Abstract
Background:
Controversy persists about whether certain antidepressants reduce tamoxifen’s effectiveness on lowering breast cancer recurrence. We investigated whether taking tamoxifen and antidepressants (in particular, paroxetine) concomitantly is associated with an increased risk of recurrence or contralateral breast cancer.
Methods:
We examined 16 887 breast cancer survivors (TNM stages 0–II) diagnosed between 1996 and 2007 and treated with tamoxifen in two California health plans. Women were followed-up through December 31, 2009, for subsequent breast cancer. The main exposure was the percent of days of overlap when both tamoxifen and an antidepressant (paroxetine, fluoxetine, other selective serotonin reuptake inhibitors, tricyclics, and other classes) were used. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using multivariable Cox regression models with time-varying medication variables.
Results:
Of the 16 887 women, half (n = 8099) used antidepressants and 2946 women developed subsequent breast cancer during the 14-year study period. We did not find a statistically significant increased risk of subsequent breast cancer in women who concurrently used paroxetine and tamoxifen. For 25%, 50%, and 75% increases in percent overlap days between paroxetine and tamoxifen, hazard ratios were 1.06 (95% CI = 0.98 to 1.14, P = .09), 1.13 (95% CI = 0.98 to 1.30, P = .09), and 1.20 (95% CI = 0.97 to 1.49, P = .09), respectively, in the first year of tamoxifen treatment but were not statistically significant. Hazard ratios decreased to 0.94 (95% CI = 0.81 to 1.10, P = .46), 0.89 (95% CI = 0.66 to 1.20, P = .46), and 0.85 (95% CI = 0.54 to 1.32, P = .46) by the fifth year (all non-statistically significantly). Absolute subsequent breast cancer rates were similar among women who used paroxetine concomitantly with tamoxifen vs tamoxifen-only users. For the other antidepressants, we again found no such associations.
Conclusions:
Using the comprehensive electronic health records of insured patients, we did not observe an increased risk of subsequent breast cancer in women who concurrently used tamoxifen and antidepressants, including paroxetine.
Tens of thousands of American women with breast cancer are taking tamoxifen to reduce their chances of developing a recurrence. Tamoxifen is recommended for five years but has notable side effects including hot flashes, night sweats, and depression (1). Because hormone replacement therapy used to alleviate these difficult symptoms is contraindicated for breast cancer survivors, antidepressants have been increasingly prescribed for relief (2). Nearly half of 2.4 million breast cancer survivors in the United States use antidepressants (3–4). However, recent studies suggested that certain antidepressants reduce tamoxifen’s effectiveness in lowering subsequent breast cancer risk (5–8).
Molecular studies suggest that certain antidepressants may interfere with tamoxifen’s effectiveness (5–9). Selective serotonin reuptake inhibitor (SSRI) antidepressants, particularly paroxetine, are powerful inhibitors of cytochrome P450 (CYP) 2D6, an enzyme system that metabolizes tamoxifen into its active form. The US Food and Drug Administration subsequently imposed a black box warning on tamoxifen not to prescribe any SSRIs concurrently with tamoxifen (10–11).
However, sparse clinical evidence exists that SSRIs diminish tamoxifen’s effectiveness and no study to date has comprehensively measured antidepressant use (12–14), although one study examined the association of breast cancer mortality with antidepressants (15). Our objective was to determine whether concomitant tamoxifen and antidepressant use among women diagnosed with a first primary early-stage breast cancer is associated with an increased risk of subsequent breast cancer.
Methods
Data Sources and Setting
This study was conducted at Kaiser Permanente Southern (KPSC) and Northern California (KPNC), two health plans that comprise nearly 40 hospitals, 14 000 physicians, and over seven million members. Data elements were captured from the electronic health records, census, and State of California mortality files. Pathology reports and the health plans’ National Cancer Institute Surveillance, Epidemiology, and End Results–affiliated tumor registries were used to identify breast cancer outcomes. The institutional review boards of all study sites approved this study.
