Abstract
Background:
Colorectal cancer (CRC) is a heterogeneous disease; current research relies on cancer cell lines and animal cancer models, which may not precisely imitate inner human tumors and guide clinical medicine. The purpose of our study was to explore and further improve the process of producing three-dimensional (3D) organoid model and impel the development of personalized therapy.
Methods:
We subcutaneously injected surgically resected CRC tissues from a patient into BALB/c-nu mice to build patient-derived xenografts (PDXs). Isolated cells from PDXs at appropriate tumor size were mingled with Matrigel, and then seeded in ultra-low attachment 96-well plates at four cell densities (500, 1000, 2000, and 4000 single cells/well). Cells were cultured with advanced Dulbecco's Modified Eagle Medium/F12 medium additional with various factors added to maintain tumor's biological traits and growth activity. The growth curves of the four cell densities were measured after 24 h of culture until 25 days. We evaluated the effects of four chemotherapeutic agents on organoid model by the CellTiter-Glo® Luminescent Cell Viability Assay. Hematoxylin and eosin (H and E) staining of 3D organoids was performed and compared with patient and CRC PDX tissues. Furthermore, immunohistochemistry was performed, in which the organoids were stained with the proliferation marker, Ki-67. During the experimental process, a phase-contrast microscope was used.
Results:
Phenotype experimental results showed that 3D organoids were tightly packed together and grew robustly over time. All four densities of cells formed organoids while that composed of 2000 cells/well provided an adequate cultivation system and grew approximately 8-fold at the 25th day. The chemosensitivity of the four conventional drugs was [s]-10-hydroxycamptothecin > mitomycin C > adriamycin > paclitaxel, which can guide clinical treatment. Histological features of CRC patient's tumor tissues and mice tumor xenograft tissues were highly similar, with high-column-like epithelium and extracellular matrix. H and E-stained sections showed heterogeneous cell populations harbored in cancer organoids and were histologically similar to tumor tissues. The proliferation index was only 8.33% within spheroids, which exhibited confined proliferative cells that might be cancer stem cells.
Conclusions:
We successfully constructed a CRC organoid model that grew robustly over 25 days and demonstrated that 2000 cells/well in 96-well plate was a prime seeding density for cells to form organoids. The results confirmed that organoid model can be used for agent screening and personalized medicine.
Keywords: Colorectal Neoplasm, Drug Therapy, Neoplasm Transplantation, Printing, Three-dimensional
Introduction
Despite the improvements in early detection, reduced prevalence of risk factors, and advances in targeted therapy, colorectal cancer (CRC) remains the third most common cancer worldwide and the leading cause of cancer-related deaths, which imposes a significant health burden globally.[1] CRC incidence rates can be several times higher in more developed countries.[2] With the growing and aging population and an increasingly modernized lifestyle, developing countries will confront with more challenges. Enhanced screening and improved therapy are being used; however, the 5-year survival rate of CRC patients with last stage disease is only 12.5%.[2,3] At present, only 5% of anticancer agents that have activity in preclinical development are licensed after phase III testing; drug attrition rates for cancer are much higher than in other therapeutic areas.[4] It is essential to find optimal strategies to evaluate novel and effective agents and develop curative chemotherapies; hence, there is an urgent need for appropriate tumor models that can physiologically replicate the key features of human tissue. Classically, cell biology research and drug development in CRC have relied on in vitro monolayer human tumor-derived cell lines and in vivo xenograft mice; however, previous clinical application has taught us that reliance on these models is limiting.[5] Recently, developed three-dimensional (3D) models can overcome these deficiencies. 3D models not only better replicate natural tissue mechanical stresses but also provide a more representative pathophysiological condition than the classic monolayer cultures; they can also be established in a shorter period and much more economically than patient-derived xenograft (PDX) models.[6] There are currently several methods to alter the growth environment for cultured cells and tissues to form 3D models. Here, we employed Matrigel, culture medium, and additional factors to compose a 3D culture system. This system can be used to set up and characterize patient-derived organoids, including growth kinetics and drug sensitivity.
