Abstract
Lysine acetylation is a post-translational modification that is carried out by acetyltransferases. The MYST proteins form the largest and most diverse family of acetyltransferases, which regulate gene expression, DNA repair, and cell cycle homeostasis, among other activities, by acetylating both histone and non-histone proteins. This chapter will describe methods for the preparation and biochemical characterization of MYST family acetyltransferases, including protocols for the preparation of recombinant protein, enzyme assays for measuring steady state parameters and binding assays to measure cofactor and inhibitor binding. We also provide details on adapting these assays for high throughput screening for small molecule MYST inhibitors. This chapter seeks to prepare researchers for some hurdles that they may encounter when studying the MYST proteins so that there may be better opportunity to plan appropriate controls and obtain high quality data.
1. Introduction
Lysine acetylation is a post-translational modification that is conserved from yeast to humans and is correlated with numerous regulatory functions in both the nucleus and cytoplasm of the cell. Of the different HAT (histone acetyltransferase) families that have been characterized to date, the MYST proteins form the largest and most diverse family (Yang, 2004). MYST family proteins regulate gene expression, DNA repair, cell cycle homeostasis and other activities (Sapountzi & Cote, 2011) by acetylating both histone and non-histone proteins (Cereseto, Manganaro, Gutierrez, Terreni, Fittipaldi, Lusic et al., 2005; Iizuka & Stillman, 1999; Lin, Lu, Zhang, Walter, Dang, Wan et al., 2009; Mellert & McMahon, 2009; Sapountzi et al., 2011).
Although MYST proteins share a structurally conserved acetyl Coenzyme A (acetyl CoA) core-binding region with other HATs, they employ several unique structural and catalytic properties within their conserved HAT domain. The MYST HAT domains contain N- and C-terminal regions that flank the core-binding region that are structurally distinct from other HAT families. These include an N-terminal C2HC zinc binding region and C-terminal helix-turn-helix motifs required for chromatin regulatory activities (Lafon, Chang, Scott, Jacobson, & Pillus, 2007; Yan, Barlev, Haley, Berger, & Marmorstein, 2000). For catalysis, the MYST proteins have been shown to employ a ping-pong catalytic mechanism (Yan et al., 2000) although one study was more consistent with a ternary complex mechanism (Berndsen, Albaugh, Tan, & Denu, 2007). In the ping-pong mechanism, the general base glutamate first deprotonates the active site cysteine so that the acetyl group from acetyl CoA can be transferred to the cysteine. The glutamate then protonates the leaving cofactor and deprotonates the substrate lysine so that the cysteine can transfer the acetyl group to the lysine. Finally both the glutamate and cysteine may react with water to return to their initial state (Figure 1) (Yan et al., 2000). More recently, MYST catalytic activity was also found to require active site lysine autoacetylation for cognate substrate acetylation (Yuan, Rossetto, Mellert, Dang, Srinivasan, Johnson et al., 2012).
Figure 1.
“Ping-pong” catalytic mechanism employed by MYST family acetyltransferases. A glutamate in the active site acts as the general base to first deprotonate the active site cysteine. Next the acetyl group from acetyl CoA is transferred to the cysteine. The glutamate then protonates the leaving cofactor. The glutamate is then able to deprotonate the substrate lysine so that the cysteine can transfer the acetyl group to the lysine. Finally both the glutamate and cysteine react with water to return to their initial state.
Given the diverse regulatory roles, it is not surprising that aberrant protein acetylation or acetyltransferase function within the MYST family is correlated with several human diseases. For example, the HAT domain of the MYST protein MOZ (monocytic leukemia zinc-finger protein) can form translocation products with the CBP (CREB-binding protein) HAT in a subset of acute myeloid leukemias (Kitabayashi, Aikawa, Yokoyama, Hosoda, Nagai, Kakazu et al., 2001). Tip60 is linked to the onset of Alzheimer’s disease (Baek, Ohgi, Rose, Koo, Glass, & Rosenfeld, 2002; Cao & Sudhof, 2001, 2004; Kinoshita, Whelan, Berezovska, & Hyman, 2002), and is down-regulated in lung and colon cancer (Lleonart, Vidal, Gallardo, Diaz-Fuertes, Rojo, Cuatrecasas et al., 2006), but up-regulated in epithelial tumors (Hobbs, Wei, DeFeo, Paul, Hayes, & Gilmour, 2006). One study has reported that loss of acetylation on the MOF (males absent on the first) target lysine 16 of histone H4 is common in many human malignancies (Fraga, Ballestar, Villar-Garea, Boix-Chornet, Espada, Schotta et al., 2005). Additionally, mutated MYST proteins can become oncogenic (Lafon et al., 2007).
Because of their connections to disease, there have been efforts to develop inhibitors of MYST family acetyltransferases. The development of a bisubstrate CoA-peptide MYST inhibitor has been reported. While this inhibitor is more potent than the natural product HAT inhibitors anacardic acid and curcumin, it exhibited only micromolar IC50 values and poor selectivity for the MYST proteins versus other families of acetyltransferases (Wu, Xie, Wu, Zhang, & Zheng, 2009). Virtual screening to identify small molecule inhibitors of Tip60 led to the identification of compounds with better potency than curcumin, however these inhibitors still exhibit poor potency with IC50 values in the mid-micromolar range and do not exhibit selectivity between Tip60 and other HAT families (Wu, Wang, Li, Yang, Wang, & Zheng, 2011). Because all the current MYST inhibitors exhibit weak selectivity and/or poor pharmacokinetic properties, it is important to identify and develop new more potent MYST HAT inhibitors that can be used both as probes to further investigate the function of MYST HATs and as lead compounds for drug development.
This chapter will describe methods for the preparation and biochemical characterization of MYST family acetyltransferases, with a particular focus on human MOF (hMOF). This chapter will include protocols on the preparation of recombinant protein, enzyme assays for measuring steady state parameters and a thermofluor assay to investigate cofactor and inhibitor binding. We will also present details on adapting these assays for high throughput screening for small molecule MYST inhibitors. We place a particular focus on how MYST family proteins may need to be treated differently from other related enzymes.
