Abstract
Background:
Perineural invasion (PNI) is a histopathological characteristic of pancreatic cancer (PanCa). The aim of this study was to observe the treatment effect of continuous low-dose-rate (CLDR) irradiation to PNI and assess the PNI-related pain relief caused by iodine-125 (125I) seed implantation.
Methods:
The in vitro PNI model established by co-culture with dorsal root ganglion (DRG) and cancer cells was interfered under 2 and 4 Gy of 125I seeds CLDR irradiation. The orthotopic models of PNI were established, and 125I seeds were implanted in tumor. The PNI-related molecules were analyzed. In 30 patients with panCa, the pain relief was assessed using a visual analog scale (VAS). Pain intensity was measured before and 1 week, 2 weeks, and 1, 3, and 6 months after 125I seed implantation.
Results:
The co-culture of DRG and PanCa cells could promote the growth of PanCa cells and DRG neurites. In co-culture groups, the increased number of DRG neurites and pancreatic cells in radiation group was significantly less. In orthotopic models, the PNI-positive rate in radiation and control group was 3/11 and 7/11; meanwhile, the degrees of PNI between radiation and control groups was significant difference (P < 0.05). At week 2, the mean VAS pain score in patients decreased by 50% and significantly improved than the score at baseline (P < 0.05). The pain scores were lower in all patients, and the pain-relieving effect was retained about 3 months.
Conclusions:
The CLDR irradiation could inhibit PNI of PanCa with the value of further study. The CLDR irradiation could do great favor in preventing local recurrence and alleviating pain.
Keywords: Continuous Low-dose-rate Irradiation, Pancreatic Cancer, Perineural Invasion, Radioactive Seeds
Introduction
Pancreatic cancer (PanCa) is an aggressive malignancy with an extremely poor prognosis. Perineural invasion (PNI) extending into the extrapancreatic nerve plexus is a histopathological characteristic in PanCa, which leads to abdominal pain and retropancreatic tumor extension.[1,2] It promotes local recurrence, and finally negatively influences the prognosis of the patients.[3,4,5] The current insights indicate that plenty of molecules participate in PNI such as neurotrophins and their receptors,[6,7] cytokines,[8,9] chemokines,[10] proteinases,[11] and cell-surface markers[12] through reciprocal signaling. Agents targeting PNI-related signaling pathway such as nerve growth factor (NGF)-tyrosine kinase receptor-A pathway were confirmed to be effective to alleviate PNI-related pain.[13,14,15] Iodine-125 (125I) seed brachytherapy has been reported as a safe and effective method for the local control of malignant pancreatic tumors.[16,17] In particular, this treatment could significantly relieve the pain.[18,19,20,21,22] Peretz et al.[20] reported that among 98 PanCa cases, pain relief was observed in 65% of cases within 10 days after 125I seeds implantation. Sun et al.[21] indicated that among 15 patients with PanCa, the short-term pain relief was observed in 30% of patients after endoscopic ultrasonography (EUS)-guided 125I seed implantation. Jin et al.[22] reported that the visual analog scale (VAS) pain score significantly dropped from 5.07 to 1.73 one week after treatment, and this score was maintained for one month. However, the molecular mechanism of pain relief caused by 125I seed irradiation remained unclear.
The proper treatment of neural invasion has not been established. According to the valuable observations in the previous clinical studies, we established the PNI models of PanCa in vivo and in vitro to observe the treatment effect of 125I seeds and conducted a preliminary study of the PNI-related pain relief caused by continuous low-dose-rate (CLDR) irradiation of 125I seeds.
Methods
Iodine-125 seeds
Type-6711 sealed 125I seeds with single-grain apparent radioactivity of 34.3–37.0 MBq (0.93–1.0 mCi) were provided by Jun-An Pharmaceutical Technology (Ningbo, China). A single seed was 0.84 mm in diameter, 4.5 mm long, and had a half-life of 60.2 days and main transmission of 27.4–31.4 Kev X-ray and 35.5 Kev γ-ray. The in vitro cell irradiation using 125I seeds was performed as described in literature.[23,24] Briefly, eight 125I seeds were uniformly distributed over a round plate 3 cm in diameter, and an additional seed was placed in the center. The cell culture dish was placed over the 125I seed plate with a 5-mm gap filled with water. Halfway through the irradiation, the cell culture dish was turned by 22.5° to make the dose distribution more homogeneous. Dose distribution was calculated using a treatment planning system (TPS, Kelinzhong Institute of Atomic Energy, Beijing, China, No. YZB/1466-70-2004) based on the American Association of Physicists in Medicine Task Group No. 43 (AAPM TG-43) formalism.[25]
In vitro model establishment and iodine-125 seed irradiation
The protocol for building this model system has been described previously.[26] The dorsal root ganglions (DRGs) from the cervical, thoracic, and lumbar areas of 4-week-old Sprague-Dawley rats were dissected under sterile conditions. The human PanCa cell lines, CaPan2 and Panc1 (American Type Culture Collection, Manassas, VA, USA), were selected. Engelbreth-Holm-Swarm Matrigel (BD Biosciences, San Jose, CA, USA) was used as an extracellular matrix. The cells and the DRG were immobilized in the Matrigel by warming the culture to 37°C. The CaPan2/DRG and Panc1/DRG were routinely cultured in serum-supplemented medium (RPMI-1640 medium; Sigma, St. Louis, MO, USA) at 37°C, containing 10% heat-inactivated fetal bovine serum (Sigma) in a humidified atmosphere of 95% O2 and 5% CO2. PanCa cells only and DRG only were cultured as controls.
When a symbiotic phenomenon was observed between nerves and PanCa cell lines, the dish was placed 5 mm above the 125I seed plane for CLDR irradiation (R+) within the incubator until the accumulation doses around the inner edge of the dish were 2 and 4 Gy. The initial dose rate on average on the cell plane was 6.08 cGy/h, and it decreased slowly with a halftime of 60.2 days. The irradiation time of different experiments varied from 50 h to 62 h for 2 Gy and from 100 h to 120 h for 4 Gy. PanCa cells only and DRG only without 125I seed irradiation (R−) were cultured as controls.
In vivo model establishment and iodine-125 seed irradiation
Cell culture
The PanCa cell line of CaPan2 was cultured in RPMI 1640 (Sigma) containing 10% heat-inactivated fetal bovine serum, 100 μg/ml ampicillin, and 100 μg/ml streptomycin. All cell cultures were done at 37°C under 5% CO2.
