Abstract
Background:
Previous studies have demonstrated interhemispheric functional connectivity alterations in schizophrenia. However, the relationship between these alterations and the disease state of schizophrenia is largely unknown. Therefore, we aimed to investigate this relationship using voxel-mirrored homotopic connectivity (VMHC) method.
Methods:
This study enrolled 36 schizophrenia patients with complete remission, 58 schizophrenia patients with incomplete remission and 55 healthy controls. The VMHC was calculated based on resting-state functional magnetic resonance imaging data. Differences in VMHC among three groups were compared using one-way analysis of variance. A brain region with a significant difference in VMHC was defined as a region of interest (ROI), and the mean VMHC value in the ROI was extracted for the post hoc analysis, i.e., pair-wise comparisons across the three groups.
Results:
VMHC in the visual region (inferior occipital and fusiform gyri) and the sensorimotor region (paracentral lobule) showed significant differences among the three groups (P < 0.05, a false discovery rate method corrected). Pair-wise comparisons in the post hoc analysis showed that VMHC of the visual and sensorimotor regions in schizophrenia patients with complete remission and incomplete remission was lower than that in healthy controls (P < 0.05, Bonferroni corrected); however, there was no significant difference between the two patient subgroups.
Conclusions:
Interhemispheric functional connectivity in the sensorimotor and visual processing pathways was reduced in patients with schizophrenia, but this reduction was unrelated to the disease state; thus, this reduction may serve as a trait marker of schizophrenia.
Keywords: Functional Magnetic Resonance Imaging, Schizophrenia, Sensorimotor Pathway, Visual Pathway
Introduction
Prior studies have confirmed the existence of extensive functional connectivity disruptions in schizophrenia patients, resulting in disorders of information processing in the brain and corresponding behavioral and psychological symptoms.[1,2] Several previous studies have shown interhemispheric functional connectivity alterations in schizophrenia patients. For example, patients with schizophrenia have shown decreased interhemispheric coherence compared to normal controls.[3,4] Studies have also shown that coordination defects in the bilateral visual pathway were associated with a reduction of the schizophrenia patients’ abilities to process vocabulary.[5,6] It has been demonstrated that antipsychotic agents can improve the functional connectivity of the brain in schizophrenia, and changes in the functional connectivity are related to improvements in psychotic symptoms, resulting in certain “normalization” of brain functional connectivity for schizophrenia patients in a state of remission.[7,8,9,10,11] However, the relationship between the disease state of schizophrenia and the interhemispheric functional connectivity of the brain is largely unknown.
In address this issue, we compared the interhemispheric functional connectivity among schizophrenia patients with complete remission, patients with incomplete remission, and healthy controls using a newly developed method of voxel-mirrored homotopic connectivity (VMHC).[12] Previous studies have shown that the corpus callosum, which connects the two hemispheres, is damaged in patients with schizophrenia, and this damage is relatively stable with little change across different disease states.[13,14,15] Accordingly, we hypothesized that VMHC would be reduced in schizophrenia patients and that the degree of this reduction may not be associated with the disease state of the schizophrenia, i.e., the reduction in VMHC may serve as one trait marker of schizophrenia.
Methods
Study objects
This study was conducted between January 2013 and October 2014, which was approved by the Ethics Committee of the Tianjin Anning Hospital (No. 2012-H-007). All participants provided signed, informed consent. A total of 94 patients with schizophrenia and 55 healthy controls were included in this study. The diagnosis of schizophrenia was determined by two experienced psychiatrists according to the Structured Clinical Interview for DSM-IV Axis I Disorders diagnostic criteria. The healthy controls were recruited from local community via advertisements. The inclusion criteria were age between 18 and 60 years, and right-handedness. The exclusion criteria included magnetic resonance imaging (MRI) contraindications, poor image quality, any neurological disorder, a history of traumatic brain injury, and a history of drug abuse. Additional exclusion criteria for healthy controls included a history of any mental disorder and a first-degree relative with any mental disorder. The severity of psychiatric symptoms in the patients with schizophrenia was quantitatively assessed using the Positive and Negative Syndrome Scale (PANSS).[16] The standard definition of clinically complete remission in this study was a PANSS score ≤60 and the absence of psychotic symptoms for at least 3 months before scanning,[17] as judged by a clinician's diagnosis. This study included 36 schizophrenia patients with complete remission and 58 patients with incomplete remission. One patient in the complete remission group and 8 patients in the incomplete remission group had never received any medication, and the rest of the patients were receiving atypical antipsychotic medications during the MRI examinations. The antipsychotic dosages are reported in Table 1 as the chlorpromazine equivalents were calculated based on clinically equivalent dosing estimates.[18] For each schizophrenia patient, the chlorpromazine equivalent was estimated according to the antipsychotic drugs and dosages used in the latest week before the MRI scan.
