Abstract
Background
Mycotic and oncotic aneurysms may result in devastating neurologic sequelae if undetected. The objectives of this study were to examine interobserver variability and accuracy of cross-sectional imaging for the detection of distal territory mycotic and oncotic aneurysms.
Methods
We searched our institutional database for all radiology reports from 2005 to 2015 with an indication or diagnosis of mycotic or oncotic aneurysm. Patients who underwent DSA and either CTA or MRA within 12 weeks of each other were identified. The cross-sectional images from each study were blinded and reviewed by two radiologists. If positive for aneurysm, location and number of aneurysms were reported. Sensitivity, specificity, positive predictive value, negative predictive value, and interobserver variability were determined for MRA and MRA/CTA.
Results
Twenty-five patients were included in this study. Ten (40%) harbored distal aneurysms. Cross-sectional imaging had a sensitivity of 45.5%, specificity of 90.0%, and kappa value of 0.29 (0.00–0.69) for the detection of cerebral mycotic and oncotic aneurysms.
Conclusions
Because of the low sensitivity and high interobserver variability of cross-sectional imaging, DSA should remain the gold standard for evaluation of suspected oncotic and mycotic aneurysms. In cases in which cross sectional imaging is negative and there is a high clinical suspicion for mycotic aneurysm, DSA should be strongly considered.
Keywords: Aneurysm, angiography, oncotic, mycotic
Introduction
Although both mycotic and oncotic aneurysms are relatively uncommon, they can result in devastating neurological consequences, as both have the potential to be complicated by ischemic and hemorrhagic infarcts. Cerebral mycotic aneurysms form as a result of embolization of septic material, most commonly from infective endocarditis, while oncotic aneurysms result from the embolization of tumor material that both weakens and infiltrates through vessel walls. In contrast to berry aneurysms, which characteristically arise around the Circle of Willis, the majority of oncotic and mycotic aneurysms are smaller, arise from more distal arterial branches—most commonly middle cerebral artery (MCA) branches—and can be fusiform, saccular, or irregular in morphology.1,2 Because mycotic and oncotic aneurysms are prone to hemorrhage, accurate detection of these lesions is essential in order to allow for appropriate treatment.3,4
Multiple studies have documented the acceptable sensitivity of magnetic resonance angiography (MRA) and computed tomography (CTA) for detecting Circle of Willis aneurysms;5,6 however, to our knowledge, there have been no systematic studies examining the sensitivity of cross-sectional imaging in detecting these smaller and more distally located cerebral mycotic and oncotic aneurysms. The current gold standard for detection of cerebral mycotic and oncotic aneurysms is catheter-based digital subtraction angiography (DSA). The objectives of this study were to examine interobserver variability and accuracy of cross-sectional imaging for the detection of distal territory mycotic and oncotic aneurysms.
Methods
Patient selection and chart review
Following institutional review board (IRB) approval, our institutional radiology reporting database was queried for all patient reports from 2005 to 2015 with a study indication or diagnosis of oncotic or mycotic aneurysm. Both positive and negative cases were included. Patients who underwent both a conventional digital subtraction cerebral angiogram and either CTA or MRA within a 12-week period of each other were identified. A retrospective chart review was then performed for each patient who met the criteria and the following pertinent clinical information was obtained: indication, age at diagnosis, gender, presenting symptom, type of cross-sectional study performed (CTA, MRA, or both), dates of the relevant imaging studies, presence of oncotic or mycotic aneurysm, number of aneurysms, and aneurysm territory. The patients that met the criteria were divided by presence or absence of oncotic or mycotic aneurysm(s) as determined by the DSA report. The clinical presentation for patients with mycotic or oncotic aneurysms were categorized as either (1) hemorrhage, (2) ischemic infarct, or (3) discovered incidentally. The presenting symptom for patients with studies negative for oncotic or mycotic aneurysm was categorized as either (1) hemorrhage, (2) ischemic infarct, or (3) neither hemorrhage nor ischemic infarct. Aneurysm territories were subdivided by (1) right distal anterior circulation, (2) left distal anterior circulation, or (3) posterior circulation.
