being a very simple method for cell disruption, osmotic shock is not widely employed as it depends highly on cell wall properties; a higher lipid recovery could be achieved with other methods, such as microwave extraction or sonication (Lee et al., 2010; Prabakaran and Ravindran, 2011). These methods can be applied in the same way to different species, while the osmotic shock procedure is species‐dependent (Yoo et al., 2012). However, if well developed for one species, an efficient osmotic shock pre‐treatment can be highly desirable as it is scalable and does not require any special equipment.
Enzymatic disruption
Cell disruption using enzymes is an alternative for lipid extraction that has been poorly studied for algal cells. Enzymatic treatment results in a good lipid recovery with the advantage of disrupting cells with minimal damage to the target product due to high selectivity of the reactions (Mercer and Armenta, 2011; Demuez et al., 2015). A successful oil extraction from plant seeds was reported by Shah et al. (2004) with a combination of sonication and enzyme treatment, while 95% of oil was recovered from borage seeds with enzymatic treatment under cold pressing conditions (Soto et al., 2007). In microalgae, enzymatic hydrolysis with immobilized cellulose was studied by Fu et al. (2010) to break cell walls of Chlorella sp. resulting in a lipid extraction efficiency of 56% (14% more than unhydrolysed microalgae). Likewise, the enzymatic hydrolysis with cellulase on C. vulgaris cultures enhanced lipid extraction by 1.73‐fold compared with unhydrolysed cultures (Cho et al., 2013). The results of Zheng et al. (2011) show that enzymatic treatment on C. vulgaris had a lipid recovery of 7%, 22% and 24% with snailase, lysozyme and cellulose respectively, while Taher et al. (2014) reported the highest extraction yield of 16.6% using lysozyme. Liang et al. (2012) achieved the highest lipid recovery (around 35%) with snailase and trypsin in comparison to cellulase (16%), neutral protease (12%) and alkaline protease (8%). Besides, several studies of enzymatic hydrolysis in microalgae to enhance bioethanol (Choi et al., 2010; Rodrigues and Bon, 2011; Kim et al., 2014) and biogas production (Ciudad et al., 2014; Mahdy et al., 2014; Ometto et al., 2014) have reported the efficiency of this method.
Oxidative stress
Oxidative stress on algal cells has been recently studied by Bai et al. (2014) by applying different concentrations of free nitrous acid (FNA) as pre‐treatment for oil extraction in order to enhance the extraction efficiency. The authors report a lipid yield 2.4‐fold higher for cultures treated with FNA (up to 2.19 mg HNO2‐N/L). This is a promising technique that requires further studies as FNA is considered a green and renewable chemical (Wang et al., 2013) that may lower production costs. Other oxidative agents or UV light have also been proposed as a suitable pre‐treatment method to improve lipid extraction efficiency (Sharma et al., 2014).
Electroporation
Electroporation of wet algal biomass can be induced by applying a pulsed electric field to the cells, creating aqueous pores in the cell walls, enhancing mass transfer across the cell membrane. It is currently well established in molecular biology, by which electroporation can be used to facilitate the transportation of drugs, chemicals and foreign DNA products into the cell (Ho and Mittal, 1996); however, there have been very few studies conducted as to whether it is an efficient method for lipid extraction in microalgae. Sommerfeld et al. (2008) determined that electroporation achieved a total lipid extraction of 92% from Pseudochlorococcum sp. in comparison to 62% using the standard Bligh and Dyer method.
Supercritical carbon dioxide extraction
The traditional use of organic solvents for lipid extraction could be displaced by supercritical carbon dioxide (SCCO2) as an alternative solvent. SCCO2 is a green technology which is also efficient at extracting TAG and other lipid components, while it has a lower toxicity and produces an organic solvent‐free extract in a shorter extraction time compared with the use of organic solvents (Andrich et al., 2005; Halim et al., 2011; Soh and Zimmerman, 2011). Halim et al. (2011) compared SCCO2 extraction with hexane extraction of lipids from the marine microalga Chlorococcum sp. concluding that hexane extraction is significantly less efficient as it required about 5 times longer to achieve a comparable lipid yield in comparison to SCCO2. However this technique is suffering from the high costs associated with its energy consumption, required infrastructure and operation (Halim et al., 2011).
