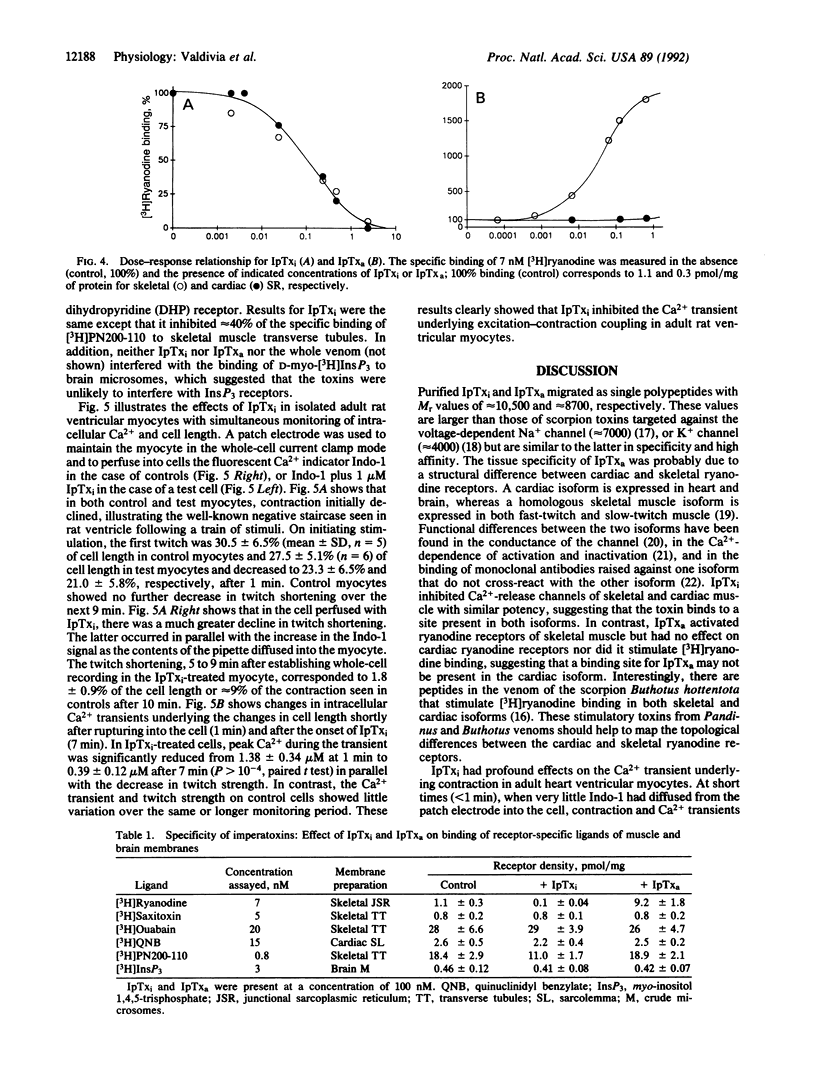
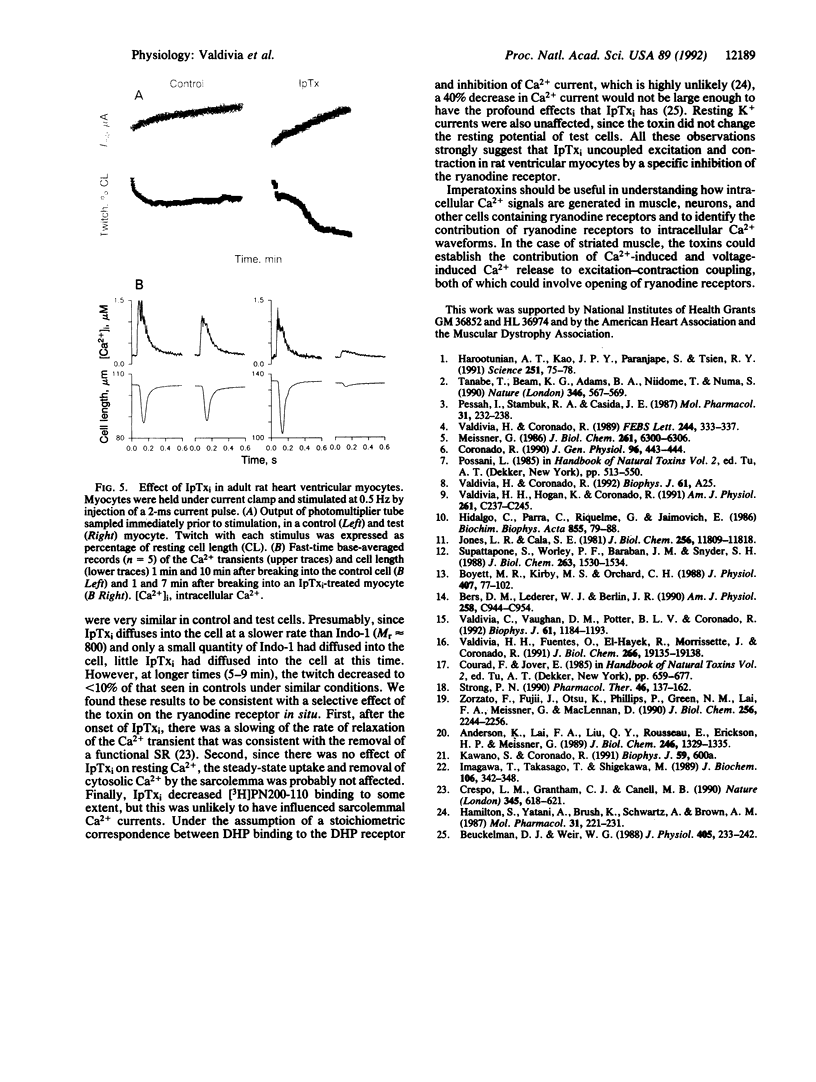
Abstract
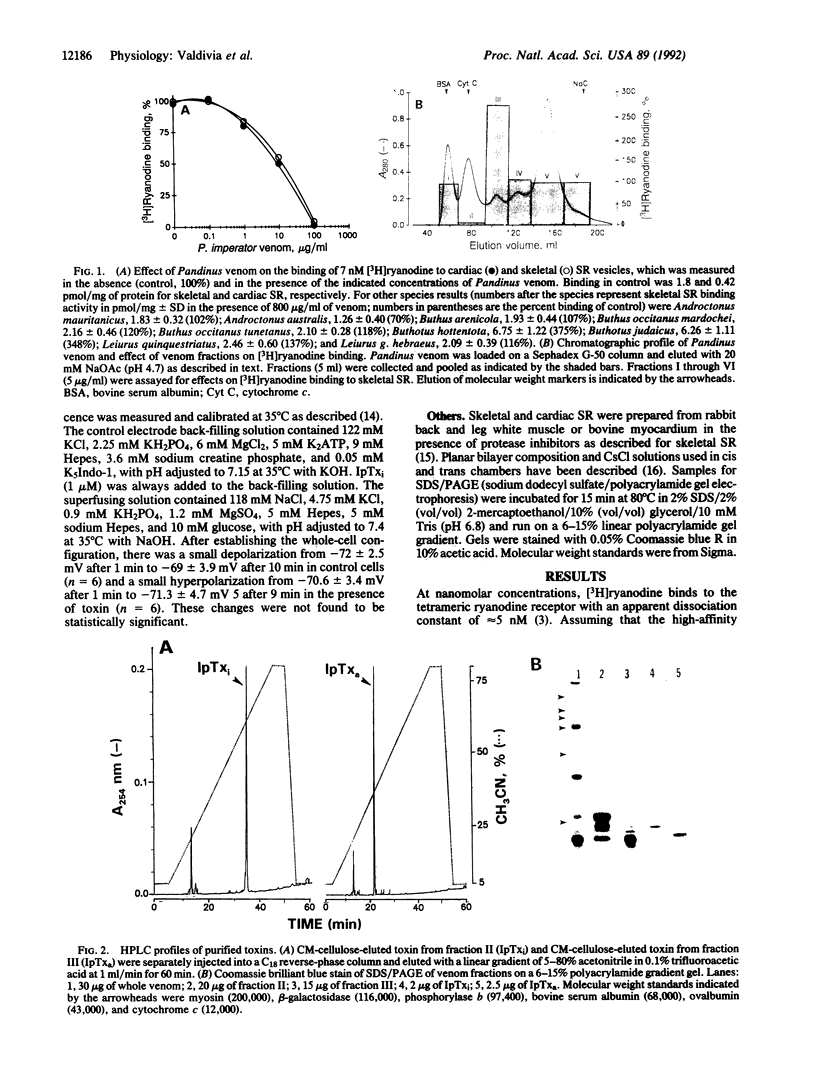
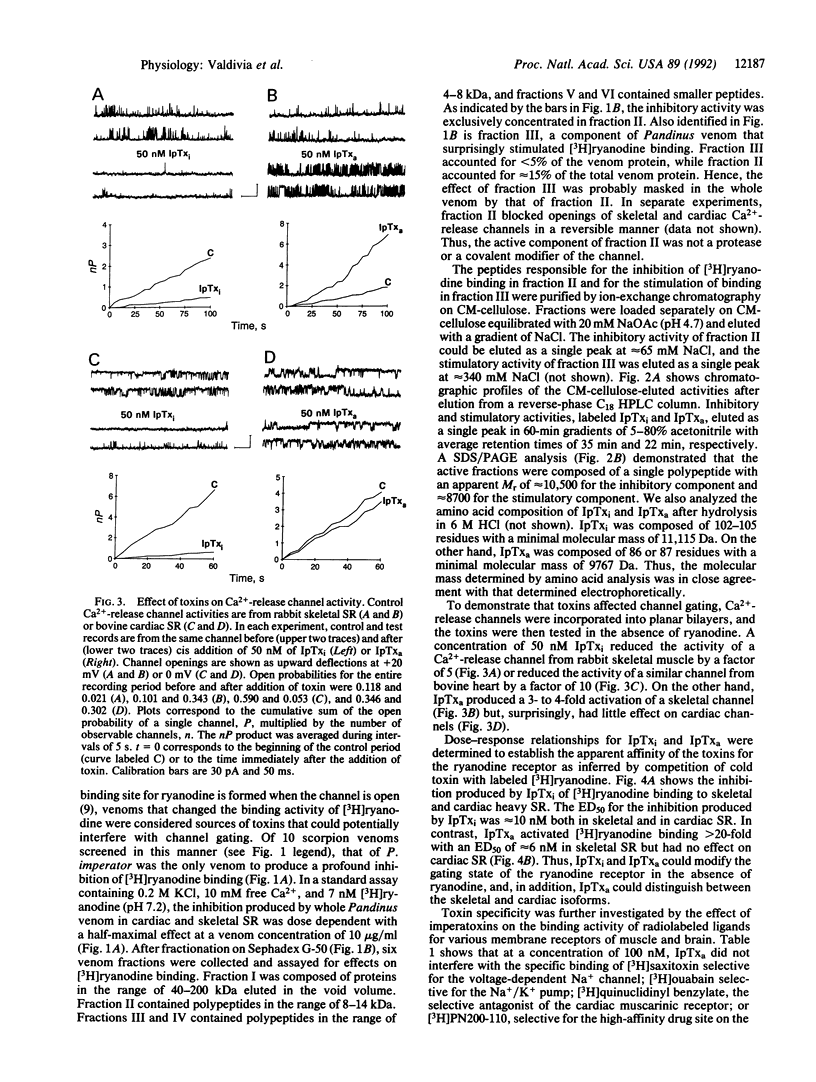
We report the purification of two peptides, called "imperatoxin inhibitor" and "imperatoxin activator," from the venom of the scorpion Pandinus imperator targeted against ryanodine receptor Ca(2+)-release channels. Imperatoxin inhibitor has a M(r) of approximately 10,500, inhibits [3H]ryanodine binding to skeletal and cardiac sarcoplasmic reticulum with an ED50 of approximately 10 nM, and blocks openings of skeletal and cardiac Ca(2+)-release channels incorporated into planar bilayers. In whole-cell recordings of cardiac myocytes, imperatoxin inhibitor decreased twitch amplitude and intracellular Ca2+ transients, suggesting a selective blockade of Ca2+ release from the sarcoplasmic reticulum. Imperatoxin activator has a M(r) of approximately 8700, stimulates [3H]ryanodine binding in skeletal but not cardiac sarcoplasmic reticulum with an ED50 of approximately 6 nM, and activates skeletal but not cardiac Ca(2+)-release channels. These ligands may serve to selectively "turn on" or "turn off" ryanodine receptors in fragmented systems and whole cells.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K., Lai F. A., Liu Q. Y., Rousseau E., Erickson H. P., Meissner G. Structural and functional characterization of the purified cardiac ryanodine receptor-Ca2+ release channel complex. J Biol Chem. 1989 Jan 15;264(2):1329–1335. [PubMed] [Google Scholar]
