Epidemiology teaches us that there is an inverse relationship between soy consumption in Asian countries and a decrease in breast cancer risk. Naturally, this observation sparked a sustained interest in a potential natural approach to the chemoprevention of breast cancer. However, there are many interconnected dimensions to the soy story, some of which are potentially bad. This is the focus of the study by Shike and colleagues (1) in this issue of the Journal.
Shike et al. (1) address the impact of short-term (7 to 30 days) soy administration to a mixed population of premenopausal and early postmenopausal women with a diagnosis of breast cancer. Soy contains genistein and diadzein that were measured as known phytoestrogens in patient sera. It has been known for three decades that phytoestrogens have the potential to induce estrogen-regulated genes through the estrogen receptor (ER) (2).
Shike et al. (1) report on a breast cancer genistein gene signature that is characterized by increases in cell cycle genes, which, considering that women only consumed soy for one to four weeks, is not good. Patients, however, may take soy for years. They will not be protected under the current treatment regimen, but they might with a different regimen.
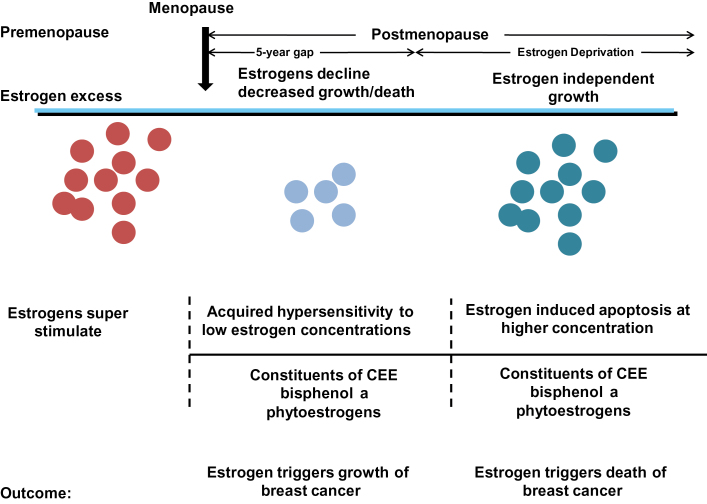
The majority of patients in the study (1) were early postmenopausal, and we know from estrogen withdrawal in ER-positive breast cancer cells that there is catastrophe-early cell death in the new estrogen austere conditions (3,4) and the population of cells is forced to adapt by environmental selection pressure. The new breast cancer cell population has adaptive hypersensitivity (5) to exogenous estrogen and scavenges any estrogen for growth through elevated ER levels (Figure 1) (3,4,6). This is a characteristic of breast cancer that must replicate to survive. However, the study by Shike and colleagues (1) comes at a fortunate moment, when the interlocking dimensions of estrogen action in breast cancer are being redefined. Our understanding is being transformed from random clinical and laboratory observations into a set of rules to test in clinical trials.
Figure 1.
Rules for the change in estrogen receptor (ER) positive breast cancer cell populations as they leave an estrogen rich environment at menopause, adapt to a declining estrogen environment over a 5 year period (referred to as Gap). Estrogen independent clones then grow out that are able to survive in an estrogen austere environment. This is modeled in the laboratory with long term estrogen deprived cells that exhibit acquired hypersensitivity to estrogen for growth(6) and then estrogen induced apoptosis(14,15). Laboratory studies illustrate that the constituents of conjugated equine estrogen (CEE)(26), the endocrine disruptor bisphenol A (27) and phytoestrogens(24) can trigger cell replication or apoptosis dependent upon the cell populations and its natural estrogen rich or austere environment.
What evidence is there in the literature to move from myths about phytoestrogens to create a foundation for future clinical study? The patient population (a total of 104) is broad, consisting of a mix or pre- and postmenopausal women (39.4% and 60.6%, respectively) with a mean age of 56.2±11.9 (SD) years. As will be examined and defined, this is an appropriate population to define the risks of soy consumption, but excludes the potential benefits. Another complicating and diluting aspect of the study is the fact that the breast cancers turn out to be 82% ER positive and 18% ER negative. This strategy, however, was not unreasonable, as the authors state it is the first study to monitor gene activation before and after the consumption of soy.
