Abstract
Background
Thrombolytic therapy with recombinant tissue plasminogen activator (rtPA) remains the only approved medication for acute ischemic stroke, but incurs significant bleeding risks. Therefore, approaches to combine lower doses of thrombolytic therapy with other effective drugs aim at improving efficacy and reducing bleeding rates. We examined the safety and therapeutic effects of various dosings of rtPA, either alone or combined with glycoprotein VI-Fc fusion protein (GPVI-Fc, Revacept) on experimental stroke in mice.
Methods and results
The effect of filament-induced intracerebral thrombus formation and embolization was investigated after a one-hour occlusion of the middle cerebral artery.
In accordance with previous studies, treatment with 10 mg/kg rtPA significantly improved functional outcome, cerebral infarct size and edema, but also resulted in markedly increased intracranial bleeding volumes. In contrast, low doses of rtPA (0.1 or 0.35 mg/kg body weight) did not change outcome parameters. However, addition of 1 mg/kg Revacept to 0.35 mg/kg rtPA led to improved reperfusion compared to rtPA alone. Moreover, these combined treatments resulted in improved grip strength, compared to the respective dose of rtPA alone. Infarct-surrounding edema improved after combined treatments, but not after respective single rtPA dosings. Intracranial bleeding volumes were below controls after all low-dose rtPA therapies, given either alone or combined with Revacept.
Conclusions
In contrast to using the equally effective full dose of rtPA, intracranial bleeding was not increased by low-dose rtPA combined with Revacept. Therefore, addition of Revacept to low-dose rtPA does not incur safety risks, but improves efficacy of treatment.
Keywords: Glycoprotein VI, Middle cerebral artery occlusion, Platelet aggregation, Stroke
Introduction
Ischemic stroke is the most frequent disabling disease and a leading cause of death above the age of 60 years (1). Among the most frequent causes is rupture of atherosclerotic plaques which leads to platelet adhesion and thrombus formation and/or embolization in cerebral arteries.
Recombinant tissue plasminogen activator (rtPA) remains the only approved therapy of acute ischemic stroke (2). Extensive clinical research has resulted in the use of rtPA for an extended time window of 4.5 hours after start of symptoms (3). However, even with fast reperfusion, a second wave of embolic events and inflammatory alterations may lead to reperfusion injury and progressive stroke (4).
Several studies investigated the use of rtPA in stroke models in rodents. Mostly, doses of 6-10 mg/kg body weight were used to treat stroke induced by occlusion of the middle cerebral artery (MCAO) in rats (5, 6). Similarly, embolic clot-induced stroke in mice after local injection of thrombin (7) was treated with doses of 10 mg/kg rtPA (8, 9). Embolic stroke was treated with 20 mg/kg in rats (6). In rats, it was also shown that 0.9 mg/kg rtPA results in some efficacy to treat MCAO, albeit less than the full rodent dose of 10 mg/kg (10). Kilic et al (11) used various doses of rtPA, ranging from 0.2 to 10 mg/kg, in the mouse MCAO model. In their studies, rtPA provoked complex hemodynamic changes which may even result in increased infarct sizes. This was in accordance with an earlier report (12). The topic was discussed in following reports – for example, investigation of tPA-/- knockout mice showed increased infarct sizes (13). Some of the damages seen with rtPA may be associated with differential kinase activation (14).
Additionally, low-dose rtPA was combined with additional drugs, testing the hypothesis that this would allow for more efficient therapy and reduced complications. A special focus was on the use of anti-von Willebrand factor (vWF) antibodies: Addition of the nanobody ALX-0081 to reduced dose rtPA (0.32 mg/kg) exerted a beneficial effect, producing comparable outcomes to full-dose rtPA after MCAO in guinea pigs (15). Addition of the antibody AJW200 (which blocks the vWF-GPIb interaction) to low-dose rtPA (0.9 mg/kg) also led to improved functional outcomes in rabbits (16).
Glycoprotein VI (GPVI) is the major signaling receptor for collagen and exclusively expressed on platelets and megakaryocytes initiating platelet recruitment at sites of vascular injury (17, 18). GPVI-mediated platelet adhesion and activation play an important role in thrombus formation and subsequent development of stroke and could be a target for pharmacological inhibition of pathological thrombus formation (18, 19). Blocking of GPVI with specific antibodies led to a reduced infarct volume and a significantly improved functional outcome in an acute stroke model in mice with one hour occlusion of the middle cerebral artery (MCA) (20). These animals did not show any increased incidence of intracranial hemorrhage nor prolonged tail bleeding time.