Patients and Design
Using a retrospective cohort design, we identified 19 877 women in the tumor registries who were diagnosed with a first primary early-stage breast cancer (American Joint Committee on Cancer TNM stage 0-II) (16) between January 1, 1996 and December 31, 2007. All women were age 18 years or older at diagnosis, had estrogen or progesterone receptor–positive tumors, complete pharmacy benefits, and were treated with tamoxifen for at least six months. We excluded women with a prior history of any invasive cancer (n = 2782) and women with bilateral breast cancer and/or bilateral breast mastectomy (n = 150), recurrent ipsilateral breast cancer (n = 21), or second nonbreast primaries (n = 37) within six months of their initial breast cancer diagnosis. The final cohort consisted of 16 887 breast cancer survivors.
Subsequent Breast Cancer Identification
Patients were followed-up until December 31, 2009. The main outcome was subsequent breast cancer defined as recurrences in the same breast, metastases, or contralateral breast cancer that occurred at least six months after initial breast cancer surgery. Contralateral breast cancers were identified from the tumor registries, and included noninvasive or invasive cancers. Recurrences included lesions occurring in the ipsilateral breast, with or without spread to regional areas (eg, nearby axillary lymph nodes), or distant metastasis.
Recurrences were identified through manual review of all available pathology reports (two-thirds of the cohort) and by electronic health records to identify utilization patterns and diagnoses indicative of recurrence or metastases when pathology reports were unavailable. Our hybrid approach of manually reviewing pathology text supplemented with an automated data algorithm that examined encounter, procedure, and clinical codes indicative of subsequent breast cancer was validated and yielded robust test characteristics (sensitivity = 96.9%; specificity = 92.4%). Our hybrid approach to identifying subsequent breast cancer has been described (17).
Pharmacy Exposures
All medications were extracted from computerized pharmacy dispensings. The main independent variable was the percent of tamoxifen-prescribed days when antidepressants (paroxetine, fluoxetine, other SSRIs, tricyclics, and other classes) were also prescribed. For the percent overlap calculation, the numerator was the number of days antidepressants and tamoxifen were coprescribed; the denominator was the number of days tamoxifen was prescribed. Both the numerator and denominator were calculated every three months through the end of follow-up; the calculation was a running average of all past use up to that time point. We calculated the percent overlap every three months because a single day increment is clinically negligible. This percent was entered as a continuous variable in the multivariable models.
For non–antidepressant users (ie, tamoxifen only), the percent overlap was set to 0%. Other covariate medications examined in the multivariable models included aromatase inhibitors, antipsychotic drugs, antihypertensives, other potential CYP2D6 drug inhibitors (eg, amniodarone, celecoxib, chloroquinone, cimetidine, haloperidol, propranolol), bisphosphonates, and oral adjuvant chemotherapy, which were also treated as time-dependent variables. We also ascertained history of oral contraceptive use and hormonal replacement therapy up to five years prior to the initial breast cancer diagnosis (yes/no). For all oral covariate medications, women had to have used the drug for at least six months to be considered exposed.
Covariate Characteristics
Tumor characteristics (hormone receptor status, stage, grade, histology), breast cancer surgery (mastectomy, lumpectomy), chemotherapy (yes, no), and radiotherapy (yes, no) were obtained from the tumor registries. Race/ethnicity, covariate medications, comorbidity status one year prior to diagnosis, and overall healthcare utilization were collected from electronic health records. Geocoded neighborhood income and education were based on California’s 2000 census (18).
Statistical Analysis
Differences in sociodemographics, tumor characteristics, cancer treatments, healthcare utilization, and other covariates by antidepressant use were examined by comparing frequency distributions and χ2 or Fisher exact tests (P values were two-sided). Covariate medications were also summarized by two time periods based on the initial breast cancer diagnosis: 1) before initial breast cancer diagnosis and 2) between initial diagnosis and one of the study’s endpoints.
Women were followed-up from initial breast cancer diagnosis until the date of subsequent breast cancer diagnosis, health plan disenrollment, death, or December 31, 2009, whichever occurred first. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated with Cox proportional hazard regression models using time-varying medication variables (19). These models included cumulative tamoxifen duration and the interaction of tamoxifen duration with the main exposure variable, ie, the percent of concomitant antidepressant and tamoxifen use. Both tamoxifen duration and the percent overlap were treated as continuous time-dependent variables. When a patient stopped taking tamoxifen, the percent overlap was carried forward with the most recent value to their study endpoint. Because one day of overlap is not clinically meaningful, the hazard ratios were presented for 25%, 50%, and 75% overlap, similar to a previously published article (15).
