Abstract
Background:
A single drilled tunnel from the lateral mastoid cortex to the cochlea via the facial recess is essential for minimally invasive cochlear implant surgery. This study aimed to explore the safety profile of this kind of new image-guided and bi-planar device-assisted surgery procedure in vitro.
Methods:
Image-guided minimally invasive cochlear implantations were performed on eight cadaveric temporal bone specimens. The main procedures were: (1) temporal bone specimens were prepared for surgery and fiducial markers were registered. (2) computed tomography (CT) scans were performed for future reference. (3) CT scan images were processed and drill path was planned to minimize cochlear damage. (4) bi-planar device-assisted drilling was performed on the specimens using the registration. (5) surgical safety was evaluated by calculating the deviation between the drill and the planned paths, and by measuring the closest distance between the drilled path and critical anatomic structures.
Results:
Eight cases were operated successfully to the basal turn of the cochlear with intact facial nerves (FNs). The deviations from target points and entrance points were 0.86 mm (0.68–1.00 mm) and 0.44 mm (0.30–0.96 mm), respectively. The angular error between the planned and the drilled trajectory was 1.74° (1.26–2.41°). The mean distance from the edge of the drilled path to the FN and to the external canal was 0.60 mm (0.35–0.83 mm) and 1.60 mm (1.30–2.05 mm), respectively. In five specimens, the chorda tympani nerves were well preserved. In all cases, no injury happened to auditory ossicles.
Conclusions:
This exploratory study demonstrated the safety of the newly developed image-guided minimally invasive cochlear implantation assisted by the bi-planar device and established the operational procedures. Further, more in vitro experiments are needed to improve the system operation and its safety.
Keywords: Cochlear Implantation, Minimally Invasive Surgical Procedures, Navigation
Introduction
Cochlear implant surgery is the only way to cure severe and profound sensorineural hearing loss. So far, over 320,000 patients with severe hearing loss have recovered through this surgery. To identify the implantation location and avoid injury of important anatomic structures, the size of open cavity in traditional cochlear implant surgery is significantly larger than that is needed for electrodes to pass through. Taking advantage of medical navigations and robotic techniques, otologic surgeons[1] and researchers in the electrical and computer engineering fields achieved submillimeter accuracy in 2005 using registration system of new fiducial markers in otologic surgeries. Based on these experiments, they hypothesized that the range of mastoidectomy could be reduced to a drill path, whose diameter was slightly larger than an electrode. This drill path was from lateral mastoid cortex to the cochlea via the facial recess. Therefore, this minimally invasive path was named as “percutaneous cochlear implantation (PCI).”
In PCI surgery, the edge of the drill hole is adjacent to important structures, such as facial nerves (FNs), chorda tympani nerves (ChTs), the back wall of the external canal, and the auditory ossicles. The distance between the edge of the drill hole and anatomic structures is the safety margin, which depends on three-dimensional (3D) anatomies of the patients and the choice of the drill path. The most important factor here is the width between FN and ChT. According to histological statistics among most people, this width on the oval window plane is typically 1.8–4.2 mm.[2] If the ChT can be sacrificed, the width might reach 3.1–4.9 mm. As a result, high accuracy is essential when the drill passes safely through the FN recess. Considering system errors and the diameter of the drill, the accuracy of cochlear pore-forming should be ±1 mm, or even better, ±0.5 mm.[3]
An early study showed that it was impossible to achieve high accuracy in temporal surgeries with the traditional image-guided mode.[4] More recently, attempts of improving accuracy by locating the implanting targets through, for instance, patient-customized microstereotactic frames achieved satisfactory results.[5,6,7] The further employment of robot-guided frames and robot-controlled implantation instrument[8,9,10,11] provided higher accuracy. In 2014, the first cadaveric feasibility study of a master-slave-assisted cochlear implant procedure in the Otolaryngology-Head and Neck Surgery field using the da Vinci Si Surgical System was reported.[12] They not only assured a more flexible operation but also avoided errors caused by manual surgical drill instability.
Aforementioned image-guided minimally invasive PCI reduced the trauma caused by mastoidectomy, but it is still necessary to drill three bone holes in advance on patients’ skull surface both for microstereotactic frames and robot-guided drilling.[13,14] Moreover, the second CT scan is required to fix the fiducial frame or fiducial markers, which imperceptibly caused additional trauma or radioactive damage during the registration. In our previous study, we demonstrated a minimal invasive registration method in which the joint registration of the bone-bed and the malleus short process was adopted to successfully obtain ±1 mm registration accuracy in deep targets.
