Abstract
Introduction:
Poor socioeconomic status and illiteracy attribute to the advanced presentation of head and neck cancer (HNC) patients in India and are candidates for palliation in our setup. We set up a palliative cancer care clinic (PCCC), and an audit of initial 153 HNC patients is presented.
Aims and Objectives:
To assess the impact of palliative cancer care services.
Methodology:
Data of advanced HNC patients suited for palliation were collected to document demography, symptomatology, cancer treatment, and supportive care.
Results:
One hundred and fifty-three patients were seen during January 2013 to March 2015 in the PCCC. Seventy-two (47%) referral cases were due to disease progression and 81 (53%) due to de novo advanced cases. Median follow-up for this group was 5.3 months. Ninety (59%) cases needed some degree of assistance for their normal activities. Sixty-seven (44%) patients belonged to poor socioeconomic status and 65 (43%) were educated up to equivalent of high school. One hundred and thirty-five (88%) patients had an adequate family support. Pain was the most common presenting symptom in 134 (87%) cases with adequate relief in 112 (84%) patients with another 13 (09%) could not be assessed. Overall median duration of symptoms was 6 months. Cancer-directed therapy was used in 143 (93%) patients. Near the end of life in 47 (73%) out of 63 documented cases, caregivers were psychologically prepared for the inevitable.
Conclusion:
The role of palliative care team in alleviating physical, psychosocial, and emotional issues of patient and family members was significant. PCCC seems to be a feasible working model in our setup.
Key words: Advanced stage, End of life care, Head and neck cancer, Palliation, Supportive care
INTRODUCTION
Head and neck cancer (HNC) is one of the most common malignancies in India constituting up to 30% of all cancers.[1,2] Out of these, 75–80% cases present in advanced stage and the 5-year overall survival (OS) is <20% in these stages.[3,4] In comparison, early stage HNC has a 5-year OS up to 60–80% depending on the exact T and N size. The advanced presentation is often due to lack of awareness, low level of education, tobacco use, and poor personal hygiene, along with poor economic status. Other factors such as medical facilities being present far away and patient's general condition also have an impact on the presentation.
Out of these advanced cases, nearly half are too advanced for curative treatment and are for palliative therapy. These are usually the tumors that have either a huge disease or large fungating nodes/growth or cutaneous fistulas, etc., Other factors for decision of radical or palliative treatment are age, associated comorbidities, and general condition of the patient. Palliative therapy focuses on maintaining or improving patient's quality of life by control of various physical and psychosocial symptoms related to disease. Control of disease and symptoms are at times attempted by adding treatment modalities with minimal toxicity.
Although palliation is an integral part of oncology practice, may not be possible in a busy oncology outpatient clinic. In addition, as oncologists, who are treating physicians to these cancer patients, zeal to treat aggressively may be high. Patients with advanced disease and/or poor general condition may find it difficult to tolerate intense treatment strategy leading to high toxicity rate without any increase in the cure rate. In January 2013, we started a dedicated palliative cancer care clinic (PCCC) attached to the oncology outdoor clinic. The team of oncologists was from the same oncology department where the outpatient clinics for all cancer patients were being run. Familiar set of doctors probably added to the comfort, apart from providing continuity of care. Another advantage of oncologists being palliative care specialist in cancer patients is the fact that, better understanding of disease process led to improved and layered psychosocial counseling of patients and their near ones. An initial audit of 153 HNC patients was done retrospectively to evaluate the demographic profile, range of symptoms, other needs that required attention, and evaluation of treatment strategies, especially for pain management. In addition, the aim was also to evaluate the response of various cancer-directed therapies (CDTs) in palliative setting.
METHODOLOGY
All patients attending the PCCC were either advanced HNCs treated radically and subsequently failed on treatment or late presentation during the natural course.