Table 1.
Demographic and clinical information of schizophrenia patients with complete or incomplete remission, and healthy controls
| Characteristics | Complete remission (n = 36) | Incomplete remission (n = 58) | Healthy controls (n = 55) | Statistics | P |
|---|---|---|---|---|---|
| Age (years) | 32.8 ± 6.2 | 34.0 ± 9.6 | 33.1 ± 9.8 | 0.249* | 0.780 |
| Sex (female/male) | 15/21 | 23/35 | 22/33 | 0.040† | 0.980 |
| Antipsychotic dosage (mg/d) (chlorpromazine equivalents) | 464.8 ± 310.5 | 461.0 ± 370.1 | - | −0.051‡ | 0.960 |
| Duration of illness (months) | 117.4 ± 80.0 | 126.7 ± 109.1 | - | 0.443‡ | 0.659 |
| PANSS | |||||
| Total | 48.6 ± 7.6 | 83.5 ± 16.7 | - | 11.731‡ | <0.001 |
| Positive score | 11.6 ± 3.9 | 20.5 ± 7.8 | - | 6.411‡ | <0.001 |
| Negative score | 12.9 ± 5.0 | 23.6 ± 7.0 | - | 7.994‡ | <0.001 |
| General score | 24.2 ± 3.8 | 39.4 ± 9.0 | - | 9.559‡ | <0.001 |
*F value; †χ2 value; ‡t value. Complete remission: These criteria comprise a severity criterion and a time criterion. With regard to the severity criterion, a score of mild (3) or less was required for all eight core symptoms of PANSS, including P1 delusions, P2 conceptual disorganization, P3 hallucinatory behavior, N1 blunted affect, N4 social withdrawal, N6 lack of spontaneity, G5 mannerisms/posturing, and G9 unusual thought concept. With regard to the time criterion, the symptom severity mentioned above must have been maintained for at least 6 months. Incomplete remission: These criteria comprise a severity criterion and a time criterion. With regard to the severity criterion, a score of (4) or more was required for all eight core symptoms of PANSS. -: Not applicable; PANSS: The Positive and Negative Syndrome Scale.
Magnetic resonance imaging data acquisition
MRI data were acquired using a 3.0-Tesla MR scanner (Discovery MR750, General Electric, Milwaukee, WI, USA). All participants underwent three-dimensional (3D) high-resolution T1-weighted structural imaging and resting-state functional magnetic resonance imaging (fMRI) scanning. The brain volume sequence was applied in the 3D high-resolution structural imaging with the following parameters: Repetition time (TR)/echo time (TE)/inversion time (TI) = 8.2/3.2/450 ms; flip angle (FA) = 12°; field of view (FOV) = 256 mm × 256 mm; matrix = 256 × 256; slice thickness = 1 mm; and no gap in 188 sagittal slices. The resting-state fMRI data were obtained using the gradient-echo single-shot echo planar imaging sequence, and the scan parameters were as follows: TR/TE = 2000/45 ms; FOV = 220 mm × 220 mm; matrix = 64 × 64; FA = 90°; slice thickness = 4 mm; gap = 0.5 mm; 32 interleaved axial slices; and 180 volumes. All participants were asked to close their eyes, relax, move as little as possible, think of nothing in particular, and not fall asleep during the resting-state fMRI scans.