Assisted by the self-developed bi-planar device, this study adopted a minimal invasive registration method to conduct the image-guided minimal invasive PCI on eight cadaveric temporal specimens and further verified the safety of this system.
Methods
Materials
Bi-planar hybrid system
We designed a bi-planar hybrid system based on the special body position and the operation path in the cochlear implant operation. It was divided into two parts including a fixing support and a hybrid mechanism.
The fixing support was used to fix the head during the operation. The chin and occiput of the head were fixed by two baffle plates. The fixing support could be adjusted to accommodate patients with different head shapes using two adjustable threaded rods.
The hybrid mechanism was composed by two manipulators in series with 7 degrees of freedom (7-DOF) for each one and a parallel mechanism with 4-DOF. These two manipulators in series provided high stiffness and large workspace at the same time for holding up the parallel mechanism. The parallel mechanism was used to grasp the otology drill by two sleeves. There were two same sets of moving units in it. For each moving unit, two sliding blocks could be moved manually along with orthogonal directions to control the pose of the otology drill. The lead screws with self-locking were adopted to ensure high precision and safety. The robot system could readily reach the planed position and fix the otology drill solidly. The feed of the otology drilling was operated by surgeons for safety issues.
Optical navigation system
In this study, the photoelectric navigation, Polaris Spectra (NDI Corporation, Canada), was used. The Polaris optical tracking solution gave medical simulator manufacturers exceptionally accurate and reliable 3D tracking of simulated medical tools (via attached markers) over a large measurement volume. The Polaris emitted infrared light to wirelessly detect and track the tool's position and orientation.
Software
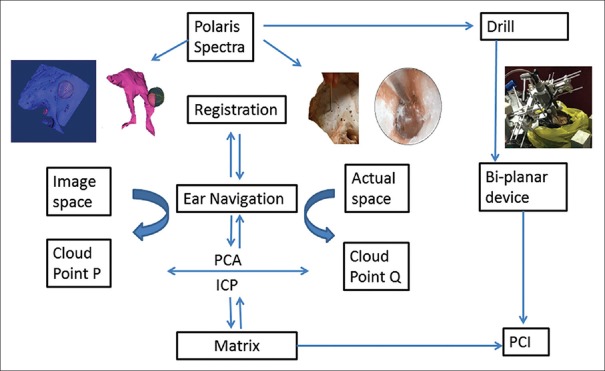
ENT navigation software system (copyright registration number: 2014SR050996, Beijing, China) was independently developed by the Beihang University, School of Mechanical Engineering and Automation and Peking University Third Hospital. The software includes functions of image-import, data-management, registration, path-planning, and real-time navigation. The overview of the study system is shown in Figure 1.
Figure 1.
The whole study system process. PCA: Principal component analysis; ICP: Iterative closest point algorithm; PCI: Percutaneous cochlear implantation.
Experiment process
Cadaveric specimens preparation
The Institutional Review Board approval was obtained by the Peking University Third Hospital Medical Ethics Committee. A total of eight formalin-fixed adult cadaveric temporal specimens were selected. Every specimen had an intact external auditory canal (EAC), intact tympanic membrane, and intact structure of ossicles with no apparent lesion in the middle ear. Before the surgery, the bones of the mastoid and temporal regions were exposed by removing the skin, subcutaneous tissue, and muscles. On each specimen, a vertical hole of 1 mm depth, which was used in the registration process later, was drilled in the transplant bed area for conventional cochlear implants in the temporal region by a 1 mm diamond drill (NSK Volvere GX, Japan).
Imaging and specimen fixation
After the preparation of each cadaveric specimen as mentioned above, a high-resolution CT (HRCT) scan was performed on its temporal bone (SIEMENS/SOMATOM Definition Flash, German, thickness = 0.6 mm, pitch = 0.3 mm).
The scanned cadaveric specimens were put into the head-fixed frame of the operation platform (the simulated operation table) to ensure the fixation during the registration and operational process.
Image processing and planning
Data obtained by CT scans were input to the image processing software Mimics 10.01 (Materialise, Leuven, Belgium) with the Digital Imaging and Communications in Medicine (DICOM) format. The related anatomic structures were extracted by this software, and the cochlear, FN, ChT; the auditory ossicles were formed as the 3D models. This reconstructed 3D model was further generated as a stereolithography (STL) file, which was imported into the ENT navigation software system. In the visualization unit of the software, the 3D positions of anatomic structures were visually analyzed.