Hazard ratios in the Cox model were adjusted for aromatase inhibitor use, bisphosphonates, and the other aforementioned covariates that were deemed important in the bivariate analyses, ie, if the dropping the variable changed the effect measure by 10% or more (20). The proportional hazards assumption was tested via graphic plots and Schoenfeld residuals. Interactions with time were further checked for this assumption. We also calculated crude annualized rates of subsequent breast cancer for non–antidepressant users (tamoxifen only) and antidepressant users (paroxetine). We used SAS 9.3 (SAS Institute, Cary, NC).
To determine if medication adherence affected our findings, we repeated the analyses on a subset of women who had a medication possession ratio (MPR) for tamoxifen of at least 80%, a recognized level that suggests fairly continuous medication usage (21). The MPR was calculated as the number of days supplied (excluding last refill) divided by the number of days between first and last dispense dates (21). All statistical tests were two-sided, and a P value of less than .05 was considered statistically significant.
Nested Case-Control Analysis
To explore potential unmeasured confounding and effect modification, we conducted a nested case-control study to abstract additional covariates from medical charts. A total of 250 case patients and 250 matched control patients were chart-reviewed. The covariates included family history of breast cancer in a first-degree relative, history of mammographically dense breasts or atypical hyperplasia, menopause status, smoking, body mass index (BMI), and alcohol history. Control patients included women who were alive on the date of the matched case patient’s subsequent breast cancer diagnosis date and who did not develop the outcome in the same interval. We matched women based on age at original diagnosis (+/- 1 year) and stage. In this subset, we fit additional proportional hazards models to explore the impact of these variables on the association. We checked the model’s assumptions using the same technique as described for the full cohort data.
Results
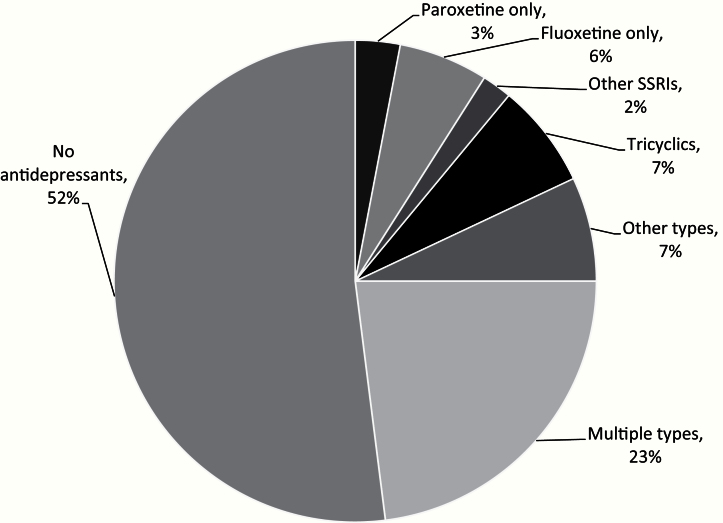
The cohort of 16 887 early-stage breast cancer survivors treated with tamoxifen was followed a maximum of 14 years (median = 6.0 years). Overall, the median tamoxifen duration was 2.7 years (interquartile range [IQR] = 1.2–4.6 years). Nearly half (48.0%, n = 8089) were prescribed antidepressants (Figure 1). The median days overlap of antidepressant and tamoxifen use was 144 days (IQR = 31–609 days). Many women (23.0%) switched antidepressants over time. Including both exclusive and nonexclusive users, fluoxetine was the most commonly used SSRI (3361 women or 19.9%), while paroxetine was used by 1784 (10.6%) women.
Figure 1.
Distribution of antidepressant use in the cohort of 16 887 breast cancer patients.
Comparison of Patients by Antidepressant Use
Demographic characteristics and healthcare utilization by initial antidepressant use categories are displayed in Table 1. The racial/ethnic diversity of the cohort was similar to the distribution in the general southern California population. Median follow-up length was higher in the antidepressant group (6.7 years) than among nonusers (5.2 years). The fraction of death was greater in antidepressant users (10.1%) than in nonusers (6.9%), though disenrollment from the health plan was lower in antidepressant users (15.1% vs 17.4%) (data not shown). Because we captured antidepressant use through the end of the study December 2009, the use of antidepressants appeared lowest in recently diagnosed patients (eg, 2006–2007).
Table 1.