2. Preparation of recombinant MYST proteins
We generally follow previously described methods for purifying the MYST proteins (hMOF specifically) (Yuan et al., 2012) but with some modifications. To study MYST activity in vitro we purify the HAT domain of hMOF, residues 174-449, with an N-terminal 6xHis tag separated by a TEV protease recognition site. The plasmid is transformed into E. coli BL21(DE3) codon plus RIL (Stratagene) cells and the protein is overexpressed in TB media by induction with 1M IPTG and grown at 15°C overnight after the O.D reaches 0.8–1.5. The cells are harvested and lysed by sonication in lysis buffer containing 50 mM HEPES (pH 7.5), 0.5 M NaCl, 2mM β-mercaptoethanol, 5 mM imidazole, 5% glycerol, 0.1% CHAPS and 0.1 mM phenylmethylsulfonyl fluoride. The lysate is cleared by centrifugation at 28,000g for 30 min at 4 °C. The supernatant is then loaded onto Nickel charged NTA resin (Qiagen) that has been equilibrated with lysis buffer. The resin is then washed with 10 column volumes of lysis buffer containing 25 mM imidazole and then eluted with lysis buffer containing 250 mM imidazole buffer in 10 mL fractions. Because we found that the 6xHis tag does not interfere with hMOF activity or crystallization, we do not cleave the tag. Instead we proceed directly from the affinity column to size exclusion chromatography, skipping ion exchange gel chromatography due to hMOF sensitivity to low salt conditions. The hMOF is concentrated and injected onto an FPLC using a HiLoad Superdex 75 16/60 gel filtration column using a gel filtration buffer composed of 20 mM HEPES (pH 7.5), 0.5 M NaCl. We then concentrate the hMOF protein to ~50 μM, aliquot to ~10 μL, flash freeze using liquid nitrogen, and store at −80°C. Once we thaw an aliquot of hMOF we discard the remainder of that aliquot as multiple flash freezings has a negative impact on enzymatic activity. In our experience hMOF expresses and purifies fairly well, and 1L of cells will yield approximately 5mg of protein (Figure 2). In the case of hMOZ and yESA1, we found that the proteins express better in LB rather than TB, but still express about 10x less protein than hMOF. Both MOZ and ESA1 tolerate ion-exchange well, and achieve a higher level of activity if sizing buffer/storage buffer is at a low pH and contains reducing agent. We use 20mM sodium citrate pH 5.5, 100mM NaCl, and 1mM DTT.
Figure 2.
Left: Chromatogram from size exclusion chromatography of hMOF174-449 using a Superdex 75 column. The collected peak is indicated with vertical lines. Right: SDS-PAGE gel showing purified hMOF174-449.
3. Kinetic analysis
3.1 Primer on steady state kinetic analysis
The steady state kinetic parameters of a protein include the Michaelis constant (Km), which is the concentration where the reaction rate of the enzyme is at one-half of its maximal velocity (Vmax) and is related to the affinity of an enzyme for its substrate, and the catalytic constant (kcat), which is the rate that an enzyme turns over its substrates (Figure 3).
Figure 3.

Michaelis-Menten equation, kcat equation and graphical representation of the Michaelis-Menten equation where ν= reaction rate, [S] = substrate concentration, Vmax= maximum reaction velocity, Km = Michaelis constant, and [ET] = total enzyme concentration.
To determine the kinetic parameters for a given enzyme very carefully conducted activity assays must be performed. The basic steps to follow to determine these parameters for a bisubstrate system such as the MYST family is to hold one substrate at a saturating concentration (~10x Km) while the other substrate is varied. About half of the concentrations of the varied substrate should be below Km and the other half above Km. Generally a minimum of eight concentrations should be used (Brooks, Geeganage, Kahl, Montrose, Sittampalam, Smith et al., 2004). Results are then graphed as substrate concentrations versus reaction rate to obtain a Michaelis-Menten curve. For a thorough overview of how to perform these experiments see (Brooks et al., 2004). Also, when performing these experiments it is important to keep the following in mind:
Maintain steady state
A kinetics reaction must be run under steady state conditions, meaning that the reaction is occurring at a linear rate and less than 10% of the substrate is consumed. These parameters ensure that substrate concentration ([S]) can be assumed to remain constant for a given point. To find steady state conditions a reaction progress curve should be obtained before starting kinetic assays. See (Brooks et al., 2004) for an explanation of how to run a reaction progress experiment.
[Enzyme]≪<[Substrate]
Enzyme concentration must be significantly lower than substrate concentration (generally at least 100x lower), this allows for enzyme concentration to be equated to zero and excluded from the Michaelis-Menten equation.
Graph as a function of reaction rate
To obtain kcat from a graph of Michaelis-Menten data, assay output signal must be converted to reaction rate. To do so, a standard curve should be generated so that output can be converted to a known amount of substrate conversion. For example, in our radioactivity assays the read out is in CPM (counts per minute) based on how much 14C-acetyl is transferred from the acetyl CoA to H4. To generate our standard curve we measure a known number of moles of 14C- acetyl CoA so that CPM can be related to molar transfer of the acetyl group in a linear equation. That value is then divided by reaction volume to obtain a reaction rate with units of M s−1.