Imaging evaluation
All images were anonymized and two radiologists, each with four or greater years of experience, viewed each exam in random order. Ground truth was determined by an experienced subspecialty trained neurointerventional radiologist reviewing the angiographic images. The readers were aware that they were searching for cerebral aneurysms, but no information was provided on patient demographics or patient history or prior interpretation. No comparison imaging studies were provided. MRA exams were performed on 1.5T and 3T scanners. No contrast was administered in any of the exams. Standard gradient-echo time of flight (TOF)-MRA sequences were used (repetition time (TR) ∼20 sec, 640 × 640 pixels, field of view (FOV) 180 mm × 180 mm). CTA exams were generally performed on 16- or 64-section helical CT scanners. Axial acquisitions were performed from the base of the C1 vertebral body to the vertex with slice thickness of 0.75 mm, kVp 120, mA 494, image size of 512 × 512 pixels and FOV 250 mm × 250 mm. The readers assessed CTA exams consisting of standard contiguous 1.5 mm thick axial CTA images with three-dimensional (3D) volume reconstructions generated at the time of the original exam. MRA exams consisted of axial slices from TOF-MRA images and the 3D volumetric reconstructions generated at the time of the exam. Imaging findings were categorized as either (1) negative for aneurysm or (2) positive for aneurysm. If positive, location was assessed as (1) left anterior circulation, (2) right anterior circulation, and/or (3) posterior circulation. If positive for any of those locations, the number of aneurysms was then requested by free response with a numeric integer.
Statistical analysis
All statistical analyses were performed using JMP 10.0. Interobserver variability was assessed with the kappa-statistic. A kappa < 0 indicated no agreement, 0–0.20 indicated slight agreement, 0.21–0.40 indicated fair agreement, 0.41–0.60 indicated moderate agreement, 0.61–0.80 indicated substantial agreement, and 0.81–1 indicated almost perfect agreement. Interobserver agreement was calculated for MRA interpretations and all cross-sectional imaging (CTA/MRA) interpretations. Because there were only five CTAs performed, no kappa was calculated for CTA interpretation. Sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV) and accuracy were calculated for all cross-sectional imaging and MRA in reference to the gold standard read by the senior neuroradiologist. These were calculated on a perpatient basis and per-aneurysm basis. Receiver operating characteristic (ROC) analyses were performed on both a per-patient and per-aneurysm basis as well.
Results
Patient population
Twenty-five patients met the inclusion criteria for chart review described above. Ten (40%) of these patients were positive for the presence of at least one distal territory mycotic or oncotic aneurysm. Fifteen (60%) of the patients were negative for the presence of aneurysm on DSA. Twenty patients (80%) were evaluated with MRA, four patients with CTA (16%) and one patient (4%) with both modalities. Eleven patients (44%) presented with hemorrhage, 11 with infarct (44%), and three with other findings (12%).
Among patients who were positive for a mycotic aneurysm, six presented with hemorrhage (60%), three with ischemic infarct (30%), and one (10%) of the aneurysms was found incidentally. Twenty-four of 30 (80%) were located in the distal anterior territory (distal to the A1 segment of the anterior cerebral artery (ACA) or distal to the M1 segment of the MCA). The mean number of aneurysms per patient was 3.0 ± 4.0 (range 1–14). Five of 10 patients (50%) had aneurysms that were mycotic in etiology, the other five (50%) oncotic. Mean aneurysm size was 2.8 ± 1.3 mm. Results are summarized in Table 1.
Table 1.