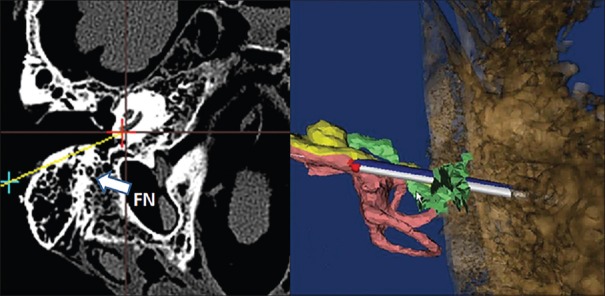
The planning and navigation unit was used to plan the PCI access. Proper PCI access met the following requirements: the target point of the access was located in the tympanic canal of the cochlear basal turn; the access was tangent to the cochlear basal turn; when the access went through the FN recess region, there was sufficient safety margins from the FN and the ChT; and the access did not penetrate posterior parties of the EAC or damage the ossicular chain (OC) [Figure 2].
Figure 2.
Proper PCI accessing plan. The red cross shows the target point. The green cross shows the entry point. The arrow shows the facial nerve. PCI: Percutaneous Cochlear Implantation; FN: Facial nerve.
Registration
In this study, Polaris Spectra navigation was employed. A minimally invasive method combined with a drilled hole-centered bone-bed and the short process of malleus was adopted.[15] The detailed illustrations were as follows.
In the 3D visual module of the ear navigation, a 3D model was reconstructed with the 2D CT data, and the temporal region of the surgery side was selected
-
In the space registration unit, a 3D point cloud (so-called point cloud P) was interactively collected on the characteristic surface of the reconstructive model in the selected regions by ray-casting algorithm. This process was called the digital CT space characteristic surface
Using the collecting ball, the point clouds were collected on the bone surface area centered around the bone hole in the temporal region with certain radius (usually, 10 mm), which was also referred as “point clouds on the bone-bed.” The STL file of the auditory ossicle was imported; the collecting ball was moved to enclose the short process of malleus and all-point clouds were collected in the ball. The coordinates of point clouds of the STL file was transformed to those of the CT coordinate system. The data mentioned above were merged and point cloud P in CT space coordinate system was acquired.
-
In the process of operation, the point data collection in the actual coordinate system was obtained. By sliding the probe on the predetermined characteristic surface of the surgical location (corresponding to characteristic surface of reconstruction model), we obtained a real-time recording of the coordinate of the end of the probe in the actual space by the position indicator. This coordinate was referred as the point cloud Q, and this process was referred as the digital actual space characteristic curve surface
Likewise, after the bone exposure, a disc area with a drilled hole center and 10 mm diameter was selected. When the Polaris Spectra navigation began to collect, the tip of the probe should contact with the bone surface and be completely well-distributed on the disc. Data of around 3000 points should be collected, so as to gain the actual space point cloud. With the endoscope, the tip of the probe was penetrated into the inside of the EAC and touched the short process of the malleus lightly to collect the point cloud. This step should be softly to avoid the injury of the tympanic membrane and the OC. Point cloud Q in the actual space coordinate system was acquired when these two-point clouds were combined.
Principal component analysis of point cloud P and point cloud Q was applied to acquire the eigenvector of the point cloud for the primary registration. Iterative closest point algorithm was applied to performing the fine registration[16,17] with a registration matrix to map locations from the actual space to the image space [Figure 3].
Figure 3.
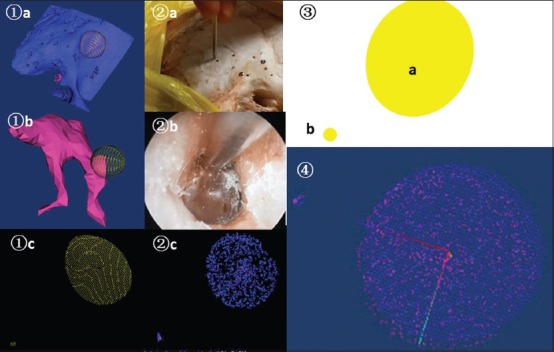
Process of collecting point cloud and navigation registration. ① point cloud P in image space ② point cloud Q in actual space ③ point cloud simulated diagram ④ registration matrix T after registering point cloud P and point cloud Q. a: Point cloud collection in bone-bed; b: Point cloud collection in short process of malleus; c: Cloud of points presented by the system.