3.2 Specific kinetic properties of MYST acetyltransferases
The MYST family of acetyltransferases exhibit high substrate Km values. For example, the Km for the yESA1 HAT domain is ~920 μM for histone H3 (Yan et al., 2000). An important consideration when studying the MYST family is that a high Km for the substrate peptide causes saturating conditions to be extremely high. For example in the case of the yESA1, 9.2 mM of H3 would be 10x Km, and thus saturating. To accommodate this MYST family characteristic, we have mainly used radioactivity based assays to measure enzyme activity. Radioactive read outs tend to be quite sensitive, and are not typically limited by binding capacities. For example if we require the attachment of the peptide substrate to a bead for a fluorescence based assay, the binding capacity of the bead might be less than saturating for the peptide and we might then not be able to achieve maximum velocity (Vmax). Furthermore, when using a synthetic peptide as the substrate, using such a high concentration may lead to trifluoroacetic acid (TFA) contamination that could destabilize the enzyme. According to Genscript’s website, synthetic peptides may contain 10–45% TFA salt (“Genscript TFA Removal Service http://www.genscript.com/tfa_removal_service.html,”). This amount of TFA could be damaging to the enzyme of interest, leading to a much lower Vmax in assays where the peptide is the saturating component, or a decrease in activity as peptide concentration increases. To avoid this issue, it may be important to remove the TFA for kinetic studies. If that option is not available, then using more stringent buffering conditions or using slightly less than 10x Km as an assumed saturating condition may be the best solution.
3.3 Controlling for chemical acetylation
Another hurdle that we have encountered in our lab is chemical acetylation of substrates. Because the acetyl group on acetyl CoA is a fairly labile bond it is possible for the acetyl transfer to occur in the absence of enzyme, especially with high concentrations of acetyl CoA in the presence of basic peptides. To control for this background chemical acetylation, it is imperative to include a control experiment that contains substrate and acetyl CoA but no enzyme. This can be especially important when testing very low activity levels such as when studying catalytic mutants. The chemical acetylation should then be subtracted from assay readouts to obtain a more accurate value for enzyme activity. For an example see (Figure 4).
Figure 4.
Progress curve showing the enzymatic activity of the hMOF174-449 K274M mutant over time as compared to a chemical acetylation control, which contains only the substrate and acetyl-CoA, but no enzyme. The acetylation that occurs in the presence of the mutant enzyme is just slightly greater than the chemical acetylation observed in the absence of enzyme, indicating that this mutant has lost much of its catalytic activity. Note, however, that the activity levels of the mutant enzyme could be misleading without the chemical acetylation control.
3.4 Kinetic assay using radioactive acetyl-CoA
To perform kinetic experiments using a radioactivity-based assay we first mix hMOF at 50 nM with H4 peptide in PCR tubes in a buffer containing 40 mM Tris (pH 8.0), 100 mM NaCl, 800 μM Cys, and 7.5μM BSA. Depending on which substrate is tested, H4 will either be at varied concentrations or saturating. We begin the assay by adding 14C-acetyl CoA (which will also be varied or saturating depending on which substrate is being examined) in a final volume of 50 μL. Because these assays are so sensitive, and we want each experiment to run for exactly the same amount of time, we space out each reaction by 30 seconds (that is, begin reaction one at time 0:00, two at 0:30, three at 1:00 etc.). We allow the reaction to proceed for eight minutes, which we have previously determined to be within the linear range for the enzyme. To stop the reaction, we spot 20 μL each of the reaction on to two P81 filter papers, which will bind the positively charged H4 peptide. This step, again, is done in 30-second intervals so that each reaction proceeds for exactly eight minutes (that is, spot reaction one at time 8:00, two at 8:30, three at 9:00 etc.). The filter papers are then washed 3x in 20 mM HEPES (pH 7.5). Because water is insoluble in some scintillation fluids and might interfere with signal readout, we dip each filter paper in acetone and lay each one out to dry on a paper towel. We then put each filter paper in a scintillation vial, add scintillation fluid and read our results on a scintillation counter where CPM will correlate with the amount of 14C-acetyl that has been transferred from the acetyl CoA to the H4 peptide and thus, enzyme activity. We can then use this data to prepare Michaelis-Menten curves for the enzyme (Figure 5).
Figure 5.
Michaelis-Menten curves and calculated steady state parameters for hMOF174-449. Note that these values vary somewhat from the aforementioned Km values for hMOF of 50 μM and 400 μM for acetyl CoA and H4 peptide, respectively, likely due to the difference in enzyme preparation and buffer conditions. To calculate the kcat for this data, Vmax was converted from CPM to M s−1 using a standard curve (data not shown) and divided by the total enzyme concentration of 50 nM.
4. Thermofluor assay to investigate cofactor and inhibitor binding
A thermofluor assay measures the melting temperature of a protein (Pantoliano, Rhind, & Salemme, 2000). If the protein is stabilized by a cofactor or ligand it will have a higher melting temperature. For example, Apo hMOF HAT domain has a Tm of about 37°C, however, upon addition of 100 μM CoA, acetyl CoA, or acetonyl CoA the Tm increases to approximately 47 °C, 50 °C and 52 °C, respectively; interestingly, however, we do not observe a strong thermal shift of hMOF in the presence of H4 peptide (Figure 6). The thermofluor assay uses SYPRO® Orange, a dye that fluoresces in a hydrophobic environment. Thus if SYPRO® Orange is present as a protein is melting it will bind the exposed hydrophobic patches and will fluoresce with increasing intensity as a protein unfolds. This data can then be plotted to produce a melting curve. All parameters for this assay can be determined empirically and may vary from protein to protein, but in general approximately 1 μM of protein (15 μL) is incubated with 100 μM of compound (1 μL) followed by addition of a 1:300 dilution of a 5000x concentrate of SYPRO® Orange (4 μL) to a 20 μL final volume. Each sample is prepared in a minimum of triplicate and read using RT-PCR measuring from 20°C to 95°C.
Figure 6.
Thermofluor assay as a readout of hMOF ligand binding. Data for apo hMOF, and hMOF in the presence of 1 mM H4 peptide, and 100 μM CoA, acetyl CoA, and acetonyl CoA , respectively. Tm is the inflection point for each curve: ~37°C for apo hMOF, ~47°C for hMOF with CoA, ~50°C for hMOF with acetyl CoA, ~52°C for hMOF with acetonyl CoA, and ~38°C for hMOF with H4 peptide.
