Abstract
Background
Research trials have shown improved short-term outcome with drug-eluting stents (DES) over bare metal stents (BMS) in saphenous vein graft (SVG) percutaneous coronary intervention (PCI), primarily by reducing target vessel revascularization (TVR) for in-stent restenosis. We compared the outcomes in patients undergoing SVG stent implantation treated with DES or BMS. In exploratory analyses we investigated the influence of stent generation and diameter.
Methods
Data were obtained from a prospective database of 657 patients who underwent PCI for SVG lesions between 2003 and 2011. A total of 344 patients had PCI with BMS and 313 with DES. Propensity scores were developed based on 15 observed baseline covariates in a logistic regression model with stent type as the dependent variable. The nearest-neighbour-matching algorithm with Greedy 5-1 Digit Matching was used to produce two patient cohorts of 313 patients each. We assessed major adverse cardiac events (MACE) out to a median of 3.3 years (interquartile range: 2.1-4.1). MACE was defined as all-cause mortality, myocardial infarction (MI), TVR and stroke.
Results
There was a significant difference in MACE between the two groups in favour of DES (17.9% DES vs. 31.2% BMS group; p = 0.0017) over the 5-year follow-up period. MACE was driven by increased TVR in the BMS group. There was no difference in death, MI or stroke. Adjusted Cox analysis confirmed a decreased risk of MACE for DES compared with BMS 0.75 (95% confidence interval (CI) 0.52-0.94), with no difference in the hazard of all-cause mortality (hazard ratio: 1.08; 95% CI: 0.77-1.68). However, when looking at stent diameters greater than 4 mm, no difference was seen in MACE rates between BMS and DES.
Conclusions
Overall in our cohort of patients who had PCI for SVG disease, DES use resulted in lower MACE rates compared with BMS over a 5-year follow-up period; however, for stent diameters over 4 mm no difference in MACE rates was seen.
Keywords: Bare metal stent, Drug-eluting stent, Percutaneous intervention, Stroke, Target vessel revascularisation, Venous graft
Introduction
Drug-eluting stents (DES) are now widely used in preference to bare metal stents (BMS) for elective and emergency percutaneous coronary intervention (PCI), with studies demonstrating their superiority to BMS in reducing major adverse cardiac events (MACE) in native coronary artery disease (1-2-3-4). However, the use of DES in saphenous vein graft (SVG) and the long-term MACE outcome has not been yet demonstrated in larger studies over longer follow-up periods. Occlusion rates of SVG over 10 years are 40%, and of those grafts that do not occlude 43% will have significant stenosis (5). Despite the fact that up to 10% of PCIs are done in SVG (6), these lesions have been either poorly represented or excluded from pivotal clinical trials (1, 2). However, the degeneration of SVG appears to be different compared with the progression of coronary artery atherosclerosis in native vessels (7, 8). In native vessels the cause of restenosis is almost exclusively due to neointima proliferation (9) while in SVGs variable degrees of contribution have been reported for thrombus formation, cellular hyperplasia and progression of atherosclerotic process (10). Due to the difference in pathophysiology of vein graft stenosis, the outcome of DES in native coronary artery disease cannot be directly translated to this different clinical setting (11). The question arises as to whether the long-term advantages of DES over BMS in reducing restenosis are offset by increased MACE driven by late stent thrombosis, whether different DES types have different outcomes and whether the size of the stent affects the outcome. To address this we studied a large cohort of consecutive patients undergoing SVG PCI with the aim of comparing long-term outcomes for those treated with DES and BMS. We also investigated the influence of stent diameter on MACE during the follow-up period.
Methods
This was a retrospective observational cohort study in a high-volume interventional centre of patients with vein graft disease treated with either DES or BMS. The study period was from January 2003 to July 2011. During this period, 15,569 consecutive patients underwent PCI, of whom 13,422 (86%) with complete database records and National Health Service numbers were available for analysis. A total of 657 patients with stable angina, unstable angina or non-ST elevation acute coronary syndrome (NSTEACS) underwent PCI for the treatment of SVG stenosis. Patients undergoing primary PCI were excluded as BMSs were routinely used for the majority of the study period as per local guidelines.