Inhibition of GPVI-mediated platelet activation can also be achieved by injecting the soluble GPVI receptor Revacept, a dimeric soluble GPVI-Fc fusion protein. Bleeding time was not altered when Revacept was combined with a number of other platelet inhibitors or anticoagulants, even in triple therapy (21). In a clinical phase I study, it was shown to be a safe and well-tolerated new antiplatelet compound with a clear dose-dependent pharmacokinetic profile. Revacept led to an inhibition of platelet aggregation but unaltered general hemostasis in all subjects (22).
Our previous study (23) showed that administration of 1 mg/kg body weight (bw) Revacept after stroke in mice results in reduced infarct size and improves functional outcome. In the same study, we also demonstrated GPVI-Fc inhibits the platelet activation which is exerted by collagen-bound vWF (23), and that a high dose of rtPA (10 mg/kg bw) improved functional outcome and infarct volumes. The current study now tests the hypothesis that the necessary doses of rtPA can be lowered, if Revacept is given concomitantly. Based on the hypothesis that a combination of a reduced dose of thrombolytic therapy together with a strong inhibitor of platelet adhesion might allow for an improved risk–benefit profile, we tested markedly lower doses of 0.1 mg/kg or 0.35 mg/kg rtPA (as taken from ref. 15) in combination with GPVI-Fc (Revacept) for its effect on cerebral damage and outcome after experimental arterial thrombosis in stroke mice.
Methods
Experimental groups and materials used
Revacept, a dimeric soluble GPVI-Fc protein, was produced as previously described (19). The Fc part from human immunoglobulin G served as a control (19). rtPA (Actilyse) was from Boehringer Mannheim (Roche, Germany). Eight animals were included in each of the intervention groups (either 0.1, 0.35 or 10 mg/kg rtPA, or the combinations of either 0.1 or 0.35 mg/kg rtPA with 1 mg/kg Revacept). As controls, 17 animals received Fc only. All parameters were measured in all animals of each group, and results reflect the mean values of these measurements.
Induction of ischemic stroke
Experiments of this study were specifically approved by the Institutional Animal Care and Use Committee (local animal welfare authority) at the government of Upper Bavaria in Munich, Germany (reference number 55.2-1-54-2531-98-09). About 6- to 9-week-old male C57Bl/6J mice weighing 21-26 g were used (Charles River, Sulzfeld, Germany) and housed under standard conditions with a 12-hour diurnal cycle and free access to food and water.
Cerebral infarction and neurological function/motor activity after cerebral ischemia were assessed in mice after occlusion of the left MCA. One hour ischemia was induced by placing a silicon-coated monofilament (Doccol, USA) in the MCA via the left common/internal carotid artery as described by Hata et al (24). Flow reduction in the MCA was monitored with a laser Doppler flow probe attached to the left temporal skull. At the beginning of reperfusion, Revacept, Fc only or rtPA or combinations of those were injected via the tail vein. After reperfusion times of 4 hours or 24 hours, mice underwent evaluation for neurological and motor dysfunction, as detailed below. A total of 24 hours after reperfusion of the MCA, mice were sacrificed, the brain was removed and the infarct areas were investigated. The histological investigator was blinded to all interventions. Additionally, brains were investigated for intracerebral hemorrhage by a spectrophotometric assay.
During all interventions, anesthesia was induced with Medetomidine 0.5 mg/kg (Domitor®, Janssen-Cilag GmbH), midazolam 5 mg/kg (Dormicum®, Roche) and fentanyl 0.05 mg/kg (Fentanyl®-Janssen, Janssen-Cilag GmbH) and maintained with isoflurane 0.2-0.8% (Isofluran CP®, CP-Pharma). Analgesia was achieved with 200 mg/kg Metamizol p.o. (Novalgin®, Sanofi-Aventis) three times within 24 hours.
Morphological and functional outcome after ischemic stroke
Assessment of neurological function and motor function was performed before MCAO, and 4 and 24 hours after reperfusion of the MCA. The motor function was evaluated with a grip strength test (Bio-GS3, Bioseb, France) in five consecutive measurements. The mean value of these measurements was determined and the percentage change compared to the value before surgery was determined. The neurological function was assessed with a modified Bederson score (25): no spontaneous movement was scored as 4 points, circling as 3, decreased resistance to lateral push without circling as 2, forelimb flexion to one side at tail lifting as 1 and no deficit as 0.