Demographics and healthcare utilization of women diagnosed with breast cancer by antidepressant drugs (mutually exclusive groups)
| Characteristics * | Paroxetine (SSRI) | Fluoxetine (SSRI) | Other SSRI | Tricyclics (amitriptyline, nortriptyline, imipramine, doxepin, desipramine) | Other types (venlafaxine, trazodone, buprion, tetracyclics) | Multiple types (SSRIs and other antidepressants) | Nonusers | Total+ |
|---|---|---|---|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |
| Age at first diagnosis, y | ||||||||
| <40 | 15 (3.1) | 42 (4.1) | 9 (3.3) | 25 (2.2) | 34 (2.7) | 120 (3.1) | 400 (4.6) | 645 (3.8) |
| 40–49 | 85 (17.7) | 200 (19.4) | 52 (19.2) | 146 (12.6) | 263 (20.9) | 748 (19.2) | 1779 (20.2) | 3273 (19.4) |
| 50–59 | 126 (26.2) | 268 (26.0) | 71 (26.2) | 275 (23.7) | 424 (33.7) | 1114 (28.6) | 2257 (25.7) | 4535 (26.9) |
| 60–69 | 130 (27.0) | 271 (26.3) | 61 (22.5) | 375 (32.2) | 302 (24.0) | 1035 (26.6) | 2234 (25.4) | 4408 (26.1) |
| 70–79 | 92 (19.1) | 183 (17.7) | 56 (20.7) | 285 (24.5) | 173 (13.7) | 672 (17.3) | 1629 (18.5) | 3090 (18.3) |
| 80+ | 33 (6.9) | 68 (6.6) | 22 (8.1) | 57 (4.9) | 63 (5.0) | 203 (5.2) | 490 (5.6) | 936 (5.5) |
| Year of diagnosis | ||||||||
| 1996–1998 | 207 (43.0) | 288 (27.9) | 73 (26.9) | 461 (39.6) | 341 (27.1) | 1371 (35.2) | 2952 (33.6) | 5693 (33.7) |
| 1999–2001 | 116 (24.1) | 218 (21.1) | 44 (16.2) | 267 (23.0) | 293 (23.3) | 926 (23.8) | 1706 (19.4) | 3570 (21.1) |
| 2002–2005 | 125 (26.0) | 416 (40.3) | 92 (34.0) | 327 (28.1) | 445 (35.4) | 1259 (32.4) | 2805 (31.9) | 5469 (32.4) |
| 2006–2007 | 33 (6.9) | 110 (10.7) | 62 (22.9) | 108 (9.3) | 180 (14.3) | 336 (8.6) | 1326 (15.1) | 2155 (12.8) |
| Race/ethnicity | ||||||||
| Non-Hispanic white | 372 (77.3) | 810 (78.5) | 212 (78.2) | 851 (73.2) | 984 (78.2) | 3140 (80.7) | 5835 (66.4) | 12 204 (72.3) |
| Hispanic | 53 (11.0) | 103 (10.0) | 30 (11.1) | 94 (8.1) | 94 (7.5) | 307 (7.9) | 758 (8.6) | 1439 (8.5) |
| African American | 29 (6.0) | 56 (5.4) | 12 (4.4) | 105 (9.0) | 100 (7.9) | 252 (6.5) | 815 (9.3) | 1369 (8.1) |
| Asian/Pacific Islander | 26 (5.4) | 58 (5.6) | 15 (5.5) | 111 (9.5) | 76 (6.0) | 179 (4.6) | 1332 (15.2) | 1797 (10.6) |
| Other/unknown | 1 (0.2) | 5 (0.5) | 2 (0.7) | 2 (0.2) | 5 (0.4) | 14 (0.4) | 49 (0.6) | 78 (0.5) |
| Geocoded median household income$, † | ||||||||
| Q1 (Lower 25%) <41 793 | 134 (28.6) | 256 (25.6) | 54 (20.9) | 311 (27.5) | 253 (20.7) | 959 (25.2) | 2134 (25.1) | 4101 (25.0) |
| Q2 (>25% - 50%) >41 793 - 56 442 | 114 (24.3) | 272 (27.2) | 61 (23.6) | 270 (23.9) | 294 (24.1) | 977 (25.6) | 2106 (24.8) | 4094 (25.0) |
| Q3 (>50% - 75%) >56 442 - 74 659 | 122 (26.0) | 246 (24.6) | 71 (27.5) | 267 (23.6) | 327 (26.8) | 952 (25.0) | 2113 (24.9) | 4098 (25.0) |