There are some caveats to this assay. Firstly, because of the nature of SYPRO® Orange fluorescence in a hydrophobic environment, detergent in the buffer can interfere with signal. In our experience, baseline fluorescence of SYPRO® Orange in the presence of detergent (0.05% Triton-X) is significantly increased, and completely masks any melting data from the protein. Decreasing to 0.01% Triton X shows some improvement but still complicates the melting curve of the protein. It is also possible that the detergent may directly affect protein unfolding properties. If detergent is absolutely required, then other dye options would need to be explored to employ this assay. Additionally, if another protein is present in the sample (a contaminant or BSA, for example) that protein will melt also, producing its own melting curve in tandem with the protein of interest and thus complicating the data.
5. Inhibitor Screening
5.1 General considerations
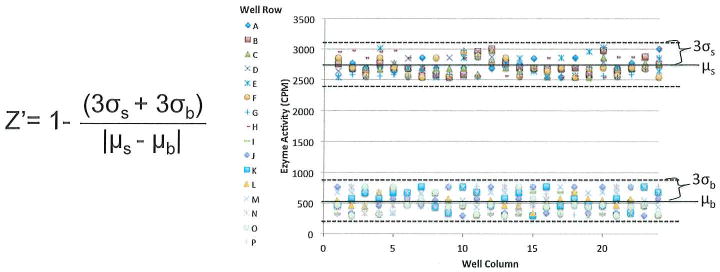
There are certain parameters that need to be considered for all high throughput screening campaigns. These are reviewed in (Williams & Scott, 2009) (Inglese, Johnson, Simeonov, Xia, Zheng, Austin et al., 2007). This chapter seeks to touch on a subset of key considerations that we encountered when developing a MYST inhibitor screening assay and should not be treated as a comprehensive screening guide. Briefly, one should consider what assay is going to be most appropriate for the system being tested and what buffer conditions are appropriate for that assay. Typically, an additive of some kind will be required to terminate all of the reactions in HTS simultaneously; for our assays we have used 3.7% HCl for this purpose. Also, a control inhibitor is often required to monitor screening quality statistics. Acetonyl-CoA is a non-hydrolyzable acetyl CoA analog that has served us well as a control inhibitor against MYST proteins. An orthogonal secondary assay must also be planned to counter screen any hits from the primary assay to eliminate any false positives that may have interfered with the detection system of the primary assay. There are also a few standard statistics for HTS that should be performed before screening compound libraries. These are discussed in (Coma, Herranz, & Martin, 2009). One important validation statistic is the Z-factor, a parameter which measures the screening window of an assay to assess assay quality (Zhang, Chung, & Oldenburg, 1999) (Figure 7).
Figure 7.
Equation and sample data for determining assay Z-factor. Z’= Z-factor, σs = standard deviation of the positive signal, σb= standard deviation of the background signal, μs =mean of the positive signal, and μb =mean of the background signal. Each point in the graph represents the readout for a single reaction within a 384 well plate.
The Z-factor can be any value from 0 to 1 where assay quality increases with increasing Z-factor, which would indicate a large signal window with low signal variability. To obtain a Z-factor a control plate should be run in the assay where half of the reactions are a control for positive signal (enzyme with no inhibitor present/ DMSO only) and the other half of the reactions are controls for background signal (either containing no enzyme, a catalytically dead mutant of the enzyme, or WT enzyme plus a saturating amount of control inhibitor, depending on what is available). The means and standard deviations of the results can then be calculated and put into the equation shown in Figure 7 to calculate Z-factor. Generally the minimum acceptable Z-factor value is 0.5, with higher values being more desirable.
In general, when screening for inhibitors each substrate concentration should be set at Km within a reaction. This allows the assay to be sensitive to competitive inhibitors while maintaining robust signal (Williams et al., 2009). However, this can pose a problem for MYST family proteins, which tend to have a very high Km for their substrates. It must be determined if enough of the substrate can be obtained to perform an entire screening campaign, which often involves a minimum of thousands of reactions. Furthermore, if the assay requires immobilization of the substrate, then the binding surface must have a high enough capacity to reach substrate Km. It may be reasonable to use less than Km of the substrate if the Km is too high to reasonably achieve in the screen. However in this case, it is important to consider that lowering the substrate concentration will slow the reaction rate. This may mean that your reaction will have to be run for a longer amount of time to obtain a detectable signal to noise ratio. In this case, it is important that the enzyme be stable enough to survive the duration of the assay. Additionally, lowering the concentration of one substrate in a bisubstrate system causes the screen to have a greater bias for finding inhibitors that are competitive with that substrate as it could then be easier to compete off of the enzyme.
5.2. Buffer Considerations
It is important to remember that when transitioning from low throughput to high throughput assays there will potentially be a transition to more forms of solution transfer which may translate into a greater variety of contact surfaces for the enzyme/ reaction mixture (tubing, transfer pins, etc.). In our experience working with the MYST protein hMOF, we have found that while we have had limited difficulty with protein stickiness in low throughput, we lose a significant portion of the protein through the course of transfer steps. To alleviate the effects of a much lower signal than expected when transitioning from low throughput to high throughput we tested the addition of bovine serum albumin (BSA) to our assay buffer, finding that it increased our signal by about 5 times.
Cysteine reactive compounds can lead to a high number of false hits in a screening campaign. In the case of MYST HATs, which have been demonstrated to employ an active site cysteine for acetyltransferase activity (Yan et al., 2000), this can be a particular problem. It may be appropriate to add some amount of reducing agent to the reaction buffer to limit the amount of inhibition due to compounds reacting with the active site cysteine. This however can lead to more problems as it has been shown that redox cycling, or production of peroxide caused by strong reducing agent interactions with some compounds, can also lead to a high false hit rate (Johnston, Soares, Shinde, Foster, Shun, Takyi et al., 2008). To circumvent this issue, we have included in some of our assays free cysteine in an attempt to outcompete cysteine reactive compounds.