- Bers D. M., Lederer W. J., Berlin J. R. Intracellular Ca transients in rat cardiac myocytes: role of Na-Ca exchange in excitation-contraction coupling. Am J Physiol. 1990 May;258(5 Pt 1):C944–C954. doi: 10.1152/ajpcell.1990.258.5.C944. [DOI] [PubMed] [Google Scholar]
- Boyett M. R., Kirby M. S., Orchard C. H. Rapid regulation of the 'second inward current' by intracellular calcium in isolated rat and ferret ventricular myocytes. J Physiol. 1988 Dec;407:77–102. doi: 10.1113/jphysiol.1988.sp017404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo L. M., Grantham C. J., Cannell M. B. Kinetics, stoichiometry and role of the Na-Ca exchange mechanism in isolated cardiac myocytes. Nature. 1990 Jun 14;345(6276):618–621. doi: 10.1038/345618a0. [DOI] [PubMed] [Google Scholar]
- Hamilton S. L., Yatani A., Brush K., Schwartz A., Brown A. M. A comparison between the binding and electrophysiological effects of dihydropyridines on cardiac membranes. Mol Pharmacol. 1987 Mar;31(3):221–231. [PubMed] [Google Scholar]
- Harootunian A. T., Kao J. P., Paranjape S., Tsien R. Y. Generation of calcium oscillations in fibroblasts by positive feedback between calcium and IP3. Science. 1991 Jan 4;251(4989):75–78. doi: 10.1126/science.1986413. [DOI] [PubMed] [Google Scholar]
- Hidalgo C., Parra C., Riquelme G., Jaimovich E. Transverse tubules from frog skeletal muscle. Purification and properties of vesicles sealed with the inside-out orientation. Biochim Biophys Acta. 1986 Feb 13;855(1):79–88. doi: 10.1016/0005-2736(86)90191-4. [DOI] [PubMed] [Google Scholar]