Orthotopic model of perineural invasion and irradiation
The experimental procedures in this study were approved by the Animal Use and Care Committee of the Second Military Medical University, China. The method for the orthotopic model of PNI was described previously.[27,28] The 8- to 12-week-old nonobese diabetes/severe combined immunodeficiency (NOD/SCID) mice (Second Military Medical University Experimental Animal Center) were used in this experiment. The NOD/SCID mice were anesthetized, and the distal pancreases were exteriorized,[27,28] and 106 viable tumor cells in 10 μl of cell suspension were injected into the pancreas using an inoculator with a 27-gauge needle. The pancreas was relocated into the abdominal cavity, and the peritoneum and skin were sutured. Six weeks after the injection, mice were then randomized into two groups: radiation group and control group (n = 11 in each group); the mice were anesthetized again and one 125I seed was implanted in the tumor, and the absorbed dose at the point of 10 mm distant from the center of the 125I seed was not <2 Gy. Two weeks after 125I seed implantation, the mice were sacrificed, and the pancreas and the nearby tissues contained the retroperitoneal nerve were harvested to examine the positive rate and the degree of PNI. The mice with nonradioactive seed implantation were selected as controls.
Image analysis
The DRGs, CaPan2, and Panc1 colonies were photographed at a magnification of ×40 using inverted microscopy (Olympus, Tokyo, Japan). Neurite outgrowth and cell colony growth were quantitated using the Optimas 6.1 image analysis system (Optimas Corp., Bothell, MA, USA). The total number of neurites was obtained by counting the neurites along the entire DRG circumference from different experiment days. The area of PanCa cell colony growth was calculated by measuring the surface area occupied by the colonies. The percentage changes of neurite outgrowth and cell colony growth after radiation were calculated by the difference between the control group and radiation group divided by the value in control group at the corresponding times.
Real-time quantitative reverse transcription-polymerase chain reaction and enzyme-linked immunosorbent assay analysis
Total RNA was extracted from PanCa cells or tissues using Trizol reagent (Invitrogen, Carlsbad, CA, USA). Real-time quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis was carried out under the same conditions. The expressions of NGF and epidermal growth factor receptor (EGFR) mRNA in PanCa cells of in vitro and in vivo models were tested, respectively. The sequences of the primer pairs used in this study were as follows: NGF forward 5’-AGCAAGCGGTCATCATCCC-3’, NGF reverse 5’- ACCACCGCCACAGACATCA-3’; EGFR forward 5’- CTACGAGCTGCCTGACG-3’, EGFR reverse 5’- AGAAGCATTTGCGGTGG-3’; and GAPDH forward 5’- GCACCG TCAAGGCTGAGAAC-3’, GAPDH reverse 5’-ATGGTGGTGAAGACGCCAGT-3’. GAPDH served as an internal control in each experiment. PCR products were run on agarose gels and visualized by ethidium bromide staining. The qRT-PCR was performed using the ABI 7500 Sequence Detection System (Applied Biosystems, USA). Each 25 μl reaction mixture contained 2.5 μl target cDNA template, primers (10 μmol/L) used for the amplification of target gene sequences, and SYBR Green real-time PCR master (Toyobo, Japan). The thermal cycling parameters were set for the following conditions: One cycle at 95°C for 1 min, and 40 cycles of 95°C for 15 s, 60°C for 15 s, and 72°C for 45 s. Normalization for the relative quantity of mRNA was accomplished by comparison to glyceraldehyde-3-phosphate dehydrogenase, using a previously reported method.[29] The qRT-PCR was done at least thrice. The concentration of NGF, EGF, and transforming growth factor-α (TGF-α) in media supernatant was examined with enzyme-linked immunosorbent assay using kits (R & D Systems, Inc., Minneapolis, MN, USA), respectively.
Histologic studies and Immunohistochemical staining
Sections were prepared by formalin-fixed, paraffin-embedded. Histologic diagnoses were made according to the World Health Organization criteria or the Japan pancreas society classification.[30] The definition and the degree of PNI were determined as described previously.[27] All the sections were assessed by a single pathologist blinded to the group information.
Immunohistochemical analysis was conducted as described previously.[31] Tissues were obtained from PanCa approximately 5 mm distant from the center of the implanted 125I seeds. The sections were incubated with rabbit anti-human NGF antibody (1:100, Abcam, USA) at room temperature overnight. Positive protein expression was visualized as nuclear localization of granular brown-yellow precipitate. The counts were performed in 3 high-power fields of vision under a high magnification (×400) for each section. The percentage of positive cells was calculated as the ratio of positive cells to the total number of cells. The scoring scale for the percentage of positive cells was as follows: 0, <1%; 1, 1%–24%; 2, 25%–50%; 3, 51%–75%; and 4, >75%. The scoring scale for staining intensity was as follows: 0, no color; 1, bright yellow; 2, yellow; 3, brown-yellow; and 4, brown. The final score was obtained by multiplying the percentage of positive cells by the staining intensity score.
Patients and perineural invasion-related pain relief
Written informed consent was obtained from all patients, and the study was approved by the Ethics Committees of 307 Hospital of PLA. Patients were of either gender, with the diagnosis of histologically proven or radiologically consistent, surgically unresectable pancreatic adenocarcinoma.[32] To be included in the study, patients were required to have a Karnofsky Performance score (KPS) of ≥50 and were expected to survive for more than 3 months after diagnosis; they were also required to have adequate bone marrow function (blood leukocytes ≥3.0 × 109 cells/L, platelet count ≥100 × 109/L, and hemoglobin ≥100 g/L). Patients with a prothrombin time of 3 s longer than the control were excluded. Patients treated by any previous irradiation or a previous course of chemotherapy were excluded. While receiving implantation treatment, the patients also received other necessary treatments such as chemotherapy or biological therapy.[22] Dose distribution was calculated using a special treatment planning system 1.0 (TPS 1.0; Second Military Medical University, China) based on EUS images. The minimal calculated dose should be not <2 Gy. Pain intensity was measured before and 1 week, 2 weeks, and 1, 3, and 6 months after 125I seed implantation, and patients were followed up until death. The tumor diameter and general condition of patients were monitored and recorded during follow-up. The short-term efficacy was determined according to the tumor response standards suggested by the World Health Organization.