Methods
Tissue specimen and clinical data
Specimen used in this study was obtained from a patient of the Peking University Cancer Hospital in December 2013 and confirmed by a pathologist. The patient had not undergone chemotherapy or radiotherapy before surgery and signed informed consent. The patient was male, 36-year-old, and was diagnosed as well-differentiated rectum adenocarcinoma without metastasis.
Establishment of patient-derived xenograft models
Surgically resected CRC tissue samples from the patient were chopped into 5-mm pieces. Tissues were washed with normal saline several times and injected subcutaneously into the flanks of 6–7-week-old BALB/c-nu mice.[7,8,9] The established PDX models were maintained at CrownBio HuPrime animal facility. Tumor growth was monitored daily. The study followed the guidelines approved by the Institutional Ethics Committee of Peking University Cancer Hospital. All animal studies were performed in accordance with the guidelines of the Peking University Institutional Animal Care and Use Committee.
Isolation of cancer cells and three-dimensional organoid culture
Growth medium was nutrient mixture F-12 (Dulbecco's Modified Eagle Medium/F12) containing 10% fetal bovine serum with additional factors. B27, GlutaMAX, and HEPES buffer were obtained from Invitrogen (Invitrogen, California, USA). Recombinant Human R-Spondin 1 and Recombinant Human Noggin were obtained from PeproTech (PeproTech, Rocky Hill, USA). Nicotinamide, SB202190, gastrin, and N-acetyl-L-cysteine were obtained from Sigma (Sigma, Missouri, USA). Other supplements and suppliers were A83-01 (Tocris, Missouri, USA), Primocin (InvivoGen, SanDiego, USA), Y-27632 (Selleck Chemicals, Houston, USA), and EGF (R&D Systems, Minneapolis, USA). Matrigel was purchased from BD (BD, New Jersey, USA).
Mouse xenografts were harvested at appropriate tumor size with sterile surgical tools.[7,8,9] Tumor tissue was minced into tiny pieces with scissors, and the tissue fragments were incubated in collagenase solution (Invitrogen, California, USA) at 37°C for up to 1 h. The cells were filtered through a cell strainer (BD Falcon, New Jersey, USA) after digestion followed by centrifugation for 5 min at the speed of 1000 r/min. The supernatant was removed and the pellets were washed with phosphate-buffered saline, and centrifuged as described above. After being mechanically dissociated by pipetting, cell pellets were further resuspended in ice-cold 1:1 mixture of growth medium and Matrigel, and then seeded in ultra-low attachment 96-well plates (Corning, Tewksbury, USA) at four cell densities (500, 1000, 2000, and 4000 single cells/well) for the following experiments. We also plated cells in culture dishes for further study. During this process, cells were counted by CountStar (Inno-Alliance Biotech, USA) and trypan blue (Sigma, Missouri, USA). The Matrigel was polymerized for 10 min at 37°C, and growth medium was added to the wells (100 μl/well in 96-well plates and 1 ml/well in 60 mm diameter culture dishes).
Cell viability and proliferation assay
The organoid model was generated by plating cells in ultra-low attachment 96-well plates at four densities as described above; this method stimulated the formation of organoids within 24 h. Fresh growth medium was applied two to three times a week. Morphologies of the Matrigel-cultivated CRC cells were captured by a phase-contrast confocal microscope (Nikon, Tokyo, Japan). The proliferation of the cells was examined by the CellTiter-Glo® (CTG) assay (Promega, Wisconsin, USA) according to the modified manufacturer's instructions for tumor spheroids after an incubation period for 24 h at 37°C and 5% CO2. On the indicated day between day 1 and day 25, the cell viability assay was performed and the luminescence was quantified by the EnVision Multilabel Plate Reader (PerkinElmer, California, USA). Throughout the experimental process, the phase-contrast microscope was used to observe and record organoids morphology.