Future directions
When considering different extraction techniques, the main consideration should be placed on costs, scalability, safety and environmental concerns (Table 2). To make microalgal lipid extraction/recovery a more economically viable option which can compete better with conventional oil industries, there is a need to introduce and develop techniques which are not only efficient but also safe to operate, safe to the environment and sustainable. Hence, a major aim for future work could be the development of safe and low‐cost mechanical (solvent‐free) lipid recovery technology (Fig. 2). A desirable solvent‐free technique is one which can be applied in situ and at large scale, making microalgal lipid extraction/recovery a continuous process directly linked to algae cultivation. A promising approach seems to be the use of mechanical rupturing without the use of organic solvents (Fig. 3). Most economical and environmentally friendly could be the use of thermal or osmotic shock pre‐treatments which, depending on the cell wall properties of the microalgae, can result in the release of lipid bodies in the surrounding liquid. Next, oil droplets from the oil‐in‐water emulsion then need to be recovered. While organic solvents are suitable for this process, other mechanical separation technologies (e.g. by ultrafiltration) can be applied. Clearly, this area deserves further development. The co‐production of microalgal oil and protein‐rich biomass for the production of biodiesel and animal feed, respectively, has been discussed as a biorefinery concept in the literature. Both products are essentially produced at the same cost as one cannot be produced without the other. However, economical feasibility has yet to be established for these two low‐value products. Therefore, the industry has focussed more on higher value products, such as high‐protein microalgal biomass, omega‐3‐rich microalgal oil and microalgae‐derived carotenoids. Recent techno‐economic analyses have indicated that with the use of a cheap source of CO2 ($40/ton), microalgal oil and biomass can be produced for as little as $2250/kL (Davis et al., 2011) and $1790/ton (Slade and Bauen, 2013), respectively, but data from actual large‐scale production demonstration farms have yet to be included.
Table 2.
Comparison of different lipid extraction techniques from microalgae
| Method | Safe and environmentally friendly | Easily scalable | Economical | Efficient on wet biomass | Purity of the final product | Efficiency | Fast operational time | Other advantages/limitations |
|---|---|---|---|---|---|---|---|---|
| Ultrasound‐assisted extraction | ✓ | □ | ✗ | ✓ | ✓ | ✓ | ✓ |
Low set‐up costs Reducing thermal denaturation of essential biomolecules Possible oxidation of lipids due to prolonged exposure |
| Microwave‐assisted extraction | ✓ | ✗ | ✓ | ✓ | ✓ |
Inefficient on non‐polar or volatile compounds and/or the solvent(s) High maintenance costs at commercial scale |
||
| Hydrothermal liquefaction | ✗ | ✗ | ✓ | ✗ | ✓ | ✓ |
Applicable on wet biomass with water content as high as 80–95% Can achieve an energy recovery from biomass to fuel up to 80% Process may lead to high NOx emissions High equipment and maintenance costs |
|
| Enzymatic disruption | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ |
Minimal damage to the target product Long process duration Species‐dependent |
| Supercritical carbon dioxide extraction | ✓ | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ |
Green technology Uncontaminated products |
| Electroporation | ✓ | ✓ | ✓ | ✓ | Insufficient studies on its application for microalgal lipid extraction | |||
| Oxidative stress | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | Long process duration |
| Use of combination solvents | ✗ | ✗ | ✗ | ✓ | ✗ | ✓ | ✓ | |
| Osmotic shock | ✓ | ✓ | ✓ | ✓ | ✓ |
No special equipment required Depends highly on cell wall properties Species‐dependent |
Figure 2.
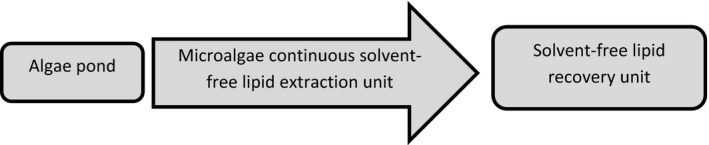
Proposed process for solvent‐free lipid extraction and recovery from wet, concentrated microalgae.
Figure 3.