| Q4 (Top 25%) >74 659 | 99 (21.1) | 226 (22.6) | 72 (27.9) | 283 (25.0) | 347 (28.4) | 925 (24.3) | 2144 (25.2) | 4096 (25.0) |
| Utilization, No. of outpatient visits up to 1 y before diagnosis | ||||||||
| None | 1 (0.2) | 4 (0.4) | 1 (0.4) | 3 (0.3) | 6 (0.5) | 5 (0.1) | 43 (0.5) | 63 (0.4) |
| Q1 (1 - 14) | 106 (22.0) | 219 (21.2) | 52 (19.2) | 213 (18.3) | 289 (23.0) | 576 (14.8) | 2945 (33.5) | 4400 (26.1) |
| Q2 (15 - 26) | 107 (22.3) | 262 (25.4) | 63 (23.3) | 261 (22.4) | 331 (26.3) | 810 (20.8) | 2350 (26.7) | 4184 (24.8) |
| Q3 (27 - 45) | 141 (29.3) | 267 (25.9) | 78 (28.8) | 321 (27.6) | 323 (25.7) | 1050 (27.0) | 1978 (22.5) | 4158 (24.6) |
| Q4 (46+) | 126 (26.2) | 280 (27.1) | 77 (28.4) | 365 (31.4) | 310 (24.6) | 1451 (37.3) | 1473 (16.8) | 4082 (24.2) |
| Mean no. of visits | 37.0 | 37.2 | 38.7 | 39.5 | 35.2 | 46.4 | 28.5 | |
| Median no. of visits | 37.0 | 28.5 | 29.0 | 31.0 | 27.0 | 35.0 | 21.0 | |
| Utilization, No. of hospitalizations up to 1 y before diagnosis | ||||||||
| 0 | 359 (74.6) | 819 (79.4) | 219 (80.8) | 909 (78.2) | 999 (79.4) | 2895 (74.4) | 7230 (82.3) | 13 430 (79.5) |
| 1+ | 122 (25.4) | 213 (20.6) | 52 (19.2) | 254 (21.8) | 260 (20.7) | 997 (25.6) | 1559 (17.7) | 3457 (20.5) |
| Charlson Index (year prior to breast cancer diagnosis) | ||||||||
| 0 | 300 (62.4) | 673 (65.2) | 182 (67.2) | 670 (57.6) | 803 (63.8) | 2299 (59.1) | 6037 (68.7) | 10 964 (64.9) |
| 1 or 2 | 150 (31.2) | 299 (29.0) | 80 (29.5) | 412 (35.4) | 393 (31.2) | 1315 (33.8) | 2391 (27.2) | 5040 (29.9) |
| 3 or more | 31 (6.4) | 60 (5.8) | 9 (3.3) | 81 (7.0) | 63 (5.0) | 278 (7.1) | 361 (4.1) | 883 (5.2) |
* P values for all variables were <.01. Probabilities based on chi-square test for homogeneity. All statistical tests were two-sided. SSRI = selective serotonin reuptake inhibitor.
† N does not add to total because of missing data.
Overall, office visits and hospitalization in the year prior to the initial breast cancer diagnosis was higher in antidepressant users (P < .01 for both) (Table 1). Roughly 65% of the cohort had no comorbidity. However, antidepressant users were more likely to have a comorbid condition.
Tumor characteristics and treatments did not vary substantially by antidepressant prescription (Table 2). The distribution of histology, grade, tumor size, lymph node status, estrogen receptor/progesterone receptor positivity, and human epidermal growth factor receptor 2 negativity varied little by antidepressant use status. The same was true for first course of cancer treatment (breast-conserving surgery or mastectomy with or with adjuvant radiation). Breast-conserving surgery with radiation was the most common treatment (42.0% both groups). Similarly, there was no difference in chemotherapy use (37.0% in both groups).
Table 2.