Detailed history of symptoms burden and psychosocial milieu was taken in all the patients. General physical examination and local examination were done to assess the patient's ability to withstand any cancer-directed palliative treatment. Pain severity assessment was done as mild, moderate, or severe degree; pain management was done according to the WHO ladder, i.e., mild pain was addressed by nonopioid ± adjuvants; in moderate degree of pain additionally weak opioids were used, and in severe pain, addition of a strong opioid was done. For instance, pain was managed primarily by the use of 2 cheap but effective drugs, i.e., paracetamol (PCM) and oral morphine. Both these drugs were readily available in the hospital pharmacy. Emphasis was to ensure compliance and timely administration to prevent “end of dose” pain. Adjunct therapies were used, as and when required, to prevent drug-related side effects; for instance, each prescription of morphine always had a laxative along with it. Other symptoms such as fungation and infection were taken care by teaching the patient/attendants cleaning and dressing. Antibiotics were used generously to ensure infection clearance.
General symptoms and signs such as swallowing difficulty and consequently malnutrition, inability to sleep, depression, and/or anxiety were also assessed and addressed. All the patients who could not take the diet orally or were aspirating were advised nasogastric intubation for enteral feeding. All patients consulted a dedicated team of dietician at regular intervals, and as and when required. Procedures such as fluid drainage (pleural or peritoneal drainage) carried out in the adjoining day care facility as and when required.
CDTs used were metronomic chemotherapy, short course palliative radiotherapy (RT), and/or targeted therapy. Single agent weekly, methotrexate 50 mg intravenously was administered at home. If required, 1–3 courses of palliative RT were planned, spaced at least 1 month apart. A dose of 20 Gy was delivered in 5 fractions by small portal and using a linear accelerator – this schedule comprised a single course of palliative RT. Tyrosine kinase inhibitor – Gefitinib tablet orally, once a day dosage, was given to the patients as per their individual requirement. Since this was a retrospective study, data collection was done from case files and telephonically. Issues regarding financial constraints were also discussed and recorded with the primary caretakers of patient and their feedback with regard to mental and physical status of their patient near the end of life was recorded over the phone after the final event. An average number of visits per patient and time duration between any treatment and death/last follow-up were also calculated.
RESULTS
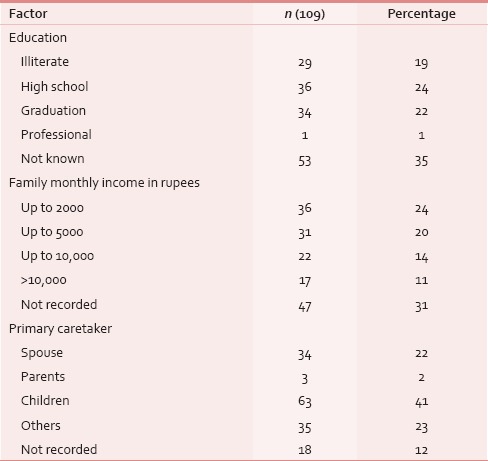
Between January 2013 and March 2015, 153 HNC patients were taken care at PCCC. One hundred and thirty-nine (91%) were males and 23 (15%) had one/multiple coexisting comorbidities; diabetes mellitus was the most common in 12 (8%) cases. Median age of this cohort was 52 years. One hundred and twenty-five (82%) patients had documented tobacco habit using either smoking or smokeless tobacco, i.e., paan, paan-masala, or tobacco quid [Table 1]. Their performance score was measured using Karnofsky performance scale and ninety (59%) of these patients scored between 50 and 60, i.e., they needed assistance of varying degree for their daily routine activities. Usual body weight, i.e. weight before the patient had developed cancer was known only in 21 (14%) cases. At the first palliative visit, median weight was 49 kg, which was recorded in 137 (90%) patients. In this cohort, oral cavity was the most common site of cancer, i.e., 66 (43%), followed by oropharynx 44 (29%) and laryngopharynx 21 (14%) [Table 2]. Nearly half (53%) the patients presented as untreated advanced HNC, whereas the rest either failed to the primary radical treatment or were having residual disease [Table 2]. The psychosocial factors were looked into and were detailed in Table 3.
Table 1.
Demographic characteristics of cohort
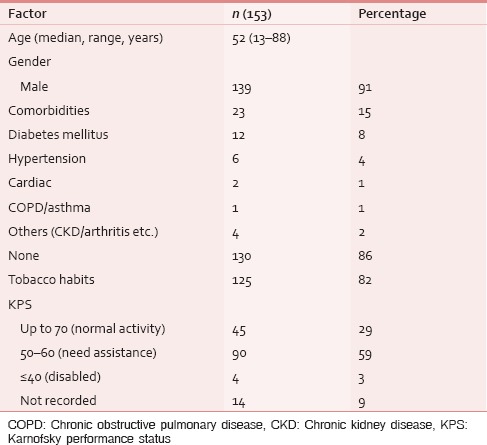
Table 2.