Image-guided drilling by bi-planar device
The first step was to fix the bi-planar robot and place the drill on the bi-planar robot. Four identifiable balls for the electrooptical navigation were fixed at the end of the drill, by which the corresponding position of the drill's tip on the image was recognized. The operator held the drill and faced the navigation display which showed the planned path and the real-time location of the drill in 3D. After ascertaining the entry point, the operator adjusted the bi-planar positions to ensure that the drill trajectory coincided with the planned path. After the manual orientation, the position of the bi-planar device was locked, and the drilling was subsequently processed following the planned pathway.
Three intermittent processes were carried on during the drilling involving three drill bits: in the mastoid, 2.3 mm cutting drill bits (Medicom, Germany); in the facial recess, 1.8 mm twist drill bits; and at the basal turn of the cochlear, 1.0 mm diamond drill bits. 10,000 r/min was used at the first two processes with the continuous irrigation, and 1000 r/min for the cochleostomy. In the operation, the real-time image was displayed to verify the coincidence between the actual trajectory and the planned path, the relationship between the extension cord of the actual trajectory and the basal turn of the cochlear, as well as the relationship between the actual trajectory and the FN and the cochlear. Meanwhile, the operator who held the drill had a real-time force feedback.
Postoperation safety analysis
The cadaveric head temporal bone was scanned by HRCT with the same scanning parameters as the preoperation, and the 3D reconstruction and the image analysis were made to observe and measure the relative location and the distance between the actual trajectory and the important anatomic structures. Routine anatomy was conducted on the drilled specimens to specify the relationship between the drill path and the surrounding anatomic structures. The cochlear was dissected and contoured to identify the location of the cochleostomy.
Postoperative CT images were projected to the coordinate system of the preoperative CT images and registered through similar methods. Since it was impossible to directly obtain the axis of the actual postoperative trajectory, using the similar planned method as the preoperation (two points to determine a straight line), the target point and the entry point of the actual trajectory were expected to be at the center of the hole in the cochlear and the temporal bone surface, respectively. Therefore, the actual trajectory was matched. This study designed a self-developed pre- and post-operation comparison procedure to calculate the distance between the actual and the planned position of the target and the entry point, as well as the deviation of the distance and the angle between the actual trajectory and the planned path, to directly obtain the location relationship between the FN and the ChT. The maximal, minimal, and median values were recorded and referred as the median (inter-quartile range).
Results
The average length of the drilling trajectory from the mastoid surface to the cochlear fenestration point was 27.69 mm (26.77–29.15 mm), and the average width of the FN recess was 2.80 mm (2.50–3.10 mm). The drilling trajectory was clearly observed in the postoperative CT scans. Eight PCI tunnels were drilled successfully in this study. Errors at the entrance and the target points were 0.86 mm (0.68–1.00 mm) and 0.44 mm (0.30–0.96 mm), respectively. The angular error between the planed and the drilled trajectory was 1.74° (1.26–2.41°). Distances to other structures were also reported as the absolute distance measured postoperatively. The statistical measures from all eight cases are compiled in Table 1.
Table 1.
Summary statistics from the drilling tests (n = 8)
| Items | Angle (°) | Distance errors (mm) | Trajectory length (mm) | Width of FNR (mm) | Distance to (mm) | ||
|---|---|---|---|---|---|---|---|
| Entrance | Target | FN | EAC | ||||
| Max | 3.29 | 1.45 | 1.29 | 30.21 | 3.50 | 1.00 | 2.60 |
| Min | 1.10 | 0.16 | 0.30 | 25.36 | 2.00 | 0.00 | 1.00 |
| MED | 1.74 | 0.44 | 0.86 | 27.69 | 2.80 | 0.60 | 1.60 |
| IOR | 1.26–2.41 | 0.30–0.96 | 0.68–1.00 | 26.77–29.15 | 2.50–3.10 | 0.35–0.83 | 1.30–2.05 |
FNR: Facial nerve recess; FN: Facial nerve; EAC: External auditory canal; Max: Maximum; Min: Minimum; MED: Median; IQR: Inter-quartile range.
Eight specimens all maintained intact FNs, with no damage in the EAC, tympanic membrane, and ossicles among specimens while five had the intact ChT. Taking one case as example, Figure 4 shows the location between the drill path between the surrounding anatomic structures with CT scans, anatomical observation, and pre- and post-operation comparison procedure.
