Abstract
Background:
The long-term outcomes of patients with autoimmune hepatitis (AIH) given the immunosuppressive treatment are considered to be preferable. However, little is known about the response of AIH patients with cirrhosis to immunosuppressive treatment. We assessed the effects of immunosuppressive therapy in Chinese AIH patients with cirrhosis from a tertiary hospital.
Methods:
Patients with a clinical diagnosis of AIH January 2000 and December 2015 were retrospectively reviewed. Two-hundred and fourteen patients who were followed up and satisfied the simplified AIH criteria were included in the study. Among these patients, 87 presented with cirrhosis when initially diagnosed for AIH. Immunosuppressive treatments were employed in 57 AIH patients who did not present with cirrhosis and 39 patients who presented with cirrhosis. Initial responses to immunosuppressive treatment of patients with and without cirrhosis were analyzed. Independent risk factors were assessed for predicting the prognosis of patients. The t-test and Cox regression statistical analysis were used.
Results:
In total, 96 AIH patients including 39 with cirrhosis and 57 without cirrhosis underwent immunosuppressive therapy. The overall complete remission after initial immunosuppressive treatment was achieved in 81/96 patients (84.4%), whereas 9/96 (9.4%) achieved incomplete response, and 6/96 (6.3%) occurred treatment failure. Compared to noncirrhotic patients, patients who presented with cirrhosis responded to treatment to a comparable extent regarding complete response (noncirrhosis 50/57 [87.7%] vs. cirrhosis 31/39 [79.5%], P = 0.275), incomplete remission (noncirrhosis 4/57 [7.0%] vs. cirrhosis 5/39 [12.8%], P = 0.338), and treatment failure (noncirrhosis 3/57 [5.3%] vs. cirrhosis 3/39 [7.7%], P = 0.629). Importantly, the remission rate was comparable (54/57 [94.7%] and 36/39 [92.3%], P = 0.629) for noncirrhotic and cirrhotic patients after immunosuppressive therapy. Compared to patients who maintained remission (n = 19) after drug withdrawal, patients who experienced relapse (n = 17) had significantly higher levels of serum immunoglobulin G at entry (15.0 ± 6.5 g/L vs. 22.3 ± 5.8 g/L, t = 2.814, P = 0.004). Moreover, cirrhosis at presentation significantly increased the risk of disease exacerbation (hazard ratio [HR]: 4.603; P = 0.002). The treatment of immunosuppressant (HR: 0.058; P = 0.005) and the level of aspartate aminotransferase at presentation (HR: 1.002; P = 0.017) also increased the risk of disease progression.
Conclusions:
The efficacy of initial immunosuppressive treatment in AIH patients with cirrhosis is comparable to that in those without cirrhosis. Cirrhotic patients not treated by immunosuppressants have poor long-term outcomes.
Keywords: Autoimmune Hepatitis, Immunosuppressive Agents, Liver Cirrhosis, Treatment Outcome
Introduction
Autoimmune hepatitis (AIH) is an immune-mediated inflammatory disease of the liver. Prednisolone alone or in combination with azathioprine is the standard therapy for AIH.[1] In general, immunosuppressive therapies are effective in inducing remission in AIH patients.[2] However, the efficacy of treatment seems to vary slightly by ethnicity. It is reported that in Caucasian patients, the remission rates after immunosuppressive treatment range from 76% to 100%,[3,4,5,6,7] and the relapse rates range from 50% to 86%.[8] Studies on Japanese patients showed that 89%–100% of patients achieved remission at 6 months after therapy as evaluated by normalized alanine aminotransferase (ALT) levels[9] whereas relapse occurred in 29.9% of patients during tapering of corticosteroid dose.[10] Patients from Saudi Arabia were reported to have a low remission rate of 54.8% after immunosuppressive therapy.[11] To date, the efficacy of immunosuppressive treatment in AIH patients of Chinese descent has rarely been reported.
Clinical indications for immunosuppressive treatment in AIH patients with liver cirrhosis, particularly those with decompensated cirrhosis and severe complications, remain difficult to specify, largely due to our limited knowledge of the long-term outcomes of these patients after treatment.[1,12,13,14,15,16] Thus far, the only literature available on comparison of remission rates between cirrhotic and noncirrhotic patients is the report by Feld et al.,[5] in which similar remission rates between cirrhotic (73.8%) and noncirrhotic (81.9%) patients after immunosuppressive treatment were recorded. However, the long-term survival of patients with cirrhosis in the presence or absence of immunosuppressive therapy remains unclear. Roberts et al.[3] reported that 10-year survival was not different between patients with and without cirrhosis (89% and 90%, respectively) after immunosuppressive treatment whereas Feld et al.[5] reported that patients with cirrhosis at presentation had worse outcomes than those without cirrhosis (61.9% vs. 94.0%, 10-year survival). Taken together, comprehensive evaluation of the effects of immunosuppressive therapy in AIH patients who present cirrhosis is called for.