Patient population.
| N (%) positive | N (%) negative | |
|---|---|---|
| Mean age (years) | 41.5 (±17.3) | 60.5 (±11.2) |
| Gender | ||
| Male | 5 (50) | 9 (60) |
| Female | 5 (50) | 6 (40) |
| Aneurysm location | ||
| Right distal anterior circulation (ACA or MCA) | 14 (46.7) | – |
| Left distal anterior circulation (ACA or MCA) | 10 (33.3) | – |
| Distal posterior circulation | 5 (16.7) | – |
| ICA | 1 (3.3) | – |
| Number with multiple aneurysms | 4 (40.0) | 0 (0.0) |
| Mean number of aneurysms (range) | 3 (1-14) | 0 |
| Initial presentation | ||
| Hemorrhage | 6 (60.0) | 5 (33.3) |
| Stroke | 3 (30.0) | 8 (53.3) |
| Other | 1 (10.0) | 2 (13.3) |
| Exam type (in addition to DSA) | ||
| CTA | 3 (30.0) | 1 (6.7) |
| MRA | 6 (60.0) | 14 (93.3) |
| Both CTA and MRA | 1 (10.0) | 0 (0.0) |
ACA: anterior cerebral artery; MCA: middle cerebral artery; ICA: internal carotid artery; DSA: digital subtraction angiography; CTA: computed tomography angiography; MRA: magnetic resonance angiography.
Combined CTA/MRA outcomes
On a per-patient basis, combined CTA/MRA had a sensitivity of 45.5%, specificity of 90.0%, an NPV of 69.2%, a PPV of 76.9%, an accuracy of 71.2%, and kappa value of 0.29 (0.00–0.69) for the detection of cerebral mycotic and oncotic aneurysms, indicating fair agreement. On ROC analysis, the area under the curve (AUC) for combined CTA/MRA was 0.62.
On a per-aneurysm basis, combined CTA/MRA had a sensitivity of 34.8%, specificity of 90.0%, an NPV of 47.4%, a PPV of 84.2%, an accuracy of 56.6%, and kappa value of 0.45 (0.08–0.82) for the detection of cerebral mycotic and oncotic aneurysms, indicating moderate agreement. On ROC analysis, the AUC for combined CTA/MRA was 0.68.
MRA outcomes
On a per-patient basis, MRA alone had a sensitivity of 37.5%, specificity of 89.3%, an NPV of 73.5%, a PPV of 62.5%, an accuracy of 71.4%, and kappa value of 0.10 (0.00–0.53) for the detection of cerebral mycotic and oncotic aneurysms, indicating slight agreement. On ROC analysis, the AUC for MRA was 0.63.
On a per-aneurysm basis, MRA alone had a sensitivity of 29.0%, specificity of 89.3%, an NPV of 48.1%, a PPV of 78.6%, an accuracy of 54.5%, and kappa value of 0.33 (0.00–0.79) for the detection of cerebral mycotic and oncotic aneurysms, indicating slight agreement. On ROC analysis, the AUC for MRA was 0.59.
Discussion
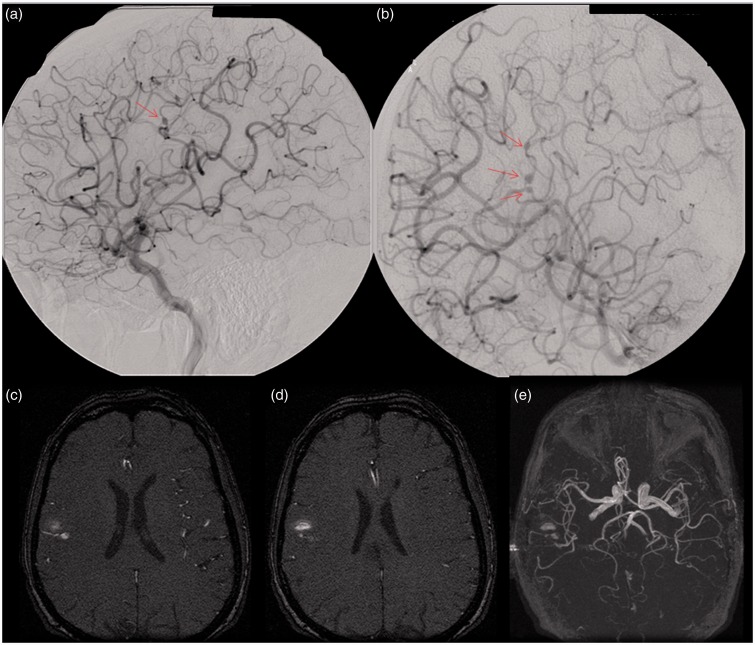
Our results demonstrate that cross-sectional imaging modalities such as CTA and MRA are not sensitive for the detection of distal territory cerebral mycotic and oncotic aneurysms. The results would indicate that in cases where there is a high clinical suspicion for mycotic aneurysm and cross-sectional imaging is negative, catheter-based angiography should be strongly considered for excluding an oncotic or mycotic cerebral aneurysm. An example of such a case is presented in Figure 1. In certain clinical settings, accurate diagnosis is critical for guiding management of such patients who are often critically ill, either due to sepsis, endocarditis, or hemodynamic instability as a result of a cardiac myxoma. Additionally, accurate assessment is important in order to reduce further morbidity and to prevent additional adverse events related to these aneurysms, such as hemorrhage.