The pain intensity was assessed for each patient using a VAS from 0 to 10 (0 is no pain and 10 is unbearable pain).[33] To enroll, the pain intensity rating (average in the last 24 h) had to be a VAS pain score of 3/10 or higher, or opioid was required for cancer-related pain control and the VAS pain score of lower than 6/10 if already optimized on opioids. An optimized opioid therapy was considered the maximum analgesia achievable without intolerable opioid-related adverse effects.[34] Pain intensity was measured before 125I seed implantation and 1 week, 2 weeks, and 1, 3, and 6 months after implantation (the intensity of pain is given as the mean of VAS points per day during the previous week). Consumption of analgesics was documented at the same intervals. Patients were excluded if they had received previous neurolytic celiac plexus block or other neurolytic blocks that could affect pain relief or had implanted epidural or intrathecal analgesic therapy. Patients with psychiatric disease affecting assessments, uncorrectable coagulopathy, or allergy to local anesthetics were excluded from the study.
Statistical analysis
All data were shown as median (range) or mean ± standard deviation (SD). Statistical analysis was performed with SPSS 13.0 software (SPSS Inc., Chicago, IL, USA). Statistical analysis was conducted using the Mann-Whitney U-test for the difference between experimental and control groups. The continuous variables were compared using the t-test (the assumption of normality was verified using the Anderson-Darling test). Variables were evaluated for an association with the use of the Pearson's Chi-squared test. A P < 0.05 was considered statistically significant.
Results
Pancreatic cancer cell colonies and neurites growth
The co-culture of DRG and PanCa cells could promote the growth of PanCa cells and DRG neurites. In co-culture group, the orientation and reciprocal growth between PanCa cells and the DRG neurites could be observed [Figure 1]. The outgrowth of neurites in co-culture group was significantly greater than that of single DRG group at the corresponding times (P < 0.05) [Figure 2]. The increase of the surface area of PanCa cell colonies in co-culture group was significantly greater than the increase in single-cultured group (P < 0.05) [Figure 3].
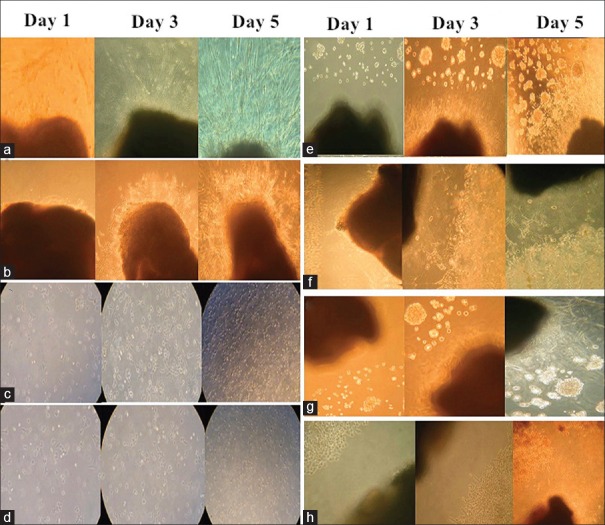
Figure 1.
The observation photos of the single-cultured groups and co-culture groups on day 1, day 3, and day 5 (original magnification × 40). In the single-cultured groups, there was no significant difference of the number of neurite between the DRG (R+) and DRG (R−) groups (a and b), and the increased surface area in CaPan2 (R−) group was significantly larger than the area in CaPan2 (R+) group (c and d). The co-culture of DRG and PanCa cells could promote the growth of PanCa cells and DRG neurites (e and g), under irradiation, the orientation and reciprocal growth still existed, but was inhibited (f and h). DRG: Dorsal root ganglion; PanCa: Pancreatic cancer; R+: Mean under radiation; R−: Mean without radiation.
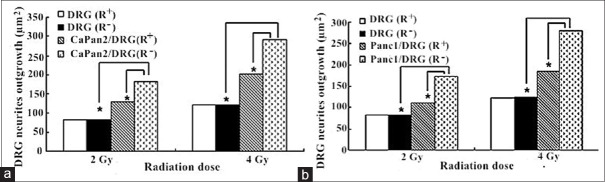
Figure 2.
The change of DRG neurites outgrowth in DRG (R+), DRG (R+), CaPan2/DRG (R−) CaPan2/DRG (R–), Panc1/DRG (R+), and Panc1/DRG (R−) groups. CaPan2 cell lines (a) and Panc1 cell lines (b) were selected in the establishment of in vitro PNI model with or without CLDR irradiation. *P < 0.05. CLDR: Continuous low-dose-rate; DRG: Dorsal root ganglion; PNI: Perineural invasion.
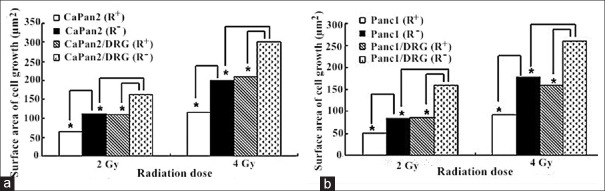
Figure 3.
The surface area of PanCa cell colony growth in four groups. CaPan2 cell lines (a) and Panc1 cell lines (b) were selected in the establishment of in vitro PNI model with or without CLDR irradiation. *P < 0.05. CLDR: Continuous low-dose-rate; PanCa: Pancreatic cancer; PNI: Perineural invasion.
Under the CLDR irradiations of the 125I seeds, the orientation and reciprocal growth still existed. There was no significant difference of the number of neurite between the DRG (R+) and DRG (R−) groups (P > 0.05). In the single-cultured groups, the increased surface area in PanCa cells (R−) group was significantly larger than the area in PanCa cells (R+) group (P < 0.05). The percentage change of surface area was 43% and 42% in CaPan-2 (R+) group under the irradiation of 2Gy and 4Gy. The percentage change in Panc1 (R+) group was 39% and 48%, respectively. In co-culture groups, the increased number of DRG neurites and pancreatic cells in PanCa cells/DRG (R−) group was significantly greater than that in PanCa cells/DRG (R+) group (P < 0.05). The percentage change of DRG neurite surface area under the irradiation of 2Gy and 4Gy was 30% and 31% in CaPan2/DRG (R+) group and 36% and 34% in Panc1/DRG (R+) group, respectively. The percentage change of pancreatic cell-surface area under the irradiation of 2Gy and 4Gy was 32% and 30% in CaPan2/DRG (R+) group and 46% and 39% in Panc1/DRG (R+) group, respectively [Figures 2 and 3].
In vitro model irradiation and perineural invasion cytokines
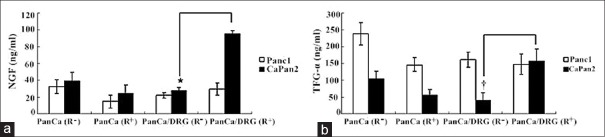
As shown in Figure 4, there was a significant difference in the level of NGF in media supernatant between CaPan2/DRG (R+) and CaPan2/DRG (R−) groups (P < 0.05). The concentration of TGF-α in CaPan2/DRG (R+) group was obviously greater than that of CaPan2/DRG (R−) group (P < 0.01). There was no obvious change of the level of EGF after 125I seed irradiation in four groups.