Data preprocessing
The resting-state fMRI data were preprocessed using the Statistical Parametric Mapping software (SPM8; http://www.fil.ion.ucl.ac.uk/spm). The preprocessing procedures were as follows: (1) exclusion of the first 10 volumes, reflecting the time needed for the BOLD signal to reach the steady state and for the participants to adapt to the environment; (2) time correction; (3) realignment; (4) nuisance covariates regression (including six head movement parameters, their first time derivations, and the signals of white matter, cerebrospinal fluid, and the whole brain); (5) the band-pass filter (frequency range of 0.01–0.08 Hz); (6) spatial normalization of the resting-state fMRI data to the standard Montreal Neurological Institute space by combination of the segmentation registration of the individual high-resolution structural images; and (7) spatial smoothing using a 6 mm × 6 mm × 6 mm Gaussian kernel.
Voxel-mirrored homotopic connectivity analysis
The VMHC was calculated using the Data Processing Assistant for Resting-state Functional MR Imaging toolkit (DPARSF, State Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University, China).[19] After preprocessing, the fMRI images were divided symmetrically into right and left brain hemispheres, with the center level as the plane of symmetry. For each case, Pearson's correlation coefficient was calculated for the time series of each voxel and its corresponding voxel in the mirrored hemisphere. To increase the normality of the distribution, Fisher's z-conversion was conducted for the correlation coefficients. The resulting correlation coefficients after the conversion were used to regenerate the VMHC map.
Statistical analysis
The statistical analysis of the demographic and clinical data was carried out using SPSS version 19.0 software (SPSS, Chicago, IL, USA). One-way analysis of variance (ANOVA) and Chi-square test were used to test differences in age and gender, respectively, among the three groups. Two-sample t-test was used to test differences in dosage of antipsychotic agent and disease duration between two patient groups. The voxel-based VMHC analysis was performed using the SPM8 software package. One-way ANOVA was used to compare the VMHC among the three groups. Multiple comparisons were corrected using a false discovery rate (FDR) method with the corrected P < 0.05. The brain region with a significant VMHC difference was defined as the region of interest (ROI), and the mean VMHC value in the ROI was extracted for post hoc analysis, i.e., pair-wise comparisons across the three groups. In addition, the ROI-based correlation analyses with PANSS scores were performed for the two patient groups using a partial correlation analysis while controlling for age and sex. Multiple comparisons were corrected using the Bonferroni method with the corrected P < 0.05.
Results
Demographic and clinical information
The demographic and clinical data of the participants are provided in Table 1. The three groups showed no significant differences in age (one-way ANOVA, F = 0.249, P = 0.780) or gender (Chi-square test, χ2 = 0.040, P = 0.980). The complete remission group and the incomplete remission group exhibited no significant differences in the dosage of antipsychotic agent (two-sample t-test, t = −0.051, P = 0.960) or the disease duration (two-sample t-test, t = 0.443, P = 0.659).
Differences in voxel-mirrored homotopic connectivity among groups
The differences in VMHC among the three groups are shown in Figure 1 and Table 2. The VMHC was significantly different in the visual region (inferior occipital and fusiform gyri) and the sensorimotor region (paracentral lobule) across the three groups (P < 0.05, FDR corrected). Pair-wise comparisons in the post hoc ROI-based analysis showed that the VMHC of the visual and sensorimotor regions in schizophrenia patients with complete remission and incomplete remission was lower than that in healthy controls; however, there was no significant difference between the two patient subgroups (P < 0.05, Bonferroni corrected) [Figure 2].
Figure 1.
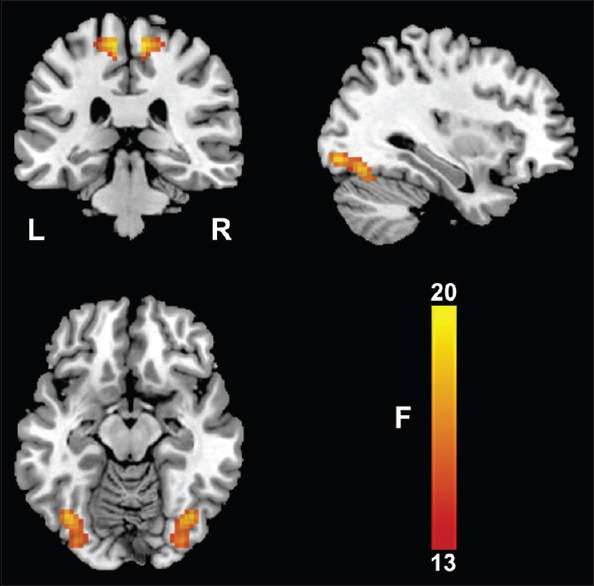
Voxel-mirrored homotopic connectivity differences across groups. One-way analysis of variance shows brain regions with voxel-mirrored homotopic connectivity differences across the three groups. L: Left; R: Right.