The more important question nowadays concerns the treatment requirements of AIH patients with cirrhosis who do not have indications for immunosuppressive treatment. It has been assumed that these patients are in an inactive phase of their hepatitis based on their lower levels of liver enzymes and serum immunoglobulin G (IgG), as well as the histological activity index scores of the liver biopsies.[5,17] The new British guidelines for AIH recommend that patients with cirrhosis even mild histological activity should be offered immunosuppressive treatment.[18] However, until now, few studies specialized on this group of patients has been reported. The study analyzed the efficacy of immunosuppressive therapy in asymptomatic AIH patients including patients with cirrhosis showing good responses to the treatment.[19] However, the long-term outcomes of cirrhotic patients remain unclear.
In the present study, we retrospectively analyzed the effects of immunosuppressive treatment in Chinese AIH patients. By comparing patients with and without cirrhosis at presentation, we analyzed the outcomes of cirrhotic patients who underwent immunosuppressive treatment, as well as the immunosuppressive requirements of cirrhotic patients who do not have indications for treatment.
Methods
Study population
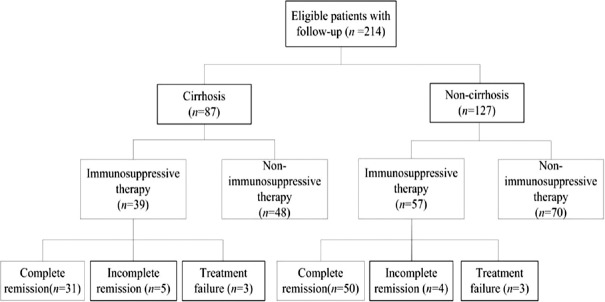
The recorded charts of 249 patients given a clinical diagnosis of AIH (including probable AIH and definite AIH) at Tianjin Medical University General Hospital January 2000 and December 2015 were retrospectively reviewed. The study was approved by the Ethics Committee of Tianjin Medical University General Hospital, which waived the need for informed written consent. Every patient provided informed consent for sample collection in accordance with the Declaration of Helsinki. Patients who also had primary biliary cirrhosis (PBC) and AIH-PBC overlap syndrome or experienced excessive alcohol consumption were excluded from the study. The 214 cases satisfied the simplified criteria of the International Autoimmune Hepatitis Group[20] (“definite” ≥7, “probable” ≥6) and had complete medical records of follow-up. Pretreatment biopsies were performed in 147 cases. Among 214 patients, the presence of liver cirrhosis was demonstrated in 87 cases at the time of AIH diagnosis. Ninety-six cases received immunosuppressive therapy following the initial AIH diagnosis, of which 39 cases were with cirrhosis [Figure 1].
Figure 1.
Flow diagram of selection and groups of patients with autoimmune hepatitis in the present study.
Diagnostic criteria and therapeutic evaluation
All patients enrolled in this study satisfied “probable” (n = 84) or “definite” (n = 130) criteria of the simplified AIH diagnostic criteria.[20,21,22] Presence or absence of liver cirrhosis at presentation was defined according to the clinical characteristics or the results of ultrasonography and blood tests.[23,24] Among the 87 patients who present of cirrhosis, 30 were confirmed by liver biopsies. All liver histology specimens were reviewed by two pathologists, and the presence or absence of cirrhosis was documented using standard histological criteria. Child–Pugh score was calculated for each patient. Decompensated cirrhosis was defined as a Child–Pugh score ≥7 or clinical evidence or history of ascites, encephalopathy, variceal bleeding, and/or impaired hepatic synthetic function.[25]
Biochemistry tests and clinical notes were carefully reviewed every month during the 1st year of treatment and then reviewed every 6 months until remission. During follow-up, patients were evaluated as having a complete remission when they satisfied all of the following four criteria: (1) disappearance of symptoms, (2) normalization of serum bilirubin and globulin (GLO) levels, (3) normal serum aminotransferase levels, and (4) normal hepatic histology or inactive cirrhosis if liver biopsies were available. Treatment failure was defined as worsened clinical, laboratory, and histological features despite compliance with therapy. Incomplete response was defined as some or no improvement in clinical, laboratory, and histological features despite compliance with therapy after 2–3 years. A relapse was indicated as an increase in the serum aminotransferase level to more than 3-fold upper limit of normal range and/or IgG levels greater than 20 g/L after cessation of treatment.[26] Disease progression was defined as the occurrence of any of the following: (1) chronic active hepatitis patients progressing to cirrhosis; (2) occurrence of esophageal and/or gastric variceal bleeding, hepatic encephalopathy, or spontaneous bacterial peritonitis; (3) died because of liver disease; (4) occurrence of hepatocarcinoma.[12]
Treatment regimens
Treatment regimens of patients were reassessed, and all recommendations were in line with the guidelines on AIH.[26,27] Immunosuppressive treatments were employed in 57 AIH patients who did not present with cirrhosis and 39 patients who presented with cirrhosis [Figure 1]. As initial treatment, all patients received high-dose prednisone alone (starting with 40–60 mg/d and tapering down to 20 mg/d within 4 weeks) or prednisone (starting with 30 mg/d and tapering down to 10 mg/d within 4 weeks) in combination with azathioprine (50 mg/d). Of the 81 patients who achieved complete remission, immunosuppressive agents were discontinued in 36 patients when remission was maintained for at least 12 months with no relapse on tapered doses. Liver histology assessment before the termination of treatment was suggested to the patient, and for those who refused, we informed them the probability of relapse. Due to the lack of treatment indications, 118 patients, including 48 with inactive cirrhosis, were not managed by immunosuppressive therapy. Most of the latter patients received conventional liver-protecting treatment, for instance, polyene phosphatidylcholine, tiopronin.