Disease status at presentation in palliative clinic
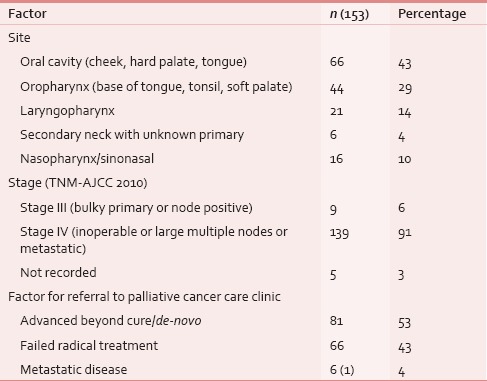
Table 3.
Patient background
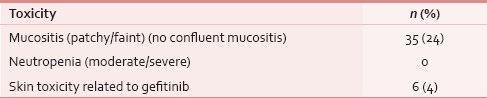
At the time of presentation, most common distressing physical symptom was pain in over 134 (87%), followed by fungating lesion (primary or node) in 47 (31%) and difficult/painful deglutition in 61 (40%), additionally 34 (22%) had features of aspiration [Table 4]; median duration of these symptoms was 4 months (range 1–48 months). Fifty-three (35%) out of these 153 patients seemed to be depressed [Table 4]. This information was gathered from the case records, where either the physicians’ perception was recorded, or patient or his attendant volunteered with this information. Out of 134 (87%) who had pain, 103 (77%) had both component, i.e. nociceptive (mostly attributed by local disease) and neuropathic pain (usually referred because of stretching associated with large mass of lymph nodes). Out of these 134 (87%) patients, nearly 95 (71%) had moderate/severe pain and needed opioids in addition to a nonopioid [Table 5]. At our center, we use PCM 500 mg to 1 g qid as the most common nonopioid, and morphine low dose (5 mg 4 hourly and double dose at bedtime) as a weak opioid. Deviation from this cocktail was seen only in cases having contraindication to these drugs. Overall, 112 (84%) patients responded effectively to this pain management strategy [Table 6]. Palliative chemotherapy, i.e., injection methotrexate 50 mg weekly, RT to locoregional disease in doses of 20 Gy in 5 fractions in 5 days (maximum three such rounds depending on the response of disease) and single fraction of 8 Gy for bony metastatic pain were given based on physician's decision. One hundred and forty-three (93%) patients received single or a combination of the above-mentioned CDT. Nearly an equal number of patients (65 [46%] vs. 70 [49%]) received methotrexate with or without RT. Out of these 143 (93%), nearly half, i.e., 80 (56%) responded at least partially, whereas 14 (10%) had stable disease; response was more in RT therapy group, i.e., 44 (63%) versus 34 (52%) [statistically not significant; Table 7]. As far as the toxicities related to CDT were concerned 35 (24%) patients had Grade I/II mucositis and 5 (3%) was having Grade I/II neutropenia which was managed conservatively; nearly 78 (55%) patients tolerated CDT without any toxicity [Table 8].
Table 4.
Symptoms assessment
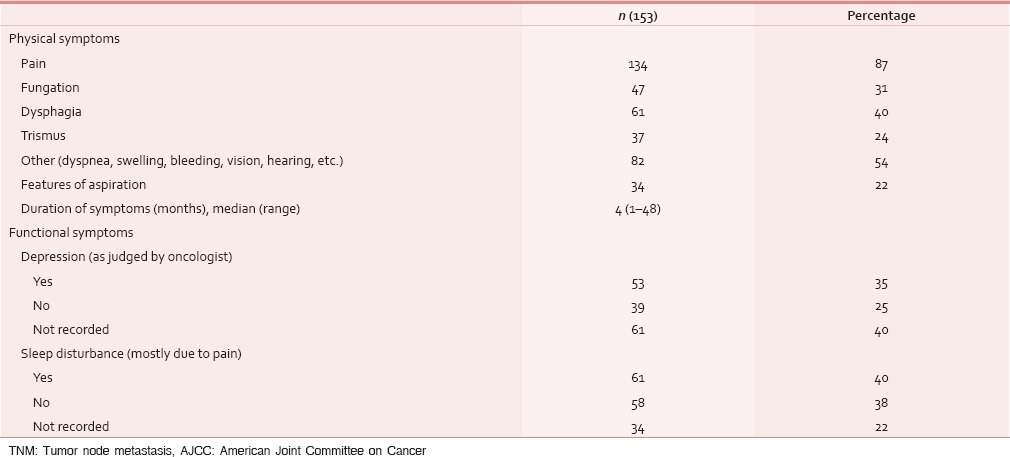
Table 5.