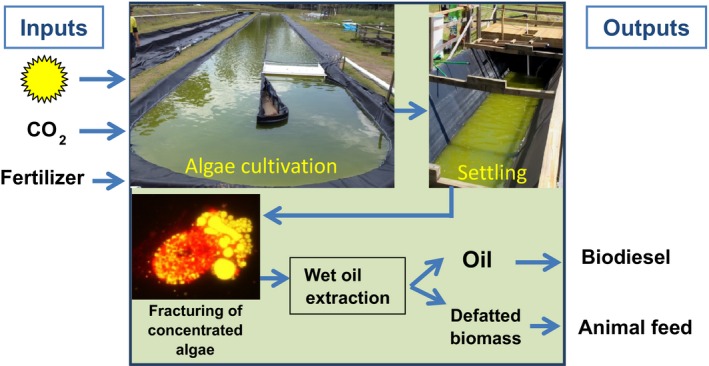
Example of a scenario for industrial production and extraction of microalgal lipids. Shown are the Algae Energy Farm at the University of Queensland with algae cultivation in raceway ponds, harvesting of microalgae by settling in a vee‐shaped pond, followed by mechanical fracturing of microalgal cells in concentrated algal slurry. The freed‐up oil droplets (shown in yellow by Nile red staining) and the remaining biomass (red) are then separated into oil and defatted biomass, which among many other applications, can be used for biodiesel and feed production respectively. Further optimization steps are required for the efficient separation of oil and defatted biomass.
To make co‐production of microalgal oil for biodiesel and biomass for feed a reality, it is essential that further cost reductions are applied to all steps. This should also include the concentration of microalgal cultures to algal slurries. The most cost‐effective way is probably settling (gravity‐assisted sedimentation; Fig. 3), while water can be recycled for further cultivation. Further cost savings can be achieved with large‐scale extraction, and the required process optimizations for wet extraction at large scale may bring further benefits. Importantly, the use of the remaining biomass after lipid extraction should be considered. Additional lipids may be recoverable from the partially defatted algal biomass by using organic solvents, as is common practice with feedstocks for vegetable oil. However, the use of organic solvents can result in residues remaining in the defatted algal biomass, therefore a solvent‐free extraction process would be preferable if the remaining biomass is to be used for human consumption or animal feed.
Acknowledgements
We thank Meat and Livestock Australia and the Australian Research Council for financial support.
Microbial Biotechnology (2016) 9(6), 718–726
FGN and LMGG contributed equally.
Funding Information
Funding for this work was provided by the Australian Research Council and Meat & Livestock Australia.
References
- Amarni, F. , and Kadi, H. (2010) Kinetics study of microwave‐assisted solvent extraction of oil from olive cake using hexane: comparison with the conventional extraction. Innov Food Sci Emerg Technol, 11: 322–7. [Google Scholar]
- Andrich, G. , Nesti, U. , Venturi, F. , Zinnai, A. , and Fiorentini, R. (2005) Supercritical fluid extraction of bioactive lipids from the microalga Nannochloropsis sp. Eur J Lipid Sci Technol, 107: 381–6. [Google Scholar]
- Bai, X. , Naghdi, F.G. , Ye, L. , Lant, P. , and Pratt, S. (2014) Enhanced lipid extraction from algae using free nitrous acid pretreatment. Bioresource Technol, 159: 36–40. [DOI] [PubMed] [Google Scholar]
- Balasubramanian, S. , Allen, J.D. , Kanitkar, A. , and Boldor, D. (2011) Oil extraction from Scenedesmus obliquus using a continuous microwave system–design, optimization, and quality characterization. Bioresource Technol, 102: 3396–403. [DOI] [PubMed] [Google Scholar]
- Barreiro, D.L. , Prins, W. , Ronsse, F. , and Brilman, W. (2013) Hydrothermal liquefaction (HTL) of microalgae for biofuel production: state of the art review and future prospects. Biomass Bioenergy, 53: 113–27. [Google Scholar]
- Biller, P. , Riley, R. , and Ross, A. (2011) Catalytic hydrothermal processing of microalgae: decomposition and upgrading of lipids. Bioresource Technol, 102: 4841–8. [DOI] [PubMed] [Google Scholar]