Figure 4.
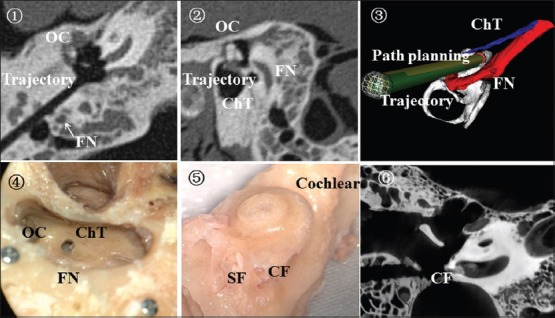
The location between the drill path and surrounding anatomic structures. ① postoperative HRCT; ② the location relationship between drill path and FN; ③ postoperative verification procedures; ④ routine temporal anatomy; ⑤ the contoured cochlear; ⑥ postoperative micro-CT. The arrow shows the facial nerve. HRCT: High-resolution computed tomography; FN: Facial nerve; OC: Ossicular chain; ChT: Chorda tympani nerve; CF: Cochlear fenestration; SF: Stapes footplate; CT: Computed tomography.
In Figure 5, we show the operation durations of each step during the complete process in PCI with assistance of image-guided bi-planar device. The operation durations were reduced significantly with the practice. At specimen 8, the total time was <60 min while the registration process consumed the longest time and the segmentation of important anatomic structures such as the cochlea was the second longest. In the actual operation, it last for 20–30 min to segment the cochlea and the plan path, which was finished before the operation. Consequently, 30–40 min was taken to register, fixed the bi-planar and drill during the process of the operation.
Figure 5.
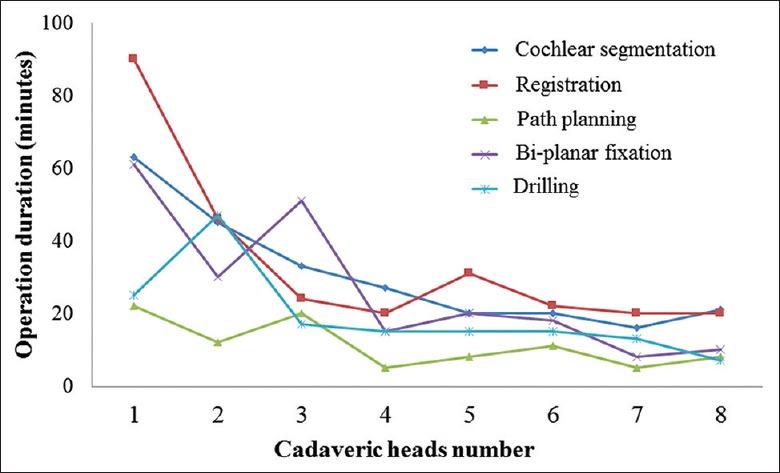
Operation duration curve of eight cadaveric heads.
Discussion
With the development of surgical techniques, otologists tried more minimally invasive methods to reduce the damage of the cochlear implantation.[18,19] The method of minimally invasive percutaneous tunnel avoided the traditional method of opening mastoid. Instead, with the computer-assisted path-planning design and the framework guidance, a tunnel with a small diameter was drilled from the mastoid outer surface to the cochlear target point. Because the anatomy variations of the temporal bone are large, the cochlear implant surgery typically demands the experience and the skill of the performer. We tried to find an easier and more precise manner to perform this surgery. On the other hand, because the target point of this tunnel was at the cochlear basal turn, any deviation in the location of the target point would fail the operation and even damage the cochlea. Meanwhile, since the tunnel was adjacent to the FN, ChT, EAC, and the ossicle, any damage of these structures, especially which of the FN, would lead to severe sequela to patients. Therefore, operation accuracy is an important indicator for the clinical application of this minimal invasive theory.
The operation accuracy was comprehensively determined by each element of the system, such as the position error from the navigation system, instrument error from the operation mechanism (the space framework, device, drill, etc.), and operation error as well. In the study, we succeeded in drilling on eight cadaveric head specimens with image-guided bi-planar operations, and the error of the target point was 0.86 mm (0.68–1.00 mm). This accuracy met the accuracy demand required by the middle ear surgery but was still lower than 0.5 mm which was reported in literature.[3] The reasons for errors and the optimization plans are as follows: the quality of the imaging data directly affects the accuracy of the image-guided surgery. With flat-panel volumetric computed tomography, the resolution at each direction reached 0.2 mm,[20] and the accuracy reached 0.46 ± 0.22 mm.[21] To evaluate the feasibility of applying this method in the current clinical settings, the temporal bone was scanned with HRCT (thickness = 0.6 mm, pitch = 0.3 mm) to acquire the accuracy of 0.86 mm (0.68–1.00 mm). With the development of the imaging technology, this accuracy will be further improved.