Tumor characteristics by ever antidepressant use*
| Characteristics | Never used | Antidepressants | Total |
|---|---|---|---|
| No. (%) | No. (%) | No. (%) | |
| Stage at diagnosis | |||
| 0 | 653 (7.4) | 407 (5.0) | 1060 (6.3) |
| I | 4364 (49.7) | 4193 (51.8) | 8557 (50.7) |
| II | 3772 (42.9) | 3498 (43.2) | 7270 (43.1) |
| P < .05 | |||
| Histology | |||
| Ductal carcinoma in situ | 273 (3.1) | 200 (2.5) | 473 (2.8) |
| Lobular Carcinoma In Situ | 8 (0.1) | 8 (0.1) | 16 (0.1) |
| Invasive Ductal Carcinoma | 5558 (63.2) | 5123 (63.3) | 10 681 (63.3) |
| Invasive Lobular Carcinoma | 758 (8.6) | 772 (9.5) | 1530 (9.1) |
| Other/Mixed category | 2192 (24.9) | 1995 (24.6) | 4187 (24.8) |
| P < .05 | |||
| Grade | |||
| 1 | 2078 (25.7) | 2153 (29.0) | 4231 (27.2) |
| 2 | 4164 (51.4) | 3706 (49.8) | 7870 (50.7) |
| 3 | 1855 (22.9) | 1577 (21.2) | 3432 (22.1) |
| P < .05 | |||
| Size of tumor, cm | |||
| No mass | 4 (0.0) | 7 (0.1) | 11 (0.1) |
| <1 | 1585 (18.9) | 1438 (18.4) | 3023 (18.6) |
| 1.00–1.99 | 3672 (43.7) | 3545 (45.4) | 7217 (44.5) |
| 2+ | 3147 (37.4) | 2814 (36.1) | 5961 (36.8) |
| P = .15† | |||
| Lymph nodes | |||
| Positive | 2422 (31.4) | 2318 (31.8) | 4740 (31.6) |
| Negative | 5289 (68.6) | 4964 (68.2) | 10 253 (68.4) |
| P = .58 | |||
| Estrogen receptor | |||
| Positive | 8673 (99.3) | 7995 (99.3) | 16 668 (99.3) |
| Negative | 58 (0.7) | 57 (0.7) | 115 (0.7) |
| P = .71 | |||
| Progesterone receptor | |||
| Positive | 6174 (94.8) | 5730 (95.2) | 11 904 (95.0) |
| Negative | 337 (5.2) | 291 (4.8) | 628 (5.0) |
| P = .54 | |||
| HER2/neu | |||
| Positive | 412 (11.9) | 330 (10.2) | 742 (11.0) |
| Negative | 3061 (88.1) | 2919 (89.8) | 5980 (89.0) |
| P < .05 | |||
| Cancer treatment | |||
| Primary therapy | |||
| Breast conserving surgery + radiation | 3623 (42.1) | 3386 (42.6) | 7009 (42.4) |
| Breast conserving surgery (no radiation) | 1555 (18.1) | 1333 (16.8) | 2888 (17.5) |
| Mastectomy (with or w/o radiation) | 3328 (38.7) | 3157 (39.8) | 6485 (39.2) |
| No primary therapy | 93 (1.1) | 65 (0.8) | 158 (1.0) |
| P < .05 | |||
| Chemotherapy | |||
| Yes | 3197 (37.1) | 3082 (38.8) | 6279 (37.9) |
| No | 5410 (62.9) | 4867 (61.2) | 10 277 (62.1) |
| P < .05 | |||
* Percentages were calculated based on known data. P values were based on chi-square test for homogeneity for known values. All statistical tests were two-sided. HER2 = human epidermal growth factor receptor 2.
† Fisher’s exact method (two-sided).