- Imagawa T., Takasago T., Shigekawa M. Cardiac ryanodine receptor is absent in type I slow skeletal muscle fibers: immunochemical and ryanodine binding studies. J Biochem. 1989 Aug;106(2):342–348. doi: 10.1093/oxfordjournals.jbchem.a122855. [DOI] [PubMed] [Google Scholar]
- Jones L. R., Cala S. E. Biochemical evidence for functional heterogeneity of cardiac sarcoplasmic reticulum vesicles. J Biol Chem. 1981 Nov 25;256(22):11809–11818. [PubMed] [Google Scholar]
- Meissner G. Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J Biol Chem. 1986 May 15;261(14):6300–6306. [PubMed] [Google Scholar]
- Pessah I. N., Stambuk R. A., Casida J. E. Ca2+-activated ryanodine binding: mechanisms of sensitivity and intensity modulation by Mg2+, caffeine, and adenine nucleotides. Mol Pharmacol. 1987 Mar;31(3):232–238. [PubMed] [Google Scholar]
- Strong P. N. Potassium channel toxins. Pharmacol Ther. 1990;46(1):137–162. doi: 10.1016/0163-7258(90)90040-9. [DOI] [PubMed] [Google Scholar]
- Supattapone S., Worley P. F., Baraban J. M., Snyder S. H. Solubilization, purification, and characterization of an inositol trisphosphate receptor. J Biol Chem. 1988 Jan 25;263(3):1530–1534. [PubMed] [Google Scholar]
- Tanabe T., Beam K. G., Adams B. A., Niidome T., Numa S. Regions of the skeletal muscle dihydropyridine receptor critical for excitation-contraction coupling. Nature. 1990 Aug 9;346(6284):567–569. doi: 10.1038/346567a0. [DOI] [PubMed] [Google Scholar]