Table 2.
Brain regions with significant differences in VMHC
| Brain regions | Voxel number | F | MNI coordinates (x, y, z) |
|---|---|---|---|
| Visual region | 143 | 21.1 | ±39, −69, −15 |
| Sensorimotor region | 68 | 19.1 | ±9, −30, 63 |
MNI: Montreal Neurological Institute; VMHC: Voxel-mirrored homotopic connectivity.
Figure 2.
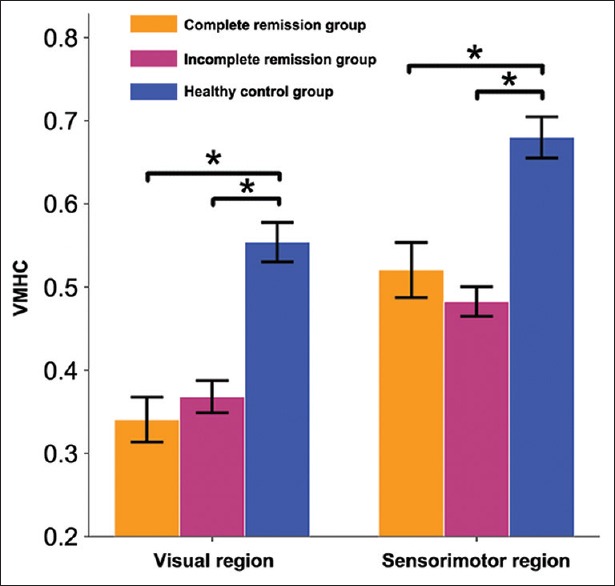
Pair-wise comparisons in the post hoc region of interest-based analysis. *P < 0.05, Bonferroni corrected. VMHC: Voxel-mirrored homotopic connectivity.
Correlations between voxel-mirrored homotopic connectivity and symptom severity
In schizophrenia patients with complete remission, there were no significant correlations between VMHC in the visual region and PANSS positive score (partial correlation coefficient [pr] =0.010, P = 0.957), negative score (pr = −0.006, P = 0.972), and general score (pr = 0.063, P = 0.725), and between VMHC in the sensorimotor region and PANSS positive score (pr = 0.349, P = 0.043), negative score (pr = 0.025, P = 0.887), and general score (pr = 0.121, P = 0.495). In schizophrenia patients with incomplete remission, we also found no significant correlations between VMHC in the visual region and PANSS positive score (pr = 0.051, P = 0.707), negative score (pr = 0.086, P = 0.530), and general score (pr = 0.158, P = 0.246), and between VMHC in the sensorimotor region and PANSS positive score (pr = 0.087, P = 0.526), negative score (pr = 0.051, P = 0.708), and general score (pr = −0.004, P = 0.976).
Discussion
In this study, we found that VMHC in the visual and sensorimotor regions was lower in schizophrenia patients with complete remission and incomplete remission compared to normal controls; however, there was no significant difference between the two patient subgroups. Our findings suggest that changes in the interhemispheric functional connectivity are unrelated to the disease state and may, therefore, serve as a trait marker of schizophrenia.
Dysconnectivity in the sensorimotor and visual pathways has been consistently reported in patients with schizophrenia, and it has been associated with behavioral and psychological symptoms of the disease. A prior study has shown that patients with schizophrenia exhibited reduced VMHC in the occipital lobes, thalamus, and cerebellum compared to normal controls, and the VMHC aberrations in the sensorimotor regions are associated with the symptoms of schizophrenia.[20] A previous study also found that VMHC was decreased in the precuneus, precentral gyrus, superior temporal gyrus, occipital gyrus, and cerebellum in patients with first-episode and drug-naive schizophrenia, and VMHC reduction in the precentral gyrus was associated with positive symptoms.[21] Chang et al.[22] reported that the altered VMHC in the default mode network, inferior frontal gyrus, and cerebellum was associated with auditory hallucination symptoms, and aberrant VMHC in the sensorimotor region has been found only in schizophrenia patients experiencing nonauditory hallucination.