Subsequent Breast Cancer According to Antidepressant Use
Of the 16 887 women, 2946 women (17.4%) developed subsequent breast cancer over the 14-year follow-up: 2512 (14.9%) were recurrences and 434 (2.5%) were contralateral breast cancer. We did not find a statistically significant effect on subsequent breast cancer with any antidepressant use in the multivariable Cox regression models (Table 3). None of the adjusted hazard ratios of subsequent breast cancer corresponding with a 25%, 50%, and 75% overlap of concomitant antidepressant and tamoxifen use (one to five years) demonstrated a statistically significant effect. For paroxetine, with 25%, 50%, and 75% of concomitant use during the first year of tamoxifen treatment we found hazard ratios of 1.06 (95% CI = 0.98 to 1.14, P = .09), 1.13 (95% CI = 0.98 to 1.30, P = .09), and 1.20 (95% CI = 0.97 to 1.49, P = .09), respectively. Furthermore, the hazard ratios decreased over time. For example, the estimated hazard ratios for paroxetine were all less than 1.00 for 25%, 50%, and 75% overlap when tamoxifen duration was five years (25%: HR = 0.94, 95% CI = 0.81 to 1.10, P = .46; 50%: HR = 0.89, 95% CI = 0.66 to 1.20, P = .46; 75%: HR = 0.85, 95% CI = 0.54 to 1.32, P = .46). However, the pattern was not statistically significant (P interaction of overlap*duration = .18). For the other antidepressant classes, we again found no statistically significant associations (Table 3). For example, the hazard ratios for fluoxetine, other SSRIs, tricyclics, and other types of antidepressants for each year of tamoxifen treatment generally paralleled the results of paroxetine, with all confidence intervals crossing the null. Additionally, in a sensitivity analysis excluding stage 0 (ductal carcinoma in situ [DCIS]) patients, we again found similar hazard ratios to that of the full cohort.
Table 3.
Adjusted HRs for subsequent breast cancer risk by percent overlap* of antidepressant and tamoxifen use by duration of tamoxifen therapy
| Tamoxifen duration |
Paroxetine | Fluoxetine | Other SSRIs | Tricyclics | Other types | |
|---|---|---|---|---|---|---|
| % overlap | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| 1 y | 0.25 | 1.06 (0.98 to 1.14) | 1.00 (0.95 to 1.07) | 0.98 (0.90 to 1.07) | 1.01 (0.95 to 1.09) | 1.08 (0.98 to 1.19) |
| 0.50 | 1.13 (0.98 to 1.30) | 1.01 (0.91 to 1.14) | 0.97 (0.81 to 1.16) | 1.04 (0.91 to 1.19) | 1.17 (0.96 to 1.43) | |
| 0.75 | 1.20 (0.97 to 1.49) | 1.03 (0.86 to 1.22) | 0.95 (0.72 to 1.25) | 1.06 (0.87 to 1.29) | 1.26 (0.94 to 1.70) | |
| 2 y | 0.25 | 1.03 (0.96 to 1.10) | 0.99 (0.94 to 1.05) | 1.00 (0.93 to 1.09) | 1.03 (0.97 to 1.09) | 1.10 (0.96 to 1.27) |
| 0.50 | 1.07 (0.94 to 1.21) | 0.98 (0.89 to 1.09) | 1.00 (0.86 to 1.18) | 1.06 (0.95 to 1.19) | 1.21 (0.93 to 1.60) | |
| 0.75 | 1.10 (0.91 to 1.33) | 0.98 (0.84 to 1.14) | 1.01 (0.79 to 1.29) | 1.09 (0.92 to 1.29) | 1.34 (0.89 to 2.03) | |
| 3 y | 0.25 | 1.01 (0.92 to 1.09) | 0.97 (0.92 to 1.04) | 1.02 (0.93 to 1.13) | 1.04 (0.97 to 1.11) | 1.13 (0.94 to 1.35) |
| 0.50 | 1.00 (0.86 to 1.18) | 0.95 (0.84 to 1.08) | 1.05 (0.86 to 1.28) | 1.08 (0.94 to 1.24) | 1.27 (0.88 to 1.83) | |
| 0.75 | 1.00 (0.79 to 1.28) | 0.93 (0.77 to 1.13) | 1.07 (0.80 to 1.45) | 1.12 (0.91 to 1.38) | 1.42 (0.83 to 2.47) | |
| 4 y | 0.25 | 0.97 (0.87 to 1.09) | 0.96 (0.87 to 1.05) | 1.04 (0.91 to 1.20) | 1.05 (0.96 to 1.15) | 1.25 (0.91 to 1.44) |
| 0.50 | 0.95 (0.75 to 1.18) | 0.92 (0.77 to 1.10) | 1.09 (0.83 to 1.44) | 1.10 (0.92 to 1.32) | 1.32 (0.83 to 2.09) | |
| 0.75 | 0.92 (0.66 to 1.29) | 0.89 (0.68 to 1.16) | 1.14 (0.76 to 1.72) | 1.16 (0.88 to 1.53) | 1.51 (0.76 to 3.03) | |
| 5 y | 0.25 | 0.94 (0.81 to 1.10) | 0.94 (0.81 to 1.06) | 1.07 (0.89 to 1.28) | 1.06 (0.94 to 1.20) | 1.17 (0.88 to 1.55) |
| 0.50 | 0.89 (0.66 to 1.20) | 0.89 (0.71 to 1.13) | 1.14 (0.79 to 1.64) | 1.13 (0.88 to 1.44) | 1.37 (0.78 to 2.40) | |
| 0.75 | 0.85 (0.54 to 1.32) | 0.84 (0.59 to 1.21) | 1.21 (0.70 to 2.09) | 1.19 (0.83 to 1.73) | 1.61 (0.69 to 3.74) |
* Percent age of days of overlap of antidepressant and tamoxifen use were modeled as continuous variables. CI = confidence interval; HR = hazard ratio; SSRI = selective serotonin reuptake inhibitor.
