The concept of response guided therapy (RGT) was developed to limit duration of exposure to interferon alpha (IFN)-based treatment in order to reduce side effects while optimizing response rates [1]. The RGT approach used statistical analysis to identify on-treatment viral load thresholds (based on HCV genotype) that predicted outcomes [2]. For example, patients with HCV genotype-1 who had viral suppression (i.e., below HCV RNA assay quantification and/or detection) 4 weeks after initiation of therapy were termed rapid viral responders and were likely to achieve cure (>90%), while those with less than a 1-2 log viral decline from pretreatment level by week 12 were advised to discontinue therapy. Viral kinetics and mathematical modeling were used to refine the RGT approach by identifying responses at earlier time points that could predict treatment outcomes [3].
The recent approval of sofosbuvir (SOF) and other DAAs has resulted in a shift in the treatment paradigm [4, 5]. Sofosbuvir-based regimens achieve high (>90%) sustained-virological response (SVR or cure) rates with limited side effects and relatively short duration of therapy (8-24 weeks). The initial clinical trials data with SOF did not indicate an association between on treatment virus levels and SVR due to high cure rates, lack of on treatment viral breakthrough, and rapid viral decline kinetics that give rise to much earlier viral suppression compared to IFN ± ribavirin (RBV) therapy [6]. In the initial clinical trials with SOF, HCV RNA suppression by week 4 was nearly universal and was not associated with cure, including patients infected with HCV genotype-3 who are currently considered the most difficult to treat [7, 8]. Subsequent studies did not find an association between early viral kinetics and treatment outcomes [9, 10]. As a result, on treatment HCV RNA measurements (weeks 2 and/or 4) are currently recommended by both EASL (www.easl.eu) and the AASLD/IDSA (www.hcvguidelines.org) only as a means to monitor adherence and a fixed duration of DAA therapy has eclipsed the RGT approach.
Detection of HCV RNA at the end of treatment (EOT) with IFN-based therapies was an indicator of treatment failure [11-13]. Recent reports document that some patients treated with all-oral DAA regimens achieved cure despite having detectable viremia at end of treatment (termed here EOT+/SVR)[14-17]. While a higher frequency of EOT+/SVR cases is expected to be identified with the use of more sensitive HCV RNA assays, a comprehensive comparison between commercial quantification assays has been lacking.
In this edition of Journal of Hepatology, Maasoumy et al [18], provide a detailed on-treatment HCV RNA kinetic analysis of patients receiving different approved SOF-based therapies. The performance of the most widely used quantification assays, i.e., Roche CobasTaqMan v2.0 (CTM) and Abbott RealTime HCV (ART) assays was compared. The CTM is based on Taqman technology with a dual probe approach using 1 forward primer and 2 staggered reverse primers from the 5’UTR region, whereas the ART is based on amplification of single stranded DNA in the 5’UTR region. The ART assay can measure significantly lower viral loads compared to the CTM assay (P<0.0001) [19]. The ART assay has the advantage of being more sensitive. However, if a small amount of residual non-infectious HCV RNA is present at EOT in some cases as hypothesized by Kohli et al [15], amplification of non-infectious virions by the ART assay could result in misclassification of patients who have cleared infectious virus as EOT+.
In the study by Maasoumy et al. [18], 298 patients infected with HCV genotypes 1-5 were treated with SOF in conjunction with RBV (n=99), pegIFN+RBV (n=51), simeprevir (SIM)±RBV (n=69) and daclatasvir (DAC)±RBV (n=79) in two German university clinics. Samples were collected at week 0, 1, 2 and 4 and then every 4 weeks until EOT (week 12 or 24). The main findings were: (i) all SOF+RBV genotype-3 treated patients with week 2 HCV RNA <45 IU/ml or <60 IU/ml based on CTM or ART, respectively, achieved SVR, while the SVR rate was only 33% among those with higher virus levels at week 2; suggesting that RGT might be applicable to genotype 3 patients, (ii) high SVR rates (88%-96%) were achieved among non-genotypes-3 patients treated with SOF-based regimens, although the data did not support the use of RGT in non-genotype 3 cases, (iii) the fraction of undetectable HCV RNA at week 4 of treatment was higher (42%-80%) by CTM compared to 5%-53% by ART, but much lower than observed in the initial clinical trials in which less sensitive quantification assay were used, as noted by Maasoumy et al, (iv) the more sensitive ART assay detected HCV RNA several weeks longer than CTM regardless of the SOF treatment regimen, suggesting a slower viral decline with ART compared to CTM, and (v) 18% of genotype-3 patients and 20% of genotype-1 patients treated with SOF/DAC±RBV and SOF/SIM±RBV were EOT+ by ART, of whom 100% and 92% were EOT+/SVR respectively.