Assessment of brain morphology was performed in mice 24 hours after MCAO. The histological investigator was blinded to all interventions. After lethal anesthesia of the mice, brains were quickly removed and seven 1-mm-thick coronal sections were cut starting from the frontal pole with a mouse brain slice matrix (Cat BSM001.1, Zivic Lab Inc) (4°C). Infarct area was visualized by staining with 2% 2,3,5-triphenyltetrazolium chloride (Sigma Aldrich No 93140) buffered in phosphate-buffered saline (Biochrom AG) with pH 7.6-7.4 at room temperature for 30 minutes. Brain slices were digitally photographed and the infarct size was quantified by image analysis software (Photoshop® CS5, Adobe) by researchers blinded to the treatment groups.
The hemoglobin content of brains was quantified with a spectrophotometric assay 24 hours after MCA occlusion. In brief, frozen brain tissue was homogenized on dry ice and consecutively dissolved with distilled water. After centrifugation, the hemoglobin-containing supernatant was collected, 80 µL of Drabkin’s reagent (Sigma) was added to a 20 µL aliquot. Cyano-methemoglobin with an absorbance peak at 540 nm was determined by measuring the optical density of the solution at ≈550 nm wavelength. The absorption values of mouse brain preparations were compared to a standard absorbance curve for quantification. This standard curve had been calculated from measurements using increasing volumes of mouse blood samples spiked with native brain tissue.
Statistical methods
Results are presented as mean ± standard errors of the mean (SEM). Statistical analysis was performed using a one-way analysis of variance (ANOVA) followed by a post hoc analysis for multiple comparisons (Fisher’s least significant difference test), where appropriate.
Results
Measurement of perfusion during and after MCAO
Blood flow in the MCA was recorded during one hour occlusion and 30 minutes reperfusion. After MCA intervention, the flow was constantly reduced by >80% during the occlusion time, as described before (23). There were no flow differences between the groups during the occlusion time. With the onset of reperfusion, a standard dose of 10 mg/kg rtPA, or low doses (0.1 or 0.35 mg/kg rtPA) either alone or combined with 1 mg/kg Revacept or the equimolar amount of Fc only were slowly injected into the tail vein. In accordance with our previous study (23), 10 mg/kg rtPA did not result in a significant effect on reperfusion flow (119.8 ± 18% at the final measurement at 30 minutes, vs. 120 ± 10% in Fc only controls, corresponding fairly well to the combined administration of 10 mg/kg rtPA and 200 IE heparin in our previous publication – compare with Figure 3 of ref. 23). Figure 1 shows intracerebral flows after administration of low doses of rtPA. During reperfusion, no effects of the administrations of either 0.1 or 0.35 mg/kg rtPA alone were observed – both treatments resulted in comparable flow patterns. In contrast, adding 1 mg/kg Revacept to 0.35 mg/kg rtPA resulted in sustained markedly better reperfusion flow.
Fig. 3 -.
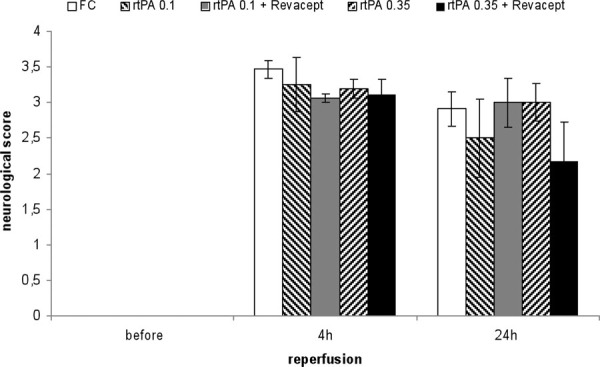
Assessment of functional outcome 24 hours after reperfusion following a 1 hour transient middle cerebral artery occlusion. The modified neurological Bederson score (0 = no deficit, 1 = forelimb flexion, 2 = decreased resistance to lateral push without circling, 3 = circling, 4 = no spontaneous movement) was compared in mice treated with Fc only, or rtPA or combinations of Revacept and rtPA, at the indicated doses. The Bederson score is shown as mean ± SEM, n = 8 per group (except 17 animals in the Fc group). rtPA = recombinant tissue plasminogen activator; SEM = standard error of the mean.
Fig. 1 -.