Pain assessment
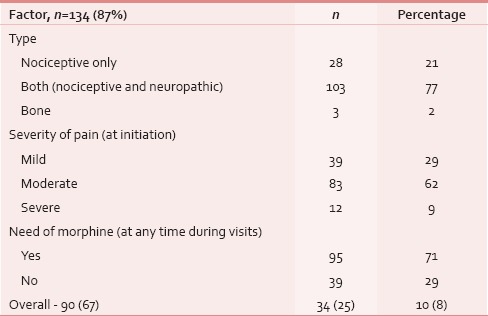
Table 6.
Pain response assessment (n=134)
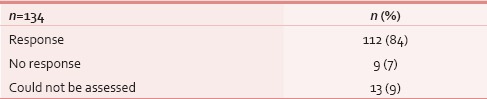
Table 7.
Tumor response assessment (clinical) following cancer-directed treatment (n=143)
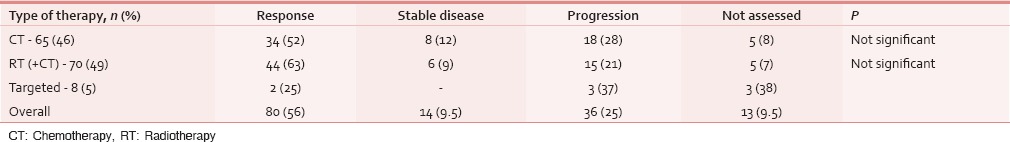
Table 8.
Treatment-related toxicities (n=143)
As regards nutrition, as a matter of routine in all the upper aerodigestive tract cancer patients, we follow a nutrition protocol that includes diet counseling at each visit, every patient had to visit a dedicated team of dieticians regularly and further supplementation by enteral feeding was provided by the palliative team in cases of poor oral intake because of dysphagia/odynophagia or features of aspiration or/and inability to take orally due to a fistulous communication. About 101 (65%) of this cohort needed nasogastric tube feeding. In spite of all these measures 80 (52%) patients had weight loss during this period. Of these patients with documented weight loss, there was a significant weight loss (>10%) in 42 (52%) patients [Table 9].
Table 9.
Weight assessment during palliation
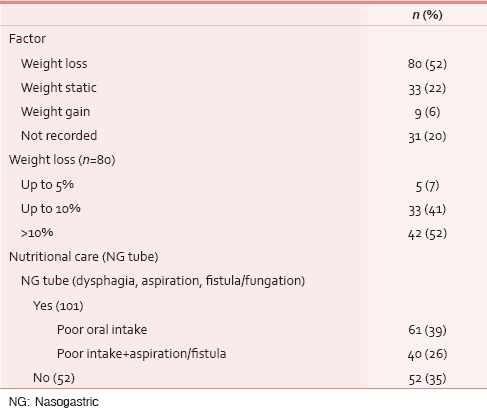
Apart from the clinical aspects, the psychosocial issues were also looked out. Sixty-five (43%) patients of this group had their formal education below or only up to high school, whereas 34 (22%) were graduate, and family monthly income was below Rs. 10,000in 89 (58%) patients [Table 3]. Sixty-three (41%) patients were being taken care of by their grown up and independent children, whereas 34 (22%) were supported and accompanied by their spouse [Table 3]. With regard to mental preparedness of the outcomes at the time of the final event, thirty (44%) out of 62 documented cases, patients were found to have some level of depression [Table 10]. Out of 63 patient's family members who were assessed over phone, 47 (73%) were mentally prepared and accepted the outcome before the final event. Peaceful death (i.e., presence of endurable, low physical, and mental symptom burden) was found in 32 (46%) patients, owing to the effective medications and procedures [Table 11].