The newly suggested RGT approach by Maasoumy and colleagues for genotype 3 patients may allow for identification of patients of as early as week 2 after initiation of SOF + RBV subjects who are likely to achieve SVR without additional antivirals such as DAC or pegIFN. This approach could help reduce drug costs and/or unnecessary side effects, pending verification in prospective trials.
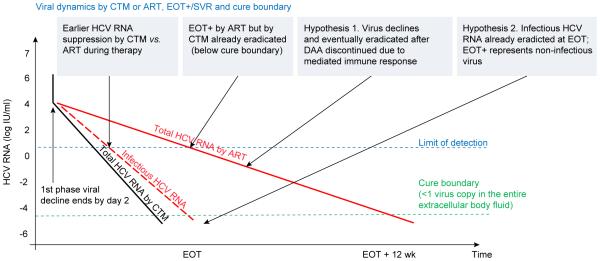
The high cure rates (>88%) shown by Maasoumy et al [18] under SOF-based regimens, suggest that cure often took place before EOT. This may explain why it is difficult to find early on-treatment virus level thresholds for a RGT approach. The use of on-treatment mathematical modeling of HCV kinetics allows for estimation of viral dynamic parameters that may predict treatment outcome and the duration of therapy needed to achieve cure. In the era of IFN-based therapies, the cure boundary concept, defined as <1 virus copy in the entire extracellular body fluid of a patient, was developed to determine the duration of treatment needed for cure, but was only tested retrospectively [3]. Real time modeling of HCV kinetics was recently used for the first time to successfully predict the length of silibinin + RBV therapy needed to achieve cure [20]. Subsequently, this modeling approach was recently applied retrospectively to data from treatment with SOF-based regimens, suggesting a potential role for on treatment viral dynamics to individualize the length (and decrease cost) of SOF therapy [21]. The study by Maasoumy et al [18] showed that use of the ART assay resulted in slower estimates of viral kinetics and EOT+ cases compared to CTM. As shown in Fig. 1, the standard biphasic modeling approach predicts the need for longer than necessary treatment duration by ART with increased costs to achieve cure compared to CTM in some cases, suggesting that the CTM assay is better suited for real time modeling of time to cure with DAAs.
Fig. 1. Viral dynamics based on CTM and ART quantification assays.
Biphasic viral kinetics in the same subject based on CTM and ART may lead to a difference in understanding cure. Measurement of HCV RNA by CTM (black solid line) indicates that viral load is suppressed (below assay detection, blue dotted line) much earlier than by ART (red solid line), suggesting via the standard biphasic model that virus level crossed the cure boundary (green dotted line) before the end of treatment, EOT, in the majority of patients, in agreement with the high SVR rates achieved by DAAs (black line). With HCV RNA measured by ART, viral load may be still detected at later stages of therapy (e.g., week 8), and in some cases at EOT (EOT+), predicting by the biphasic model longer treatment duration than is actually necessary (>12 weeks). To explain slow viral decline including EOT+/SVR cases observed by ART using the biphasic model, a continuous viral decline and eradication after discontinuation of therapy mediated by an immunologic response was hypothesized. An alternative hypothesis is that infectious virus (red dashed line), a fraction of the measured total HCV RNA, might already be eradicated before EOT while residual non-infectious virus is still detected by ART (red solid line). The two hypotheses are not mutually exclusive.