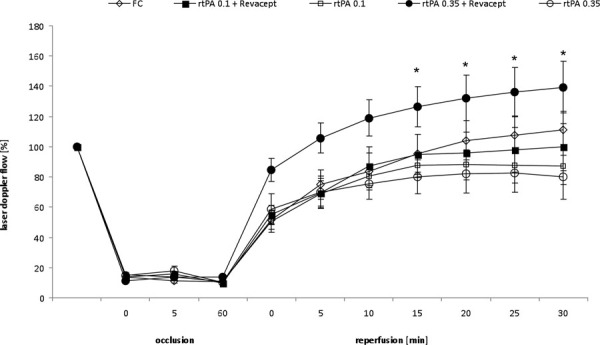
Flow in the middle cerebral artery (MCA) was measured after insertion of the catheter/filament, during occlusion of the common carotid artery (=100%), and during 30 minutes of reperfusion, as detected by a flexible Laser Doppler flow probe attached to the temporal skull. A total of 1 mg/kg body weight Revacept, or the equimolar amount of Fc only (0.33 mg/kg), or rtPA (0.1 or 0.35 mg/kg) or combinations of these doses of Revacept and rtPA were injected intravenously during reperfusion of the MCA. Mean ± SEM are given, n = 8 per group (except 17 animals in the Fc group). *Statistical significance of p<0.05 compared to the same dose of rtPA only. rtPA = recombinant tissue plasminogen activator; SEM = standard error of the mean.
Effect of rtPA and revacept on functional outcome in mice after stroke induced by MCAO
Functional outcome was assessed 4 and 24 hours after the onset of reperfusion. Figure 2 shows that the combination of Revacept with either 0.1 or 0.35 mg/kg rtPA resulted in improved grip strength compared to that with the same dose of rtPA only 4 hours after stroke. Grip strength after combined administration of Revacept and 0.35 mg/kg rtPA amounted to similar values as those observed after a full dose of 10 mg/kg rtPA: 46 ± 7% after 4 hours and 80 ± 8% after 24 hours, corresponding again to the combined administration of 10 mg/kg rtPA and 200 IE heparin in our previous publication (23).
Fig. 2 -.
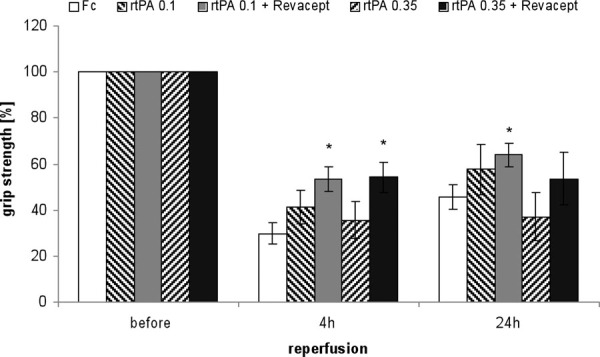
Assessment of functional outcome 24 hours after reperfusion following a 1 hour transient middle cerebral artery occlusion. The grip strength was compared in mice treated with Fc only, or rtPA or combinations of Revacept and rtPA, at the indicated doses. The mean change in grip strength in percent compared to the values before surgery and induction of stroke (100%) is shown as mean ± SEM, n = 8 per group (except 17 animals in the Fc group). *Significant differences of p<0.05, compared to the same dose of rtPA only, or the group treated with Fc only. rtPA = recombinant tissue plasminogen activator; SEM = standard error of the mean.
The results for the neurological Bederson scores are shown in Figure 3. There were no statistically significant differences between the groups (as determined by ANOVA), but we observed a trend toward better functional outcome (=lower score) in neurological function at 24 hours for the combination of Revacept with 0.35 mg/kg rtPA, compared to the same dose of rtPA alone (p = 0.17). Again, the neurological score observed after combined administration of Revacept and 0.35 mg/kg rtPA amounted to similar values as those observed after a full dose of 10 mg/kg rtPA (3.31 ± 0.27 after 4 hours, 2.31 ± 0.43 after 24 hours – corresponding to the effects observed after combined administration of 10 mg/kg rtPA and 200 IE heparin in our previous publication) (23).
Morphological effects on ischemic cerebral stroke by MCAO
After MCAO, the cerebral infarct volume of mice after treatment with 0.35 mg/kg rtPA together with Revacept tended toward lower values, compared to either 0.1 or 0.35 mg/kg rtPA alone (Fig. 4).
Fig. 4 -.