The accuracy of the registration algorithm and the procedure directly affects that of the entire navigation system which is crucial to the image-guided surgery. Given the required target accuracy and the minimal invasion, a registration method, which was the joint registration of the bone bed malleus short process, was employed in this study. This method used the full use of bone surface of the temporal implant bone bed to avoid the additional exposure and used the short process of malleus as an obvious anatomic marker. The registration accuracy was at the submillimeter level, which was of applicable significance. However, due to the high demand of the accuracy in the cochlear implantation surgery, the algorithm of the method requires more improvements to further reduce the artificial error. Subsequent clinical experiments are needed to prove its stability.
In the current drilling operation, the common devices for holding and locating the drill were microstereotactic frames[6,22,23] or robot.[8,9,10] The former had higher accuracy dispensing with real-time navigation, but it lacked the flexibility ascribing to the one-time designed path, which was hardly adjusted in the operation. However, the industrial robot with the high accuracy was too large to apply in operation settings. In recent years, robots have been specially designed for the cochlear surgery featuring the higher accuracy, smaller size, and more convenient for operation.[10,24] In this study, a passive bi-planar mechanism, with 4-DOF, was specially designed for holding an electric drill to move freely in the operational space. Its advantages were as follows: (1) this mechanism was passively driven and was image-guided by doctors to finish locating, and it reduced operation errors and improved clinical safety; (2) the special design of the parallel mechanism maintained the adequate intensity and rigidity but reduced the size and mass as much as possible, so that it could be easily fixed, moved, and applied in operation rooms; (3) this bi-planar device had a fixed electric drill leading tunnel, which could restrict the drill within the planned path to get rid of artificial factors, such as hand vibrations; (4) while the drill was moving forward, the operator could feel the change in force generated by different bone density. Particularly, at the moment when the drill penetrated the wall of cochlear, the operator might have a sense of loss. The force feedback could mutually verify the trajectory on the navigation display to guarantee the safety of the trajectory.
The operator's learning curve shows that at the early stage of the experiment, more than 4–5 h was taken. However, when the operator became familiar with this system and took relevant trainings, only 1 h was consumed. At the beginning of the PCI study, researchers optimistically hypothesized that the duration of the cochlear implant surgery could be reduced to within 50 min.[5] This duration is comparable to the time needed for an experienced surgeon but is significantly shorter for an inexperienced surgeon. So far, in the first clinical report about PCI, the average duration from the incision to the completion was 186 ± 36 min,[13] which was far longer than 80–90 min that was needed in the traditional cochlear surgery.[25] As there was a process from the exploration to the perfection for the clinical application of any new technology, there was a process from acquaintance to proficiency for humans.
There are some limitations in the study demanding further improvement. The recognition and segmentation of the important anatomic structures were manually completed, which consumed most of the surgical time and led to artificial errors. The automated program should be developed to save more time. An automated robot should be developed. During the electronic drilling process, the robot's adaptive path was manually controlled. An automated module is required to real-time monitor any path deviation, and to cease the operation automatically once it happens. In the study, there were only eight specimens, and all the specimens were from the adults. No children cadaveric temporal specimens were used in this study while most cochlear implantation was performed in children. The small sample size allowed preliminary research on feasibility of this system, rather than fully verifies the safety, which will be fulfilled later by a larger sample-sized study.
Above all, we established the navigation and drilling guide system in terms of the minimally invasive cochlear implant surgery. Based on this system, there was a preliminary attempt in PCI with target accuracy ±1 mm, which met the accuracy requirements of this surgery. The later research will further improve the accuracy of the registration and the navigation system and optimize the path planning to meet the accuracy requirement of the cochlear implant surgery.