The study by Maasoumy et al [18], shows that the sensitive ART assay identifies EOT+ cases, that appear to be EOT− by the CTM assay. Interestingly, not a single EOT+ case was detected in more than 3000 French patients who were treated with SOF-based therapies using CTM (Halfon, unpublished data). Moreover, in another study of 89 patients who received SOF-based therapies, five patients (6%), all with genotype-1, were EOT+ by ART and each achieved SVR (submitted). Two explanations have been offered for the phenomenon of EOT+/SVR, an immunologic mediated clearance that occurs after treatment is completed [22], and/or that DAAs promote the production of non-infectious viral particles [15] (Fig. 1). A deep sequence analysis of the whole virus genome will be needed to explore the possibility of residual non-infectious virus at EOT and to clarify why EOT+/SVR cases are identified by ART.
In conclusion, the important findings of Maasoumy et al [18], suggest a resurrection of the RGT approach in difficult to treat genotype 3 patients treated with SOF + RBV and indicate that CTM may be the most suitable quantification assay to explore further RGT and viral dynamics approaches to optimize SVR and cost. The study by Maasoumy et al. provides further evidence that EOT+/SVR cases are identified by ART and that EOT+ does not equal treatment failure in the DAA era.
Acknowledgments
The manuscript was supported in part by the U.S. National Institute of Health (NIH) grant R01-AI078881.
Footnotes
Conflict of interest
The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
References
Author names in bold designate shared co-first authorship
- [1].Lawitz EJ, Membreno FE. Response-guided therapy in patients with genotype 1 hepatitis C virus: current status and future prospects. J Gastroenterol Hepatol. 2014;29:1574–1581. doi: 10.1111/jgh.12632. [DOI] [PubMed] [Google Scholar]
- [2].Fried MW, Hadziyannis SJ, Shiffman ML, Messinger D, Zeuzem S. Rapid virological response is the most important predictor of sustained virological response across genotypes in patients with chronic hepatitis C virus infection. J Hepatol. 2011;55:69–75. doi: 10.1016/j.jhep.2010.10.032. [DOI] [PubMed] [Google Scholar]
- [3].Dahari H, Guedj J, Perelson AS, Layden TJ. Hepatitis C Viral Kinetics in the Era of Direct Acting Antiviral Agents and IL28B. Curr Hepat Rep. 2011;10:214–227. doi: 10.1007/s11901-011-0101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, Weiland O, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1594–1603. doi: 10.1056/NEJMoa1315722. [DOI] [PubMed] [Google Scholar]
- [5].Koff RS. Review article: the efficacy and safety of sofosbuvir, a novel, oral nucleotide NS5B polymerase inhibitor, in the treatment of chronic hepatitis C virus infection. Aliment Pharmacol Ther. 2014;39:478–487. doi: 10.1111/apt.12601. [DOI] [PubMed] [Google Scholar]
- [6].Pawlotsky JM. New hepatitis C therapies: the toolbox, strategies, and challenges. Gastroenterology. 2014;146:1176–1192. doi: 10.1053/j.gastro.2014.03.003. [DOI] [PubMed] [Google Scholar]
- [7].Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- [8].Wyles DL, Nelson DR, Swain MG, Gish RG, Ma J, McNally J, Ggggxx h. On treatment HCV RNA as a predictor of virologic response in sofosbuvir-containing regimens for genotype 2/3 HCV infection: Analysis of the FISSION, POSITRON, and FUSION studies. Hepatology. 2013;58:140A. [Google Scholar]
- [9].Zeuzem S, Dusheiko GM, Colombo M, Flisiak R, Hyland RH, Illeperuma A, Brainard DM, et al. Early viral kinetics do not predict treatment outcome with sofosbuvir+ribavirin for 12 or 24 weeks in HCV genotype 2/3 patients in the Valence trial. J Hepatol. 2014;60:S452. [Google Scholar]