Laboratory methods
All patients were tested for antibodies known to be associated with AIH. Assessment for antinuclear antibody, antimitochondrial, and anti-smooth muscle antibodies was performed by indirect immunofluorescence, and a titer of 1:40 or greater was considered positive; liver kidney microsomal antibody-1 and anti-soluble liver antigen antibody were detected by enzyme-linked immunosorbent assay (ELISA), and a titer of 1:100 or greater was considered positive; IgG level was determined by the nephelometer method (normal range: 7.2–15.6 g/L). Baseline and follow-up blood work included parameters of liver function, serum IgG, and complete blood count. During follow-up, serum liver function tests were determined at 4-week intervals among the withdrawal process and every 3 months on maintenance therapy. When remission was sustained for at least 12 months with no relapse on tapered doses, serum liver function tests were determined at 3-month intervals. Hepatitis serology was performed by ELISA for examination of hepatitis B virus surface antigen and hepatitis C virus (HCV) antibodies. Patients with anti-HCV antibodies were subsequently screened for HCV RNA using polymerase chain reaction. When regarded appropriate, the results of hepatitis A virus antibody and hepatitis E virus antibody were obtained.
For the Cox regression analysis, 188 of the 214 patients were assessed. Twenty-six were excluded because of censoring before the occurrence of death. By univariate analysis, age, sex, ALT, alkaline phosphatase (ALP), GLO, and total bilirubin levels had no effect on prognosis of the patients and were, therefore, removed from the model. The final multivariate model was created by whether the presence of cirrhosis, level of aspartate aminotransferase (AST), and use of immunosuppressive therapy or not.
Statistical analysis
Statistical analysis was performed using statistical software SPSS 17.0 software (IBM, Armonk, NY, USA). Continuous data were summarized as mean ± standard deviations (SD), and categorical data were expressed as proportions. The detection of significant differences between continuous variables was performed using the t-test; Discrete variables such as sex and event of positive antibodies were analyzed by the Chi-square test between patients with and without cirrhosis at presentation. Initial laboratory data may be characterized in part by skewness of the distribution, were summarized by median and range and analyzed using the Mann–Whitney U-test. The association of several variables with prognosis was assessed by Cox regression analysis. P < 0.05 was considered statistically significant. GraphPad Prism 6.0 (GraphPad SanDiego, CA, USA) was used for the above-mentioned data analysis.
Results
Clinical features of autoimmune hepatitis patients with cirrhosis
Of the 214 patients who had been given a diagnosis of AIH, cirrhosis was found in 87 patients (40.7%) at the time of diagnosis, comprising 20 patients (23.0%) with compensated cirrhosis and 67 patients (77.0%) with decompensated cirrhosis. Analysis of initial laboratory data shows that patients with cirrhosis at presentation had lower white blood cell counts, platelet counts, and lower levels of serum ALT, AST, and gamma-glutamyl transferase (GGT) [Table 1].
Table 1.
Clinical and laboratory characteristics of patients with and without cirrhosis at presentation
| Variables | Without cirrhosis (n = 127) | With cirrhosis (n = 87) | Statistics | P |
|---|---|---|---|---|
| Age (years) | 58.1 ± 13.2 | 60.5 ± 13.5 | 0.764* | 0.447 |
| Male/female | 1:6.5 (17/110) | 1:8.7 (9/78) | 0.110† | 1.000 |
| Duration of follow-up (months) | 38 (6–60) | 36 (8–60) | 0.159‡ | 0.835 |
| ANA, n (%) | 118 (92.9) | 81 (93.1) | 0.006† | 1.000 |
| SMA, n (%) | 20 (15.7) | 19 (21.8) | 0.407† | 0.544 |
| LKM-1, n (%) | 3 (2.4) | 3 (3.5) | 0.118† | 1.000 |
| WBC (×109/L) | 5.6 ± 2.5 | 3.6 ± 1.4 | 3.452* | 0.001 |
| Hb (g/L) | 113.3 ± 21.4 | 101.9 ± 24.8 | 1.783* | 0.081 |
| PLT (×109/L) | 195 (66–360) | 94 (32–187) | 3.672‡ | 0.001 |
| GLO (U/L) | 40.6 ± 8.4 | 40.3 ± 13.0 | 0.111* | 0.912 |
| ALT (U/L) | 351 (23–786) | 118 (21–324) | 2.909‡ | 0.005 |
| AST (U/L) | 350 (28–714) | 152 (26–372) | 2.396‡ | 0.019 |
| ALP (U/L) | 243 (132–394) | 192 (106–307) | 1.149‡ | 0.254 |
| GGT (U/L) | 347 (72–683) | 183 (64–325) | 2.047‡ | 0.044 |
| IgG (g/L) | 19.4 ± 6.5 | 18.7 ± 7.7 | 0.273* | 0.787 |
Values are presented as n (%), median (range), or mean ± SD. *compared by t-test, †analyzed by Chi-square test, ‡analyzed by the Mann–Whitney U-test. ANA: Anti-nuclear antibody; SMA: Smooth muscle antibody; LKM-1: Liver kidney microsomal antibody-1; WBC: White blood count; Hb: Hemoglobin; PLT: Platelet count; GLO: Globulin; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; ALP: Alkaline phosphatase; GGT: γ-glutamyl transpeptidase; IgG: Immunoglobulin G; SD: Standard deviation.