- Biller, P. , Ross, A.B. , Skill, S. , Lea‐Langton, A. , Balasundaram, B. , Hall, C. , et al (2012) Nutrient recycling of aqueous phase for microalgae cultivation from the hydrothermal liquefaction process. Algal Res, 1: 70–6. [Google Scholar]
- de Boer, K. , Moheimani, N.R. , Borowitzka, M.A. , and Bahri, P.A. (2012) Extraction and conversion pathways for microalgae to biodiesel: a review focused on energy consumption. J Appl Phycol, 24: 1681–98. [Google Scholar]
- Chemat, F. , Grondin, I. , Costes, P. , Moutoussamy, L. , Sing, A.S.C. , and Smadja, J. (2004) High power ultrasound effects on lipid oxidation of refined sunflower oil. Ultrasonics Sonochem, 11: 281–5. [DOI] [PubMed] [Google Scholar]
- Chen, M. , Liu, T. , Chen, X. , Chen, L. , Zhang, W. , Wang, J. , et al (2012) Subcritical co‐solvents extraction of lipid from wet microalgae pastes of Nannochloropsis sp. Eur J Lipid Sci Technol, 114: 205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, Y. , Zhou, W. , Gao, C. , Lan, K. , Gao, Y. , and Wu, Q. (2009) Biodiesel production from Jerusalem artichoke (Helianthus tuberosus L.) tuber by heterotrophic microalgae Chlorella protothecoides . J Chem Technol Biotech, 84: 777–81. [Google Scholar]
- Chisti, Y. (2007) Biodiesel from microalgae. Biotech Adv, 25: 294–306. [DOI] [PubMed] [Google Scholar]
- Chiu, S.‐Y. , Kao, C.‐Y. , Tsai, M.‐T. , Ong, S.‐C. , Chen, C.‐H. , and Lin, C.‐S. (2009) Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresource Technol, 100: 833–8. [DOI] [PubMed] [Google Scholar]
- Cho, H.‐S. , Oh, Y.‐K. , Park, S.‐C. , Lee, J.‐W. , and Park, J.‐Y. (2013) Effects of enzymatic hydrolysis on lipid extraction from Chlorella vulgaris . Renewable Energy, 54: 156–60. [Google Scholar]
- Choi, S.P. , Nguyen, M.T. , and Sim, S.J. (2010) Enzymatic pretreatment of Chlamydomonas reinhardtii biomass for ethanol production. Bioresource Technol, 101: 5330–6. [DOI] [PubMed] [Google Scholar]
- Ciudad, G. , Rubilar, O. , Azócar, L. , Toro, C. , Cea, M. , Torres, Á. , et al (2014) Performance of an enzymatic extract in Botrycoccus braunii cell wall disruption. J Biosci Bioeng, 117: 75–80. [DOI] [PubMed] [Google Scholar]
- Cooney, M. , Young, G. , and Nagle, N. (2009) Extraction of bio‐oils from microalgae. Separ Purific Rev, 38: 291–325. [Google Scholar]
- Davis, R. , Aden, A. , and Pienkos, P.T. (2011) Techno‐economic analysis of autotrophic microalgae for fuel production. Appl Energy, 88: 3524–31. [Google Scholar]
- Demuez, M. , Mahdy, A. , Tomás‐Pejó, E. , González‐Fernández, C. , and Ballesteros, M. (2015) Enzymatic cell disruption of microalgae biomass in biorefinery processes. Biotechnol Bioeng, 112: 1955–66. [DOI] [PubMed] [Google Scholar]
- Eskilsson, C.S. , and Björklund, E. (2000) Analytical‐scale microwave‐assisted extraction. J Chromatography A, 902: 227–50. [DOI] [PubMed] [Google Scholar]
- Fu, C.‐C. , Hung, T.‐C. , Chen, J.‐Y. , Su, C.‐H. , and Wu, W.‐T. (2010) Hydrolysis of microalgae cell walls for production of reducing sugar and lipid extraction. Bioresource Technol, 101: 8750–4. [DOI] [PubMed] [Google Scholar]
- Ganzler, K. , Salgo, A. , and Valkó, K. (1986) Microwave extraction: a novel sample preparation method for chromatography. J Chromatography A, 371: 299–306. [DOI] [PubMed] [Google Scholar]
- Garcia Alba, L. , Torri, C. , Samorì, C. , van der Spek, J. , Fabbri, D. , Kersten, S.R. , and Brilman, D.W. (2011) Hydrothermal treatment (HTT) of microalgae: evaluation of the process as conversion method in an algae biorefinery concept. Energy Fuels, 26: 642–57. [Google Scholar]