- Valdivia C., Vaughan D., Potter B. V., Coronado R. Fast release of 45Ca2+ induced by inositol 1,4,5-trisphosphate and Ca2+ in the sarcoplasmic reticulum of rabbit skeletal muscle: evidence for two types of Ca2+ release channels. Biophys J. 1992 May;61(5):1184–1193. doi: 10.1016/S0006-3495(92)81927-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia H. H., Coronado R. Inhibition of dihydropyridine-sensitive calcium channels by the plant alkaloid ryanodine. FEBS Lett. 1989 Feb 27;244(2):333–337. doi: 10.1016/0014-5793(89)80557-5. [DOI] [PubMed] [Google Scholar]
- Valdivia H. H., Fuentes O., el-Hayek R., Morrissette J., Coronado R. Activation of the ryanodine receptor Ca2+ release channel of sarcoplasmic reticulum by a novel scorpion venom. J Biol Chem. 1991 Oct 15;266(29):19135–19138. [PubMed] [Google Scholar]
- Valdivia H. H., Hogan K., Coronado R. Altered binding site for Ca2+ in the ryanodine receptor of human malignant hyperthermia. Am J Physiol. 1991 Aug;261(2 Pt 1):C237–C245. doi: 10.1152/ajpcell.1991.261.2.C237. [DOI] [PubMed] [Google Scholar]
- Zorzato F., Fujii J., Otsu K., Phillips M., Green N. M., Lai F. A., Meissner G., MacLennan D. H. Molecular cloning of cDNA encoding human and rabbit forms of the Ca2+ release channel (ryanodine receptor) of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1990 Feb 5;265(4):2244–2256. [PubMed] [Google Scholar]