Figure 1.
This patient presented with a headache and was found to have a subarachnoid hemorrhage and native mitral valve endocarditis. Images of a right carotid injection from a right cerebral digital subtraction angiogram ((a) and (b)) demonstrate three fusiform aneurysms along the frontoparietal opercular branch of the right middle cerebral artery (arrows), best appreciated on the more zoomed-in image (b). Axial ((c) and (d)) and three-dimensional reformatted (e) images from a time-of-flight magnetic resonance angiography (MRA) demonstrate signal abnormality within the subcortical white matter of the posterior right frontal operculum secondary to hemorrhage; however, an M3 branch aneurysm is difficult to appreciate.
The poor sensitivity of cross-sectional imaging for detection of distal territory mycotic and oncotic aneurysms demonstrated in this study are in keeping with a recent small study of seven patients by Hui et al. that demonstrated a 42.9% sensitivity for CTA and a 33.3% sensitivity for MRA for the detection of cerebral mycotic aneurysms.4 Unlike our study, however, Hui et al did not examine interobserver agreement and were not able to evaluate other outcomes such as specificity, PPV, NPV and accuracy.
Results of studies examining the sensitivity of CTA and MRA for the detection of all types of aneurysms vary, but there is a trend, as one would expect, that sensitivity progressively decreases with smaller aneurysm size.7 In one of the larger blinded studies performed with 162 patients, the sensitivity of CTA to detect aneurysms <3 mm was 40%, compared with 92% for aneurysms between 5 and 10 mm.8 In the same study, MRA was reported to have a sensitivity of 0.35 for aneurysms less than 5 mm and 0.86 for aneurysms greater than 5 mm. The interobserver variability was not considered significant for either CTA or MRA in this study, however. In a study with 133 patients evaluated by DSA and MRA, 59% of cases demonstrated markedly different results, and 38% of those cases were false positives, usually in aneurysms less than 5 mm.9 A separate study with 142 patients reported a 71% sensitivity for CTA to detect aneurysms 3 mm or less and 100% sensitivity for aneurysms greater than 3 mm.10 Since mycotic and oncotic aneurysms are typically smaller, arise in more tortuous vessels, and are located more distally compared to aneurysms from other causes, the low sensitivity of cross-sectional imaging modalities demonstrated in our study is not surprising.