Phytoestrogens display estrogenic effects in laboratory tests (2); therefore, it may be instructive to draw upon both clinical and laboratory data about the actions of estrogen on breast cancers. In this way, a logical strategy for deploying phytoestrogens in future clinical trials can be formulated.
The pharmacology of estrogen action changes in relation to the time from menopause in postmenopausal women with either metastatic breast cancer or occult disease in the breast. Haddow (7) first reported a small series of patients with metastatic breast cancer that had a 30% response rate to high-dose estrogen therapy. He used these data to complete the first multi-institutional clinical trial and reported his retrospective observations (8).
The beneficial responses were three times more frequent in women over the age of 60 years than in those under that age; that estrogens may, on the contrary, accelerate the course of mammary cancer in younger women, and that their therapeutic use should be restricted to cases 5 years beyond the menopause. (8)
Similarly, Stoll (9) noted that the objective remission rate from estrogen treatment in 407 patients with metastatic breast cancer was higher in women more than five years past menopause (35%) when compared with women who were less than five years postmenopause (9%).
The second data set is the estrogen replacement therapy literature with the Million Women’s Study (10) and the Women’s Health Initiative (WHI) (11).
In the Million Women’s Study, that accrued 4.05 million women years of follow up, 15750 incident breast cancers were noted with a total of 7107 breast cancer in current users of hormone therapy.
The principal conclusion relevant to our current considerations of timing and estrogen therapy was that current users of estrogen alone (ERT) had little increase in breast cancer if use was started more than five years after menopause (relative risk [RR] = 1.05, 95% confidence interval [CI] = 0.89 to 1.24), but if ERT was initiated straight after menopause there was increase in breast cancer (RR = 1.43, 95% CI = 1.35 to 1.51) (10).
The WHI recruited 10739 hysterectomized postmenopausal women into a randomized trial to receive either CEE (0.625mg daily) or placebo. Women were between ages 50 and 79 years. The treatment phase of the trial was a median of 5.9 years, as stop rules for stroke were invoked, but an overall follow-up had a median of 11.8 years. The first surprise was a finding of a lower incidence of breast cancer. At the latest analysis with 11.8 years median follow-up (11), there was a lower incidence of invasive breast cancer (151 case patients) compared with placebo (199 case patients). Fewer women died from breast cancer in the estrogen group (six deaths) compared with placebo (16 deaths). Indeed, few women died of any cause in the estrogen group after breast cancer diagnosis (30 deaths) than did those in the placebo group (50 deaths).
The breakthrough in understanding how one hormone estradiol, can be responsible for either the growth or death of breast cancer occurred with the realization that tumor cell populations adapt and evolve over years in response to long-term tamoxifen treatment (12). Acquired resistance to tamoxifen occurs in MCF-7 tumors implanted into tamoxifen-treated athymic mice within about a year. The acquired resistance mimics acquired resistance in metastatic breast cancer and tumors grow with either estrogen or tamoxifen. However, continuing growth for years of retransplanted tumors into tamoxifen-treated athymic mice exposes a vulnerability in evolving tumor cell populations: physiologic estrogen-induced apoptosis (13). This occurs not only in animal models in vivo but also in response to long-term estrogen deprivation in vitro (14,15). Estrogen is no longer perceived as a survival signal through cell replication but as a trigger of apoptosis. An understanding of the ER-mediated mechanism of estrogen-induced apoptosis in vulnerable estrogen deprived breast cancer cells has been defined (14–16), refined (17), and interrogated (18–21). It is the timing of estrogen administration after menopause that determines tumor growth or cancer cell death (Figure 1). Clinical translation validates the importance of the new biology of estrogen-induced apoptosis.
Lonning and colleagues (22) conducted a small study on 32 patients exhaustively treated with antihormones and then high-dose estrogen. Four complete remissions occurred, with a 30% overall response rate. Ellis and colleagues (23) evaluated the benefits of high- (30mg daily) and low (6mg daily)- dose estrogen treatment as a salvage therapy following failure of aromatase inhibitors. There was approximately a 30% clinical benefit for both dosage groups, but a lower side effect rate for the low-dose estrogen. In this context, the decrease in breast cancer, in the estrogen-alone trial in the WHI trial with women in their 60s illustrates the value of low-dose estrogen treatment on prepared and vulnerable estrogen-deprived nascent breast cancer (11).