Financial support and sponsorship
This work was supported by grants from the China Capital Health Development Project (No. 2011-4023-03 and No. 2016-2-4094).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
References
- 1.Labadie RF, Chodhury P, Cetinkaya E, Balachandran R, Haynes DS, Fenlon MR, et al. Minimally invasive, image-guided, facial-recess approach to the middle ear: Demonstration of the concept of percutaneous cochlear access in vitro. Otol Neurotol. 2005;26:557–62. doi: 10.1097/01.mao.0000178117.61537.5b. doi: 10.1097/01.mao.0000178117.61537.5b. [DOI] [PubMed] [Google Scholar]
- 2.Bielamowicz SA, Coker NJ, Jenkins HA, Igarashi M. Surgical dimensions of the facial recess in adults and children. Arch Otolaryngol Head Neck Surg. 1988;114:534–7. doi: 10.1001/archotol.1988.01860170064020. doi: 10.1001/archotol.1988.01860170064020. [DOI] [PubMed] [Google Scholar]
- 3.Schipper J, Aschendorff A, Arapakis I, Klenzner T, Teszler CB, Ridder GJ, et al. Navigation as a quality management tool in cochlear implant surgery. J Laryngol Otol. 2004;118:764–70. doi: 10.1258/0022215042450643. doi: 10.1258/0022215042450643. [DOI] [PubMed] [Google Scholar]
- 4.Schipper J, Klenzner T, Aschendorff A, Arapakis I, Ridder GJ, Laszig R. Navigation-controlled cochleostomy. Is an improvement in the quality of results for cochlear implant surgery possible? HNO. 2004;52:329–35. doi: 10.1007/s00106-004-1057-5. doi: 10.1007/s00106-004-1057-5. [DOI] [PubMed] [Google Scholar]
- 5.Labadie RF, Noble JH, Dawant BM, Balachandran R, Majdani O, Fitzpatrick JM. Clinical validation of percutaneous cochlear implant surgery: Initial report. Laryngoscope. 2008;118:1031–9. doi: 10.1097/MLG.0b013e31816b309e. doi: 10.1097/MLG.0b013e31816b309e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labadie RF, Mitchell J, Balachandran R, Fitzpatrick JM. Customized, rapid-production microstereotactic table for surgical targeting: Description of concept and in vitro validation. Int J Comput Assist Radiol Surg. 2009;4:273–80. doi: 10.1007/s11548-009-0292-3. doi: 10.1007/s11548-009-0292-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warren FM, Balachandran R, Fitzpatrick JM, Labadie RF. Percutaneous cochlear access using bone-mounted, customized drill guides: Demonstration of concept in vitro. Otol Neurotol. 2007;28:325–9. doi: 10.1097/01.mao.0000253287.86737.2e. doi: 10.1097/01.mao.0000253287.86737.2e. [DOI] [PubMed] [Google Scholar]
- 8.Majdani O, Rau TS, Baron S, Eilers H, Baier C, Heimann B, et al. Arobot-guided minimally invasive approach for cochlear implant surgery: Preliminary results of a temporal bone study. Int J Comput Assist Radiol Surg. 2009;4:475–86. doi: 10.1007/s11548-009-0360-8. doi: 10.1007/s11548-009-0360-8. [DOI] [PubMed] [Google Scholar]
- 9.Baron S, Eilers H, Munske B, Toennies JL, Balachandran R, Labadie RF, et al. Percutaneous inner-ear access via an image-guided industrial robot system. Proc Inst Mech Eng H. 2010;224:633–49. doi: 10.1243/09544119JEIM781. doi: 10.1243/09544119JEIM781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bell B, Stieger C, Gerber N, Arnold A, Nauer C, Hamacher V, et al. Aself-developed and constructed robot for minimally invasive cochlear implantation. Acta Otolaryngol. 2012;132:355–60. doi: 10.3109/00016489.2011.642813. doi: 10.3109/00016489.2011.642813. [DOI] [PubMed] [Google Scholar]
- 11.Klenzner T, Ngan CC, Knapp FB, Knoop H, Kromeier J, Aschendorff A, et al. New strategies for high precision surgery of the temporal bone using a robotic approach for cochlear implantation. Eur Arch Otorhinolaryngol. 2009;266:955–60. doi: 10.1007/s00405-008-0825-3. doi: 10.1007/s00405-008-0825-3. [DOI] [PubMed] [Google Scholar]