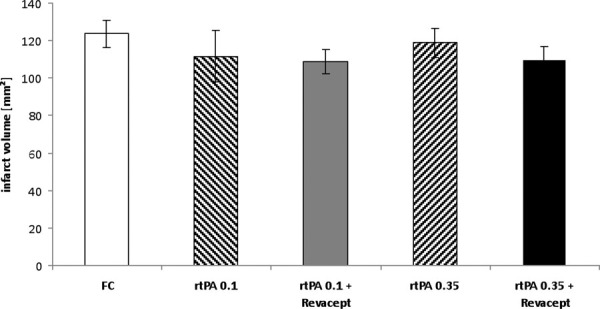
Assessment of infarct volumes by postmortem 2,3,5-triphenyltetrazolium chloride staining in brain slices from mice 24 hours after reperfusion following a 1 hour transient middle cerebral artery occlusion. Bars show mean infarct volumes of mice treated with Fc only, or rtPA or combinations of Revacept and rtPA, at the indicated doses. Mean ± SEM are given, n = 8 per group (except 17 animals in the Fc group). rtPA = recombinant tissue plasminogen activator; SEM = standard error of the mean.
Similarly, intracerebral edema volumes tended toward lower values after administration of Revacept together with 0.35 mg/kg rtPA compared to the administration of the respective doses of rtPA alone in these mice with ischemic stroke (Fig. 5). Consequently, the combination of 1 mg/kg Revacept with either 0.1 or 0.35 mg/kg rtPA resulted in a statistically significant effect compared to the control Fc only group, whereas the administration of the respective doses of rtPA alone did not.
Fig. 5 -.
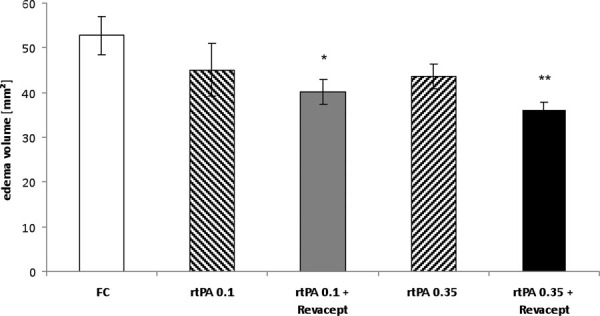
Assessment of intracerebral edema volume by postmortem 2,3,5-triphenyltetrazolium chloride staining in brain slices from mice 24 hours after reperfusion following a 1 hour transient middle cerebral artery occlusion. Bars show mean edema volumes of mice treated with Fc only, or rtPA or combinations of Revacept and rtPA, at the indicated doses. Mean ± SEM are given. *Significant differences of p<0.05, and **p<0.01, compared to the group treated with Fc only. rtPA = recombinant tissue plasminogen activator; SEM = standard error of the mean.
Also treatment with 10 mg/kg rtPA resulted in improvements of infarct volumes (to 68 ± 17 vs. 123 ± 7 µL in Fc only controls) and edema volumes (25 ± 4.5 vs. 52 ± 4 µL in Fc controls).
Effects on ischemic cerebral bleeding volumes
Upon measuring cyano-methemoglobin content in minced mouse brain sections, no signs of increased intracranial hemorrhage after Revacept treatment occurred, as assessed 24 hours after MCA occlusion and stroke in a previous study (23): There was no difference between the groups treated with saline, up to 1 mg/kg Revacept or Fc only. In contrast, treatment with 10 mg/kg rtPA led to a marked increase in intracerebral bleeding volume to 11.7 ± 3 vs. 2.0 ± 0.83 µL in the Fc only control group, corresponding to the combined administration of 10 mg/kg rtPA and 200 IE heparin in our previous publication (23). The current study showed that administration of low doses of 0.1 or 0.35 mg/kg rtPA alone or in a combination with Revacept did not result in any excess bleeding. The mean bleeding volumes did not exceed the values measured in the control group: 1.38 ± 0.45 µL for rtPA 0.1 mg/kg alone, 0.037 ± 0.03 µL for the combination of rtPA 0.1 and Revacept, 0.23 ± 0.08 µL for 0.35 mg/kg rtPA and 1.16 ± 0.73 µL for rtPA 0.35 + Revacept.