Small amounts of non-ionic detergent can also help to decrease a false hit rate (Feng, Simeonov, Jadhav, Babaoglu, Inglese, Shoichet et al., 2007). This is especially true when screening MYST family acetyltransferases where screening at a higher compound concentration may lead to colloidal aggregation, which will be discussed in more detail in the “Eliminating Promiscuous Inhibitors” section of this chapter.
5.3 Assays
In the case of the MYST acetyltransferases, we have developed a radioactivity based assay and an ELISA assay to use in high throughput. We have also developed a thermofluor assay to be used as a secondary orthogonal assay in low throughput.
5.3.1 Radioactivity based assay
Because of the sensitivity and robustness of radioactivity assays, we decided to adapt the radioactivity based P81 filter paper assay to be used in high throughput as a primary assay. The assay is performed by dispensing hMOF (or inactive mutant hMOF E350Q as a negative control) into each well of a 384 well plate followed by the addition of compound and then an H4 peptide/ 14C-acetyl CoA solution to final concentrations of 50 nM hMOF, 50 μM 14C- acetyl CoA, and 400 μM H4 peptide (both substrates are used at ~Km) in 40 mM Tris (pH 8.0), 100 mM NaCl, 800 μM Cys, and 7.5 μM BSA. The reaction is allowed to proceed for 1 hour and then 3.7% HCl is added to each well to stop the reaction. The reaction is then transferred from the 384 well plate to four 96 well p81 paper embedded mesh bottom plates. A vacuum manifold is used to pull excess liquid from the wells. Next, each well is washed with 10 mM HEPES (pH 7.5), followed by addition of acetone, which is also vacuumed off. The plate bottoms are then sealed and scintillation fluid is added to each well. The plates are then sealed and read on a TopCount plate reader. The Z-factor for this assay has remained >0.6 for all optimized trials and we have confirmed the ability of the assay to detect inhibition by acetonyl CoA.
5.3.2 ELISA Assay
We also developed an ELISA assay, which is performed in automation by first binding H4-GST to a 384-well glutathione coated plate. We have found that hMOF acetylates H4-GST almost as well as H4 peptide, whereas GST-H4 barely becomes acetylated at all. 50 nM enzyme, followed by test compounds are added to the plate followed by 50 μM acetyl CoA. The reaction is allowed to react for 3 hours to obtain a suitable signal window. Primary anti-H4AcK antibody is added and allowed to bind for 30 minutes. Next, the secondary antibody conjugated to horseradish peroxidase (HRP) is added and allowed to bind for 30 minutes. Chemiluminescent substrate is added and is oxidized by HRP to produce light that can be detected on a plate reader. The plate is washed between each step. The Z-factor has remained reproducibly above 0.6.
This assay provides a good example of how requiring a binding surface may limit how much substrate can be included in the assay, thus slowing reaction time. In the case of this assay, because screening could take multiple hours, the stability of the assay was tested as a function of time. Another option is to perform the full reaction in a separate plate keeping the H4-GST concentration at Km and then transferring the full reaction to the glutathione plate second. This however adds an extra step, so it is important to weigh the pros and cons that adding the extra step would introduce to the assay and the screening campaign.
Another important aspect of this assay to note is that not all primary antibodies developed to recognize the acetylated substrate will perform equally well. In the case of this assay, multiple antibodies were characterized via a dot blot as well as screening within the assay to ensure we could achieve the highest signal to noise ratio with the best performing antibody. In our experience, Histone H4 tetra-acetyl antibody (pAb) (Cat# 39179, 39228), and Histone H4ac (pan-acetyl) antibody (pAb) (Cat# 39243, 39244) from Active Motif have performed relatively well in our assay giving signal windows of approximately 10x and 4x, respectively.
5.3.3 Other Assays
There are a number of other assays that we have considered or used that despite their successes with other systems, we have not yet been able to optimize to work effectively with the MYST family of acetyltransferases. Some methods such as Alpha (Amplified Luminescent Proximity Homogeneous Assay) and LANCE® TR-FRET (time resolution- fluorescence resonance energy transfer) assays rely on both substrate binding to beads, as well as antibody recognition of the substrate. Briefly, for these assays there are two beads, one donor bead and one acceptor bead. For our purposes, one bead is conjugated to H4 peptide, and the other bead is conjugated to an antibody that recognizes acetylated H4K16. Thus, when hMOF acetylates the H4 peptide on one bead, the antibody on the other bead will bind the acetylated peptide, bringing the donor and acceptor beads in close proximity so that a signal can be detected. In the case of the Alphascreen® when the beads are in close proximity and illuminated with 680nm light, singlet oxygen is transferred from the donor bead to the acceptor bead, which will react to produce 520–620nm light (“User's Guide to Alpha Assays Protein:Protein Interactions,” 2011). In the case of the LANCE® TR-FRET assay, when the beads are in close proximity irradiated europium chelate molecules from the donor beads transfer energy to the acceptor bead which contain a dye that converts that energy to light which emits at 665nm (Legault, Roby, Beaudet, & Rouleau, 2006). These assays can be particularly tricky to use with MYST proteins because, as mentioned before, substrate peptide Km tends to be very high, meaning that the binding capacity of the beads would also need to be quite high. If the beads are unable to bind a high level of the H4 peptide either the level of peptide will have to be decreased, slowing reaction rate, or the assays will display what is known as a “hooking effect” where the free peptide outcompetes the peptide that is bound to the bead to artifactually lower signal (“User's Guide to Alpha Assays Protein:Protein Interactions,” 2011). Furthermore, the success of the assay depends on the antibody conjugated bead being specific for a given substrate, and it could take excessive amounts of time and money to test several antibody conjugated beads to find the best one for optimum signal, if multiple antibodies are even available.