Initial responses to immunosuppressive treatment of patients with and without cirrhosis
After diagnosis, immunosuppressive treatment (prednisone alone or prednisone in combination with azathioprine) was prescribed to 96 patients, including 39 patients with cirrhosis and 57 patients without cirrhosis. In total, 81 patients (84.4%) reached complete remission, including 31 patients (35.6%) with cirrhosis and 50 patients (39.4%) without cirrhosis (P = 0.580) [Figure 1]. Incomplete remission was achieved in 9 patients (9.4%) in total, including 5 patients (5.7%) with cirrhosis and 4 patients (3.1%) without cirrhosis (P = 0.352). Treatment failure occurred in 6 patients (6.2%), including 3 patients (3.4%) with cirrhosis and 3 patients (2.4%) without cirrhosis (P = 0.636). There was no significant difference in remission rate of AIH patients with and without cirrhosis.
Relapses after drug withdrawal of patients with immunosuppressive therapy
Of the 81 patients who achieved complete remission, immunosuppressive therapy sustained for at least 12 months was discontinued in 36 patients after remission, consisting of 10 patients with cirrhosis and 26 patients without cirrhosis. Relapse rates were similar between patients with cirrhosis and without cirrhosis (6/10 vs. 11/26, P = 0.341) with a median duration of 4 months (range: 1.2–96.0 months).
However, the total relapse rates of patients with drug withdrawal after remission occurred in 17 of 36 patients (47.2%). In contrast, 45 patients continued immunosuppressive therapy, and the aggravation rates were 17.8% (8/45), fewer than before (P = 0.004).
Notably, 17 patients (47.2%) experienced disease relapse within a median duration of 4 months (range: 1.2–96.0 months) after treatment termination. This relapse rate is significantly higher than that in patients who continued immunosuppressive therapy (n = 45) after remission (47.2% vs. 17.8%, P < 0.001). Moreover, we observed that 5 patients (29.4%) experienced relapse within 1 year of drug withdrawal whereas 12 patients (70.6%) experienced relapse after 1 year.
Risk factors predicting responsiveness of immunosuppressive treatment
After treatment withdrawal, our patients were followed up for 2 years. Compared to patients who maintained remission (n = 19) after drug withdrawal, patients who experienced relapse (n = 17) had significantly higher levels of serum IgG at entry (15.0 ± 6.5 g/L vs. 22.3 ± 5.8 g/L, P = 0.004) [Table 2], suggesting that IgG is a marker of relapse/compromised response to the therapy. No association was observed with the age at diagnosis, duration of treatment, treatment regimens, and other laboratory parameters.
Table 2.
Association between sustained remission and relapse in various features after therapy
| Features at entry | Sustained remission (n = 19) | Relapse (n = 17) | Statistics | P |
|---|---|---|---|---|
| Age (years) | 56.3 ± 13.9 | 62.2 ± 10.7 | 0.842* | 0.162 |
| AST (U/L) | 303 (112–537) | 153 (27–298) | 1.271† | 0.068 |
| Bilirubin (µmol/L) | 1090 (510–3890) | 820 (340–1570) | 0.713† | 0.395 |
| GLO (U/L) | 42.2 ± 9.5 | 42.2 ± 12.5 | 0.094* | 0.999 |
| Immunoglobulin G (g/L) | 15.0 ± 6.5 | 22.3 ± 5.8 | 2.814* | 0.004 |
| Prednisone only, n | 12 | 9 | 0.385‡ | 0.535 |
| Prednisone and azathioprine, n | 7 | 8 | ||
| Duration of treatment (months) | 23 (6–96) | 20 (1–96) | 0.578† | 0.437 |
Values are presented as n (%), median (range), or mean ± SD. *compared by t-test. †analyzed by the Mann–Whitney U-test. ‡Initial responses to treatment of patients in prednisone only and combined with prednisone and azathioprine were compared by Chi-square test. AST: Aspartate aminotransferase; GLO: Globulin; SD: Standard deviation.
Effects of immunosuppressive treatment on outcomes of patients
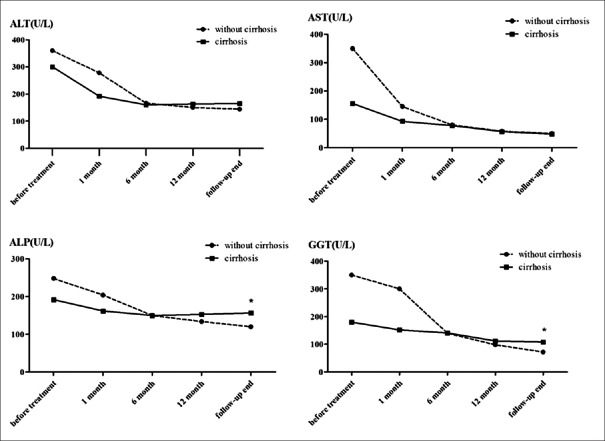
We next depicted the trends of serum enzyme levels (ALT, AST, ALP, and GGT) of patients who received immunosuppressive therapy [Figure 2]. Even the presence of cirrhosis and the levels of ALT and AST decreased dramatically in the 1st month of treatment whereas reductions of ALP and GGT took longer. Importantly, the levels of ALP and GGT at the end of follow-up in patients with cirrhosis were significantly higher than those in noncirrhotic patients (P < 0.001 for ALP, P < 0.001 for GGT).