- Ghasemi Naghdi, F. , Thomas‐Hall, S.R. , Durairatnam, R. , Pratt, S. , and Schenk, P.M. (2014) Comparative effects of biomass pre‐treatments for direct and indirect transesterification to enhance microalgal lipid recovery. Front Energy Res, 2: 57. [Google Scholar]
- Gouveia, L. , and Oliveira, A.C. (2009) Microalgae as a raw material for biofuels production. J Industr Microbiol Biotechnol, 36: 269–74. [DOI] [PubMed] [Google Scholar]
- Greenwell, H. , Laurens, L. , Shields, R. , Lovitt, R. , and Flynn, K. (2009) Placing microalgae on the biofuels priority list: a review of the technological challenges. J Royal Soc Interface, 7: 703–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halim, R. , Gladman, B. , Danquah, M.K. , and Webley, P.A. (2011) Oil extraction from microalgae for biodiesel production. Bioresource Technol, 102: 178–85. [DOI] [PubMed] [Google Scholar]
- Harun, R. , Singh, M. , Forde, G.M. , and Danquah, M.K. (2010) Bioprocess engineering of microalgae to produce a variety of consumer products. Renewable Sustain Energy Rev, 14: 1037–47. [Google Scholar]
- Ho, S. , and Mittal, G. (1996) Electroporation of cell membranes: a review. Critic Rev Biotechnol, 16: 349–62. [DOI] [PubMed] [Google Scholar]
- Ho, S.‐H. , Chen, W.‐M. , and Chang, J.‐S. (2010) Scenedesmus obliquus CNW‐N as a potential candidate for CO2 mitigation and biodiesel production. Bioresource Technol, 101: 8725–30. [DOI] [PubMed] [Google Scholar]
- Hu, Q. , Sommerfeld, M. , Jarvis, E. , Ghirardi, M. , Posewitz, M. , Seibert, M. , and Darzins, A. (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J, 54: 621–39. [DOI] [PubMed] [Google Scholar]
- Kim, J. , Yoo, G. , Lee, H. , Lim, J. , Kim, K. , Kim, C.W. , et al (2013) Methods of downstream processing for the production of biodiesel from microalgae. Biotechnol Adv, 31: 862–76. [DOI] [PubMed] [Google Scholar]
- Kim, K.H. , Choi, I.S. , Kim, H.M. , Wi, S.G. , and Bae, H.‐J. (2014) Bioethanol production from the nutrient stress‐induced microalga Chlorella vulgaris by enzymatic hydrolysis and immobilized yeast fermentation. Bioresource Technol, 153: 47–54. [DOI] [PubMed] [Google Scholar]
- Kita, K. , Okada, S. , Sekino, H. , Imou, K. , Yokoyama, S. , and Amano, T. (2010) Thermal pre‐treatment of wet microalgae harvest for efficient hydrocarbon recovery. Appl Energy, 87: 2420–3. [Google Scholar]
- Lee, J.‐Y. , Yoo, C. , Jun, S.‐Y. , Ahn, C.‐Y. , and Oh, H.‐M. (2010) Comparison of several methods for effective lipid extraction from microalgae. Bioresource Technol, 101: S75–7. [DOI] [PubMed] [Google Scholar]
- Lee, A.K. , Lewis, D.M. , and Ashman, P.J. (2012) Disruption of microalgal cells for the extraction of lipids for biofuels: processes and specific energy requirements. Biomass Bioenergy, 46: 89–101. [Google Scholar]
- Levine, R.B. , Pinnarat, T. , and Savage, P.E. (2010) Biodiesel production from wet algal biomass through in situ lipid hydrolysis and supercritical transesterification. Energy Fuels, 24: 5235–43. [Google Scholar]
- Liang, Y. , Sarkany, N. , and Cui, Y. (2009) Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett, 31: 1043–9. [DOI] [PubMed] [Google Scholar]
- Liang, K. , Zhang, Q. , and Cong, W. (2012) Enzyme‐assisted aqueous extraction of lipid from microalgae. J Agricult Food Chem, 60: 11771–6. [DOI] [PubMed] [Google Scholar]
- Liu, J. , Huang, J. , Sun, Z. , Zhong, Y. , Jiang, Y. , and Chen, F. (2011) Differential lipid and fatty acid profiles of photoautotrophic and heterotrophic Chlorella zofingiensis: assessment of algal oils for biodiesel production. Bioresource Technol, 102: 106–10. [DOI] [PubMed] [Google Scholar]