Limitations
Several limitations in this study include the broad time span throughout which the imaging was obtained, which inherently leads to varied quality as imaging resolution, and therefore the ability to detect more subtle aneurysms, has improved over the past 10 years. In addition, studies were obtained using a variety of equipment, as we are a large referral center and many patients bring imaging studies from elsewhere for our radiologists to interpret. It is also possible that the use of gadolinium-enhanced MRA or 320-slice multidetector CTA would be more sensitive in detecting these aneurysms. Additionally, the DSAs were performed by a number of different angiographers with diverse training and therefore techniques may not be identical to the individuals interpreting the studies, had they themselves performed the angiogram. A large component of DSA is dynamic evaluation, which cannot be adequately captured on a series of still images. Another limitation is that the readers were provided with only axial slices and the 3D multiplanar reformat (MPR) images created at the time of the initial study. At our institution, MRA and CTA examinations are routinely reconstructed in the axial, coronal and sagittal planes, with 3D reconstructions of the bilateral anterior and posterior circulations provided. Furthermore, in daily practice, the radiologist is able to create his or her own reconstructions. The readers in this study were unable to alter the images that they were provided. Also, mycotic aneurysms can spontaneously thrombose, which can make detection difficult on CTA or MRA. Lastly, there is selection bias in that patients with a negative result on cross-sectional imaging that went on to DSA inherently had a high index of clinical suspicion for oncotic or mycotic aneurysm, whereas patients with a negative cross-sectional imaging study and low index of suspicion were less likely to have undergone DSA, and were therefore excluded from this study.
Conclusions
Our small study demonstrated low sensitivity and low interobserver agreement in the use of cross-sectional imaging for detecting oncotic and mycotic aneurysms. These findings suggest that in settings in which there is a high suspicion for these aneurysms, DSA should strongly be considered if the cross-sectional imaging examination is negative. Larger studies are needed to confirm our results.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Zimmerman R, Gibby W, Carmody R. Neuroimaging: Clinical and physical principles, New York: Springer, 2012. p.826. [Google Scholar]
- 2.Sabolek M, Bachus-Banaschak K, Bachus R, et al. Multiple cerebral aneurysms as delayed complication of left cardiac myxoma: A case report and review. Acta Neurol Scand 2005; 111: 345–350. [DOI] [PubMed] [Google Scholar]
- 3.Salgado A, Furlan A, Keys T, et al. Mycotic aneurysm, subarachnoid hemorrhage, and indications for cerebral angiography in infective endocarditis. Stroke 1987; 18: 1057–1060. [DOI] [PubMed] [Google Scholar]
- 4.Hui F, Bain M, Obuchowski N, et al. Mycotic aneurysm detection rates with cerebral angiography in patients with infective endocarditis. J NeuroIntervent Surg 2015; 7: 449–452. [DOI] [PubMed] [Google Scholar]
- 5.Kouskouras C, Charitanti A, Giavroglou C, et al. Intracranial aneurysms: Evaluation using CTA and MRA. Correlation with DSA and intraoperative findings. Neuroradiology 2004; 46: 842–850. [DOI] [PubMed] [Google Scholar]
- 6.van Gelder JM. Computed tomographic angiography for detecting cerebral aneurysms: Implications of aneurysm size distribution for the sensitivity, specificity, and likelihood ratios. Neurosurgery 2003; 53: 597–606. [DOI] [PubMed] [Google Scholar]
- 7.Goddard A, Tan G, Becker J, et al. Computed tomography angiography for the detection and characterization of intra-cranial aneurysms: Current status. Clin Radiol 2005; 60: 1221–1236. [DOI] [PubMed] [Google Scholar]
- 8.White PM, Teasdale EM, Wardlaw JM, et al. Intracranial aneurysms: CT angiography and MR angiography for detection prospective blinded comparison in a large patient cohort. Radiology 2001; 219: 739–749. [DOI] [PubMed] [Google Scholar]
- 9.Schwab K, Gailloud P, Wyse G. Limitations of magnetic resonance imaging and magnetic resonance angiography in the diagnosis of intracranial aneurysms. Neurosurgery 2008; 63: 29–35. [DOI] [PubMed] [Google Scholar]
- 10.Seruga T, Bunc G, Klein G. Helical high-resolution volume-rendered 3-dimensional computer tomography angiography in the detection of intracranial aneurysms. J Neuroimaging 2001; 11: 280–286. [DOI] [PubMed] [Google Scholar]