Figure 2.
The decreasing trends of ALT, AST, ALP, and GGT levels in patients receiving immunosuppressive treatment including both AIH with cirrhosis (n = 39) and without cirrhosis (n = 57), especially during the first 6 months. The levels of ALP and GGT at the end of follow-up in patients with cirrhosis were significantly higher than those in noncirrhotic patients (P < 0.0001 for ALP, P < 0.0001 for GGT). *P < 0.05 was considered statistically significant. ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; ALP: Alkaline phosphatase; GGT: γ-glutamyl transpeptidase; AIH: Autoimmune hepatitis.
With disease progression for clinical endpoint events, we analyzed the factors leading to the progression of the disease. The univariate Cox regression analysis of factors affecting the prognosis of AIH patients showed only the level of AST, presence of cirrhosis, and treatment affected outcome [Table 3]. The multivariate Cox regression analysis further suggested that cirrhosis at presentation significantly increases the risk of disease exacerbation (hazard ratio [HR]: 4.603; P = 0.002). The level of AST at presentation also increases the risk of disease progression (P = 0.017), but the HR is smaller (HR: 1.002). Besides, the treatment of immunosuppressants markedly reduced disease progression (HR: 0.058; P = 0.005) [Table 4].
Table 3.
Univariate Cox regression analysis for prognosis of AIH patients experienced disease relapse (n = 17)
| Variables | HR | 95% CI | P |
|---|---|---|---|
| Age | 1.035 | 0.975–1.098 | 0.261 |
| Sex | 6.490 | 0.729–57.749 | 0.094 |
| ALT | 1.000 | 0.994–1.007 | 0.999 |
| AST | 1.002 | 0.996–1.008 | 0.043 |
| ALP | 1.001 | 0.996–1.006 | 0.677 |
| GLO | 0.980 | 0.932–1.031 | 0.436 |
| TBIL | 1.002 | 0.994–1.010 | 0.606 |
| Cirrhosis at presentation | 4.095 | 1.422–11.793 | 0.009 |
| Treatment | 0.048 | 0.004–0.531 | 0.013 |
A univariate Cox regression analysis of factors affecting prognosis of AIH patients. Only the level of AST, presence of cirrhosis and treatment affected outcome. ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; ALP: Alkaline phosphatase; GLO: Globulin; TBIL: Total bilirubin; AIH: Autoimmune hepatitis; HR: Hazard ratio; CI: Confidence interval.
Table 4.
Multivariate Cox regression analysis of prognosis of AIH patients (n = 17)
| Variables | HR | 95% CI | P |
|---|---|---|---|
| Cirrhosis at presentation | 4.603 | 1.757–12.059 | 0.002 |
| Treatment | 0.058 | 0.008–0.423 | 0.005 |
| AST | 1.002 | 1.000–1.004 | 0.017 |
A multivariate Cox regression analysis of factors affecting prognosis of AIH patients. The presence of cirrhosis, level of AST and treatment affected outcome. AST: Aspartate aminotransferase; AIH: Autoimmune hepatitis; HR: Hazard ratio; CI: Confidence interval.
Moreover, we analyzed disease progression of patients in these four groups. Among 57 noncirrhotic patients who received immunosuppressive therapy, disease progression was noted in 13 patients (22.8%), including 12 who developed cirrhosis and 1 who died of hepatic failure. Of the 70 noncirrhotic patients who did not receive immunosuppressive therapy, disease progression occurred in 12 patients (17.1%), including 4 patient who developed cirrhosis and 8 patients died of hepatic failure. In 39 cirrhotic patients who received immunosuppressive therapy, disease progression occurred in 8 patients (20.5%), including 4 patients who developed complications of decompensated cirrhosis, 2 patients developed hepatocarcinoma, and 2 patients died of hepatic failure. In 48 cirrhotic patients who did not receive immunosuppressive therapy, disease progression occurred in 26 patients (54.2%), including 12 patients developed complications of decompensated cirrhosis and 14 patients died of hepatic failure. Taken together, disease progression was comparable between noncirrhotic (22.8%) and cirrhotic (20.5%) patients who received immunosuppressive treatment (P = 0.789) whereas cirrhotic patients who did not receive immunosuppressive therapy (54.2%) showed a higher rate of disease progression than cirrhotic patients who have been treated (20.5%) (P = 0.001).