Figure 4.
The different concentration of NGF and TGF-α in media supernatant in four groups. The iodine-125 irradiation altered the level of NGF (a) and TGF-α (b) in CaPan2/DRG co-culture group. *P < 0.05. †P < 0.01. NGF: Nerve growth factor; TGF-α: Transforming growth factor-α; DRG: Dorsal root ganglion; PanCa: Pancreatic cancer.
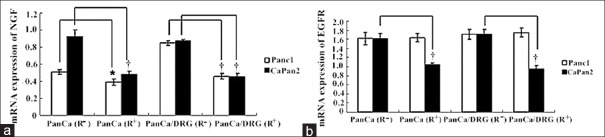
The NGF mRNA expression in Panc1 (R-) and CaPan2 (R−) groups was significantly greater than those in Panc1 (R+) and CaPan2 (R+) groups, respectively (P < 0.05 in Panc1 cells; and P < 0.01 in CaPan2 cells). The expression of NGF mRNA in Panc1/DRG group and CaPan2/DRG group was significantly reduced after CLDR irradiation, respectively (P < 0.01). Moreover, EGFR mRNA expression of CaPan2 cells in single and co-culture groups was significantly reduced after irradiation [P < 0.01; Figure 5].
Figure 5.
The different expression of NGF and EGFR mRNA in PanCa cells in four groups. Iodine-125 irradiation reduced the expression of NGF mRNA in two cell lines (a) and only altered EGFR mRNA expression in CaPan2 cells (b). *P < 0.05; †P < 0.01. NGF: Nerve growth factor; EGFR: Epidermal growth factor receptor; PanCa: Pancreatic cancer.
In vivo model irradiation and perineural invasion cytokines
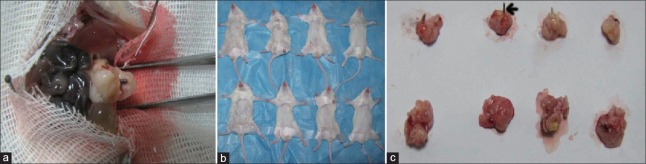
In orthotopic models, the PNI-positive rate in radiation group and control group was 3/11 and 7/11, respectively [Figures 6 and 7], and there was no significant difference in PNI-positive rate between the radiation group and control group; however, the degrees of PNI between the radiation group and control group was significant difference (P < 0.05). In radiation group, the degree of PNI was low, and the scores were all 0–1 (3/3). In control group, the high degree (score was 2–3) of PNI was 5/7, and the low degree (score was 0–1) of perineural invasion was only 2/7.
Figure 6.
In orthotopic model, the iodine-125 seed was implanted in the tumor (a), and the tumor sizes in radiation group and control group were different (b). (c) The above row represented the radiation group (the iodine-125 seed: black arrow) and the below row represented the control group.
Figure 7.
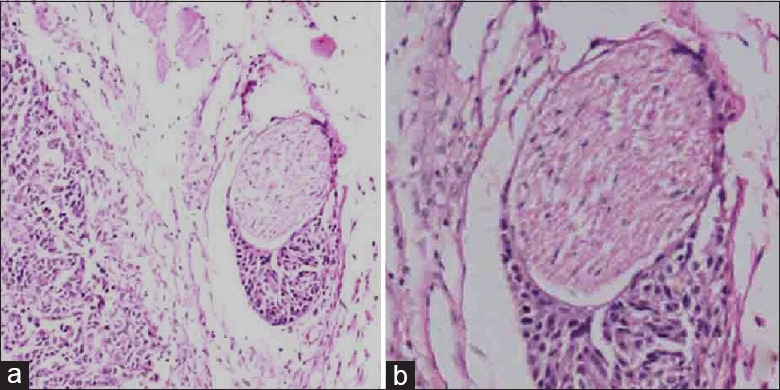
Hematoxylin and eosin staining image of perineural invasion (a: original magnification ×100; b: original magnification ×200).
The expression of NGF mRNA and EGFR mRNA in radiation group was significantly reduced compared with the control group (P < 0.01) [Figure 8]. In immunohistochemical detection, the positive scores of NGF in control group were significantly higher than that in the radiation group [171.4 ± 9.2 vs. 99.9 ± 40.0, P < 0.01; Figure 9].
Figure 8.
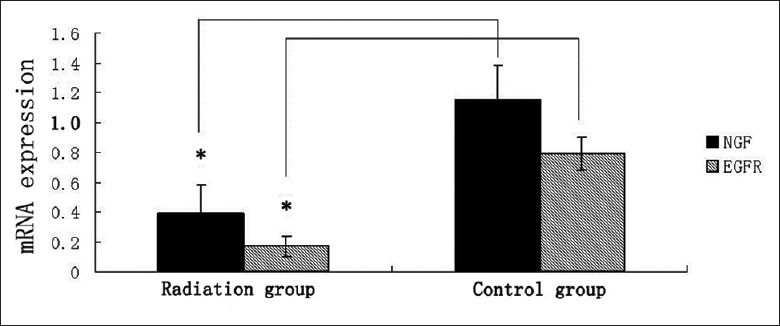
Iodine-125 irradiation reduced the expression of NGF and EGFR. In radiation group, mRNA expression was significantly lower than the control group. *P < 0.01. NGF: Nerve growth factor; EGFR: Epidermal growth factor receptor.
Figure 9.
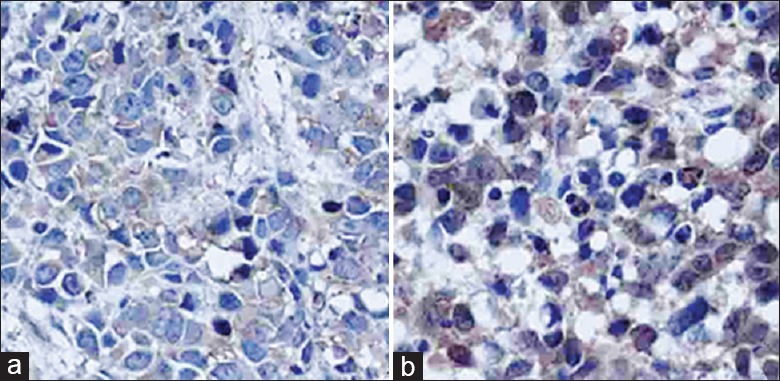
Immunohistochemical staining image of nerve growth factor. (a) Radiation group; (b) control group (original magnification ×400).