Drug treatment
To evaluate the cytotoxicity and proliferation of the organoids for anticancer agents’ treatment, four chemotherapeutic agents, such as adriamycin (ADM), mitomycin C (MMC), paclitaxel, and [s]-10-hydroxycamptothecin (HCPT), were used. Anticancer drug ADM, MMC, paclitaxel, and HCPT were purchased from Dalian Meilun Biotechnology (Dalian Meilun Biotechnology, Dalian, China). Paclitaxel was obtained from Selleck Chemicals (Selleck Chemicals, Huston, USA). The stock solution of four drugs – 1 mmol/L in ddH2O for ADM, 1 mmol/L in ddH2O for MMC, 1 mmol/L in dimethyl sulfoxide (DMSO) for HCPT, and 10 mmol/L in DMSO for paclitaxel – was prepared. After cultivating for 48 h, the organoids seeded at 2000 cells/well were treated. A range of nine drug concentrations with 3-point 6-fold dilution series of each compound was exposed to 3D organotypic spheroids: ADM (0.01–100 μmol/L), MMC (0.01–100 μmol/L), HCPT (0.001–10 μmol/L), and paclitaxel (0.01–100 μmol/L). On the same plate, a medium control and a DMSO control were included. After 72 h, the number of living cells was assessed with CTG assay according to the manufacturer's protocol. The fluorescent signal was quantified with EnVision Multilabel Plate Reader. Every condition was repeated three times in independent tests.
Hematoxylin and eosin staining and immunohistochemistry
Organoids were fixed with 10% formalin for 24 h at room temperature and were embedded in paraffin. After rinsing with running water (~4 h), the specimen was transferred to the Vacuum Tissue Processor Leica ASP200S (Leica Biosystems, Frankfurt, Germany) for dehydration, and then embedded into formalin-fixed paraffin-embedded (FFPE) blocks with tissue embedding center Leica EG1150 (Leica Biosystems, Frankfurt, Germany). The FFPE blocks were then sectioned at 3 μm using a manual rotary microtome Leica RM2235 (Leica Biosystems, Frankfurt, Germany) and were stained with hematoxylin and eosin (H and E) solution. For further immunohistochemistry analysis, we used Rabbit antihuman Ki-67 antibody (Vector Laboratories, California, USA) at a final concentration of 1:500. The secondary antibodies used were UltraVision LP Detection System (Thermo, Massachusetts, USA) including Ultra V Block, Primary Antibody Enhancer, and HRP Polymer.
Statistical analysis
For compound effects, data were analyzed using GraphPad Prism version 5 (GraphPad Software, California, USA) to fit a dose-response curve. Functional analyses were performed in triplicate. Results are presented as the mean ± standard error (SE).
Results
Morphology of three-dimensional organoid cultures and cell proliferation
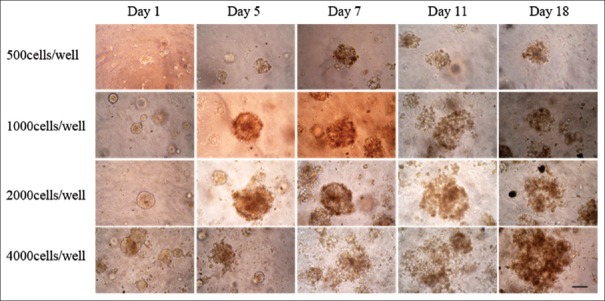
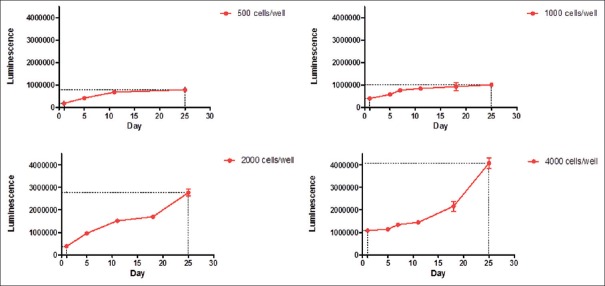
We monitored the formation and growth of tumor organoids for 25 days. The morphology on day 1, day 5, day 7, day 11, and day 18 is shown in Figure 1. The isolated cells formed compact 3D structures within 24 h, and the organoids increased in size along with the cultivation time. It is worth mentioning that the organoid diameter did not always increase remarkably with time, possibly because of cellular reorganization. The spheroid in a 3D system contains inner dead cells and living cells in the outer layers. To determine the optimal seeding density for the establishment of physiological gradients for pH, oxygen, nutrients, and waste, we set different densities.[10] Under these growth conditions, the cells all formed organoids and grew with time while organoids of 2000 cells/well provided an adequate cultivate system and grew approximately 8-fold at the 25th day [Figures 1 and 2].