The general principle that emerges from both laboratory and clinical studies is that estrogen enhances growth in breast cancer cell populations maintained in estrogen but triggers apoptosis in cell populations adapted to long-term estrogen deprivation. The study by Shike et al. (1) illustrates the dangers of phytoestrogen consumption too soon, around menopause, but the biology of estrogen in estrogen-deprived conditions suggests that phytoestrogen could have a benefit a decade after menopause. Recent laboratory studies support this (24), but there are two issues. First, appropriate dosing of soy to create high levels of circulating phytoestrogen is needed to trigger apoptosis. Ten nanomolar concentrations with phytoestrogens stimulate cell replication in culture, but a hundred nanomolar are necessary to trigger apoptosis (24). The Shike et al. study (1) shows a huge range of cumulating levels of genestein (0–400ng/mL), but this may be because of compliance problems or different durations of treatment (one to four weeks). The second and most important issue is that women often take soy products to ameliorate menopausal symptoms. It is now clear they should not. The clinical findings of Shike and colleagues (1) are consistent with the current rules of estrogen action in estrogen-replete or estrogen-deprived breast cancer cells, both in the laboratory and the clinic (Figure 1) (25). No estrogen is good around menopause. However, a growing body of laboratory (24,26) and clinical (11) evidence has now created an opportunity for evidence-based clinical studies of chemoprevention with some form of estrogen, perhaps given intermittently, a decade following menopause.
Funding
VCJ is supported by the Department of Defense Breast Program under Award number W81XWH-06-1-0590 Center of Excellence, subcontract under the SU2C (AACR) Grant number SU2C-AACR-DT0409, the Susan G Komen For The Cure Foundation under Award number SAC100009, GHUCCTS CTSA (Grant # UL1RR031975), and the Lombardi Comprehensive Cancer Center Support Grant (CCSG) Core Grant NIH P30 CA051008.
The author has no conflict of interest to declare.
References
- 1. Shike M, Doane A, Russo L, et al. The effects of soy supplementation on gene expression in breast cancer: a randomized placebo-controlled study. J Natl Cancer Inst. 2014: In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jordan VC, Koch R, Lieberman ME. Structure-activity relationships of nonsteroidal estrogens and antiestrogens. In: Jordan VC, ed. Estrogen/Antiestrogen Action and Breast Cancer Therapy. Madison, WI: University of Wisconsin Press; 1986:19–41. [Google Scholar]
- 3. Welshons WV, Jordan VC. Adaptation of estrogen-dependent MCF-7 cells to low estrogen (phenol red-free) culture. Eur J Cancer Clin Oncol. 1987;23(12):1935–9. [DOI] [PubMed] [Google Scholar]
- 4. Katzenellenbogen BS, Kendra KL, Norman MJ, et al. Proliferation, hormonal responsiveness, and estrogen receptor content of MCF-7 human breast cancer cells grown in the short-term and long-term absence of estrogens. Cancer Res. 1987;47(16):4355–4360. [PubMed] [Google Scholar]
- 5. Masamura S, Santner SJ, Heitjan DF, et al. Estrogen deprivation causes estradiol hypersensitivity in human breast cancer cells. J Clin Endocrinol Metab. 1995;80(10):2918–2925. [DOI] [PubMed] [Google Scholar]
- 6. Jeng MH, Shupnik MA, Bender TP, et al. Estrogen receptor expression and function in long-term estrogen-deprived human breast cancer cells. Endocrinology. 1998;139(10):4164–4174. [DOI] [PubMed] [Google Scholar]
- 7. Haddow A, Watkinson JM, Paterson E, et al. Influence of synthetic oestrogens on advanced malignant disease. Br Med J. 1944;2(4368):393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haddow A., David A. Karnofsky memorial lecture. Thoughts on chemical therapy. Cancer. 1970;26(4):737–754. [DOI] [PubMed] [Google Scholar]
- 9. Stoll B. Palliation by castration or hormone ablation. In: Stoll BA, ed. Breast Cancer Management Early and Late. London, UK: William Herman Medical Books Ltd; 1977:135–149. [Google Scholar]