Patients and perineural invasion-related pain relief
Between October 2009 and November 2010, a total of 30 PanCa patients were enrolled. There were 17 men and 13 women, with a median age of 69 years (range 54–79 years), and the median KPS score of 83 (range 60–90). Nine patients were in Stage III with locally advanced cancer, and 21 patients were in Stage IV with liver metastasis. In 23/30 patients, the lesions were located in the body/tail part, with mean tumor diameter of 3.5 cm. All patients were generally well tolerated without any complication throughout the study. The mean number of implanted seeds (0.8 mCi) was 18 per patient (range 5–35 per patient). The median survival was 6.8 months. Nineteen patients survived more than 3 months. One and two weeks after 125I seed implantation (ISI), the mean absorbed dose at the tumor edge was 2.17 Gy and 3.64 Gy, respectively. At 2 months after the first seed implantation, the proportion of complete and partial remission (progressive disease [PD]), stable disease, and partial development among the 9 Stage III patients were 0/9, 4/9, 4/9, and 1/9, respectively.
As shown in Figure 10, the mean VAS pain score was 8.0 ± 1.2 before the implantation. At week 2, the mean VAS pain score decreased by 50%, and significantly improved than the score at baseline (P < 0.05). At week 2, 2 patients were pain-free, and 10 patients with VAS pain score of 2 points. The VAS pain scores were lower in all patients, and the pain-relieving effect was retained about 3 months. Only 5 patients have returned to their preoperative VAS scores with PD. The decreased analgesic consumption at the corresponding follow-up is shown in Table 1. After 1 month, only 5 (16.7%) patients were satisfied with opioids, which was significantly <46.7% observed from baseline (P < 0.05).
Figure 10.
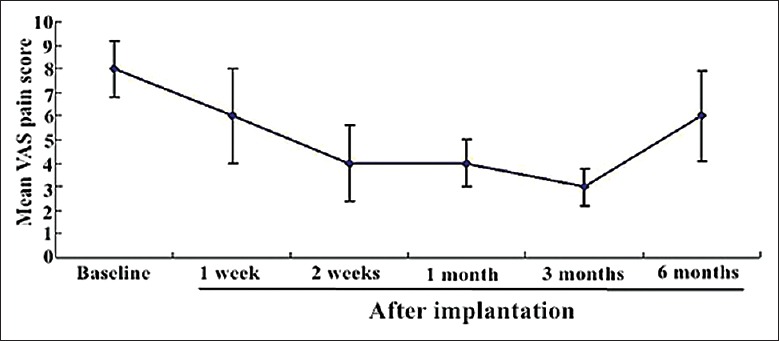
The mean VAS pain score before and 1 week, 2 weeks, and 1, 3, and 6 months after implantation. VAS: Visual analog scale.
Table 1.
Follow-up of opioid use and analgesic consumption in thirty patients with pancreatic cancer
| Time points | Number of patients | Opioid use, n (%) | Positive response of analgesic consumption, n (%) |
|---|---|---|---|
| Baseline | 30 | 14 (46.7) | – |
| After 1 week | 30 | 12 (40.0) | 6 (20.0) |
| After 2 weeks | 30 | 5 (16.7)* | 15 (50.0) |
| After 1 month | 30 | 5 (16.7)* | 17 (56.7) |
| After 3 months | 30 | 6 (20.0) | 12 (40.0) |
| After 6 months | 18† | 8 (44.4) | 4 (22.2) |
Patients were followed up weekly until death. The decreased analgesic consumption (≥50% reduction compared with baseline consumption) was regarded as positive response. *P<0.05, compared with the baseline; †Declining sample sizes for the observed data reflect attrition due to patient death and occasional failure to respond to individual follow-up questions.
Discussion
PNI was an important pathological feature of PanCa. Tumor cells not only infiltrate peripancreatic nerve plexus but also reach distant metastases through the perineural space.[3,4,19,20] PNI is usually associated with poor prognosis, likely because neoplastic cells hidden in the perineural space cannot be removed during tumor resection, leading to disease relapse.[4] The treatment of PNI had become a key in the PanCa.
The approach of building PNI models, including in vitro and in vivo, had gradually matured. Dai et al.[26] cocultured rat DRGs and PanCa cell lines, successfully established the PNI in vitro model. Koide et al.[27] implanted the celiac plexus tissue of human in NOD/SCID mice subcutaneously. Then, the PanCa cells were injected nearby the survived celiac plexus tissue, and a PNI process model in human was successfully built. They also implanted PanCa cells CaPan2 in NOD/SCID mice subcutaneously and successfully established the mouse nerve PNI model. Eibl et al.[35] introduced a recurrence PanCa model in nude mice. The tumor was removed in 4 weeks, 6 weeks, and 8 weeks after orthotopic transplantation. The recurrence was found in 80% of cases 6 weeks after the resection of PanCa, accompanied by extensive infiltration of retroperitoneal nerve.
In our study, two PanCa cell lines, CaPan-2 cell line with high nerve invasion and Panc-1 cell line with low nerve invasion, were selected. Using the same way with Dai, the co-culture models of PanCa cells and DRGs were successfully established. The in vitro models were evaluated under the intervention of CLDR irradiation of 125I seeds source. The results showed that, under the radiation exposure of different doses, the promotion of the growth between the PanCa cells and neurites was obviously inhibited.
In animal study, the PNI orthotopic transplantation model in NOD/CID mice were successfully established according to the previous reporting.[27,36] In orthotopic transplantation models, although there were no significant differences of PNI-positive rate between the two groups, the degree of PNI was significantly reduced in radiation group. The results suggested that 125I seeds implantation could effectively inhibit the neural invasion of PanCa cells.
NGF as a member of the NGF family is a signaling factor participating in the survival, growth, and differentiation of neuronal cells and cancer cells.[37] EGFR as the cytokine TGF-α receptor involves in the growth signaling to increase the affinity of PDA cells for pancreatic nerves and vice versa.[8] The overexpressions of NGF and EGFR in PanCa cells were markers of poor prognosis.[38] In vivo and in vitro models, the expressions of NGF and EGFR in CaPan-2 cells were obviously inhibited under the exposure of CLDR irradiation. In panc1/DRG co-culture group, the irradiation of 125I seeds could significantly reduce the expression of NGF. In CaPan2/DRG co-culture model, the levels of NGF and TGF-α in supernatant after irradiation were significantly increased. However, this difference was not detected in panc-1/DRG co-culture model. These results concluded that CLDR irradiation could inhibit the NGF and TGF-α in panc1/DRG co-culture system. The PNI models of different PanCa cell lines were exposed under the same irradiation condition, which might lead to the different changes in local microenvironment. Further study is necessary to demonstrate that CLDR irradiation could alleviate PNI-related pain by targeting the NGF and TGF-α signaling pathway.