Figure 1.
Three-dimensional organoids morphological changes observed by a phase-contrast microscope on day 1, day 5, day 7, day 11, and day 18. The isolated cells formed compact three-dimensional structures and the spheroids increased in size along with the cultivation time (scale bar 50 μm).
Figure 2.
Growth curve of CR0010 organoid spheroids within 25 days. We set four cell densities. Under these growth conditions, cells all formed organoids and grew over time. With densities of 500 cells/well and 1000 cells/well in ultra-low attachment 96-well plates, the cells barely grew and formed spheroids with effective diameter. The other two densities, 2000 cells/well and 4000 cells/well, displayed vigorous growth rate. The organoids with 2000 cells/well showed double the growth of 4000 cells/well (8 vs. 4).
Microtissue drug response
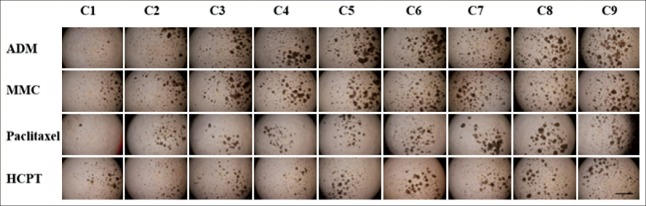
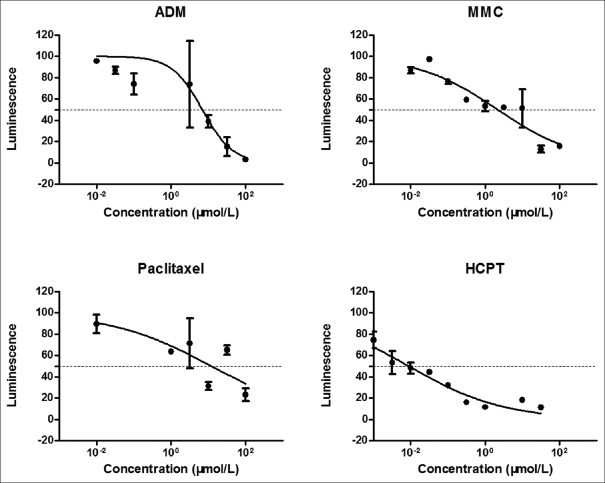
We observed the effect of four chemotherapeutic agents, such as ADM, MMC, paclitaxel, and HCPT, on the morphology of the micro tumors; we assessed pharmacological function after 72 h of the drug exposure via the CTG assay. There was obvious change in the organoid structural organization and integrity under the impact of drug treatment. The organoids suffered obvious deterioration with an increase in drug concentration [Figure 3]. Figure 4 shows a curve of cells viability at various drug concentrations determined by the CTG assay, and the agents with their corresponding IC50 values are: ADM 7.55, MMC 6.27, HCPT 0.16, paclitaxel 13.00. The CRC model is highly sensitive to HCPT, and the chemosensitivity of the four conventional drugs is HCPT > MMC > ADM > paclitaxel.
Figure 3.
