Abstract
Infection by certain human papillomavirus types is regarded as the major risk factor in the development of cervical cancer, one of the most common cancers of women worldwide. Analysis of the immunogenic and structural features of papillomavirus virions has been hampered by the inability to efficiently propagate the viruses in cultured cells. For instance, it has not been established whether the major capsid protein L1 alone is sufficient for virus particle assembly. In addition, it is not known whether L1, L2 (the minor capsid protein), or both present the immunodominant epitopes required for induction of high-titer neutralizing antibodies. We have expressed the L1 major capsid proteins of bovine papillomavirus type 1 and human papillomavirus type 16 in insect cells via a baculovirus vector and analyzed their conformation and immunogenicity. The L1 proteins were expressed at high levels and assembled into structures that closely resembled papillomavirus virions. The self-assembled bovine papillomavirus L1, in contrast to L1 extracted from recombinant bacteria or denatured virions, also mimicked intact bovine papillomavirus virions in being able to induce high-titer neutralizing rabbit antisera. These results indicate that L1 protein has the intrinsic capacity to assemble into empty capsid-like structures whose immunogenicity is similar to infectious virions. This type of L1 preparation might be considered as a candidate for a serological test to measure antibodies to conformational virion epitopes and for a vaccine to prevent papillomavirus infection.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
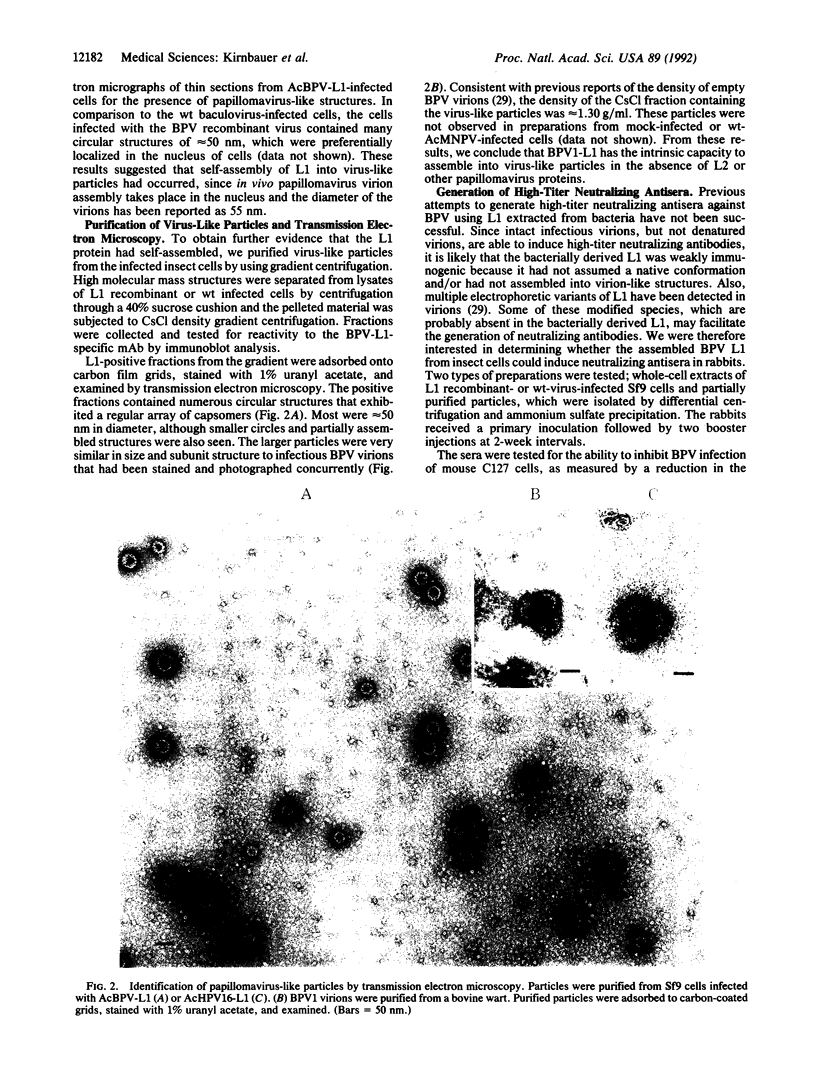
- Baker T. S., Newcomb W. W., Olson N. H., Cowsert L. M., Olson C., Brown J. C. Structures of bovine and human papillomaviruses. Analysis by cryoelectron microscopy and three-dimensional image reconstruction. Biophys J. 1991 Dec;60(6):1445–1456. doi: 10.1016/S0006-3495(91)82181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAWFORD L. V., CRAWFORD E. M. A COMPARATIVE STUDY OF POLYOMA AND PAPILLOMA VIRUSES. Virology. 1963 Oct;21:258–263. doi: 10.1016/0042-6822(63)90265-4. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Howley P. M., Levinson A. D., Seeburg P. H. The primary structure and genetic organization of the bovine papillomavirus type 1 genome. Nature. 1982 Oct 7;299(5883):529–534. doi: 10.1038/299529a0. [DOI] [PubMed] [Google Scholar]