Consistent with previous findings, we found aberrant VMHC in the sensorimotor and visual-related pathways in patients with schizophrenia. In regard to the association between VMHC and psychotic symptoms, previous studies have uniformly demonstrated correlations between VMHC and schizophrenia symptoms, but the brain regions associated with symptoms are not consistently reported. In this study, we compared the VMHC differences between schizophrenia patients with complete remission and those with incomplete remission. We found no VMHC difference between the two patient groups, suggesting that reduced VMHC may be a trait marker of schizophrenia.
Several limitations should be acknowledged in this study. First, patients with first-episode and drug-naive schizophrenia were not available in this study; therefore, our findings need to be confirmed by future studies investigating interhemispheric connectivity differences between patients with first-episode and drug-naive schizophrenia and recovered patients after medication administration. Second, most of the enrolled patients were chronic and had followed long-term medication regimens, which may affect our results. Finally, the criteria to distinguish between complete and incomplete remission were whether patients had experienced psychotic symptoms in the past 3 months. However, the time for determining clinical outcomes was relatively short, which may weaken the applicability of our study's inferences. Therefore, a long-term follow-up study should be adopted in the future study.
In conclusion, interhemispheric functional connectivity in the sensorimotor and visual processing pathways was reduced in patients with schizophrenia. More importantly, the reduction of interhemispheric functional connectivity was unrelated to the remission state of the disease; therefore, it may serve as a trait marker of schizophrenia.
Financial support and sponsorship
This study was supported by the grants from the National Basic Research Program of China (973 program, No. 2011CB707801); the Natural Science Foundation of China (No. 81501451, No. 91332113 and No. 81271551); and the China Postdoctoral Science Foundation (No. 2012M520585).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yuan-Yuan Ji
References
- 1.Jiang T, Zhou Y, Liu B, Liu Y, Song M. Brainnetome-wide association studies in schizophrenia: The advances and future. Neurosci Biobehav Rev. 2013;37(s10 Pt 2):2818–35. doi: 10.1016/j.neubiorev.2013.10.004. doi: 10.1016/j.neubiorev.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Friston KJ, Frith CD. Schizophrenia: A disconnection syndrome? Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- 3.Stark DE, Margulies DS, Shehzad ZE, Reiss P, Kelly AM, Uddin LQ, et al. Regional variation in interhemispheric coordination of intrinsic hemodynamic fluctuations. J Neurosci. 2008;28:13754–64. doi: 10.1523/JNEUROSCI.4544-08.2008. doi: 10.1523/JNEUROSCI.4544-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribolsi M, Koch G, Magni V, Di Lorenzo G, Rubino IA, Siracusano A, et al. Abnormal brain lateralization and connectivity in schizophrenia. Rev Neurosci. 2009;20:61–70. doi: 10.1515/revneuro.2009.20.1.61. doi: 10.1515/REVNEURO.2009.20.1.61. [DOI] [PubMed] [Google Scholar]
- 5.Mohr B, Pulvermüller F, Cohen R, Rockstroh B. Interhemispheric cooperation during word processing: Evidence for callosal transfer dysfunction in schizophrenic patients. Schizophr Res. 2000;46:231–9. doi: 10.1016/s0920-9964(00)00020-7. doi: 10.1016/S0920-9964(00) 00020-7. [DOI] [PubMed] [Google Scholar]