- 12.Liu WP, Azizian M, Sorger J, Taylor RH, Reilly BK, Cleary K, et al. Cadaveric feasibility study of da Vinci Si-assisted cochlear implant with augmented visual navigation for otologic surgery. JAMA Otolaryngol Head Neck Surg. 2014;140:208–14. doi: 10.1001/jamaoto.2013.6443. doi: 10.1001/jamaoto.2013.6443. [DOI] [PubMed] [Google Scholar]
- 13.Labadie RF, Balachandran R, Noble JH, Blachon GS, Mitchell JE, Reda FA, et al. Minimally invasive image-guided cochlear implantation surgery: First report of clinical implementation. Laryngoscope. 2014;124:1915–22. doi: 10.1002/lary.24520. doi: 10.1002/lary.24520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balachandran R, Reda FA, Noble JH, Blachon GS, Dawant BM, Fitzpatrick JM, et al. Minimally invasive image-guided cochlear implantation for pediatric patients: Clinical feasibility study. Otolaryngol Head Neck Surg. 2014;150:631–7. doi: 10.1177/0194599813519050. doi: 10.1177/0194599813519050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ke J, Zhang SX, Li CS, Zhu YF, Hu L, Ma FR. Application of Bone bed-Malleus Short Process Based Registration in Minimally Invasive Cochlear Implantation: A Preliminary Study. [Accepted 2016];The 5thInternational Conference on Biomedical Engineering and Biotechnology (ICBEB 2016). August, 1st to 4th China. [Google Scholar]
- 16.Wang JC, Wang TM, Xu Y, Fang LM. Registration method based on ICP algorithm for 3D surgical navigation. J Beijing Univ Aeronaut A. 2009;35:434–8. [Google Scholar]
- 17.Nolte LP, Beutler T. Basic principles of CAOS. Injury. 2004;35(Suppl 1):6–16. doi: 10.1016/j.injury.2004.05.005. doi: 10.1016/j.injury.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Cui D, Shi Y, Su Q, Liu T, Han D, Li Y. Minimal incision access for pediatric and adult cochlear implantation. Chin Med J. 2014;127:2434–7. doi: 10.3760/cma.j.issn.0366-6999.20140106. [PubMed] [Google Scholar]
- 19.Wang LE, Xia J, Shen XX, Wang ZX, Wang W, Zhang DX. Retaining chorda tympani nerve integrity during cochlear implant surgery. Chin Med J. 2015;128:2115–8. doi: 10.4103/0366-6999.161399. doi: 10.4103/0366-6999.161399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalender WA. The use of flat-panel detectors for CT imaging. Radiologe. 2003;43:379–87. doi: 10.1007/s00117-003-0897-4. doi: 10.1007/s00117-003-0897-4. [DOI] [PubMed] [Google Scholar]
- 21.Bartling SH, Leinung M, Graute J, Rodt T, Dullin C, Becker H, et al. Increase of accuracy in intraoperative navigation through high-resolution flat-panel volume computed tomography: Experimental comparison with multislice computed tomography-based navigation. Otol Neurotol. 2007;28:129–34. doi: 10.1097/01.mao.0000244364.16826.09. doi: 10.1097/01.mao.0000244364.16826.09. [DOI] [PubMed] [Google Scholar]
- 22.Balachandran R, Mitchell JE, Blachon G, Noble JH, Dawant BM, Fitzpatrick JM, et al. Percutaneous cochlear implant drilling via customized frames: An in vitro study. Otolaryngol Head Neck Surg. 2010;142:421–6. doi: 10.1016/j.otohns.2009.11.029. doi: 10.1016/j.otohns.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labadie RF, Balachandran R, Mitchell JE, Noble JH, Majdani O, Haynes DS, et al. Clinical validation study of percutaneous cochlear access using patient-customized microstereotactic frames. Otol Neurotol. 2010;31:94–9. doi: 10.1097/MAO.0b013e3181c2f81a. doi: 10.1097/MAO.0b013e3181c2f81a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bell B, Gerber N, Williamson T, Gavaghan K, Wimmer W, Caversaccio M, et al. In vitro accuracy evaluation of image-guided robot system for direct cochlear access. Otol Neurotol. 2013;34:1284–90. doi: 10.1097/MAO.0b013e31829561b6. doi: 10.1097/MAO.0b013e31829561b6. [DOI] [PubMed] [Google Scholar]
- 25.Semaan MT, Fredman ET, Shah JR, Fares SA, Murray GS, Megerian CA. Surgical duration of cochlear implantation in an academic university-based practice. Am J Otolaryngol. 2013;34:382–7. doi: 10.1016/j.amjoto.2013.01.013. doi: 10.1016/j.amjoto.2013.01.013. [DOI] [PubMed] [Google Scholar]