- Christensen N. D., Kreider J. W., Cladel N. M., Patrick S. D., Welsh P. A. Monoclonal antibody-mediated neutralization of infectious human papillomavirus type 11. J Virol. 1990 Nov;64(11):5678–5681. doi: 10.1128/jvi.64.11.5678-5681.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen N. D., Kreider J. W., Kan N. C., DiAngelo S. L. The open reading frame L2 of cottontail rabbit papillomavirus contains antibody-inducing neutralizing epitopes. Virology. 1991 Apr;181(2):572–579. doi: 10.1016/0042-6822(91)90890-n. [DOI] [PubMed] [Google Scholar]
- Cowsert L. M., Lake P., Jenson A. B. Topographical and conformational epitopes of bovine papillomavirus type 1 defined by monoclonal antibodies. J Natl Cancer Inst. 1987 Nov;79(5):1053–1057. [PubMed] [Google Scholar]
- Dollard S. C., Wilson J. L., Demeter L. M., Bonnez W., Reichman R. C., Broker T. R., Chow L. T. Production of human papillomavirus and modulation of the infectious program in epithelial raft cultures. OFF. Genes Dev. 1992 Jul;6(7):1131–1142. doi: 10.1101/gad.6.7.1131. [DOI] [PubMed] [Google Scholar]
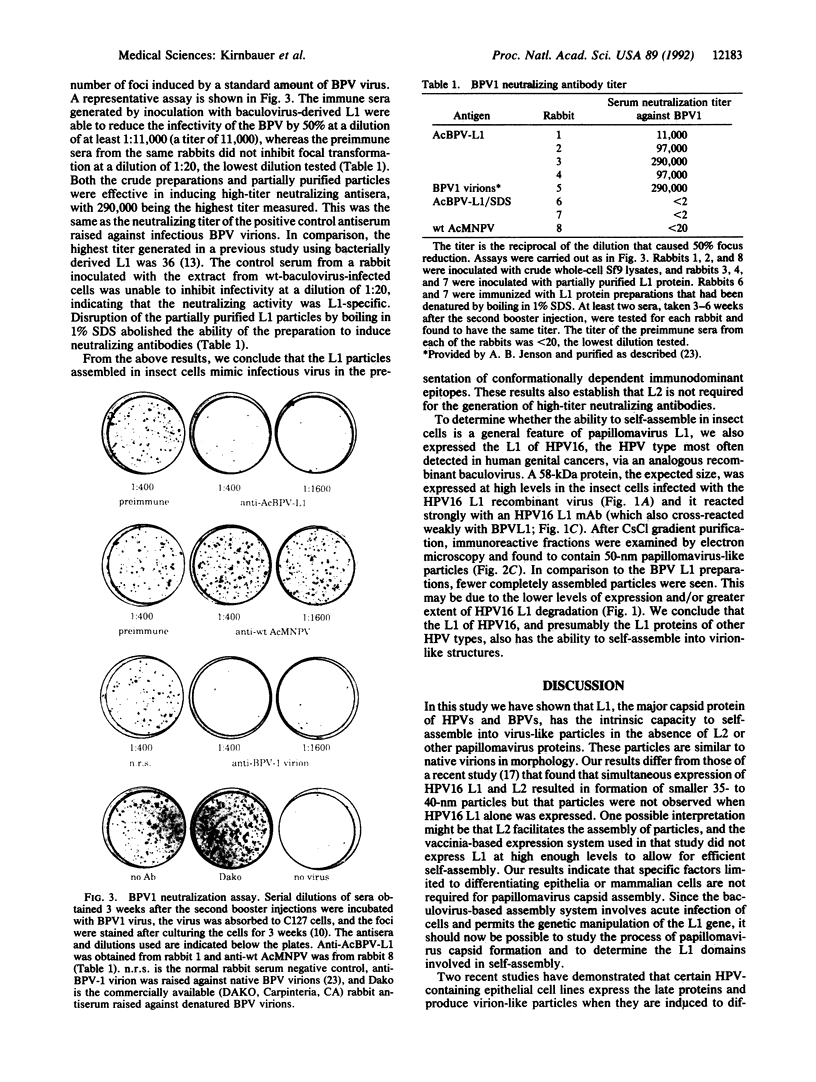
- Dvoretzky I., Shober R., Chattopadhyay S. K., Lowy D. R. A quantitative in vitro focus assay for bovine papilloma virus. Virology. 1980 Jun;103(2):369–375. doi: 10.1016/0042-6822(80)90195-6. [DOI] [PubMed] [Google Scholar]
- Ghim S., Christensen N. D., Kreider J. W., Jenson A. B. Comparison of neutralization of BPV-1 infection of C127 cells and bovine fetal skin xenografts. Int J Cancer. 1991 Sep 9;49(2):285–289. doi: 10.1002/ijc.2910490224. [DOI] [PubMed] [Google Scholar]
- Griffith J. P., Griffith D. L., Rayment I., Murakami W. T., Caspar D. L. Inside polyomavirus at 25-A resolution. Nature. 1992 Feb 13;355(6361):652–654. doi: 10.1038/355652a0. [DOI] [PubMed] [Google Scholar]
- Hartig P. C., Cardon M. C., Kawanishi C. Y. Generation of recombinant baculovirus via liposome-mediated transfection. Biotechniques. 1991 Sep;11(3):310, 312-3. [PubMed] [Google Scholar]
- Jarrett W. F., O'Neil B. W., Gaukroger J. M., Laird H. M., Smith K. T., Campo M. S. Studies on vaccination against papillomaviruses: a comparison of purified virus, tumour extract and transformed cells in prophylactic vaccination. Vet Rec. 1990 May 5;126(18):449–452. [PubMed] [Google Scholar]
- Jarrett W. F., Smith K. T., O'Neil B. W., Gaukroger J. M., Chandrachud L. M., Grindlay G. J., McGarvie G. M., Campo M. S. Studies on vaccination against papillomaviruses: prophylactic and therapeutic vaccination with recombinant structural proteins. Virology. 1991 Sep;184(1):33–42. doi: 10.1016/0042-6822(91)90819-w. [DOI] [PubMed] [Google Scholar]