The morphology of CR0010 organoid spheroids after drug treatment for 72 h. The C1–C9 concentration of adriamycin (ADM), mitomycin C (MMC), and paclitaxel is 100 μmol/L–0.01 μmol/L with serial 3.16-fold dilution. The C1–C9 concentration of (s)-10-hydroxycamptothecin (HCPT) is 10 μmol/L–0.001 μmol/L. There was obvious change in the spheroid structural organization and integrity under the impact of treatment. The spheroids suffered obvious deterioration with increases in agents’ concentration (scale bar 200 μm).
Figure 4.
Dose-response curves to assess effect of adriamycin (ADM), mitomycin C (MMC), paclitaxel, and [s]-10-hydroxycamptothecin (HCPT) on three-dimensional organoids. There was a linear relationship between the fluorescence intensity and cell vitality. Error bars indicate standard error.
Hematoxylin and eosin staining and immunohistochemistry
Figure 5 shows H and E staining as well as immunohistochemical Ki-67 characterization of CRC patient tissue, xenograft tissue, and xenograft-derived organoid sections. Histological features of CRC patient's tumor tissues [Figure 5a] and mice tumor xenograft tissues [Figure 5b] are highly similar with high-column-like epithelium and extracellular matrix and classic histologic features of human moderately differentiated colorectal adenocarcinoma. We fixed the organoid sample after seeding for 7 days. Observed microscopically, spheroid cells were tightly packed together as shown in Figure 5c and 5d. H and E-stained sections of organoids clearly illustrate viable cells with atypical nuclei and cytoplasm throughout the spheroids which were histologically similar with xenografts [Figure 5c and 5d]. To assess proliferative activity, organoids were stained with the proliferation marker, Ki-67 [Figure 5e and 5f]. ImageJ (National Institute of Health, Maryland, USA) software was used for Ki-67 assessment to identify and evaluate IHC scoring. The percentage of positive Ki-67 cells in the organoid based on brown color was detected. At least three sections’ samples were assessed and averaged in our experiments. The ratio of Ki-67-positive cells in the total cells contacting Matrigel in the cross-section of 3D structures at maximum diameter was calculated. The proliferation index was only 8.33% within spheroids suggesting that 7-day-old organotypic tumor pheroids contain few cycling cells, which might be cancer stem cells.
Figure 5.
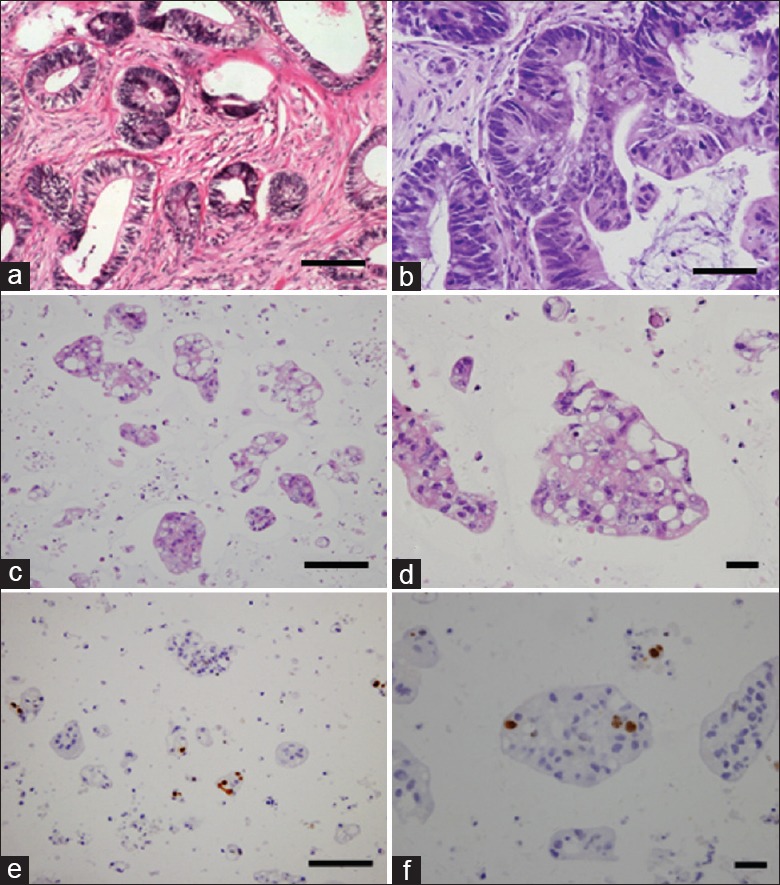
Immunohistochemical staining of colorectal cancer patient's tumor tissues, the mice tumor xenograft tissues and organoid sections. (a) Hematoxylin and eosin (H and E) staining of colorectal cancer patient's tumor tissues. (b) H and E staining of mice tumor xenograft tissues. (c and d) H and E staining of three-dimensional organoids at day 7. These organoids were composed of carcinoma cells and stromal cells and histologically similar with xenografts. (e and f) Ki-67 staining of three-dimensional organoids at day 7. The brown color indicates proliferating cells (scale bar a, b, c, and e: 50 μm; d and f: 20 μm).