Abdominal and/or back pain was a common symptom of PanCa. The present findings showed that the incidence of PanCa pain was related to its neurotropic characteristics. The traditional high-dose-rate (HDR) radiation therapy could kill tumor cells, reduce tumor volume, and thus relieve pain symptoms.[39] However, in the preclinical practice of EUS-guided 125I seeds implantation, the radiation therapy was CLDR irradiation with different biologic effects compared with traditional HDR. Wang et al. found that a CLDR irradiation of 125I seeds was more effective in inducing cell apoptosis of Panc-1 cells than an acute HDR 60Co irradiation. Interestingly, CLDR irradiation by 125I seeds can cause Panc-1 cell-cycle arrest in the G2/M phase and induce apoptosis.[40] Otherwise, in our preclinical practice, the method of EUS-guided radioactive seed implantation was not matured and the therapy dose at the tumor edge varied widely.[22] In some patients, the therapy dose was obviously lower than the recommended dose. Therefore, the median survival time was only 9 months with a partial remission rate of 13.6%, which was far below the rate of pain relief. The high rate of pain relief could not be explained by the traditional theory of radiation-related pain relief.
Celiac plexus neurolysis (CPN) and celiac plexus block (CPB) have been considered the first-line adjuvant therapies for the treatment of pain in PanCa patients.[41] Gunaratnam et al. reported that EUS-guided CPN reduced pain in 78% of PanCa patients.[42] However, CPN could only relieve the pain to a limited degree, and lasted a short period, and the analgesic effect was inversely correlated to the extent of invasion of celiac ganglia.[43,44,45] In conventional methods of CPN or CPB, pain scores were significantly reduced within 7 days, then entered a relatively stable plateau. However, after the implantation of radioactive seeds in advanced PanCa, the pain scores gradually decreased and the change was most obvious on 0.5 or 1 month. The long-term effect of pain relief was significantly better than the response of short term.[21,22] Therefore, according to the previous experimental results, we speculated that the inhibition on PNI by CDLR irradiation after radioactive seed implantation might be a major mechanism of pain relief in advanced PanCa and was defined as the PNI-related pain relief.
Knowledge of patterns of the development and extension of neural invasion might improve the treatment of PanCa. The pain relief in advanced PanCa related to a variety of factors, and PNI was only one aspect of them. In this study, we confirmed that the CLDR irradiation of 125I seeds could inhibit PNI of PanCa cells with the value of the further study.
In summary, our study proves that the CLDR irradiation could inhibit the PanCa cell growth. The CLDR irradiation could reduce the expression of NGF mRNA in PanCa cell by in vitro and in vivo. However, the further study is necessary to discuss the related signaling pathway. The CLDR irradiation could do a great favor in alleviating PNI-related pain.
Financial support and sponsorship
This study was supported by the grants from the Beijing Municipal Science and Technology Commission (No. Z141107002514184), the National Natural Science Foundation of China (No. 81272667), and the Beijing Municipal Science and Technology Commission (No. Z151100004015213).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
References
- 1.Demir IE, Friess H, Ceyhan GO. Neural plasticity in pancreatitis and pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2015;12:649–59. doi: 10.1038/nrgastro.2015.166. doi: 10.1038/nrgastro.2015.166. [DOI] [PubMed] [Google Scholar]
- 2.Li XB, Zhao L, Liao Q, Xu Q, Zhang TP, Cong L, et al. Gastroesophageal varices (bleeding) and splenomegaly: the initial manifestations of some pancreatic body and tail carcinoma. Chin Med J. 2015;128:558–61. doi: 10.4103/0366-6999.151118. doi: 10.4103/0366-6999.151118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Roest MH, Gouw AS, Peeters PM, Porte RJ, Slooff MJ, Fidler V, et al. Results of pancreaticoduodenectomy in patients with periampullary adenocarcinoma: Perineural growth more important prognostic factor than tumor localization. Ann Surg. 2008;248:97–103. doi: 10.1097/SLA.0b013e31817b6609. doi: 10.1097/SLA.0b013e31817b6609. [DOI] [PubMed] [Google Scholar]
- 4.Marchesi F, Piemonti L, Mantovani A, Allavena P. Molecular mechanisms of perineural invasion, a forgotten pathway of dissemination and metastasis. Cytokine Growth Factor Rev. 2010;21:77–82. doi: 10.1016/j.cytogfr.2009.11.001. doi: 10.1016/j.cytogfr.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Reddy S, Wolfgang CL, Cameron JL, Eckhauser F, Choti MA, Schulick RD, et al. Total pancreatectomy for pancreatic adenocarcinoma: Evaluation of morbidity and long-term survival. Ann Surg. 2009;250:282–7. doi: 10.1097/SLA.0b013e3181ae9f93. doi: 10.1097/SLA.0b013e3181ae9f93. [DOI] [PubMed] [Google Scholar]
- 6.Gil Z, Cavel O, Kelly K, Brader P, Rein A, Gao SP, et al. Paracrine regulation of pancreatic cancer cell invasion by peripheral nerves. J Natl Cancer Inst. 2010;102:107–18. doi: 10.1093/jnci/djp456. doi: 10.1093/jnci/djp456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao J, Hu XF, Feng XS, Gao SG. Pleiotrophin promotes perineural invasion in pancreatic cancer. World J Gastroenterol. 2013;19:6555–8. doi: 10.3748/wjg.v19.i39.6555. doi: 10.3748/wjg.v19.i39.6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Y, Chen H, Wang D, Luo W, Zhu B, Zhang Z. Diagnosis of pancreatic carcinoma based on combined measurement of multiple serum tumor markers using artificial neural network analysis. Chin Med J. 2014;127:1891–6. doi: 10.3760/cma.j.issn.0366-6999.20133101. [PubMed] [Google Scholar]