- Jenison S. A., Yu X. P., Valentine J. M., Koutsky L. A., Christiansen A. E., Beckmann A. M., Galloway D. A. Evidence of prevalent genital-type human papillomavirus infections in adults and children. J Infect Dis. 1990 Jul;162(1):60–69. doi: 10.1093/infdis/162.1.60. [DOI] [PubMed] [Google Scholar]
- Jin X. W., Cowsert L. M., Pilacinski W. P., Jenson A. B. Identification of L2 open reading frame gene products of bovine papillomavirus type 1 using monoclonal antibodies. J Gen Virol. 1989 May;70(Pt 5):1133–1140. doi: 10.1099/0022-1317-70-5-1133. [DOI] [PubMed] [Google Scholar]
- Jin X. W., Cowsert L., Marshall D., Reed D., Pilacinski W., Lim L. Y., Jenson A. B. Bovine serological response to a recombinant BPV-1 major capsid protein vaccine. Intervirology. 1990;31(6):345–354. doi: 10.1159/000150171. [DOI] [PubMed] [Google Scholar]
- Kajigaya S., Fujii H., Field A., Anderson S., Rosenfeld S., Anderson L. J., Shimada T., Young N. S. Self-assembled B19 parvovirus capsids, produced in a baculovirus system, are antigenically and immunogenically similar to native virions. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4646–4650. doi: 10.1073/pnas.88.11.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P. M., Storgaard L., Fey S. J. Proteins present in bovine papillomavirus particles. J Virol. 1987 Nov;61(11):3596–3601. doi: 10.1128/jvi.61.11.3596-3601.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddington R. C., Yan Y., Moulai J., Sahli R., Benjamin T. L., Harrison S. C. Structure of simian virus 40 at 3.8-A resolution. Nature. 1991 Nov 28;354(6351):278–284. doi: 10.1038/354278a0. [DOI] [PubMed] [Google Scholar]
- Lin Y. L., Borenstein L. A., Selvakumar R., Ahmed R., Wettstein F. O. Effective vaccination against papilloma development by immunization with L1 or L2 structural protein of cottontail rabbit papillomavirus. Virology. 1992 Apr;187(2):612–619. doi: 10.1016/0042-6822(92)90463-y. [DOI] [PubMed] [Google Scholar]
- McLean C. S., Churcher M. J., Meinke J., Smith G. L., Higgins G., Stanley M., Minson A. C. Production and characterisation of a monoclonal antibody to human papillomavirus type 16 using recombinant vaccinia virus. J Clin Pathol. 1990 Jun;43(6):488–492. doi: 10.1136/jcp.43.6.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers C., Frattini M. G., Hudson J. B., Laimins L. A. Biosynthesis of human papillomavirus from a continuous cell line upon epithelial differentiation. Science. 1992 Aug 14;257(5072):971–973. doi: 10.1126/science.1323879. [DOI] [PubMed] [Google Scholar]