- Mahdy, A. , Mendez, L. , Ballesteros, M. , and González‐Fernández, C. (2014) Enhanced methane production of Chlorella vulgaris and Chlamydomonas reinhardtii by hydrolytic enzymes addition. Energy Convers Managem, 85: 551–7. [Google Scholar]
- Mercer, P. , and Armenta, R.E. (2011) Developments in oil extraction from microalgae. Eur J Lipid Sci Technol, 113: 539–47. [Google Scholar]
- Metherel, A.H. , Taha, A.Y. , Izadi, H. , and Stark, K.D. (2009) The application of ultrasound energy to increase lipid extraction throughput of solid matrix samples (flaxseed). Prostaglandins Leukot Essent Fatty Acids, 81: 417–23. [DOI] [PubMed] [Google Scholar]
- Ometto, F. , Quiroga, G. , Pšenička, P. , Whitton, R. , Jefferson, B. , and Villa, R. (2014) Impacts of microalgae pre‐treatments for improved anaerobic digestion: thermal treatment, thermal hydrolysis, ultrasound and enzymatic hydrolysis. Water Res, 65: 350–61. [DOI] [PubMed] [Google Scholar]
- Pal, D. , Khozin‐Goldberg, I. , Cohen, Z. , and Boussiba, S. (2011) The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Applied Microbiol Biotechn, 90: 1429–41. [DOI] [PubMed] [Google Scholar]
- Passell, H. , Dhaliwal, H. , Reno, M. , Wu, B. , Amotz, A.B. , Ivry, E. , et al (2013) Algae biodiesel life cycle assessment using current commercial data. J Environm Managem, 129: 103–11. [DOI] [PubMed] [Google Scholar]
- Prabakaran, P. , and Ravindran, A. (2011) A comparative study on effective cell disruption methods for lipid extraction from microalgae. Lett Appl Microbiol, 53: 150–4. [DOI] [PubMed] [Google Scholar]
- Ranjith Kumar, R. , Hanumantha Rao, P. , and Arumugam, M. (2015) Lipid extraction methods from microalgae: a comprehensive review. Front Energy Res, 2: 61. [Google Scholar]
- Refaat, A. , El Sheltawy, S. , and Sadek, K. (2008) Optimum reaction time, performance and exhaust emissions of biodiesel produced by microwave irradiation. Intern J Environm Sci Technol, 5: 315–22. [Google Scholar]
- Rodolfi, L. , Chini Zittelli, G. , Bassi, N. , Padovani, G. , Biondi, N. , Bonini, G. , and Tredici, M.R. (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low‐cost photobioreactor. Biotechnol Bioeng, 102: 100–12. [DOI] [PubMed] [Google Scholar]
- Rodrigues, M.A. , and Bon, E.P.d.S. (2011) Evaluation of Chlorella (Chlorophyta) as source of fermentable sugars via cell wall enzymatic hydrolysis. Enzyme Res, 2011: 405603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, A. , Biller, P. , Kubacki, M. , Li, H. , Lea‐Langton, A. , and Jones, J. (2010) Hydrothermal processing of microalgae using alkali and organic acids. Fuel, 89: 2234–43. [Google Scholar]
- Ryckebosch, E. , Muylaert, K. , and Foubert, I. (2012) Optimization of an analytical procedure for extraction of lipids from microalgae. J Amer Oil Chemists' Soc, 89: 189–98. [Google Scholar]
- Shah, S. , Sharma, A. , and Gupta, M. (2004) Extraction of oil from Jatropha curcas L. seed kernels by enzyme assisted three phase partitioning. Industr Crops Products, 20: 275–9. [Google Scholar]
- Sharma, K. , Li, Y. , and Schenk, P.M. (2014) UV‐C‐mediated lipid induction and settling, a step change towards economical microalgal biodiesel production. Green Chem, 16: 3539–48. [Google Scholar]
- Singh, N.K. , and Dhar, D.W. (2011) Microalgae as second generation biofuel. A review. Agron Sustain Develop, 31: 605–29. [Google Scholar]
- Slade, R. , and Bauen, A. (2013) Micro‐algae cultivation for biofuels: cost, energy balance, environmental impacts and future prospects. Biomass Bioenergy, 53: 29–38. [Google Scholar]