Table 10.
Psychological symptoms
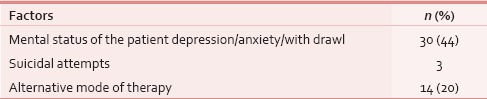
Table 11.
End of life

Details about financial constraints were recorded in 68 patient's families out of which 23 (34%) has to borrow money from either their relatives or has taken loan from banks, 7 (10%) has had to sell their property for the sake of the continuity of treatment, this expenditure included the cost of care throughout the treatment (including radical treatment if offered) [Table 12]. Out of 68 patients, those were contacted over phone, 14 (20%) had received some or other form of alternative medicinal support during end of life.
Table 12.
Psychosocial support to patient and family

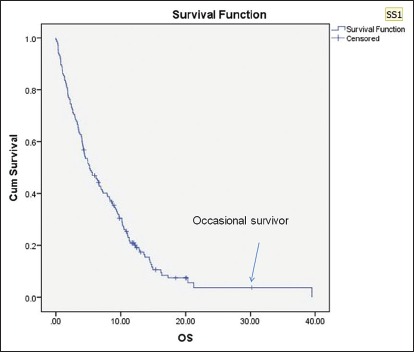
At the time of analysis, nearly 109 (71%) were dead, 27 (17%) were lost to follow-up, and 17 (11%) were alive. Twenty-three (15%) patients required indoor admissions for some form of supportive care and 13 (8%) had to take emergency admission for the distressing symptoms such as respiratory distress and bleeding, average length of hospital stay was calculated to be 7.4 days. Fifty-four (50%) patients had breathed their last at their home, near their close ones, and nearly half of them (47%) had a peaceful death. Most distressing symptom for the near ones of the patients at the time of death was inability to eat (usually due to trismus) in nearly one-third of 25 (36%) patients; pain and fungation were primary distressing symptoms in another 17 (25%) patients [Table 13]. Median follow-up of this cohort of patients was 5.3 months, and survival was 12.1 months (95% confidence interval [CI]: 9.7–14.5 months) [Figure 1]. Average time elapsed between last CDT and their last visit/death was nearly 2.8 months On an average, the patient made 11 visits to PCCC (this is excluding the daily visits while the patient was taking palliative RT) during his/her natural course of illness.
Table 13.
Preterminal symptoms

Figure 1.
Overall survival
DISCUSSION
Palliative care is still an emerging specialty in the developing world and in the northern part of India, very few palliative care centers exist.[5,6,7,8] This study is a retrospective analysis of the patients managed in PCCC. HNC patients contributed around 50% of the total patients who attended the palliative care clinic in first 2ݫ years time period. Oral cavity and oropharynx malignancies were the most common sites. Our patient population was similar to other reports, i.e., predominantly male population and in the fourth/fifth decade of life.[5,7,9] We had nearly an equal proportion of de novo patients as well as radical treatment failed patients. Expectedly, pain was the predominant feature in these patients.[5,7] Pain was managed according to the WHO 3-step ladder in most cases. Nearly one-fourth of the patients had clinical features of aspiration and needed nasogastric tube to bypass the leaking passage and therefore were able to prevent aspiration-related morbidity and mortality. In a previously published report, we had reported that HNCs in our setup carried out a risk of nearly 30% of developing aspirations as documented on video fluorography. This risk could be fatal in nearly half of the cases during the natural course of illness.[10] This may have been the reason for patients reporting dead, despite no overt progression of disease in the present analysis as well.