Discussion
In this study, we demonstrate that combining 1 mg/kg Revacept (recombinant dimeric GPVI-Fc) with a low dose of 0.35 mg/kg rtPA leads to significantly improved flow patterns during reperfusion. Investigation of neurological function (grip test) showed significant improvement for the combined treatments compared to the respective single rtPA doses at short term, and trends toward improvement upon longer observation. Some discrepancy between the functional data and those of flow measurements became obvious, since flow improved only after combined therapy with 0.35 mg/kg rtPA, whereas grip strength improved with both low rtPA doses. Histological investigation of cerebral infarction showed that surrounding edema volumes were significantly improved after combination therapy with Revacept, but not after single application of low doses of rtPA.
In summary, efficacy parameters of low-dose rtPA with Revacept equal those obtained after single full dose of rtPA on a numerical basis. In contrast to the equally effective treatment with full-dose rtPA, this anti-ischemic effect is, however, achieved without increasing the risk of intracerebral hemorrhage.
GPVI-mediated platelet activation is effectively inhibited by the soluble GPVI fusion protein GPVI-Fc (Revacept). Administration of Revacept led to a reduction in platelet adhesion to the injured vessel wall in healthy mice (19) as well as in cholesterol-fed ApoE-/- mice (19, 26) and reduced neointima formation (26), and exerts an inhibitory effect on platelet–collagen interaction in an arterial wall injury model (23). Revacept also led to an improvement in motor function, reduction in infarct volume and edema in ischemic stroke, without an increased risk of intracerebral hemorrhage (23).
In a previous phase I study in humans, Revacept was proven safe with regard to bleeding time, general coagulation and platelet counts in healthy volunteers (22). Thus, the lesion-specific binding of Revacept to collagen at the injured vessel wall does not impact on the general platelet function including its receptors, and presumably does not incur a risk of bleeding complications or intracerebral hemorrhages during the treatment of stroke, since adenosine 5′-diphosphate- and thrombin-induced platelet activation was unaffected (22).
Compared to previously published results with filament MCAOs, rtPA had a strong beneficial effect on infarction volume and functional outcome. Some investigators (11, 12) found a paradoxical increase of the infarction volume in rtPA-treated mice after MCAO, whereas others (5, 7) could not confirm these findings in different ischemia models. Recently it was reported that the effect of rtPA was dramatically different between awake and anesthetized mice and that ketamine plus rtPA has largely reduced the cerebral infarct volume in MCAO in mice (8).
In conclusion, many of the benefits, risks and problems associated with rtPA therapy of stroke can be reproduced in the mouse animal model. Therefore, many experimental and clinical studies have investigated the option to mitigate the negative side effects of a therapy with rtPA, for example, by adding compounds such as activated protein C (27), metalloproteinase inhibitors (5), glutamate N-methyl-D-aspartate receptor antagonists such as memantine (28) or statins (14, 29). Alternatively, studies sought to identify combination therapies which would allow for reduced rtPA dosing. Among the most promising recent approaches, anti-vWF antibodies were used. Addition of the nanobody ALX-0081 to reduced dose rtPA (0.32 mg/kg) exerted a beneficial effect, producing comparable outcomes to full-dose rtPA after MCAO in guinea pigs (15). Addition of another anti-vWF antibody, AJW200, to low-dose rtPA (0.9 mg/kg) also led to improved functional outcomes in rabbits (16). Despite these promising preclinical results, clinical development of these compounds, however, was halted because of the inherent bleeding risks (30, 31) (http://hugin.info/137912/R/1562875/484367.pdf). As Revacept also inhibits local collagen-induced vWF activation (23), the concept of this study was to use this at least partially similar anti-vWF potency in the absence of any known bleeding risk.
Limitations of the study
There are certainly limitations of extrapolating the results of this study to the clinical setting, since the data have been obtained in an experimental model system. In contrast, treatment of human patients is always more complicated, and effects of therapies have to be reconfirmed in clinical studies.
Conclusion
If these experimental results are confirmed in clinical studies, Revacept should be a promising, effective and safe drug for the treatment of ischemic complications in stroke and reperfusion, by improving the thrombolytic efficacy of low-dose rtPA, or during intravascular cerebral interventions, such as thrombectomy, by ameliorating the low reflow phenomenon after successful recanalization.
Acknowledgment
We acknowledge the excellent technical assistance of Isabel Fodor.
Disclosures
Financial support: This study was supported by a grant from the Bavarian Research Foundation (Bayerische Forschungsstiftung), grant # 1145-14.