- 9.Yao J, Li WY, Li SG, Feng XS, Gao SG. Midkine promotes perineural invasion in human pancreatic cancer. World J Gastroenterol. 2014;20:3018–24. doi: 10.3748/wjg.v20.i11.3018. doi: 10.3748/wjg.v20.i11.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchesi F, Piemonti L, Fedele G, Destro A, Roncalli M, Albarello L, et al. The chemokine receptor CX3CR1 is involved in the neural tropism and malignant behavior of pancreatic ductal adenocarcinoma. Cancer Res. 2008;68:9060–9. doi: 10.1158/0008-5472.CAN-08-1810. doi: 10.1158/0008-5472.CAN-08-1810. [DOI] [PubMed] [Google Scholar]
- 11.Okada Y, Eibl G, Guha S, Duffy JP, Reber HA, Hines OJ. Nerve growth factor stimulates MMP-2 expression and activity and increases invasion by human pancreatic cancer cells. Clin Exp Metastasis. 2004;21:285–92. doi: 10.1023/b:clin.0000046131.24625.54. doi: 10.1023/B: CLIN.0000046131.24625.54. [DOI] [PubMed] [Google Scholar]
- 12.Singh PK, Wen Y, Swanson BJ, Shanmugam K, Kazlauskas A, Cerny RL, et al. Platelet-derived growth factor receptor beta-mediated phosphorylation of MUC1 enhances invasiveness in pancreatic adenocarcinoma cells. Cancer Res. 2007;67:5201–10. doi: 10.1158/0008-5472.CAN-06-4647. doi: 10.1158/0008-5472.CAN-06-4647. [DOI] [PubMed] [Google Scholar]
- 13.Hefti FF, Rosenthal A, Walicke PA, Wyatt S, Vergara G, Shelton DL, et al. Novel class of pain drugs based on antagonism of NGF. Trends Pharmacol Sci. 2006;27:85–91. doi: 10.1016/j.tips.2005.12.001. doi: 10.1016/j.tips.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Wood JN. Nerve growth factor and pain. N Engl J Med. 2010;363:1572–3. doi: 10.1056/NEJMe1004416. doi: 10.1056/NEJM.e1004416. [DOI] [PubMed] [Google Scholar]
- 15.Bapat AA, Hostetter G, Von Hoff DD, Han H. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer. 2011;11:695–707. doi: 10.1038/nrc3131. doi: 10.1038/nrc3131. [DOI] [PubMed] [Google Scholar]
- 16.Bakst RL, Lee N, He S, Chernichenko N, Chen CH, Linkov G, et al. Radiation impairs perineural invasion by modulating the nerve microenvironment. PLoS One. 2012;7:e39925. doi: 10.1371/journal.pone.0039925. doi: 10.1371/journal.pone.0039925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang ZM, Lu J, Zhang LY, Lin XZ, Chen KM, Chen ZJ, et al. Biological effects of low-dose-rate irradiation of pancreatic carcinoma cells in vitro using 125I seeds. World J Gastroenterol. 2015;21:2336–42. doi: 10.3748/wjg.v21.i8.2336. doi: 10.3748/wjg.v21.i8.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhongmin W, Yu L, Fenju L, Kemin C, Gang H. Clinical efficacy of CT-guided iodine-125 seed implantation therapy in patients with advanced pancreatic cancer. Eur Radiol. 2010;20:1786–91. doi: 10.1007/s00330-009-1703-0. doi: 10.1007/s00330-009-1703-0. [DOI] [PubMed] [Google Scholar]
- 19.Wang JJ, Yuan HS, Li JN, Jiang WJ, Jiang YL, Tian SQ. Interstitial permanent implantation of 125I seeds as salvage therapy for re-recurrent rectal carcinoma. Int J Colorectal Dis. 2009;24:391–9. doi: 10.1007/s00384-008-0628-4. doi: 10.1007/s00384-008-0628-4. [DOI] [PubMed] [Google Scholar]
- 20.Peretz T, Nori D, Hilaris B, Manolatos S, Linares L, Harrison L, et al. Treatment of primary unresectable carcinoma of the pancreas with I-125 implantation. Int J Radiat Oncol Biol Phys. 1989;17:931–5. doi: 10.1016/0360-3016(89)90138-7. [DOI] [PubMed] [Google Scholar]
- 21.Sun S, Xu H, Xin J, Liu J, Guo Q, Li S. Endoscopic ultrasound-guided interstitial brachytherapy of unresectable pancreatic cancer: Results of a pilot trial. Endoscopy. 2006;38:399–403. doi: 10.1055/s-2006-925253. doi: 10.1055/s-2006-925253. [DOI] [PubMed] [Google Scholar]
- 22.Jin Z, Du Y, Li Z, Jiang Y, Chen J, Liu Y. Endoscopic ultrasonography-guided interstitial implantation of iodine 125-seeds combined with chemotherapy in the treatment of unresectable pancreatic carcinoma: A prospective pilot study. Endoscopy. 2008;40:314–20. doi: 10.1055/s-2007-995476. doi: 10.1055/s-2007-995476. [DOI] [PubMed] [Google Scholar]
- 23.Aird EG, Folkard M, Mayes CR, Bownes PJ, Lawson JM, Joiner MC. A purpose-built iodine-125 irradiation plaque for low dose rate low energy irradiation of cell lines in vitro. Br J Radiol. 2001;74:56–61. doi: 10.1259/bjr.74.877.740056. doi: 10.1259/bjr.74.877.740056. [DOI] [PubMed] [Google Scholar]
- 24.Chen HH, Jia RF, Yu L, Zhao MJ, Shao CL, Cheng WY. Bystander effects induced by continuous low-dose-rate 125I seeds potentiate the killing action of irradiation on human lung cancer cells in vitro. Int J Radiat Oncol Biol Phys. 2008;72:1560–6. doi: 10.1016/j.ijrobp.2008.07.038. doi: 10.1016/j.ijrobp.2008.07.038. [DOI] [PubMed] [Google Scholar]
- 25.Furhang EE, Anderson LL. Functional fitting of interstitial brachytherapy dosimetry data recommended by the AAPM Radiation Therapy Committee Task Group 43. American Association of Physicists in Medicine. Med Phys. 1999;26:153–60. doi: 10.1118/1.598497. doi: 10.1118/1.598497. [DOI] [PubMed] [Google Scholar]