In India, majority of palliative aspects of medicine are taken care by either oncologists (radiation oncologist) or anesthetists. At All India Institute of Medical Sciences, New Delhi, palliative care clinic is led by anesthetists, and on the other hand, at Postgraduate Institute of Medical Sciences and Research, Chandigarh, an oncologist led palliative care clinic is operational.[11] At our center, we have both yet independent anesthetists led pain clinic and oncologist led palliative care clinic where we take advantage of both specialties through cross referrals. There is another model of palliative care services which exists in some part of Kerala, where highly motivated, trained community workers along with physicians are involved in taking care of these patients, who need some form of medical assistant along with good domiciliary care; this model has its own advantage of involving the community itself in taking care of patients.[12]
Oncologists, as seen in literature, do try to incorporate CDT in their palliative care practice. They have frequently shown meaningful clinical response with CDT in their series.[13] CDT was used for symptoms control and growth restraint of tumor as – either palliative chemotherapy alone in the form of weekly injection methotrexate (metronomic therapy) which is effective, safe, cheap, quick, and easy to administer or sequence it with palliative RT to a dose of 20 Gy in 5 fractions in 5 days. Targeted therapy (Gefitinib) alone was also used in 8 (5%) patients. A good number of studies supported CDT in palliative care of advanced HNC patients such as the study by Anuradha et al. in 68 patients concluded that 75% patients had a meaningful clinical response which has further effect on symptom improvement with weekly methotrexate ± geftinib.[9] Other study by Banipal and Mahajan showed a very good clinical response with decrease in the tumor bulk by more than 50% in nearly 40% patients and further 40% has stable disease with weekly injection methotrexate.[14] A study by Patil et al. from Tata Memorial Centre in 110 patients showed that weekly oral methotrexate with celecoxib has significantly better progression-free survival and OS (P = 0.014 and 0.02, respectively) in comparison to single agent cisplatin; additionally, the combination was much better tolerated with much lower toxicities.[15] In the present study as well, in methotrexate group, we also reported nearly half had a clinical response with one-tenth stable disease of the patients who received weekly methotrexate. In fact, a study from rural India has shown a good clinical activity with fortnightly use of oral methotrexate in elderly oral cancer population.[16]
The clinical response increased to two-third of the patients with 9% stable disease when palliative RT was added to weekly methotrexate. Hypofractionated palliative RT has been used as a single modality for locally advanced HNC, and there are various short course RT schedules such as 20 Gy in 5 fractions in 5 days that have been practiced in the literature. A study by Mohanti et al. in 505 patients showed that the dose schedule of 20 Gy/5# showed 60% symptom relief with a median survival of 6.5 months, 1 year OS of 30%.[17] Another study by Ghoshal et al. showed more than 50% clinical response in 13 out of 15 patients with 14 Gy dose in 2 days, two fractions per day, 6 h apart ("Quad Shot" earlier used by Corry et al.), and further these 13 patients received the second course of same radiation dose-schedule after 3 weeks.[18,19] Grade II/III mucositis was the only morbidity with these hypofractionated schedule ranging from 15% to 100% which was managed conservatively.[15,17,18] We also got 25% Grade I/II mucositis in the present study and managed conservatively.
In the present report, we had adopted the sequence of metronomic weekly methotrexate with or without palliative short course RT (if no obvious contraindication to RT like fistula) was given in these patients. Apart from providing symptom relief and tumor growth restraint, these CDTs also gave the patient and their family members, a sense of something being done to take care of cancer, which meant that the sense of hopelessness was kept at bay during this period. Median OS of our study was 12 months (95% CI: 9.7–14.5 months) after the patient had been referred to palliative care, whereas other published studies reported median OS around 6–7 months.[14,15,17,20]
Das et al. showed that 60% patients had the improvement of performance status and reduction of pain in 88% patients using radiation dose of 40 Gy in 10 fractions in 2 weeks time.[20] Same results were reported by Anuradha et al. with the use of weekly methotrexate by stabilization of symptoms with minimal toxicities, and at the same time, it is an economical treatment.[9] A study by Ghoshal et al. with “Quad Shot” also reported a decrease in pain score in 10 out of 15 patients.[18] We also reported the improvement in pain and further overall quality of life with CDT nearly in three-fourth of them.
A combination of PCM and morphine was effective in taking care of pain in 2/3 cases in the present study. However, with morphine, the major issue in suboptimal pain relief was the “end of dose” pain. As our experience grew, and so did our documentation and by repeated reinforcement and training of the family toward timely drug administration along with individualizing the morphine schedule, we obtained greater pain relief in the past 1 year. Weekly methotrexate is an effective metronomic therapy till date, apart from the time-tested hypofractionation RT.[21] To conclude, effective pain management is the backbone of palliative care clinic, but its strength can be further increased by adding appropriate palliative CDTs which are effective, safe, economical, and easy to use.