- 10. Beral V, Reeves G, Bull D, et al. Breast cancer risk in relation to the interval between menopause and starting hormone therapy. J Natl Cancer Inst. 2011;103(4):296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13(5):476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jordan VC. Selective estrogen receptor modulation: concept and consequences in cancer. Cancer Cell. 2004;5(3):207–213. [DOI] [PubMed] [Google Scholar]
- 13. Yao K, Lee ES, Bentrem DJ, et al. Antitumor action of physiological estradiol on tamoxifen-stimulated breast tumors grown in athymic mice. Clin Cancer Res. 2000;6(5):2028–2036. [PubMed] [Google Scholar]
- 14. Song RX, Mor G, Naftolin F, et al. Effect of long-term estrogen deprivation on apoptotic responses of breast cancer cells to 17beta-estradiol. J Natl Cancer Inst. 2001;93(22):1714–1723. [DOI] [PubMed] [Google Scholar]
- 15. Lewis JS, Meeke K, Osipo C, et al. Intrinsic mechanism of estradiol-induced apoptosis in breast cancer cells resistant to estrogen deprivation. J Natl Cancer Inst. 2005;97(23):1746–1759. [DOI] [PubMed] [Google Scholar]
- 16. Obiorah I, Sengupta S, Fan P, et al. Delayed triggering of oestrogen induced apoptosis that contrasts with rapid paclitaxel-induced breast cancer cell death. Br J Cancer. 2014;110(6):1488–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ariazi E, Cunliffe H, Lewis-Wambi JS, et al. Estrogen induces apoptosis in estrogen deprivation-resistant breast cancer through stress responses as identified by global gene expression across time. Proc Natl Acad Sci USA. 2011;108(47):18879–18886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fan P, Agboke FA, McDaniel RE, et al. Inhibition of c-Src blocks oestrogen-induced apoptosis and restores oestrogen-stimulated growth in long-term oestrogen-deprived breast cancer cells. Eur J Cancer. 2014;50(2):457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fan P, Griffith OL, Agboke FA, et al. c-Src modulates estrogen-induced stress and apoptosis in estrogen-deprived breast cancer cells. Cancer Res. 2013;73(14):4510–4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Obiorah I, Sengupta S, Curpan R, et al. Defining the conformation of the estrogen receptor complex that controls estrogen induced apoptosis in breast cancer. Molecular Pharmacology. 2014: In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Obiorah IE, Jordan VC. Differences in the rate of oestrogen-induced apoptosis in breast cancer by estradiol and the triphenylethylene bisphenol. Br J Pharmacol. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lonning PE, Taylor PD, Anker G, et al. High-dose estrogen treatment in postmenopausal breast cancer patients heavily exposed to endocrine therapy. Breast Cancer Res Treat. 2001;67(2):111–116. [DOI] [PubMed] [Google Scholar]
- 23. Ellis MJ, Gao F, Dehdashti F, et al. Lower-dose vs high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer: a phase 2 randomized study. JAMA. 2009;302(7):774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Obiorah IE, Fan P, Jordan VC. Breast cancer cell apoptosis with phytoestrogens is dependent on an estrogen deprived state. Cancer Prev Res (Phila). 2014: In Press. [DOI] [PubMed] [Google Scholar]
- 25. Jordan VC. The 38th David A. Karnofsky lecture: the paradoxical actions of estrogen in breast cancer--survival or death? J Clin Oncol. 2008;26(18):3073–3082. [DOI] [PubMed] [Google Scholar]
- 26. Obiorah I, Jordan VC. 2012 NAMS/PFIZER- Wulf H. Utian endowed lecture. The scientific rationale for a delay after menopause in the use of conjugated equine estrogens in postmenopausal women that causes a reduction in breast cancer incidence and mortality. Menopause. 2013;20(4):372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sengupta S, Obiorah I, Maximov PY, et al. Molecular mechanism of action of bisphenol and bisphenol A mediated by oestrogen receptor alpha in growth and apoptosis of breast cancer cells. Br J Pharmacol. 2013;169(1):167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]