Data were prospectively entered into a clinical PCI database at the time of the procedure. Data collected included patient characteristics (age, prior myocardial infarction (MI), PCI and coronary artery bypass grafting (CABG), hypertension, diabetes mellitus, hypercholesterolaemia, peripheral vascular disease (PVD), New York Heart Association class, smoking status, chronic renal impairment (chronic renal failure (CRF): creatinine >200 µmol/l or on renal replacement therapy), left ventricular (LV) function and cardiogenic shock) and procedure-related data (indications for PCI, target vessel, number of diseased vessels, use of intravascular ultrasound (IVUS)/pressure wire, use of DES and glycoprotein (GP) IIb/IIIa inhibitor).
Interventional strategy was at the discretion of the operator, including the use of direct stenting, pre/post-dilatation, IVUS, adjunctive antiplatelet therapy and use of ablative devices. Angiographic success was defined as residual stenosis <30% with Thrombolysis In Myocardial Infarction (TIMI) flow grade 3. All patients received aspirin 300 mg and either clopidogrel 300 mg or 600 mg prior to the procedure. All patients were prescribed 75 mg aspirin and 75 mg clopidogrel maintenance therapy. Clopidogrel maintenance therapy was recommended for 1 month in the BMS group, 12 months in the DES group and 12 months for patients treated for NSTEACS. Unfractionated heparin was given during the procedure at a loading dose of 70 u/kg and the activated clotting time was maintained >250 sec. Glycoprotein IIb/IIIa inhibitors were used at the operator’s discretion and according to local guidelines.
Procedural complications and MACE were recorded prospectively. MACE was defined as death, MI (new pathologic Q waves in the distribution of the treated coronary artery with an increase of creatine kinase MB to ≥2 times the reference value or significant rise in Troponin T values) and target vessel revascularization (TVR). These MACE rates were adjudicated by three independent physicians who were not involved in the procedure and were unaware of the patient’s stent type. Procedural complications recorded included MI, emergency CABG, arterial complications, aortic/coronary dissection, side branch occlusion and arrhythmia. Procedural complications were recorded at the time of the procedure and in-hospital complications were entered into the database at the time of discharge. Stent thromboses were defined according to the Academic Research Consortium (ARC) definition as angiographic or pathologic confirmation of partial or total thrombotic occlusion within the peri-stent region plus at least one of acute ischaemic symptoms, ischaemic electrocardiogram changes or elevated cardiac biomarkers (12). Repeat PCI rates due to TVR were identified from the PCI database. All-cause mortality data were recorded as of 10 August 2011 and obtained via the British Cardiovascular Intervention Society national database, part of the National Institute of Cardiovascular Outcomes Research. This national database is periodically linked to the UK Office of National Statistics and provides live/death status of treated patients. Only patients who had complete database records and National Health Service unique numbers (allowing live/death status to be assessed) were included in the analysis. A retrospective data quality audit of 100 randomly selected medical records established that 94.8% of data fields, including complications, were entered correctly into the database.
Ethics
Data were collected as part of a national cardiac audit and all patient-identifiable fields were removed prior to analysis. The local ethics committee advised that formal ethical approval was not required.
Statistical analysis
Baseline patient, procedural and post-procedural characteristics were compared between the two groups. Categorical data are summarized using absolute values (percentage). Normally distributed, continuous data are presented as mean ± standard deviation or, where skewed, as median (inter-quartile range). Normally distributed continuous variables were compared using Student t-tests, and the Mann–Whitney U test was used to compare non-normally distributed continuous variables. Categorical data were compared using the Pearson chi-squared test.
Propensity matching
Baseline comorbidity was unbalanced between the DES and BMS groups. A non-parsimonious logistic regression model with stent type as the dependent variable (c-statistic, 0.785) was constructed to adjust for the confounding of baseline comorbidity and surgical complexity. Covariates in the model included age, sex, previous MI, hypertension, previous stroke, PVD, LV ejection fraction, diabetes mellitus, CRF, acute coronary syndrome (ACS) presentation, cardiogenic shock, stent length and GP IIb/IIIa use. To balance comorbidity between the study groups, a greedy matching SPSS macro was used to match the 313 patients who underwent DES insertion with the 344 patients from the BMS group with similar comorbidity. This created a “propensity-matched BMS” population.
Midterm survival was described using the Kaplan–Meier method, and comparisons were made using the log-rank statistic. Estimations of risk were calculated using Cox regression analysis. Potential independent predictors of outcome were identified by univariate Cox regression analyses, and all significant univariate predictors (p<0.05) were then entered into the multivariate Cox regression model.
Influence of stent diameter and DES type on outcome
Subgroup analysis was performed based on the diameter of stent inserted, with patients split into above and below 4 mm with further subgroup analysis based on the type of DES used.