Discussion
The responses to immunosuppressive therapy of AIH patients have been reported to be good but show geographical variation.[28,29] The remission rate of AIH patients after initial therapy ranged from 76% to 100% in Caucasian patients[3,4,5,6,7] but was about 54.8% in patients from Saudi Arabia.[11] Our study showed a complete remission rate 84.4% (81/96) in AIH patients after therapy, which is similar to the remission rates of 89%–100% observed in Japanese patients.[10] Moreover, we showed that 47.2% (17/36) of patients experienced relapse after drug withdrawal or treatment termination, and retreatment effectively induced remission again, whereas the reported relapse rates were 50%–86% in Caucasian,[8] and 29.9% in Japanese during decrement to corticosteroid therapy. Distinct genetic backgrounds may play a role in the variation among ethnicities; in general, AIH patients of Chinese descent respond well to immunosuppressive treatment, with remission and relapse rates comparable to those of Caucasian patients.
To date, the only data available on comparison of remission rates between cirrhotic and noncirrhotic AIH patients were reported by Feld et al.,[5] who showed similar remission rates between noncirrhotic (81.9%) and cirrhotic (73.8%) patients after immunosuppressive treatment. In our study, complete remission after initial therapy was achieved in 87.7% (50/57) of noncirrhotic patients and 79.5% (31/39) of cirrhotic patients (P = 0.275). Furthermore, we showed that relapse occurred in 40% (4/10) of noncirrhotic patients and 50% (13/26) of cirrhotic patients (P = 0.590) after drug withdrawal, suggesting relapse rates between the two groups have no significant difference. Taken together, AIH patients who present with cirrhosis respond well to the initial immunosuppressive treatment, with remission and relapse rates comparable to those without cirrhosis.
The long-term outcomes of AIH patients with cirrhosis treated by immunosuppressive therapy remain unclear. Roberts et al.[3] reported that 10-year survival was not different between patients with and without cirrhosis (89% vs. 90%) after immunosuppressive treatment whereas Feld et al. reported that AIH patients with cirrhosis at presentation had a worse 10-year survival than those without cirrhosis (61.9% vs. 94.0%) after treatment.[5] In our study, we observed similar to the progression of the disease (20.5% vs. 22.8%, P = 0.789) and remission rate (94.7% vs. 92.3%, P = 0.629) for patients with and without cirrhosis at presentation who underwent immunosuppressive treatment, supporting the notion that the presence of cirrhosis has nothing to do with the long-term prognosis of AIH.
Importantly, we compared the disease progression of cirrhotic patients treated and not treated by immunosuppressants. It appears that patients not treated by immunosuppressants had significantly poor long-term outcomes. In our study, no immunosuppressant therapy patients were those that do not match the treatment indications according to the American Association for the Study of Liver Diseases (AASLD) guidelines for AIH. Current treatment indicators in the guidelines are determined by the clinical features of patients, for instance, symptoms, serum levels of ALT, and IgG. Due to the poor prognostic features, the new British guidelines for AIH recommends that AIH patients with cirrhosis and even mild histological activity should be treated.[18] We speculate that asymptomatic cirrhotic patients with normal levels of ALT and IgG may have subclinical levels of liver dysfunction. Therefore, we propose that AIH patients with cirrhosis who do not meet the treatment indications should undergo liver biopsy, by which the unnoted active inflammation may be identified to compensate for absence of treatment indicators. Nevertheless, further studies are urgent to uncover the reasons related to the poor long-term outcomes of those patients. Xu et al.[30] reported that splenectomy might be an option to prevent AIH relapse in some patients with high-risk factors.
It has been reported that risk factors for relapse include a high level of γ-globulin, time to initial remission, and failure to have consistently normal transaminases during remission.[8,31] In our study, high levels of IgG were associated with relapse and treatment failure [Table 3]. IgG antibodies are involved predominantly in the secondary immune response, binding to many kinds of pathogens, for example, viruses, bacteria, and fungi. The higher level of serum IgG may reflect a more vigorous immune and inflammatory response in patients, which, in turn, is associated with difficulties in suppressing inflammation. Therefore, patients with high levels of IgG, used as a risk marker of relapse, may need a longer or larger dose of immunosuppressive treatment.
In conclusion, our data suggest that AIH patients with cirrhosis of Chinese descent respond well to immunosuppressive treatment. The efficacy of immunosuppressive therapy in patients with liver cirrhosis is similar to those without cirrhosis. Cirrhotic patients not treated by immunosuppressants according to the management guidelines have poor long-term outcomes, which may be caused by unnoted active inflammation.
Financial support and sponsorship
This study was supported by the grants from the National Natural Science Foundation of China (No. 81200282, and No. 81470834).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank Rui Lin, Mi-Mi Yang, and Li-Li Luo, who reviewed and edited the manuscript.