A significant proportion of our patients was poor and from a rural background, semi-illiterate (school level education). In addition, on average, they had to travel 108 km to reach our center. Therefore, the advanced presentation was not unexpected.[22] As previously discussed, financial issues related to patients care have its own impact on the family of these patients since most of them belong to low- and lower-middle socioeconomic status, expenditure in the form of travelling, and staying had taken its toll on them, almost half of these patients and their relatives had to borrow, take loan, or sell their properties to meet the expenditure, these issues related to finances have become more relevant to the caretakers of the patients at a time when they are under extreme mental agony and fear of losing their near one.
Many times, physicians land up in a situation where we have to face the agony and denial of the patient's near ones at the time of their patient's death.[23] It has been seen that majority of these situations can be overcome with structured and multilayered psychosocial counseling. In this study, two-third of the patients, family members were mentally prepared for the inevitable and had accepted the outcome before the final event. We have also tried to take a glimpse into the mental status of these patients during their end of life and found that with repeated motivation and proper counseling, we succeeded to keep almost half these patients in the peaceful state of mind, without depression during their end of life. Based on several reports, it is clear that it is not only the medical care that the patients and their family members expect from their treating physician but also understanding their psychology, unrevealed mental agony can lead to better outcomes in the form of level of mental satisfaction in these patients.[24]
In India, family continues to take care and support their sick relative till the very last. This practice provides emotional support to the patient till the end. The patient experiences a greater degree of satisfaction and the feeling of being wanted as compared to Western societies where the patients are often managed at hospices/nursing homes/assisted living areas by professionals who are strangers to these patients.[25]
CONCLUSION
Oncologist led PCCC is a very feasible option in our community, which addresses not only the physical symptoms of these patients but taking care of all other aspects (psychosocial, emotional and financial) of patients and their families, resulting in a holistic care.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kulkarni MR. Head and neck cancer burden in India. Int J Head Neck Surg. 2013;4:29–35. [Google Scholar]
- 2.More Y, D’Cruz AK. Oral cancer: Review of current management strategies. Natl Med J India. 2013;26:152–8. [PubMed] [Google Scholar]
- 3.Coelho KR. Challenges of the oral cancer burden in India. J Cancer Epidemiol. 2012;2012:701932. doi: 10.1155/2012/701932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trivedi NP, Kekatpure VD, Trivedi NN, Kuriakose MA. Head and neck cancer in India: Need to formulate uniform national treatment guideline? Indian J Cancer. 2012;49:6–10. doi: 10.4103/0019-509X.98907. [DOI] [PubMed] [Google Scholar]
- 5.Lal M, Raheja S, Kale S, Das N, Gogia AR, Bhowmik KT. An experience with 156 patients attending a newly organized pain and palliative care clinic in a tertiary hospital. Indian J Cancer. 2012;49:293–7. doi: 10.4103/0019-509X.104491. [DOI] [PubMed] [Google Scholar]
- 6.Dastidar AG, Saha S, Srivastava A, Chakroborty D, Sardar B. Management of unresectable head and neck cancers – A retrospective analysis at a rural medical college of India. Indian J Otolaryngol Head Neck Surg. 2010;62:49–54. doi: 10.1007/s12070-010-0006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma K, Mohanti BK, Rath GK, Bhatnagar S. Pattern of palliative care, pain management and referral trends in patients receiving radiotherapy at a tertiary cancer center. Indian J Palliat Care. 2009;15:148–54. doi: 10.4103/0973-1075.58462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devi BC, Tang TS, Corbex M. Setting up home-based palliative care in countries with limited resources: A model from Sarawak, Malaysia. Ann Oncol. 