Results
A total of 657 patients underwent PCI for SVG lesions, 344 patients who underwent PCI with BMS and 313 treated with DES. The DES used was broken down into Taxus 128 (paclitaxel), Cypher (sirolimus) 70, Resolute 20 (zotarolimus), Endeavor 122 (zotarolimus), Promus (everolimus) 37.
Patient and procedural characteristics (Tab. I)
TABLE I -.
Baseline patient characteristics according to stent type
| DES n = 313 | BMS n = 344 | Significance | |
|---|---|---|---|
| p<0.05. | |||
| ACS = acute coronary syndrome; BMS = bare metal stent; DES = drug-eluting stent; LVEF = left ventricular ejection fraction; MI = myocardial infarction; PVD = peripheral vascular disease. | |||
| Age (mean) | 66.8 ± 10 | 68.6 ± 7.4 | 0.13 |
| Gender (female) (%) | 45 (13.9) | 47 (14.1) | 0.20 |
| Diabetes (%) | 102 (31.6) | 70 (21.0) | 0.007 |
| Hypertension (%) | 176 (54.5) | 182 (54.5) | 0.98 |
| Current smoker (%) | 43 (13.3) | 47 (14.1) | 0.82 |
| Hypercholesterolaemia (%) | 143 (44.3) | 137 (41.0) | 0.34 |
| Previous MI (%) | 151 (46.7) | 147 (44) | 0.54 |
| PVD (%) | 4 (1.2) | 6(1.8) | 0.05 |
| ACS Presentation | 71 (22.0) | 105 (31.4) | 0.02 |
| LVEF | 50.44 ± 0.17 | 48.73 ± 0.19 | 0.52 |
| Chronic renal failure | 8 (2.5) | 7 (2.1) | 0.65 |
Full unmatched study population
Baseline characteristics for both groups were similar apart from there being more patients with diabetes in the DES group (31.6% vs. 21.0%, p = 0.007) and more patients with ACS in the BMS group (31.4% vs. 22.0%, p = 0.02). Angiographic success rates were similar for both groups (93.8% vs. 92.8%, p = 0.78). More stents per lesion were used in the DES group (1.5 ± 0.7 vs. 1.3 ± 0.6, p<0.0001), with a longer average length (22.0 ± 5.4 vs. 18.8 ± 3.9, p<0.0001). Average stent width was higher in the BMS group (3.7 ± 0.5 vs. 3.2 ± 0.4, p<0.001).
Propensity matched population
After propensity matching, all baseline patient and procedural characteristics were balanced between the two groups.
Outcomes after propensity matching (Figs. 1 and 2)
Fig. 1 -.
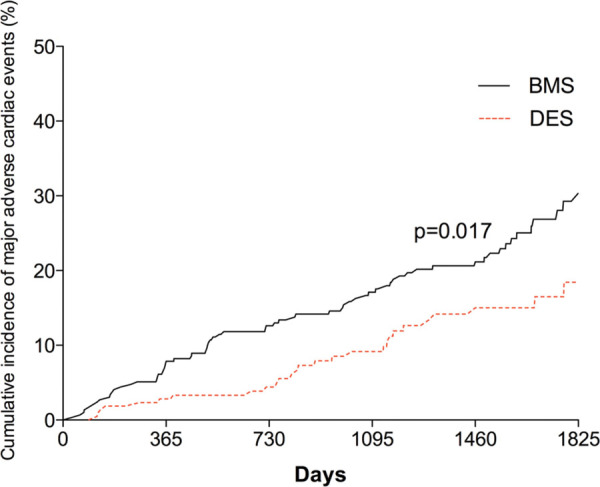
Kaplan–Meier curve showing cumulative probability of MACE after PCI according to stent group. BMS = bare metal stent; DES = drug-eluting stent; MACE = major adverse cardiac events; PCI = percutaneous coronary intervention.
Fig. 2 -.