Conflict of interest: Drs. Reimann, Li, Göbel, Fassbender, Holthoff, Münch, Ungerer are employees of the biotech company AdvanceCOR (formerly Procorde). Meinrad Gawaz is a cofounder of AdvanceCOR, owns shares of AdvanceCOR and is Professor at the Cardiology Department of the University of Tübingen. He further received honoraria payments from Lilly, Bristol-Myers Squibb and Bayer-Schering and is also consultant for Bayer-Schering.
References
- 1.Go AS, Mozaffarian D, Roger VL et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics – 2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NINDS rtPA stroke study group. rtPA for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 3.Hacke W, Kaste M, Bluhmki E et al. ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 4.Molina CA. Reperfusion therapies for acute ischemic stroke: current pharmacological and mechanical approaches. Stroke. 2011;42(1 Suppl):S16–S19. doi: 10.1161/STROKEAHA.110.598763. [DOI] [PubMed] [Google Scholar]
- 5.Yagi K, Kitazato KT, Uno M et al. Edaravone, a free radical scavenger, inhibits MMP-9-related brain hemorrhage in rats treated with tissue plasminogen activator. Stroke. 2009;40(2):626–631. doi: 10.1161/STROKEAHA.108.520262. [DOI] [PubMed] [Google Scholar]
- 6.Eriksen N, Rasmussen RS, Overgaard K, Johansen FF, Pakkenberg B. Comparison of quantitative estimation of intracerebral hemorrhage and infarct volumes after thromboembolism in an embolic stroke model. Int J Stroke. 2014;9(6):802–810. doi: 10.1111/j.1747-4949.2012.00870.x. [DOI] [PubMed] [Google Scholar]
- 7.Orset C, Macrez R, Young AR et al. Mouse model of in situ thromboembolic stroke and reperfusion. Stroke. 2007;38(10):2771–2778. doi: 10.1161/STROKEAHA.107.487520. [DOI] [PubMed] [Google Scholar]
- 8.Gakuba C, Gauberti M, Mazighi M, Defer G, Hanouz JL, Vivien D. Preclinical evidence toward the use of ketamine for recombinant tissue-type plasminogen activator-mediated thrombolysis under anesthesia or sedation. Stroke. 2011;42(10):2947–2949. doi: 10.1161/STROKEAHA.111.620468. [DOI] [PubMed] [Google Scholar]
- 9.García-Yebenes I, Sobrado M, Zarruk JG et al. A mouse model of hemorrhagic transformation by delayed tPA administration after in situ thromboembolic stroke. Stroke. 2011;42:196–203. doi: 10.1161/STROKEAHA.110.600452. [DOI] [PubMed] [Google Scholar]
- 10.Haelewyn B, Risso JJ, Abraini JH. Human recombinant tissue-plasminogen activator (alteplase): why not use the ‘human’ dose for stroke studies in rats? J Cereb Blood Flow Metab. 2010;30(5):900–903. doi: 10.1038/jcbfm.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kilic E, Bähr M, Hermann DM. Effects of recombinant tissue plasminogen activator after intraluminal thread occlusion in mice: role of hemodynamic alterations. Stroke. 2001;32(11):2641–2647. doi: 10.1161/hs1101.097381. [DOI] [PubMed] [Google Scholar]
- 12.Wang YF, Tsirka SE, Strickland S, Stieg PE, Soriano SG, Lipton SA. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat Med. 1998;4(2):228–231. doi: 10.1038/nm0298-228. [DOI] [PubMed] [Google Scholar]
- 13.Tabrizi P, Wang L, Seeds N et al. Tissue plasminogen activator (tPA) deficiency exacerbates cerebrovascular fibrin deposition and brain injury in a murine stroke model: studies in tPA-deficient mice and wild-type mice on a matched genetic background. Arterioscler Thromb Vasc Biol. 1999;19(11):2801–2806. doi: 10.1161/01.atv.19.11.2801. [DOI] [PubMed] [Google Scholar]
- 14.Kilic E, Kilic Ü, Matter CM, Lüscher TF, Bassetti CL, Hermann DM. Aggravation of focal cerebral ischemia by tPA is reversed by 3-HMGCoA reductase inhibitor but does not depend on eNOS. Stroke. 2005;36:332–336. doi: 10.1161/01.STR.0000152273.24063.f7. [DOI] [PubMed] [Google Scholar]
- 15.Momi S, Tantucci M, Van Roy M, Ulrichts H, Ricci G, Gresele P. Reperfusion of cerebral artery thrombosis by the GPIb-VWF blockade with the Nanobody ALX-0081 reduces brain infarct size in guinea pigs. Blood. 2013;121(25):5088–5097. doi: 10.1182/blood-2012-11-464545. [DOI] [PubMed] [Google Scholar]