- Soh, L. , and Zimmerman, J. (2011) Biodiesel production: the potential of algal lipids extracted with supercritical carbon dioxide. Green Chem, 13: 1422–9. [Google Scholar]
- Sommerfeld, M. , Chen, W. , Hu, Q. , Giorgi, D. , Navapanich, T. , Ingram, M. , and Erdman, R. (2008) Application of Electroporation for Lipid Extraction from Microlalgae. Seattle, WA, USA: Algae Biomass Summit. [Google Scholar]
- Šoštarič, M. , Klinar, D. , Bricelj, M. , Golob, J. , Berovič, M. , and Likozar, B. (2012) Growth, lipid extraction and thermal degradation of the microalga Chlorella vulgaris . New Biotechnol, 29: 325–31. [DOI] [PubMed] [Google Scholar]
- Soto, C. , Chamy, R. , and Zuniga, M. (2007) Enzymatic hydrolysis and pressing conditions effect on borage oil extraction by cold pressing. Food Chem, 102: 834–40. [Google Scholar]
- Suslick, K.S. , and Flannigan, D.J. (2008) Inside a collapsing bubble: sonoluminescence and the conditions during cavitation. Ann Rev Phys Chem, 59: 659–83. [DOI] [PubMed] [Google Scholar]
- Taher, H. , Al‐Zuhair, S. , Al‐Marzouqi, A.H. , Haik, Y. , and Farid, M. (2014) Effective extraction of microalgae lipids from wet biomass for biodiesel production. Biomass Bioenergy, 66: 159–67. [Google Scholar]
- Toor, S.S. , Rosendahl, L. , and Rudolf, A. (2011) Hydrothermal liquefaction of biomass: a review of subcritical water technologies. Energy, 36: 2328–42. [Google Scholar]
- Toor, S.S. , Reddy, H. , Deng, S. , Hoffmann, J. , Spangsmark, D. , Madsen, L.B. , et al (2013) Hydrothermal liquefaction of Spirulina and Nannochloropsis salina under subcritical and supercritical water conditions. Bioresource Technol, 131: 413–9. [DOI] [PubMed] [Google Scholar]
- Torres, C.M. , Ríos, S.D. , Torras, C. , Salvadó, J. , Mateo‐Sanz, J.M. , and Jiménez, L. (2013) Microalgae‐based biodiesel: a multicriteria analysis of the production process using realistic scenarios. Bioresource Technol, 147: 7–16. [DOI] [PubMed] [Google Scholar]
- Uduman, N. , Qi, Y. , Danquah, M.K. , Forde, G.M. , and Hoadley, A. (2010) Dewatering of microalgal cultures: a major bottleneck to algae‐based fuels. J Renewable Sustain Energy, 2: 012701. [Google Scholar]
- Vinatoru, M. , Toma, M. , Radu, O. , Filip, P. , Lazurca, D. , and Mason, T. (1997) The use of ultrasound for the extraction of bioactive principles from plant materials. Ultrasonics Sonochem, 4: 135–9. [DOI] [PubMed] [Google Scholar]
- Wang, L. , and Weller, C.L. (2006) Recent advances in extraction of nutraceuticals from plants. Trends Food Sci Technol, 17: 300–12. [Google Scholar]
- Wang, Q. , Ye, L. , Jiang, G. , Jensen, P.D. , Batstone, D.J. , and Yuan, Z. (2013) Free nitrous acid (FNA)‐based pretreatment enhances methane production from waste activated sludge. Environ Sci Technol, 47: 11897–904. [DOI] [PubMed] [Google Scholar]
- Xu, H. , Miao, X. , and Wu, Q. (2006) High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J Biotechnol, 126: 499–507. [DOI] [PubMed] [Google Scholar]
- Yoo, G. , Park, W.‐K. , Kim, C.W. , Choi, Y.‐E. , and Yang, J.‐W. (2012) Direct lipid extraction from wet Chlamydomonas reinhardtii biomass using osmotic shock. Bioresource Technol, 123: 717–22. [DOI] [PubMed] [Google Scholar]
- Zheng, H. , Yin, J. , Gao, Z. , Huang, H. , Ji, X. , and Dou, C. (2011) Disruption of Chlorella vulgaris cells for the release of biodiesel‐producing lipids: a comparison of grinding, ultrasonication, bead milling, enzymatic lysis, and microwaves. Appl Biochem Biotechnol, 164: 1215–24. [DOI] [PubMed] [Google Scholar]