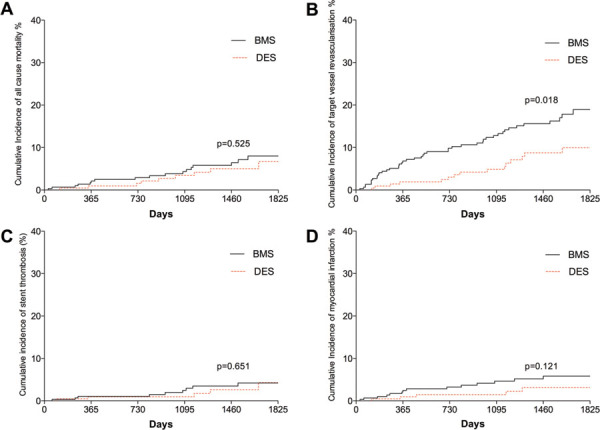
Kaplan–Meier curves showing cumulative probability of (A) all-cause mortality; (B) target vessel revascularization; (C) stent thrombosis; (D) myocardial infarction after PCI according to stent group. BMS = bare metal stent; DES = drug-eluting stent; PCI = percutaneous cornary intervention.
MACE after 5 years were less frequent with DES compared with BMS (17.9%, 95% confidence interval (CI) 9.3-14.2% vs. 31.2%, 95% CI 16.9-25.1%, p = 0.017), driven largely by decreased TVR in the DES group (9.9%, 95% CI 6.0-11.5% vs. 18.8%, 95% CI 13.0-22.3%, p = 0.018). Rates of target lesion revascularization were also significantly lower in the DES group (8.3%, 95% CI 5.2-10.1% vs. 17.2%, 95% CI 10.8-19.4%, p = 0.020). There was no difference in death, MI or stroke between the stent types (Fig. 2). Rates of definite/confirmed stent thrombosis were comparable for BMS and DES (3.2%, 95% CI: 1.7-4.6% vs. 3.3%, 95% CI 1.4-4.4%, p = 0.6). There was no difference in the timing of stent thrombosis between the two groups with similar rates of late (1.6% vs. 1.3%) and very late thrombosis (1.6% vs. 1.5%). Procedural characteristics according to stent type are illustrated in Table II.
TABLE II -.
Procedural characteristics according to stent type
| DES (n = 313) | BMS (n = 344) | Significance | |
|---|---|---|---|
| p<0.05. | |||
| BMS = bare metal stent; DES = drug-eluting stent; GP = glycoprotein. | |||
| Distal protection (%)f | 98 (30.3) | 115 (34.4) | 0.08 |
| GP IIb/IIIA use (%) | 175 (54.2) | 166 (49.7) | 0.06 |
| Number stents used | 1.68 ± 0.7 | 1.66 ± 0.6 | 0.56 |
| Stent length | 29.05 ± 7.4 | 19.0 ± 5.9 | 0.19 |
| Stent width | 3.20 ± 0.4 | 3.70 ± 0.5 | <0.0001 |
| Angiographic success | 303 (93.8%) | 310 (92.8%) | 0.78 |
Cox analysis (Fig. 3)
Fig. 3 -.
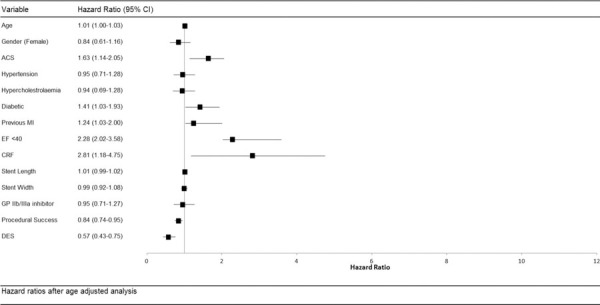
Hazard ratios after multivariate analysis for baseline and procedural characteristics. ACS = acute coronary syndrome; CI = confidence interval; CRF = chronic renal failure; DES = drug-eluting stent; EF = ejection fraction; GP = glycoprotein; MI = myocardial infarction.
Adjusted Cox analysis confirmed a decreased risk of MACE for DES compared with BMS 0.75 (95% CI 0.52-0.94) with no difference in the hazard of all-cause mortality (hazard ratio (HR) 1.08; 95% CI 0.77-1.68). The above Cox proportional hazard model was repeated with the year of procedure included as a categorical variable to allow for improvements in PCI technique and technology over the long study period. This confirmed the association between DES use and improved MACE rates (HR 0.79; 95% CI 0.53-0.96) Table III illustrates the Cox proportional model of univariate and multivariate analysis of predictors of major adverse cardiac events after PCI.
TABLE III -.