- 16.Lapchak PA, Doyan S, Fan X, Woods CM. Synergistic effect of AJW200, a von Willebrand factor neutralizing antibody with low dose (0.9 mg/kg) thrombolytic therapy following embolic stroke in rabbits. J Neurol Neurophysiol. 2013;4(2):2. doi: 10.4172/2155-9562.1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nieswandt B, Watson SP. Platelet-collagen interaction: is GPVI the central receptor? Blood. 2003;102(2):449–461. doi: 10.1182/blood-2002-12-3882. [DOI] [PubMed] [Google Scholar]
- 18.Massberg S, Gawaz M, Grüner S et al. A crucial role of glycoprotein VI for platelet recruitment to the injured arterial wall in vivo. J Exp Med. 2003;197(1):41–49. doi: 10.1084/jem.20020945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Massberg S, Konrad I, Bültmann A et al. Soluble glycoprotein VI dimer inhibits platelet adhesion and aggregation to the injured vessel wall in vivo. FASEB J. 2004;18(2):397–399. doi: 10.1096/fj.03-0464fje. [DOI] [PubMed] [Google Scholar]
- 20.Kleinschnitz C, Pozgajova M, Pham M, Bendszus M, Nieswandt B, Stoll G. Targeting platelets in acute experimental stroke: impact of glycoprotein Ib, VI, and IIb/IIIa blockade on infarct size, functional outcome, and intracranial bleeding. Circulation. 2007;115(17):2323–2330. doi: 10.1161/CIRCULATIONAHA.107.691279. [DOI] [PubMed] [Google Scholar]
- 21.Ungerer M, Li Z, Baumgartner C et al. The GPVI-Fc fusion protein Revacept reduces thrombus formation and improves vascular dysfunction in atherosclerosis without any impact on bleeding times. PLoS One. 2013;8(8):e71193. doi: 10.1371/journal.pone.0071193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ungerer M, Rosport K, Bültmann A et al. Novel antiplatelet drug revacept (Dimeric Glycoprotein VI-Fc) specifically and efficiently inhibited collagen-induced platelet aggregation without affecting general hemostasis in humans. Circulation. 2011;123(17):1891–1899. doi: 10.1161/CIRCULATIONAHA.110.980623. [DOI] [PubMed] [Google Scholar]
- 23.Goebel S, Li Z, Vogelmann J et al. The GPVI-Fc fusion protein Revacept improves cerebral infarct volume and functional outcome in stroke. PLoS One. 2013;8(7):e66960. doi: 10.1371/journal.pone.0066960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hata R, Maeda K, Hermann D, Mies G, Hossmann KA. Evolution of brain infarction after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2000;20(6):937–946. doi: 10.1097/00004647-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17(3):472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 26.Bültmann A, Li Z, Wagner S et al. Impact of glycoprotein VI and platelet adhesion on atherosclerosis – a possible role of fibronectin. J Mol Cell Cardiol. 2010;49(3):532–542. doi: 10.1016/j.yjmcc.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Zhang Z, Chow N et al. An activated protein C analog with reduced anticoagulant activity extends the therapeutic window of tissue plasminogen activator for ischemic stroke in rodents. Stroke. 2012;43(9):2444–2449. doi: 10.1161/STROKEAHA.112.658997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montagne A, Hébert M, Jullienne A et al. Memantine improves safety of thrombolysis for stroke. Stroke. 2012;43(10):2774–2781. doi: 10.1161/STROKEAHA.112.669374. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Chopp M, Jia L, Cui Y, Lu M, Zhang ZG. Atorvastatin extends the therapeutic window for tPA to 6 h after the onset of embolic stroke in rats. J Cereb Blood Flow Metab. 2009;29(11):1816–1824. doi: 10.1038/jcbfm.2009.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ungerer M, Münch G. Novel antiplatelet drugs in clinical development. Thromb Haemost. 2013;110(5):868–875. doi: 10.1160/TH13-02-0084. [DOI] [PubMed] [Google Scholar]
- 31.Markus HS, McCollum C, Imray C, Goulder MA, Gilbert J, King A. The von Willebrand inhibitor ARC1779 reduces cerebral embolization after carotid endarterectomy: a randomized trial. Stroke. 2011;42(8):2149–2153. doi: 10.1161/STROKEAHA.111.616649. [DOI] [PubMed] [Google Scholar]