- [10].Hezode C, Chevaliez S, Scoazec G, Bouvier-Alias M, Ruiz I, Francois M, Mallat A, et al. On-treatment viral kinetics do not predict SVR in patients with advanced liver disease receiving sofosbuvir in combination with daclatasvir or simeprevir for 12 weeks. J Hepatol. 2015;62:S654–S655. [Google Scholar]
- [11].Ferenci P, Laferl H, Scherzer TM, Gschwantler M, Maieron A, Brunner H, Stauber R, et al. Peginterferon alfa-2a and ribavirin for 24 weeks in hepatitis C type 1 and 4 patients with rapid virological response. Gastroenterology. 2008;135:451–458. doi: 10.1053/j.gastro.2008.04.015. [DOI] [PubMed] [Google Scholar]
- [12].Poordad F, McCone J, Jr., Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jacobson IM, Dore GJ, Foster GR, Fried MW, Radu M, Rafalsky VV, Moroz L, et al. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2014;384:403–413. doi: 10.1016/S0140-6736(14)60494-3. [DOI] [PubMed] [Google Scholar]
- [14].Sarrazin C, Wedemeyer H, Cloherty G, Cohen DE, Chevaliez S, Herman C, Bernstein B, et al. Importance of very early HCV RNA kinetics for prediction of treatment outcome of highly effective all oral direct acting antiviral combination therapy. J Virol Methods. 2015;214:29–32. doi: 10.1016/j.jviromet.2014.11.027. [DOI] [PubMed] [Google Scholar]
- [15].Kohli A, Osinusi A, Sims Z, Nelson A, Meissner EG, Barrett LL, Bon D, et al. Virological response after 6 week triple-drug regimens for hepatitis C: a proof-of-concept phase 2A cohort study. Lancet. 2015;385:1107–1113. doi: 10.1016/S0140-6736(14)61228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sidharthan S, Kohli A, Sims Z, Nelson A, Osinusi A, Masur H, Kottilil S. Utility of hepatitis C viral load monitoring on direct-acting antiviral therapy. Clin Infect Dis. 2015;60:1743–1751. doi: 10.1093/cid/civ170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Harrington PR, Deming DJ, Komatsu TE, Naeger LK. Hepatitis C Virus RNA Levels During Interferon-Free Combination Direct-Acting Antiviral Treatment in Registrational Trials. Clin Infect Dis. 2015;61:666–667. doi: 10.1093/cid/civ402. [DOI] [PubMed] [Google Scholar]
- [18].Maasoumy B, Vermehren J, Welker MW, Bremer B, Perner D, Zu Siederdissen CH, Deterding K, et al. Clinical value of on-treatment HCV RNA levels during different approved sofosbuvir-based antiviral regimens. J Hepatol. 2016 doi: 10.1016/j.jhep.2016.04.006. in press. [DOI] [PubMed] [Google Scholar]
- [19].Kessler HH, Cobb BR, Wedemeyer H, Maasoumy B, Michel-Treil V, Ceccherini-Nelli L, Bremer B, et al. Evaluation of the COBAS((R)) AmpliPrep/COBAS((R)) TaqMan((R)) HCV Test, v2.0 and comparison to assays used in routine clinical practice in an international multicenter clinical trial: The ExPECT study. J Clin Virol. 2015;67:67–72. doi: 10.1016/j.jcv.2015.03.023. [DOI] [PubMed] [Google Scholar]
- [20].Dahari H, Shteingart S, Gafanovich I, Cotler SJ, D'Amato M, Pohl RT, Weiss G, et al. Sustained virological response with intravenous silibinin: individualized IFN-free therapy via real-time modelling of HCV kinetics. Liver Int. 2015;35:289–294. doi: 10.1111/liv.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dahari H, Canini L, Graw F, Uprichard SL, Araujo ES, Penaranda G, Coquet E, et al. HCV kinetic and modeling analyses indicate similar time to cure among sofosbuvir combination regimens with daclatasvir, simeprevir or ledipasvir. J Hepatol. 2016;64:1232–1239. doi: 10.1016/j.jhep.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Meissner EG, Nelson A, Marti M, Masur H, Osinusi A, Kottilil S. Sustained Virologic Response for Chronic Hepatitis C Infection after 27 Days of Treatment with Sofosbuvir and Ribavirin. Open Forum Infect Dis. 2014;1:013. doi: 10.1093/ofid/ofu013. [DOI] [PMC free article] [PubMed] [Google Scholar]