Cox proportional model of univariate and multivariate analysis of predictors of major adverse cardiac events after PCI
| Variable | Comparator | Univariate | Multivariate* |
|---|---|---|---|
| ACS = acute coronary syndrome; BMS = bare metal stent; CRF = chronic renal failure; DES = drug-eluting stent; DM = diabetes mellitus; EF = ejection fraction; GP = glycoprotein; MI = myocardial infarction; PCI = percutaneous coronary intervention. | |||
| * Adjusted for age, DM, previous MI, CRF, EF, ACS presentation and procedural success; * = significant association in multivariate analysis. | |||
| Age | 1.01 (1.00-1.03) | 1.01 (1.00-1.02) | |
| Female | Male | 0.84 (0.61-1.16) | NA |
| ACS | Non-ACS | 1.63 (1.14-2.05) | 1.35 (1.08-1.95) |
| Hypertension | No hypertension | 0.95 (0.71-1.28) | NA |
| Hypercholesterolaemia | No hypercholesterolaemia | 0.94 (0.69-1.28) | NA |
| Diabetic | Non-diabetic | 1.41 (1.03-1.93) | 1.26 (1.06-2.01) |
| Previous MI | No previous MI | 1.24 (1.03-2.00) | 1.37 (0.97-1.93) |
| EF<40 | EF>40 | 2.28 (2.02-3.58) | 1.70 (1.47-3.00) |
| CRF | No CRF | 2.81 (1.18-4.75) | 2.28 (1.38-3.40) |
| Stent length | 1.01 (0.99-1.02) | NA | |
| Stent width | 0.99 (0.92-1.08) | NA | |
| GP IIb/IIIa | No GP IIb/IIIa | 0.95 (0.71-1.27) | NA |
| Procedural success | Failure | 0.84 (0.74-0.95) | 0.68 (0.60-0.79) |
| DES | BMS | 0.57 (0.43-0.75) | 0.75 (0.52-0.94) |
Subgroup analysis
On subgroup analysis comparing different types of DES there was no significant difference in MACE rates between old- and new-generation DES (198 stents were old-generation DES, and 179 were new-generation DES). Neither stent thrombosis (relative risk (RR) 1.19, 95% CI 0.70-1.80, p = 0.48), restenosis (RR 0.95, 95% CI 0.55-1.49, p = 0.54) nor death (RR 1.13, 95% CI 0.74-1.29, p = 0.44) were statistically significantly improved with new-generation vs. old-generation DES.
Influence of stent diameter (Fig. 4)
Fig. 4 -.
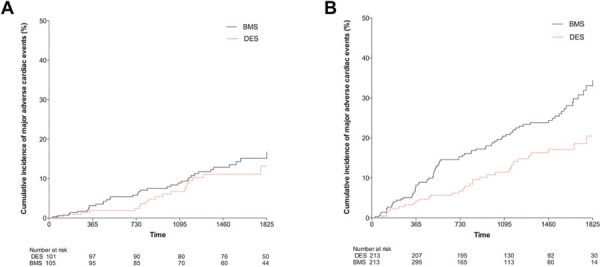
Kaplan–Meier curves showing cumulative probability of major adverse cardiac events for (A) stents ≥4 mm diameter and (B) stents <4 mm diameter according to stent group. BMS = bare metal stent; DES = drug-eluting stent.
In the study cohort there were 206 patients who had stent diameters ≥4 mm with the remaining 426 patients having stent diameters <4 mm. The larger diameter (≥4 mm) cohort were split into 101 DES and 105 BMS. In this cohort there were no differences in MACE after 5 years with DES compared with BMS (11.9%, 95% CI 8.1-14.2% vs. 15.2%, 95% CI: 9.4-12.7%, p = 0.317) (Fig. 4A), with no difference in rates of TVR, death, MI or stroke between the stent types. Rates of definite/confirmed stent thrombosis were comparable for BMS and DES in this larger stent cohort (0.6%, 95% CI: 0.2-1.9% vs. 0.9%, 95% CI 0.1-1.7%, p = 0.8).
In the smaller stent diameter cohort (<4 mm diameter) MACE after 5 years were significantly less frequent with DES compared with BMS (20.5%, 95% CI 10.8-13.8% vs. 38.2%, 95% CI: 19.9-45.1%, p = 0.0007), driven by decreased TVR in the DES group (14.9%, 95% CI 9.0-16.5% vs. 28.8%, 95% CI 15.0-32.3%, p = 0.0008). There was no difference in death, MI, stent thrombosis or stroke between the stent types in the smaller diameter cohort (Fig. 4B).