Footnotes
Edited by: Ning-Ning Wang
References
- 1.Dhaliwal HK, Hoeroldt BS, Dube AK, McFarlane E, Underwood JC, Karajeh MA, et al. Long-term prognostic significance of persisting histological activity despite biochemical remission in autoimmune hepatitis. Am J Gastroenterol. 2015;110:993–9. doi: 10.1038/ajg.2015.139. doi: 10.1038/ajg.2015.139. [DOI] [PubMed] [Google Scholar]
- 2.van Gerven NM, Verwer BJ, Witte BI, van Hoek B, Coenraad MJ, van Erpecum KJ, et al. Relapse is almost universal after withdrawal of immunosuppressive medication in patients with autoimmune hepatitis in remission. J Hepatol. 2013;58:141–7. doi: 10.1016/j.jhep.2012.09.009. doi: 10.1016/j.jhep.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Roberts SK, Therneau TM, Czaja AJ. Prognosis of histological cirrhosis in type 1 autoimmune hepatitis. Gastroenterology. 1996;110:848–57. doi: 10.1053/gast.1996.v110.pm8608895. doi: 10.1053/gast.1996.v110.pm8608895. [DOI] [PubMed] [Google Scholar]
- 4.Seo S, Toutounjian R, Conrad A, Blatt L, Tong MJ. Favorable outcomes of autoimmune hepatitis in a community clinic setting. J Gastroenterol Hepatol. 2008;23:1410–4. doi: 10.1111/j.1440-1746.2008.05365.x. doi: 10.1111/j.1440-1746.2008.05365.x. [DOI] [PubMed] [Google Scholar]
- 5.Feld JJ, Dinh H, Arenovich T, Marcus VA, Wanless IR, Heathcote EJ. Autoimmune hepatitis: Effect of symptoms and cirrhosis on natural history and outcome. Hepatology. 2005;42:53–62. doi: 10.1002/hep.20732. doi: 10.1002/hep.20732. [DOI] [PubMed] [Google Scholar]
- 6.Mendizabal M, Marciano S, Videla MG, Anders M, Zerega A, Balderramo DC, et al. Changing etiologies and outcomes of acute liver failure: perspectives from 6 transplant centers in Argentina. Liver Transpl. 2014;20:483–9. doi: 10.1002/lt.23823. doi: 10.1002/lt.23823. [DOI] [PubMed] [Google Scholar]
- 7.Kanzler S, Löhr H, Gerken G, Galle PR, Lohse AW. Long-term management and prognosis of autoimmune hepatitis (AIH): A single center experience. (344-8).Z Gastroenterol. 2001;39:339–41. doi: 10.1055/s-2001-13708. doi: 10.1055/s-2001-13708. [DOI] [PubMed] [Google Scholar]
- 8.Verma S, Gunuwan B, Mendler M, Govindrajan S, Redeker A. Factors predicting relapse and poor outcome in type I autoimmune hepatitis: Role of cirrhosis development, patterns of transaminases during remission and plasma cell activity in the liver biopsy. Am J Gastroenterol. 2004;99:1510–6. doi: 10.1111/j.1572-0241.2004.30457.x. doi: 10.1111/j.1572-0241.2004.30457.x. [DOI] [PubMed] [Google Scholar]
- 9.Yoshizawa K, Matsumoto A, Ichijo T, Umemura T, Joshita S, Komatsu M, et al. Long-term outcome of Japanese patients with type 1 autoimmune hepatitis. Hepatology. 2012;56:668–76. doi: 10.1002/hep.25658. doi: 10.1002/hep.25658. [DOI] [PubMed] [Google Scholar]
- 10.Yokokawa J, Kanno Y, Saito H, Abe K, Takahashi A, Yokokawa H, et al. Risk factors associated with relapse of type 1 autoimmune hepatitis in Japan. Hepatol Res. 2011;41:641–6. doi: 10.1111/j.1872-034X.2011.00812.x. doi: 10.1111/j.1872-034X.2011.00812.x. [DOI] [PubMed] [Google Scholar]
- 11.Fallatah HI, Akbar HO, Qari YA. Autoimmune hepatitis: Single-center experience of clinical presentation, response to treatment and prognosis in Saudi Arabia. Saudi J Gastroenterol. 2010;16:95–9. doi: 10.4103/1319-3767.61235. doi: 10.4103/1319-3767.61235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kil JS, Lee JH, Han AR, Kang JY, Won HJ, Jung HY, et al. Long-term treatment outcomes for autoimmune hepatitis in Korea. J Korean Med Sci. 2010;25:54–60. doi: 10.3346/jkms.2010.25.1.54. doi: 10.3346/jkms.2010.25.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang F, Wang Q, Bian Z, Ren LL, Jia J, Ma X. Autoimmune hepatitis: East meets west. J Gastroenterol Hepatol. 2015;30:1230–6. doi: 10.1111/jgh.12952. doi: 10.1111/jgh.12952. [DOI] [PubMed] [Google Scholar]
- 14.Ngu JH, Gearry RB, Frampton CM, Stedman CA. Predictors of poor outcome in patients w ith autoimmune hepatitis: A population-based study. Hepatology. 2013;57:2399–406. doi: 10.1002/hep.26290. doi: 10.1002/hep.26290. [DOI] [PubMed] [Google Scholar]
- 15.Migita K, Watanabe Y, Jiuchi Y, Nakamura Y, Saito A, Yagura M, et al. Evaluation of risk factors for the development of cirrhosis in autoimmune hepatitis: Japanese NHO-AIH prospective study. J Gastroenterol. 2011;46(Suppl 1):56–62. doi: 10.1007/s00535-010-0337-y. doi: 10.1007/s00535-010-0337-y. [DOI] [PubMed] [Google Scholar]