- 6.Mohr B, Pulvermüller F, Rockstroh B, Endrass T. Hemispheric cooperation – A crucial factor in schizophrenia? Neurophysiological evidence. Neuroimage. 2008;41:1102–10. doi: 10.1016/j.neuroimage.2007.12.032. doi: 10.1016/j.neuroimage.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 7.Abbott CC, Jaramillo A, Wilcox CE, Hamilton DA. Antipsychotic drug effects in schizophrenia: A review of longitudinal FMRI investigations and neural interpretations. Curr Med Chem. 2013;20:428–37. doi: 10.2174/0929867311320030014. doi: 10.2174/0929867311320030014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gur RE, Gur RC. Functional magnetic resonance imaging in schizophrenia. Dialogues Clin Neurosci. 2010;12:333–43. doi: 10.31887/DCNS.2010.12.3/rgur. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Rossi P, Chiapponi C, Spalletta G. Brain functional effects of psychopharmacological treatments in schizophrenia: A network-based functional perspective beyond neurotransmitter systems. Curr Neuropharmacol. 2015;13:435–44. doi: 10.2174/1570159X13666150507223542. doi: 10.2174/1570159x13666150507223542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Duan M, Xie Q, Lai Y, Dong L, Cao W, et al. Functional disconnection between the visual cortex and the sensorimotor cortex suggests a potential mechanism for self-disorder in schizophrenia. Schizophr Res. 2015;166:151–7. doi: 10.1016/j.schres.2015.06.014. doi: 10.1016/j.schres.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Sarpal DK, Argyelan M, Robinson DG, Szeszko PR, Karlsgodt KH, John M, et al. Baseline striatal functional connectivity as a predictor of response to antipsychotic drug treatment. Am J Psychiatry. 2016;173:69–77. doi: 10.1176/appi.ajp.2015.14121571. doi: 10.1176/appi.ajp.2015.14121571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuo XN, Kelly C, Di Martino A, Mennes M, Margulies DS, Bangaru S, et al. Growing together and growing apart: Regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci. 2010;30:15034–43. doi: 10.1523/JNEUROSCI.2612-10.2010. doi: 10.1523/JNEUROSCI.2612-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rotarska-Jagiela A, Schönmeyer R, Oertel V, Haenschel C, Vogeley K, Linden DE. The corpus callosum in schizophrenia-volume and connectivity changes affect specific regions. Neuroimage. 2008;39:1522–32. doi: 10.1016/j.neuroimage.2007.10.063. doi: 10.1016/j.neuroimage.2007.10.063. [DOI] [PubMed] [Google Scholar]
- 14.Lungu O, Stip E. Agenesis of corpus callosum and emotional information processing in schizophrenia. Front Psychiatry. 2012;3:1. doi: 10.3389/fpsyt.2012.00001. doi: 10.3389/fpsyt.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mueller S, Keeser D, Reiser MF, Teipel S, Meindl T. Functional and structural MR imaging in neuropsychiatric disorders, Part 2: Application in schizophrenia and autism. AJNR Am J Neuroradiol. 2012;33:2033–7. doi: 10.3174/ajnr.A2800. doi: 10.3174/ajnr.A2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 17.Andreasen NC, Carpenter WT, Jr, Kane JM, Lasser RA, Marder SR, Weinberger DR. Remission in schizophrenia: Proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162:441–9. doi: 10.1176/appi.ajp.162.3.441. doi: 10.1176/appi.ajp.162.3.441. [DOI] [PubMed] [Google Scholar]
- 18.Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167:686–93. doi: 10.1176/appi.ajp.2009.09060802. doi: 10.1176/appi.ajp.2009.09060802. [DOI] [PubMed] [Google Scholar]
- 19.Chao-Gan Y, Yu-Feng Z. DPARSF: A MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front Syst Neurosci. 2010;4:13. doi: 10.3389/fnsys.2010.00013. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoptman MJ, Zuo XN, D’Angelo D, Mauro CJ, Butler PD, Milham MP, et al. Decreased interhemispheric coordination in schizophrenia: A resting state fMRI study. Schizophr Res. 2012;141:1–7. doi: 10.1016/j.schres.2012.07.027. doi: 10.1016/j.schres.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo W, Jiang J, Xiao C, Zhang Z, Zhang J, Yu L, et al. Decreased resting-state interhemispheric functional connectivity in unaffected siblings of schizophrenia patients. Schizophr Res. 2014;152:170–5. doi: 10.1016/j.schres.2013.11.030. doi: 10.1016/j.schres.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 22.Chang X, Xi YB, Cui LB, Wang HN, Sun JB, Zhu YQ, et al. Distinct inter-hemispheric dysconnectivity in schizophrenia patients with and without auditory verbal hallucinations. Sci Rep. 2015;5:11218. doi: 10.1038/srep11218. doi: 10.1038/srep11218. [DOI] [PMC free article] [PubMed] [Google Scholar]