Discussion
This is a large observational study with one of the longest follow-up periods yet reported. Propensity matching is utilized, which is only the case in few other studies (13-14-15). We specifically compared outcomes for patients with SVG disease treated by PCI with drug eluting as compared with BMS. DESs were associated with significantly lower 5-year MACE rates compared with BMSs in both elective and ACS subgroups. This difference was primarily driven by lower rates of repeat TVR by PCI. Rates of late stent thrombosis and subsequent AMI were low, with no difference between the BMS and DES groups. Therefore, the use of DES in the proximal SVG was not associated with an increase in late-stent thrombosis or long-term mortality compared with BMS. Due to the fact that this difference was driven by TVR we also looked into the effect of stent diameter on MACE. Interestingly there was no difference comparing BMS and DES for stents above 4 mm in diameter.
The degeneration of SVG appears to be a different phenomenon compared with the progression of coronary artery atherosclerosis in native vessels (7, 8). In native vessels the cause of restenosis is almost exclusively due to neointima proliferation (9), while in SVGs exposed to the arterial circulation variable degrees of contribution have been reported for thrombus formation, cellular hyperplasia and progression of atherosclerotic process (10). Acute thrombosis is the dominant aetiology in the early postoperative period. This is related to the size of the target vessel and distal run-off, size mismatch between the graft and the target vessel, graft ischemia and disruption of the endothelial layer as a result of mechanical trauma and manual distention. Initially intimal hyperplasia is seen, which is caused by the graft’s adaptation to higher arterial pressures and loss of inhibition from the endothelial layer. Later on atherosclerosis becomes the major reason for graft stenosis and occlusion. As in native coronary arteries, vein graft atheromas can rupture and cause thrombotic occlusion of the graft (16). Vein graft atheromas are also more diffuse and concentric. They are less calcified and have poorly developed or absent fibrous caps (17). Vein graft failure is also associated with worse clinical outcomes. A study on 1,243 patients, who previously underwent CABG surgery and were followed up for a median of 6.7 years, reports a significant increase in the composite end point of death, nonfatal MI or revascularization in patients who had critical or occlusive vein graft disease on angiography compared with patients who had noncritical or no vein graft disease. This was primarily driven by TVR (18). Due to the difference in the pathophysiology of vein graft stenosis, investigating the long-term outcomes of these patients is essential.
Our finding of better outcomes for DES compared with BMS in a representative PCI population with SVG disease bears comparison with the selected populations included in recent clinical trials of DES vs. BMS. The ISAR-CABG trial, a randomized controlled superiority trial, reported decreased MACE rates for DES compared with BMS in subgroups undergoing SVG stenting after 1 year follow-up (19). Again it was TVR that drove MACE in both groups, although the MACE rates for DES in our study were slightly higher (17.9% DES vs. 31.2% BMS group (p = 0.04) over the 5-year follow-up than with the rate in ISAR-CABG (19) (15% for DES group and 22% for BMS group (p = 0.02)). A recent meta-analysis on 22 studies concluded that DES in vein graft PCI was associated with a decreased reintervention rates and all-cause mortality compared with BMS. There was no difference in the risk of stent thrombosis and MI. No difference in mortality was found in this study as was the case in the other major studies. The reasons for this are not outrightly apparent, but may be related to case selection and heterogeneity of the studies in the meta-analysis leading to bias. Interestingly, no difference in mortality was reported in randomized controlled trials in the sensitivity analysis (20). A further study investigating newer generation DES reveals no significant difference as compared to early-generation stents over a follow-up period of four years (21), data consistent with our study where no difference between older and newer generation DES was seen.
Some safety concerns persist regarding stent thrombosis with DES implantation (1, 22, 23). In contrast to restenosis, which is considered to have a relatively benign clinical course, stent thrombosis is consistently associated with acute MI and the mortality is high (24). We documented low and comparable rates of stent thrombosis for BMS and DES similar to the rates reported in other patient cohorts (23, 25). Importantly, we found no difference in long-term mortality between the DES and BMS groups. Our data show that DES deployment in the SVG is safe and exposes the patient to no greater risk than is associated with BMS deployment.
Importantly, the significant difference between the BMS and DES is not evident for stent diameters above 4 mm. This has not been reported in the literature as yet. Operators can therefore consider implanting BMS with large diameter without substantial safety concerns. This is especially important for patients with contraindications to long-term antiplatelet therapy.
Strengths and limitations of this study
As all patients were treated at a single centre with standardized care protocols and pathways, the effect of bias due to different treatment strategies is limited. Our cohort has large patient numbers. The long-term follow-up based on all-cause mortality and the investigation of both acute and elective cases add to the study strengths. The univariate, multivariate and propensity analysis highlights the quality of the data with well-recognized predictors of mortality associated with adverse outcome in our data set. Although this was not a randomized study the two patient groups appear well matched with respect to baseline and procedural characteristics.