Another assay that has been troublesome for us due to antibody recognition of substrate is fluorescence polarization. To perform this assay we ordered the H4 peptide conjugated to a FITC dye. To our surprise, none of the antibodies we tested were able to recognize the acetylated peptide in the presence of the FITC tag. We have also tried to use a CoA detection assay. Briefly this assay uses a sulfur reactive dye that reacts with CoA after acetyl CoA is cleaved in the enzymatic assay (Bulfer, McQuade, Larsen, & Trievel, 2011; Trievel, Li, & Marmorstein, 2000). This assay has been ineffective with the MYST proteins for multiple reasons. Firstly, there are eight cysteines in our construct of the hMOF HAT domain. Despite the low levels of enzyme used in activity assays (50 nM), the apparent interaction between these cysteines and the dye appears to cause high background signal. Furthermore, the sensitivity of this assay has not been robust enough to detect the generally low levels of activity of MYST proteins.
5.4 Thermofluor Binding Assay
We have taken advantage of the thermofluor assay as a secondary orthogonal screen to perform in low throughput to validate compounds identified in the primary assay. We have also begun efforts to translate this assay from low throughput to high throughput for fragment screening. We currently perform the high throughput assay by adding 15 μL of 10 μM hMOF in 20 mM HEPES pH 7.5, 300 mM NaCl to each well, followed by 1 μL of 20 mM fragment compound to a final concentration of 1 mM compound (or 100 μM CoA as a positive control). After allowing the compounds to sit with the protein for 30 minutes, 4 μL of a 1:300 dilution of 5000x SYPRO® Orange dye is added to each well. The plate is then sealed and read on a real-time qPCR instrument. For this assay, we have had some difficulty with deviations in control Tm and obtaining quality screening statistics. Further optimization will be required to determine if this assay will be suitable for high-throughput screening of MYST proteins.
5.5 Eliminating Promiscuous Inhibitors
After completing a screening campaign, it is important to focus on the highest quality hits that have the potential to be developed into probes or therapeutics. In general, a good inhibitor should display reproducible dose-dependent inhibition, have a Hill slope of approximately 1.0, bind reversibly to the protein (although covalent inhibitors can be beneficial under certain circumstances, see (Copeland, 2005), chapter 8), and also be selective for the protein of interest. When a compound identified through screening does not meet these criteria, it becomes important to scrutinize it carefully to identify and eliminate compounds that are disrupting enzymatic activity through nonspecific interactions. For example, MYST family acetyltransferases were found to be particularly sensitive towards cysteine reactive compounds, mercury containing compounds, and copper containing compounds. As mentioned earlier, MYST proteins have an active site cysteine responsible for acetyl transfer, thus cysteine reactive compounds may nonspecifically form disulfide bonds to inhibit the protein. Similarly, mercury-containing compounds are also able to interact nonspecifically with the active site cysteine. Additionally, in our experience, copper-containing compounds are especially active against the MYST family proteins. We hypothesize that this occurs because either the copper interferes with the active site cysteine, or otherwise may be displacing the zinc in the zinc finger or both. These three types of compounds are poor candidates for further optimization.
5.6 PAINS
Pan-assay interference compounds (PAINS) are a class of compounds that mimic genuine inhibitors by interfering with assay readouts or undergoing nonspecific reactions with proteins. About half of PAINS known today fall into just 16 structural categories (Baell & Walters, 2014), and learning these structures can save a lot of time and effort. However, because about half of PAINS do not fit into these categories it is also prudent to check the literature for other reports of a selected compound hitting unrelated proteins as this could be a red flag (Baell et al., 2014).
In our experience with MYST proteins, it is especially important to verify the identity and purity of the identified hits in the library. Compounds within a library can break down over time into reactive products (Baell et al., 2014). Through the course of our assays, we have found that the MYST protein hMOF had numerous hits where mass spec revealed a plethora of compounds were present in the sample when there should have only have been one. This leads us to believe that MYST proteins may be particularly sensitive to these sorts of reactive breakdown products.
Testing for the reversibility of a compound can be a fairly straightforward way to determine if it is interacting with the protein of interest through desirable interactions. If a compound is specifically binding a region of a protein in a dose-dependent manner, then the enzyme should regain activity when the compound is diluted (Copeland, 2005). Briefly, to perform this experiment (in the context of the MYST protein, hMOF), 5 μM enzyme is first incubated with 10x IC50 of a compound for ~30 minutes. The solution is then diluted 100x such that the enzyme concentration is at 50 nM and the compound is at 0.1x IC50, where little to no activity should be expected from an inhibitor. A reaction is then started by adding H4 peptide and acetyl CoA at Km and time points are taken every five minutes for an hour. As a positive control for reversibility, we measure enzyme activity with no inhibitor added (DMSO control) as well as enzyme activity with the reversible inhibitor acetonyl CoA. As a negative control for reversibility, we used merbromin, a mercury-containing compound that interacts with the active site cysteine. If a compound reversibly binds to the enzyme then the activity of the enzyme should increase with time aligning with the positive controls, and if a compound is irreversible, the enzyme will remain inhibited and little activity will be seen over time, aligning with the negative control (Figure 8). Compounds that are found to be irreversible inhibitors may be nonspecifically modifying the protein of interest and are much less likely to serve as good candidates for optimization.
Figure 8.

Sample data for an enzyme recovery assay with a MYST protein. Note that both of the test compounds display irreversible inhibition, as they inhibit enzyme activity after dilution, aligning with the control irreversible inhibitor merbromin, a mercury containing compound that interacts with the active site cysteine. Also note that acetonyl CoA, a reversible inhibitor, aligns very closely to the DMSO control.