Discussion
Cancer research has relied on 2D cell lines and mouse models commonly. Conventional cell cultures are being widely used; however, the unselected long-term, high-passage in vitro maintenance of established cancer cell lines leading to continuous accumulation of genomic and epigenomic changes and the cells’ genotypes and phenotypes tend to be homogeneous because of the selection of dominant subpopulations. Plentiful malignant phenotypes in internal tumors are lost in 2D condition because most cell lines are derived from advanced cancers.[11] Furthermore, monolayer culture cells lack the spatial structure and the interaction between tumor cells and extracellular matrix. These facts illustrate that established cell lines have deficiency in the study of genomic alterations and novel anticancer drugs. They tend to be hypersensitive to chemotherapy and fail to imitate the cellular characteristics and signaling pathways in vivo, which leads to inaccurate and limited data for clinical application.[5,12] CRC is a heterogeneous disease in that tumor with the same subtype can exhibit different traits and generate considerable variability in response to drug effects. Confronted with these problems, better tumor models that can precisely imitate inner human tumors should be utilized to evaluate novel agents, develop drug targets, and find optimal curative methods for specific individuals. In vivo cancer models are essential to cancer research for their tumor microenvironment, angiogenesis, and drug metabolism. In this area, PDXs have been used extensively and proven invaluable.[9] In PDX models, stroma can grow along with the tumor cells, allowing for tumor–stroma interactions.[9] PDX models preserve primary CRC features including tumor histological characteristics, vascularization, and structure. Above all, they can mirror the heterogeneity of CRC. In this way, PDX can be applied to personalized therapy to determine the optimum approach to treat an individual patient.[13] Nevertheless, PDX models carry important disadvantages. The entire process of xenograft establishment and drug screening requires six or more months and is expensive for clinical application. These limitations highlight the need for improved models to simulate CRC and bridge the gap between preclinical work and clinical trials.
Several reports have suggested that 3D culture affects cell morphology, upregulates stem cell surface marker expression, and presents a hypoxic/necrotic center that possesses functional relevance with in vivo features compared to 2D cell lines.[5,14,15,16,17] However, in the absence of stromal cell types including fibroblasts, mesenchymal stromal cells, and immune cells, 3D models formed by these cell lines have slow proliferation, relatively short survival time, and limited effectiveness. Furthermore, in consideration of interpatient heterogeneity in CRC, establishing 3D in vitro organoids specific to a particular patient may significantly contribute to the development of personalized cancer medicine.