- Nakai Y., Lancaster W. D., Lim L. Y., Jenson A. B. Monoclonal antibodies to genus- and type-specific papillomavirus structural antigens. Intervirology. 1986;25(1):30–37. doi: 10.1159/000149652. [DOI] [PubMed] [Google Scholar]
- OLSON C., SEGRE D., SKIDMORE L. V. Further observations on immunity to bovine cutaneous papillomatosis. Am J Vet Res. 1960 Mar;21:233–242. [PubMed] [Google Scholar]
- Pilacinski W. P., Glassman D. L., Glassman K. F., Reed D. E., Lum M. A., Marshall R. F., Muscoplat C. C., Faras A. J. Immunization against bovine papillomavirus infection. Ciba Found Symp. 1986;120:136–156. doi: 10.1002/9780470513309.ch10. [DOI] [PubMed] [Google Scholar]
- Salunke D. M., Caspar D. L., Garcea R. L. Self-assembly of purified polyomavirus capsid protein VP1. Cell. 1986 Sep 12;46(6):895–904. doi: 10.1016/0092-8674(86)90071-1. [DOI] [PubMed] [Google Scholar]
- Schiffman M. H. Recent progress in defining the epidemiology of human papillomavirus infection and cervical neoplasia. J Natl Cancer Inst. 1992 Mar 18;84(6):394–398. doi: 10.1093/jnci/84.6.394. [DOI] [PubMed] [Google Scholar]
- Seedorf K., Krämmer G., Dürst M., Suhai S., Röwekamp W. G. Human papillomavirus type 16 DNA sequence. Virology. 1985 Aug;145(1):181–185. doi: 10.1016/0042-6822(85)90214-4. [DOI] [PubMed] [Google Scholar]
- Stevens C. E., Taylor P. E., Tong M. J., Toy P. T., Vyas G. N., Nair P. V., Weissman J. Y., Krugman S. Yeast-recombinant hepatitis B vaccine. Efficacy with hepatitis B immune globulin in prevention of perinatal hepatitis B virus transmission. JAMA. 1987 May 15;257(19):2612–2616. doi: 10.1001/jama.257.19.2612. [DOI] [PubMed] [Google Scholar]
- Valenzuela P., Medina A., Rutter W. J., Ammerer G., Hall B. D. Synthesis and assembly of hepatitis B virus surface antigen particles in yeast. Nature. 1982 Jul 22;298(5872):347–350. doi: 10.1038/298347a0. [DOI] [PubMed] [Google Scholar]
- Wang X. Z., Ooi B. G., Miller L. K. Baculovirus vectors for multiple gene expression and for occluded virus production. Gene. 1991 Apr;100:131–137. doi: 10.1016/0378-1119(91)90358-i. [DOI] [PubMed] [Google Scholar]
- Zhou J., Sun X. Y., Stenzel D. J., Frazer I. H. Expression of vaccinia recombinant HPV 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion-like particles. Virology. 1991 Nov;185(1):251–257. doi: 10.1016/0042-6822(91)90772-4. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Viruses in human cancers. Science. 1991 Nov 22;254(5035):1167–1173. doi: 10.1126/science.1659743. [DOI] [PubMed] [Google Scholar]