There are a number of important limitations common to observational studies of this type. Importantly this study has all the limitations of a registry and all the potential bias and unmeasured confounding associated with non-randomized studies. In addition we cannot exclude the possibility of under-reporting of complications although the tracking of mortality is robust and we only included patients who had definitive mortality data in our study cohort. We cannot account for the effects of residual confounding or of selection bias caused by exclusion of 14% of patients with missing data or no NHS unique number. However, this is unlikely as the distribution of SVG disease and use of DES or BMS was the same in the excluded and analysed cohorts.
Conclusions
In this long-term observational study of PCI for SVG disease, DES was associated with a lower MACE rate than BMS due to a decreased need for repeat revascularization with no differences in rates of stent thrombosis, MI or all-cause mortality between the groups. However, for stents with a diameter above 4 mm no difference was seen between stent types. This suggests that although DES deployment in SVGs is both safe and clinically more effective than BMS for vessels ≥4 mm, BMSs are a viable alternative.
Disclosures
Financial support: No grants or funding have been received for this study.
Conflict of interest: None.
References
- 1.Kastrati A, Mehilli J, Pache J et al. Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N Engl J Med. 2007;356(10):1030–1039. doi: 10.1056/NEJMoa067484. [DOI] [PubMed] [Google Scholar]
- 2.Moses JW, Leon MB, Popma JJ et al. SIRIUS Investigators. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349(14):1315–1323. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 3.Cheng JM, Onuma Y, Piazza N, Nuis RJ, Van Domburg RT, Serruys PW. Interventional Cardiologists of Thoraxcenter (2000-2009). Comparison of five-year outcome of octogenarians undergoing percutaneous coronary intervention with drug-eluting versus bare-metal stents (from the RESEARCH and T-SEARCH Registries). Am J Cardiol. 2010;106(10):1376–1381. doi: 10.1016/j.amjcard.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Weisz G, Leon MB, Holmes DR, Jr et al. Five-year follow-up after sirolimus-eluting stent implantation results of the SIRIUS (Sirolimus-Eluting Stent in De-Novo Native Coronary Lesions) Trial. J Am Coll Cardiol. 2009;53(17):1488–1497. doi: 10.1016/j.jacc.2009.01.050. [DOI] [PubMed] [Google Scholar]
- 5.Paradis JM, Bélisle P, Joseph L et al. Drug-eluting or bare metal stents for the treatment of saphenous vein graft disease: a Bayesian meta-analysis. Circ Cardiovasc Interv. 2010;3(6):565–576. doi: 10.1161/CIRCINTERVENTIONS.110.949735. [DOI] [PubMed] [Google Scholar]
- 6.Brilakis ES, Wang TY, Rao SV et al. Frequency and predictors of drug-eluting stent use in saphenous vein bypass graft percutaneous coronary interventions: a report from the American College of Cardiology National Cardiovascular Data CathPCI registry. JACC Cardiovasc Interv. 2010;3(10):1068–1073. doi: 10.1016/j.jcin.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 7.John LC. Biomechanics of coronary artery and bypass graft disease: potential new approaches. Ann Thorac Surg. 2009;87(1):331–338. doi: 10.1016/j.athoracsur.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Ribichini F, Pugno F, Ferrero V et al. Long-term histological and immunohistochemical findings in human venous aorto-coronary bypass grafts. Clin Sci (Lond) 2008;114(3):211–220. doi: 10.1042/CS20070243. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann R, Mintz GS, Popma JJ et al. Chronic arterial responses to stent implantation: a serial intravascular ultrasound analysis of Palmaz-Schatz stents in native coronary arteries. J Am Coll Cardiol. 1996;28(5):1134–1139. doi: 10.1016/S0735-1097(96)00278-1. [DOI] [PubMed] [Google Scholar]
- 10.Depre C, Havaux X, Wijns W. Pathology of restenosis in saphenous bypass grafts after long-term stent implantation. Am J Clin Pathol. 1998;110(3):378–384. doi: 10.1093/ajcp/110.3.378. [DOI] [PubMed] [Google Scholar]