- 16.Wang SB, Wang JH, Chen J, Giri RK, Chen MH. Natural history of liver cirrhosis in south China based on a large cohort study in one center: A follow-up study for up to 5 years in 920 patients. Chin Med J. 2012;125:2157–62. doi: 10.3760/cma.j.issn.0366-6999.2012.12.014. [PubMed] [Google Scholar]
- 17.Wang Y, Hou JL. Current strategies for quantitating fibrosis in liver biopsy. Chin Med J. 2015;128:252–8. doi: 10.4103/0366-6999.149223. doi: 10.4103/0366-6999.149223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gleeson D, Heneghan MA British Society of Gastroenterology. British Society of Gastroenterology (BSG) guidelines for management of autoimmune hepatitis. Gut. 2011;60:1611–29. doi: 10.1136/gut.2010.235259. doi: 10.1136/gut.2010.235259. [DOI] [PubMed] [Google Scholar]
- 19.Bouma G, van Nieuwkerk CM. Treatment withdrawal in autoimmune hepatitis. Dig Dis. 2015;33(Suppl 2):88–93. doi: 10.1159/000440756. doi: 10.1159/000440756. [DOI] [PubMed] [Google Scholar]
- 20.Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169–76. doi: 10.1002/hep.22322. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]
- 21.Qiu D, Wang Q, Wang H, Xie Q, Zang G, Jiang H, et al. Validation of the simplified criteria for diagnosis of autoimmune hepatitis in Chinese patients. J Hepatol. 2011;54:340–7. doi: 10.1016/j.jhep.2010.06.032. doi: 10.1016/j.jhep.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 22.Yeoman AD, Westbrook RH, Al-Chalabi T, Carey I, Heaton ND, Portmann BC, et al. Diagnostic value and utility of the simplified International Autoimmune Hepatitis Group (IAIHG) criteria in acute and chronic liver disease. Hepatology. 2009;50:538–45. doi: 10.1002/hep.23042. doi: 10.1002/hep.23042. [DOI] [PubMed] [Google Scholar]
- 23.Yo IK, Kwon OS, Park JW, Lee JJ, Lee JH, Won IS, et al. The factors associated with longitudinal changes in liver stiffness in patients with chronic hepatitis B. Clin Mol Hepatol. 2015;21:32–40. doi: 10.3350/cmh.2015.21.1.32. doi: 10.3350/cmh.2015.21.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macías-Rodríguez MA, Rendón-Unceta P, Ramos-Clemente-Romero MT, Troiteiro-Carrasco LM, Serrano-León MD. Prospective validation of two models for ultrasonographic diagnosis of cirrhosis. Rev Esp Enferm Dig. 2011;103:232–7. doi 1130-0108/2011/103/5/232-237. [PubMed] [Google Scholar]
- 25.Acevedo J, Fernández J, Prado V, Silva A, Castro M, Pavesi M, et al. Relative adrenal insufficiency in decompensated cirrhosis: Relationship to short-term risk of severe sepsis, hepatorenal syndrome, and death. Hepatology. 2013;58:1757–65. doi: 10.1002/hep.26535. doi: 10.1002/hep.26535. [DOI] [PubMed] [Google Scholar]
- 26.Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D, et al. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:2193–213. doi: 10.1002/hep.23584. doi: 10.1002/hep.23584. [DOI] [PubMed] [Google Scholar]
- 27.Lamers MM, van Oijen MG, Pronk M, Drenth JP. Treatment options for autoimmune hepatitis: A systematic review of randomized controlled trials. J Hepatol. 2010;53:191–8. doi: 10.1016/j.jhep.2010.01.037. doi: 10.1016/j.jhep.2010.01.037. [DOI] [PubMed] [Google Scholar]
- 28.Zachou K, Muratori P, Koukoulis GK, Granito A, Gatselis N, Fabbri A, et al. Review article: Autoimmune hepatitis –Current management and challenges. Aliment Pharmacol Ther. 2013;38:887–913. doi: 10.1111/apt.12470. doi: 10.1111/apt.12470. [DOI] [PubMed] [Google Scholar]
- 29.Liberal R, Vergani D. Effect of ethnicity on the clinical presentation and outcome of autoimmune hepatitis. Expert Rev Gastroenterol Hepatol. 2012;6:267–9. doi: 10.1586/egh.12.17. doi: 10.1586/egh.12.17. [DOI] [PubMed] [Google Scholar]
- 30.Xu YT, Liu DJ, Meng FY, Li GB, Liu J. Possible benefit of splenectomy in liver transplantation for autoimmune hepatitis. Hepatobiliary Pancreat Dis Int. 2014;13:328–31. doi: 10.1016/s1499-3872(14)60256-3. doi: 10.1016/S1499-3872(14)60256-3. [DOI] [PubMed] [Google Scholar]
- 31.Hartl J, Ehlken H, Weiler-Normann C, Sebode M, Kreuels B, Pannicke N, et al. Patient selection based on treatment duration and liver biochemistry increases success rates after treatment withdrawal in autoimmune hepatitis. J Hepatol. 2015;62:642–6. doi: 10.1016/j.jhep.2014.10.018. doi: 10.1016/j.jhep.2014.10.018. [DOI] [PubMed] [Google Scholar]