5.7 Colloidal aggregators
Colloidal aggregators are compounds that aggregate and sequester a protein to limit its activity (McGovern, Helfand, Feng, & Shoichet, 2003). This can be a particular problem when screening MYST proteins as compounds tend to be screened at higher inhibitor concentrations (low micromolar range) where they can have a higher tendency to aggregate. In general, if a compound is reversibly interacting with an enzyme in a 1:1 stoichiometry then the Hill slope for that compound with the enzyme should be ~1 (Feng et al., 2007), and if the Hill slope is higher than 1, this may indicate that the compound is acting through colloidal aggregate inhibition. Furthermore, colloidal aggregates can be solubilized by the addition of small amounts of non ionic detergents (~0.01% Triton-X) (McGovern et al., 2003). If running an activity assay in the presence of detergent causes the inhibitory activity of a compound to be lost or significantly decreased, this may be a sign that the compound is acting via aggregation rather than by a specific interaction. To avoid initial identification of colloidal aggregators in a primary screen it can be helpful to include small amounts of non-ionic detergent in the assay buffer. However, if detergent interferes with the assay readout of the primary screen, it is important to then include detergent in secondary screens to eliminate compounds acting through colloidal aggregation.
6. Prospects and Conclusions
The MYST family proteins remain one of the more understudied families of the acetyltransferases. This is likely, at least in part, due to their weak affinity for their substrates, which can make them especially challenging to study. Furthermore, the MYST proteins appear to be only weakly inhibited by validated inhibitors, and significantly sensitive to nonspecific inhibitors further complicating efforts to further probe their activity and find new inhibitors. This chapter seeks to prepare researchers for some hurdles that they may encounter when studying the MYST proteins so that there may be better opportunity to plan stringent controls and obtain high quality data.
Acknowledgments
This work was supported by National Institutes of Health grants R01 GM060293, R01 GM098910 and P01 AG031862 to R.M, and T32 GM008275 to C.E.M. We acknowledge support of the molecular screening facility at the Wistar Institute (NIH P30 CA010815) and the DNA Sequencing Facility at the Perelman School of Medicine, University of Pennsylvania (NIH P30 CA016520).
References
- Baek SH, Ohgi KA, Rose DW, Koo EH, Glass CK, Rosenfeld MG. Exchange of N-CoR corepressor and Tip60 coactivator complexes links gene expression by NF-kappaB and beta-amyloid precursor protein. Cell. 2002;110(1):55–67. doi: 10.1016/s0092-8674(02)00809-7. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12150997. [DOI] [PubMed] [Google Scholar]
- Baell J, Walters MA. Chemistry: Chemical con artists foil drug discovery. Nature. 2014;513(7519):481–483. doi: 10.1038/513481a. [DOI] [PubMed] [Google Scholar]
- Berndsen CE, Albaugh BN, Tan S, Denu JM. Catalytic mechanism of a MYST family histone acetyltransferase. Biochemistry. 2007;46(3):623–629. doi: 10.1021/bi602513x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks HB, Geeganage S, Kahl SD, Montrose C, Sittampalam S, Smith MC, Weidner JR. Basics of Enzymatic Assays for HTS. In: Sittampalam GS, Coussens NP, Nelson H, Arkin M, Auld D, Austin C, Bejcek B, Glicksman M, Inglese J, Iversen PW, Li Z, McGee J, McManus O, Minor L, Napper A, Peltier JM, Riss T, Trask OJ Jr, Weidner J, editors. Assay Guidance Manual. Bethesda (MD): 2004. [Google Scholar]
- Bulfer SL, McQuade TJ, Larsen MJ, Trievel RC. Application of a High-throughput Fluorescent Acetyltransferase Assay to Identify Inhibitors of Homocitrate Synthase. Analytical biochemistry. 2011;410(1):133–140. doi: 10.1016/j.ab.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Sudhof TC. A transcriptionally [correction of transcriptively] active complex of APP with Fe65 and histone acetyltransferase Tip60. Science. 2001;293(5527):115–120. doi: 10.1126/science.1058783. [DOI] [PubMed] [Google Scholar]
- Cao X, Sudhof TC. Dissection of amyloid-beta precursor protein-dependent transcriptional transactivation. J Biol Chem. 2004;279(23):24601–24611. doi: 10.1074/jbc.M402248200. [DOI] [PubMed] [Google Scholar]
- Cereseto A, Manganaro L, Gutierrez MI, Terreni M, Fittipaldi A, Lusic M, … Giacca M. Acetylation of HIV-1 integrase by p300 regulates viral integration. EMBO J. 2005;24(17):3070–3081. doi: 10.1038/sj.emboj.7600770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coma I, Herranz J, Martin J. Statistics and decision making in high-throughput screening. Methods Mol Biol. 2009;565:69–106. doi: 10.1007/978-1-60327-258-2_4. [DOI] [PubMed] [Google Scholar]
- Copeland RA. Evaluation of enzyme inhibitors in drug discovery. A guide for medicinal chemists and pharmacologists. Methods Biochem Anal. 2005;46:1–265. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16350889. [PubMed] [Google Scholar]
- Feng BY, Simeonov A, Jadhav A, Babaoglu K, Inglese J, Shoichet BK, Austin CP. A high-throughput screen for aggregation-based inhibition in a large compound library. J Med Chem. 2007;50(10):2385–2390. doi: 10.1021/jm061317y. [DOI] [PubMed] [Google Scholar]
- Fraga MF, Ballestar E, Villar-Garea A, Boix-Chornet M, Espada J, Schotta G, … Esteller M. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat Genet. 2005;37(4):391–400. doi: 10.1038/ng1531. [DOI] [PubMed] [Google Scholar]
- Genscript TFA Removal Service. http://www.genscript.com/tfa_removal_service.html. Retrieved from http://www.genscript.com/tfa_removal_service.html.