In this study, we successfully established a CRC 3D in vitro model with the utility of Matrigel from CRC patient-derived tissues, which can be called “organoid”. The tumor microenvironment not only consist of tumor cells but also various stromal cells which played a supporting role in cancer development. Matrigel is a suitable matrix that includes laminins, collagen IV, entactin, heparan sulfate proteoglycan, as well as various growth factors, which can highly representative of the tumor microenvironment.[18] With the purpose of long-term proliferation of the organoids, multifold factors were added to the overlaid medium.[19] Wnt signals are essential for the proliferation of intestinal epithelium, and the Wnt agonist R-spondin 1 induces crypt hyperplasia in vivo.[20] Signaling by epidermal growth factor (EGF) is associated with intestinal proliferation.[21] Transgenic expression of Noggin induces an expansion of crypt numbers.[22] We added R-spondin-1, EGF, and Noggin to Matrigel to sustain ever-increasing CRC organoids. Furthermore, we built a long-term expansion system by adding nicotinamide, SB202190, A83-01, and Y-27632.[23] We observed that these organoids grew as morphologically compact and round spheres after culturing for 24 h. Viable determination with CTG assay showed that the organoids robustly grew for more than 25 days with optimal density. We present 2000 cells/well in a 96-well plate which may serve as an important reference for other organoid models. The immunohistochemical staining showed heterogeneous cell populations harbored in cancer organoids.
Drug resistance to chemotherapy represents an important challenge in treating solid tumors. Therefore, we performed drug exposure studies on the organoids. This system can be used for screening agents in high-throughput methods and for selecting appropriate chemotherapy regimens for patients with malignant disease.
In conclusion, our study constructs a CRC 3D in vitro organoid model which can grow robustly over 25 days. The model was built from patient-derived tissues with extracellular matrix formed by multiple stromal cells. Furthermore, we propose 2000 cells/well in a 96-well plate as a prime seeding density for cells to form organoid, which can be used as a reference for similar research.
Financial support and sponsorship
This study was supported by the grant from the Beijing Health System of High-tech Health and Technical Personnel 215 Project Funding (No. 2013-3-085).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank Dr. Jin-Ying Ning, Dr. Xiao-Xi Xu, Dr. Zheng-Zheng Bao, Dr. Song-Ling Zhang, Dr. Feng Hao and scientist Wen-Na Zhang from Crown Bioscience, Inc., for their technical support.
Footnotes
Edited by: Ning-Ning Wang
References
- 1.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics 2014. CA Cancer J Clin. 2014;64:104–17. doi: 10.3322/caac.21220. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Tang JC, Feng YL, Liang X, Cai XJ. Autophagy in 5-fluorouracil therapy in gastrointestinal cancer: Trends and challenges. Chin Med J. 2016;129:456–63. doi: 10.4103/0366-6999.176069. doi: 10.4103/0366-6999.176069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hutchinson L, Kirk R. High drug attrition rates – Where are we going wrong? Nat Rev Clin Oncol. 2011;8:189–90. doi: 10.1038/nrclinonc.2011.34. doi: 10.1038/nrclinonc.2011.34. [DOI] [PubMed] [Google Scholar]
- 5.Pampaloni F, Reynaud EG, Stelzer EH. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol. 2007;8:839–45. doi: 10.1038/nrm2236. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- 6.Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7:211–24. doi: 10.1038/nrm1858. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 7.Rygaard J, Povlsen CO. Heterotransplantation of a human malignant tumour to “Nude” mice. Acta Pathol Microbiol Scand. 1969;77:758–60. doi: 10.1111/j.1699-0463.1969.tb04520.x. doi: 10.1111/j.16990463.1969.tb04520.x. [DOI] [PubMed] [Google Scholar]