2008;19:2061–6. doi: 10.1093/annonc/mdn422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anuradha V, Anand BB, Suresh AV, Sinha S, Babu SC, Suresh K. Palliative chemotherapy in head and neck squamous cell cancer – What is best in Indian population? A time without symptoms, treatment toxicity score based study. Indian J Med Paediatr Oncol. 2013;34:11–5. doi: 10.4103/0971-5851.113404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lal P, Nautiyal V, Chaudhuri T, Verma M, Das KJ, Kumar S. Is aspiration as detected on pretreatment video fluorography, a harbinger of poor quality of life and early mortality in cancers of the upper aerodigestive tract treated with radiotherapy? South Asian J Cancer. 2014;3:209–12. doi: 10.4103/2278-330X.142968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bansal M, Patel FD, Mohanti BK, Sharma SC. Setting up a palliative care clinic within a radiotherapy department: A model for developing countries. Support Care Cancer. 2003;11:343–7. doi: 10.1007/s00520-002-0418-4. [DOI] [PubMed] [Google Scholar]
- 12.Kumar SK. Kerala, India: A regional community-based palliative care model. J Pain Symptom Manage. 2007;33:623–7. doi: 10.1016/j.jpainsymman.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Archer VR, Billingham LJ, Cullen MH. Palliative chemotherapy: No longer a contradiction in terms. Oncologist. 1999;4:470–7. [PubMed] [Google Scholar]
- 14.Banipal RP, Mahajan MK. Methotrexate revisited-in recurrent head and neck cancer. Palliat Care. 2011;5:9–13. [Google Scholar]
- 15.Patil VM, Noronha V, Joshi A, Muddu VK, Dhumal S, Bhosale B, et al. A prospective randomized phase II study comparing metronomic chemotherapy with chemotherapy (single agent cisplatin), in patients with metastatic, relapsed or inoperable squamous cell carcinoma of head and neck. Oral Oncol. 2015;51:279–86. doi: 10.1016/j.oraloncology.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Chakraborty S, Geetha M, Sujith KM, Biji MS, Sateeshan B. Palliative low dose fortnightly methotrexate in oral cancers: Experience at a rural cancer centre from India. South Asian J Cancer. 2014;3:166–70. doi: 10.4103/2278-330X.136798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohanti BK, Umapathy H, Bahadur S, Thakar A, Pathy S. Short course palliative radiotherapy of 20 Gy in 5 fractions for advanced and incurable head and neck cancer: AIIMS study. Radiother Oncol. 2004;71:275–80. doi: 10.1016/j.radonc.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Ghoshal S, Chakraborty S, Moudgil N, Kaur M, Patel FD. Quad shot: A short but effective schedule for palliative radiation for head and neck carcinoma. Indian J Palliat Care. 2009;15:137–40. doi: 10.4103/0973-1075.58460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corry J, Peters LJ, Costa ID, Milner AD, Fawns H, Rischin D, et al. The “QUAD SHOT” – A phase II study of palliative radiotherapy for incurable head and neck cancer. Radiother Oncol. 2005;77:137–42. doi: 10.1016/j.radonc.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Das S, Thomas S, Pal SK, Isiah R, John S. Hypofractionated palliative radiotherapy in locally advanced inoperable head and neck cancer: CMC Vellore experience. Indian J Palliat Care. 2013;19:93–8. doi: 10.4103/0973-1075.116709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lutz ST, Chow EL, Hartsell WF, Konski AA. A review of hypofractionated palliative radiotherapy. Cancer. 2007;109:1462–70. doi: 10.1002/cncr.22555. [DOI] [PubMed] [Google Scholar]
- 22.Kumar S, Heller RF, Pandey U, Tewari V, Bala N, Oanh KT. Delay in presentation of oral cancer: A multifactor analytical study. Natl Med J India. 2001;14:13–7. [PubMed] [Google Scholar]
- 23.Rousseau P. Death denial. J Clin Oncol. 2000;18:3998–9. doi: 10.1200/JCO.2000.18.23.3998. [DOI] [PubMed] [Google Scholar]
- 24.Nieuwmeyer S, Hosking M. Conselling the terminally Ill – Can we prepare for death? S Afr Fam Pract. 2006;48:20–2. [Google Scholar]
- 25.Joad AS, Mayamol T, Chaturvedi M. What does the informal caregiver of a terminally ill cancer patient need? A study from a cancer centre. Indian J Palliat Care. 2011;17:191–6. doi: 10.4103/0973-1075.92335. [DOI] [PMC free article] [PubMed] [Google Scholar]