- Hobbs CA, Wei G, DeFeo K, Paul B, Hayes CS, Gilmour SK. Tip60 protein isoforms and altered function in skin and tumors that overexpress ornithine decarboxylase. Cancer Res. 2006;66(16):8116–8122. doi: 10.1158/0008-5472.CAN-06-0359. [DOI] [PubMed] [Google Scholar]
- Iizuka M, Stillman B. Histone acetyltransferase HBO1 interacts with the ORC1 subunit of the human initiator protein. J Biol Chem. 1999;274(33):23027–23034. doi: 10.1074/jbc.274.33.23027. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10438470. [DOI] [PubMed] [Google Scholar]
- Inglese J, Johnson RL, Simeonov A, Xia M, Zheng W, Austin CP, Auld DS. High-throughput screening assays for the identification of chemical probes. Nat Chem Biol. 2007;3(8):466–479. doi: 10.1038/nchembio.2007.17. [DOI] [PubMed] [Google Scholar]
- Johnston PA, Soares KM, Shinde SN, Foster CA, Shun TY, Takyi HK, … Lazo JS. Development of a 384-well colorimetric assay to quantify hydrogen peroxide generated by the redox cycling of compounds in the presence of reducing agents. Assay Drug Dev Technol. 2008;6(4):505–518. doi: 10.1089/adt.2008.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita A, Whelan CM, Berezovska O, Hyman BT. The gamma secretase-generated carboxyl-terminal domain of the amyloid precursor protein induces apoptosis via Tip60 in H4 cells. J Biol Chem. 2002;277(32):28530–28536. doi: 10.1074/jbc.M203372200. [DOI] [PubMed] [Google Scholar]
- Kitabayashi I, Aikawa Y, Yokoyama A, Hosoda F, Nagai M, Kakazu N, … Ohki M. Fusion of MOZ and p300 histone acetyltransferases in acute monocytic leukemia with a t(8;22)(p11;q13) chromosome translocation. Leukemia. 2001;15(1):89–94. doi: 10.1038/sj.leu.2401983. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11243405. [DOI] [PubMed] [Google Scholar]
- Lafon A, Chang CS, Scott EM, Jacobson SJ, Pillus L. MYST opportunities for growth control: yeast genes illuminate human cancer gene functions. Oncogene. 2007;26(37):5373–5384. doi: 10.1038/sj.onc.1210606. [DOI] [PubMed] [Google Scholar]
- Legault M, Roby P, Beaudet L, Rouleau N. Comparison of LANCE Ultra TR-FRET to PerkinElmer’s Classical LANCE TR-FRET Platform for Kinase Applications: PerkinElmer Life and Analytical Sciences 2006 [Google Scholar]
- Lin Y-y, Lu J-y, Zhang J, Walter W, Dang W, Wan J, … Zhu H. Protein Acetylation Microarray Reveals that NuA4 Controls Key Metabolic Target Regulating Gluconeogenesis. Cell. 2009;136(6):1073–1084. doi: 10.1016/j.cell.2009.01.033. http://dx.doi.org/10.1016/j.cell.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lleonart M, Vidal F, Gallardo D, Diaz-Fuertes M, Rojo F, Cuatrecasas M, … Ramon y Cajal S. New p53 related genes in human tumors: significant downregulation in colon and lung carcinomas. Oncol Rep. 2006;16(3):603–608. doi: 10.3892/or.16.3.603. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16865262. [DOI] [PubMed] [Google Scholar]
- McGovern SL, Helfand BT, Feng B, Shoichet BK. A specific mechanism of nonspecific inhibition. J Med Chem. 2003;46(20):4265–4272. doi: 10.1021/jm030266r. [DOI] [PubMed] [Google Scholar]
- Mellert HS, McMahon SB. hMOF, a KAT(8) with many lives. Mol Cell. 2009;36(2):174–175. doi: 10.1016/j.molcel.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Pantoliano MW, Rhind AW, Salemme FR. Microplate thermal shift assay for ligand development and multi-variable protein chemistry optimization: Google Patents 2000 [Google Scholar]
- Sapountzi V, Cote J. MYST-family histone acetyltransferases: beyond chromatin. Cell Mol Life Sci. 2011;68(7):1147–1156. doi: 10.1007/s00018-010-0599-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trievel RC, Li FY, Marmorstein R. Application of a Fluorescent Histone Acetyltransferase Assay to Probe the Substrate Specificity of the Human p300/CBP-Associated Factor. Analytical biochemistry. 2000;287(2):319–328. doi: 10.1006/abio.2000.4855. http://dx.doi.org/10.1006/abio.2000.4855. [DOI] [PubMed] [Google Scholar]
- User's Guide to Alpha Assays Protein:Protein Interactions. PerkinElmer Life and Analytical Sciences. 2011. [Google Scholar]
- Williams KP, Scott JE. Enzyme assay design for high-throughput screening. Methods Mol Biol. 2009;565:107–126. doi: 10.1007/978-1-60327-258-2_5. [DOI] [PubMed] [Google Scholar]
- Wu J, Wang J, Li M, Yang Y, Wang B, Zheng YG. Small molecule inhibitors of histone acetyltransferase Tip60. Bioorg Chem. 2011;39(1):53–58. doi: 10.1016/j.bioorg.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Xie N, Wu Z, Zhang Y, Zheng YG. Bisubstrate Inhibitors of the MYST HATs Esa1 and Tip60. Bioorg Med Chem. 2009;17(3):1381–1386. doi: 10.1016/j.bmc.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Yan Y, Barlev NA, Haley RH, Berger SL, Marmorstein R. Crystal structure of yeast Esa1 suggests a unified mechanism for catalysis and substrate binding by histone acetyltransferases. Mol Cell. 2000;6(5):1195–1205. doi: 10.1016/s1097-2765(00)00116-7. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11106757. [DOI] [PubMed] [Google Scholar]
- Yang XJ. The diverse superfamily of lysine acetyltransferases and their roles in leukemia and other diseases. Nucleic Acids Res. 2004;32(3):959–976. doi: 10.1093/nar/gkh252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Rossetto D, Mellert H, Dang W, Srinivasan M, Johnson J, … Marmorstein R. MYST protein acetyltransferase activity requires active site lysine autoacetylation. EMBO J. 2012;31(1):58–70. doi: 10.1038/emboj.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JH, Chung TD, Oldenburg KR. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J Biomol Screen. 1999;4(2):67–73. doi: 10.1177/108705719900400206. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10838414. [DOI] [PubMed] [Google Scholar]