- 8.Julien S, Merino-Trigo A, Lacroix L, Pocard M, Goéré D, Mariani P, et al. Characterization of a large panel of patient-derived tumor xenografts representing the clinical heterogeneity of human colorectal cancer. Clin Cancer Res. 2012;1(18):5314–28. doi: 10.1158/1078-0432.CCR-12-0372. doi: 10.1158/1078-0432.CCR-12-0372. [DOI] [PubMed] [Google Scholar]
- 9.Rosfjord E, Lucas J, Li G, Gerber HP. Advances in patient-derived tumor xenografts: From target identification to predicting clinical response rates in oncology. Biochem Pharmacol. 2014;91:135–43. doi: 10.1016/j.bcp.2014.06.008. doi: 10.1016/j.bcp.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Hirschhaeuser F, Menne H, Dittfeld C, West J, Mueller-Klieser W, Kunz-Schughart LA. Multicellular tumor spheroids: An underestimated tool is catching up again. J Biotechnol. 2010;148:3–15. doi: 10.1016/j.jbiotec.2010.01.012. doi: 10.1016/j.jbiotec.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Masters JR. Human cancer cell lines: Fact and fantasy. Nat Rev Mol Cell Biol. 2000;1:233–6. doi: 10.1038/35043102. doi: 10.1038/35043102. [DOI] [PubMed] [Google Scholar]
- 12.Bissell MJ. The differentiated state of normal and malignant cells or how to define a “normal” cell in culture. Int Rev Cytol. 1981;70:27–100. doi: 10.1016/s0074-7696(08)61130-4. doi: 10.1016/S0074-7696(08)61130-4. [DOI] [PubMed] [Google Scholar]
- 13.Hidalgo M, Bruckheimer E, Rajeshkumar NV, Garrido-Laguna I, De Oliveira E, Rubio-Viqueira B, et al. Apilot clinical study of treatment guided by personalized tumorgrafts in patients with advanced cancer. Mol Cancer Ther. 2011;10:1311–6. doi: 10.1158/1535-7163.MCT-11-0233. doi: 10.1158/1535-7163.MCT-11-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh S, Spagnoli GC, Martin I, Ploegert S, Demougin P, Heberer M, et al. Three-dimensional culture of melanoma cells profoundly affects gene expression profile: A high density oligonucleotide array study. J Cell Physiol. 2005;204:522–31. doi: 10.1002/jcp.20320. doi: 10.1002/jcp.20320. [DOI] [PubMed] [Google Scholar]
- 16.Gómez-Lechón MJ, Jover R, Donato T, Ponsoda X, Rodriguez C, Stenzel KG, et al. Long-term expression of differentiated functions in hepatocytes cultured in three-dimensional collagen matrix. J Cell Physiol. 1998;177:553–62. doi: 10.1002/(SICI)1097-4652(199812)177:4<553::AID-JCP6>3.0.CO;2-F. doi: 10.1002/(SICI)1097-4652(199812) 177: 4<553: AID-JCP6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 17.Stuelten CH, Mertins SD, Busch JI, Gowens M, Scudiero DA, Burkett MW, et al. Complex display of putative tumor stem cell markers in the NCI60 tumor cell line panel. Stem Cells. 2010;28:649–60. doi: 10.1002/stem.324. doi: 10.1002/stem.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleinman HK, Martin GR. Matrigel: Basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–86. doi: 10.1016/j.semcancer.2005.05.004. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 19.van de Wetering M, Francies HE, Francis JM, Bounova G, Iorio F, Pronk A, et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161:933–45. doi: 10.1016/j.cell.2015.03.053. doi: 10.1016/j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim KA, Kakitani M, Zhao J, Oshima T, Tang T, Binnerts M, et al. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science. 2005;309:1256–9. doi: 10.1126/science.1112521. doi: 10.1126/science.1112521. [DOI] [PubMed] [Google Scholar]
- 21.Dignass AU, Sturm A. Peptide growth factors in the intestine. Eur J Gastroenterol Hepatol. 2001;13:763–70. doi: 10.1097/00042737-200107000-00002. doi: 10.1097/00042737-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Haramis AP, Begthel H, van den Born M, van Es J, Jonkheer S, Offerhaus GJ, et al. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science. 2004;303:1684–6. doi: 10.1126/science.1093587. doi: 10.1126/science.1093587. [DOI] [PubMed] [Google Scholar]
- 23.Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 2011;141:1762–72. doi: 10.1053/j.gastro.2011.07.050. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]