- 11.Bittl JA. Drug-eluting stents for saphenous vein graft lesions: the limits of evidence. J Am Coll Cardiol. 2009;53(11):929–930. doi: 10.1016/j.jacc.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Dangas G, Ellis SG, Shlofmitz R et al.; TAXUS-IV Investigators. Outcomes of paclitaxel-eluting stent implantation in patients with stenosis of the left anterior descending coronary artery. J Am Coll Cardiol. 2005;45(8):1186–1192. doi: 10.1016/j.jacc.2004.10.077. [DOI] [PubMed] [Google Scholar]
- 13.Applegate RJ, Sacrinty M, Kutcher M, Santos R, Gandhi S, Little W. Late outcomes of drug-eluting versus bare metal stents in saphenous vein grafts: propensity score analysis. Catheter Cardiovasc Interv. 2008;72(1):7–12. doi: 10.1002/ccd.21566. [DOI] [PubMed] [Google Scholar]
- 14.Brodie BR, Wilson H, Stuckey T et al.; STENT Group. Outcomes with drug-eluting versus bare-metal stents in saphenous vein graft intervention results from the STENT (strategic transcatheter evaluation of new therapies) group. JACC Cardiovasc Interv. 2009;2(11):1105–1112. doi: 10.1016/j.jcin.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 15.Ko DT, Guo H, Wijeysundera HC et al. Long-term safety and effectiveness of drug-eluting stents for the treatment of saphenous vein grafts disease: a population-based study. JACC Cardiovasc Interv. 2011;4(9):965–973. doi: 10.1016/j.jcin.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Cox JL, Chiasson DA, Gotlieb AI. Stranger in a strange land: the pathogenesis of saphenous vein graft stenosis with emphasis on structural and functional differences between veins and arteries. Prog Cardiovasc Dis. 1991;34(1):45–68. doi: 10.1016/0033-0620(91)90019-i. [DOI] [PubMed] [Google Scholar]
- 17.Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation. 1998;97(9):916–931. doi: 10.1161/01.cir.97.9.916. [DOI] [PubMed] [Google Scholar]
- 18.Halabi AR, Alexander JH, Shaw LK et al. Relation of early saphenous vein graft failure to outcomes following coronary artery bypass surgery. Am J Cardiol. 2005;96(9):1254–1259. doi: 10.1016/j.amjcard.2005.06.067. [DOI] [PubMed] [Google Scholar]
- 19.Mehilli J, Pache J, Abdel-Wahab M et al.; Is Drug-Eluting-Stenting Associated with Improved Results in Coronary Artery Bypass Grafts? (ISAR-CABG) Investigators. Drug-eluting versus bare-metal stents in saphenous vein graft lesions (ISAR-CABG): a randomised controlled superiority trial. Lancet. 2011;378(9796):1071–1078. doi: 10.1016/S0140-6736(11)61255-5. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez-Recalde A, Jiménez Valero S, Moreno R et al. Safety and efficacy of drug-eluting stents versus bare-metal stents in saphenous vein grafts lesions: a meta-analysis. EuroIntervention. 2010;6(1):149–160. [PubMed] [Google Scholar]
- 21.Taniwaki M, Räber L, Magro M et al. Long-term comparison of everolimus-eluting stents with sirolimus- and paclitaxel-eluting stents for percutaneous coronary intervention of saphenous vein grafts. EuroIntervention. 2014;9(12):1432–1440. doi: 10.4244/EIJV9I12A241. [DOI] [PubMed] [Google Scholar]
- 22.Cutlip DE, Baim DS, Ho KK et al. Stent thrombosis in the modern era: a pooled analysis of multicenter coronary stent clinical trials. Circulation. 2001;103(15):1967–1971. doi: 10.1161/01.cir.103.15.1967. [DOI] [PubMed] [Google Scholar]
- 23.Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007;356(10):1020–1029. doi: 10.1056/NEJMoa067731. [DOI] [PubMed] [Google Scholar]
- 24.Urban P, Gershlick AH, Guagliumi G et al.; e-Cypher Investigators. Safety of coronary sirolimus-eluting stents in daily clinical practice: one-year follow-up of the e-Cypher registry. Circulation. 2006;113(11):1434–1441. doi: 10.1161/CIRCULATIONAHA.104.532242. [DOI] [PubMed] [Google Scholar]
- 25.Ong AT, Hoye A, Aoki J et al. Thirty-day incidence and six-month clinical outcome of thrombotic stent occlusion after bare-metal, sirolimus, or paclitaxel stent implantation. J Am Coll Cardiol. 2005;45(6):947–953. doi: 10.1016/j.jacc.2004.09.079. [DOI] [PubMed] [Google Scholar]