- 26.Dai H, Li R, Wheeler T, Ozen M, Ittmann M, Anderson M, et al. Enhanced survival in perineural invasion of pancreatic cancer: An in vitro approach. Hum Pathol. 2007;38:299–307. doi: 10.1016/j.humpath.2006.08.002. doi: 10.1016/j.humpath.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Koide N, Yamada T, Shibata R, Mori T, Fukuma M, Yamazaki K, et al. Establishment of perineural invasion models and analysis of gene expression revealed an invariant chain (CD74) as a possible molecule involved in perineural invasion in pancreatic cancer. Clin Cancer Res. 2006;12:2419–26. doi: 10.1158/1078-0432.CCR-05-1852. doi: 10.1158/1078-0432.CCR-05-1852. [DOI] [PubMed] [Google Scholar]
- 28.Jones-Bolin S, Ruggeri B. Orthotopic model of human pancreatic ductal adenocarcinoma and cancer cachexia in nude mice. Curr Protoc Pharmacol. 2007 Jun;(Chapter 14) doi: 10.1002/0471141755.ph1403s37. Unit 14.3. doi: 10.1002/0471141755.ph1403s37. [DOI] [PubMed] [Google Scholar]
- 29.Chuma M, Sakamoto M, Yasuda J, Fujii G, Nakanishi K, Tsuchiya A, et al. Overexpression of cortactin is involved in motility and metastasis of hepatocellular carcinoma. J Hepatol. 2004;41:629–36. doi: 10.1016/j.jhep.2004.06.018. doi: 10.1016/j.jhep.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 30.Kawarada Y. New classification of pancreatic carcinoma –Japan Pancreas Society. Nihon Shokakibyo Gakkai Zasshi. 2003;100:974–80. [PubMed] [Google Scholar]
- 31.Sato O, Wada T, Kawai A, Yamaguchi U, Makimoto A, Kokai Y, et al. Expression of epidermal growth factor receptor, ERBB2 and KIT in adult soft tissue sarcomas: A clinicopathologic study of 281 cases. Cancer. 2005;103:1881–90. doi: 10.1002/cncr.20986. doi: 10.1002/cncr.20986. [DOI] [PubMed] [Google Scholar]
- 32.Bilimoria KY, Bentrem DJ, Ko CY, Ritchey J, Stewart AK, Winchester DP, et al. Validation of the 6th edition AJCC Pancreatic Cancer Staging System: Report from the National Cancer Database. Cancer. 2007;110:738–44. doi: 10.1002/cncr.22852. doi: 10.1002/cncr.22852. [DOI] [PubMed] [Google Scholar]
- 33.Wells N, Pasero C, McCaffery M. Advances in patient safety improving the quality of care through pain assessment and management. In: Hughes RG, editor. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008. [PubMed] [Google Scholar]
- 34.Ventafridda V, Tamburini M, Caraceni A, De Conno F, Naldi F. A validation study of the WHO method for cancer pain relief. Cancer. 1987;59:850–6. doi: 10.1002/1097-0142(19870215)59:4<850::aid-cncr2820590432>3.0.co;2-1. doi: 10.1002/1097-0142(19⇾15)59: 4<850: : AID-CNCR2820590432>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 35.Eibl G, Reber HA. A xenograft nude mouse model for perineural invasion and recurrence in pancreatic cancer. Pancreas. 2005;31:258–62. doi: 10.1097/01.mpa.0000175176.40045.0f. doi: 10.1097/01.mpa.0000175176.40045. [DOI] [PubMed] [Google Scholar]
- 36.Ueda S, Ogata S, Tsuda H, Kawarabayashi N, Kimura M, Sugiura Y, et al. The correlation between cytoplasmic overexpression of epidermal growth factor receptor and tumor aggressiveness: Poor prognosis in patients with pancreatic ductal adenocarcinoma. Pancreas. 2004;29:e1–8. doi: 10.1097/00006676-200407000-00061. doi: 10.1097/00006676-200407000-00061. [DOI] [PubMed] [Google Scholar]
- 37.Miknyoczki SJ, Lang D, Huang L, Klein-Szanto AJ, Dionne CA, Ruggeri BA. Neurotrophins and Trk receptors in human pancreatic ductal adenocarcinoma: Expression patterns and effects on in vitro invasive behavior. Int J Cancer. 1999;81:417–27. doi: 10.1002/(sici)1097-0215(19990505)81:3<417::aid-ijc16>3.0.co;2-6. doi: 10.1002/(SICI)1097-0215(19990505)81: 3<417: : AID-IJC16>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 38.Morganti AG, Trodella L, Valentini V, Barbi S, Macchia G, Mantini G, et al. Pain relief with short-term irradiation in locally advanced carcinoma of the pancreas. J Palliat Care. 2003;19:258–62. [PubMed] [Google Scholar]
- 39.Malik K, Benzon HT. Radiofrequency applications to dorsal root ganglia: A literature review. Anesthesiology. 2008;109:527–42. doi: 10.1097/ALN.0b013e318182c86e. doi: 10.1097/ALN.0b013e318182c86e. [DOI] [PubMed] [Google Scholar]
- 40.Wang J, Jiang Y, Li J, Tian S, Ran W, Xiu D. Intraoperative ultrasound-guided iodine-125 seed implantation for unresectable pancreatic carcinoma. J Exp Clin Cancer Res. 2009;28:88. doi: 10.1186/1756-9966-28-88. doi: 10.1186/1756-9966-28-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michaels AJ, Draganov PV. Endoscopic ultrasonography guided celiac plexus neurolysis and celiac plexus block in the management of pain due to pancreatic cancer and chronic pancreatitis. World J Gastroenterol. 2007;13:3575–80. doi: 10.3748/wjg.v13.i26.3575. doi: 10.3748/wjg.v13.i26.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gunaratnam NT, Sarma AV, Norton ID, Wiersema MJ. A prospective study of EUS-guided celiac plexus neurolysis for pancreatic cancer pain. Gastrointest Endosc. 2001;54:316–24. doi: 10.1067/mge.2001.117515. doi: 10.1016/j.gie.2011.07.073. [DOI] [PubMed] [Google Scholar]
- 43.Yan BM, Myers RP. Neurolytic celiac plexus block for pain control in unresectable pancreatic cancer. Am J Gastroenterol. 2007;102:430–8. doi: 10.1111/j.1572-0241.2006.00967.x. doi: 10.1111/j.1572-0241.2006.00967.x. [DOI] [PubMed] [Google Scholar]
- 44.Wiersema MJ, Wiersema LM. Endosonography-guided celiac plexus neurolysis. Gastrointest Endosc. 1996;44:656–62. doi: 10.1016/s0016-5107(96)70047-0. doi: 10.1016/S0016-5107(96)70047-0. [DOI] [PubMed] [Google Scholar]
- 45.Akhan O, Ozmen MN, Basgun N, Akinci D, Oguz O, Koroglu M, et al. Long-term results of celiac Ganglia block: Correlation of grade of tumoral invasion and pain relief. AJR Am J Roentgenol. 2004;182:891–6. doi: 10.2214/ajr.182.4.1820891. doi: 10.2214/ajr.182.4.1820891. [DOI] [PubMed] [Google Scholar]