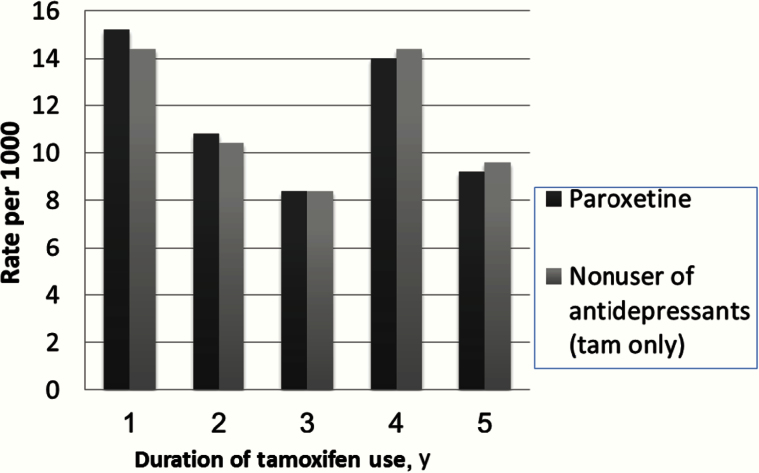
In Figure 2, we translated the overall hazard ratios into absolute rates to address clinical significance if the hazard ratios had been statistically significant. Given that paroxetine came the closest to statistical significance in our study, we compared the annualized rates of subsequent breast cancer among women who concomitantly used the two drugs vs antidepressant nonusers for each year of tamoxifen therapy. Among those with 25% overlap, Figure 2 shows that the annualized rates of subsequent breast cancer were similar among paroxetine users who used tamoxifen concomitantly vs nonusers of antidepressants. For example, for one year of 25% of days overlap of tamoxifen and paroxetine, we found the non-statistically significant hazard ratio of 1.06 (95% CI = 0.98 to 1.14, P = .09) for subsequent breast cancer corresponded to an annualized rate of developing subsequent breast cancer of 15.20/1000 PY for paroxetine users and 14.40/1000 PY for non–antidepressant users. Similarly, with five years of tamoxifen the rate of developing subsequent breast cancer was 9.20/1000 PY for paroxetine users and 9.60/1000 PY in antidepressant nonusers, corresponding with a hazard ratio of 0.94 (95% CI = 0.81 to 1.10, P = .46). Among those with 50% and 75% overlap, we again found no difference in the annualized rates among women who used the two medications concurrently. The annualized crude rates in patients who used tamoxifen at least four years appears higher than those who used three years possibly because those who completed four years of tamoxifen treatment survived long enough to develop a recurrence.
Figure 2.
Annualized rates of subsequent breast cancer by year of tamoxifen treatment.
In sensitivity analysis, we further examined this association in a subset of women with high tamoxifen adherence (MPR ≥ 80% for tamoxifen, n = 12 076). We again found no association among women who used paroxetine concurrently with tamoxifen (Supplementary Materials, available online).
In other sensitivity analyses, we examined the effect of any SSRIs combined vs no antidepressants. In these analyses, the risk of subsequent breast cancer was similar to those who used tamoxifen alone, with a hazard ratio close to 1.00 regardless of duration. Greater risk of subsequent breast cancer was associated with grade 3 cancers (HR = 1.50, 95% CI = 1.26 to 1.80, P < .001 vs grade 0), LCIS histology (HR = 4.04, 95% CI = 1.21 to 13.52. P = .02 vs DCIS), positive lymph nodes (HR = 1.51, 95% CI = 1.34 to 1.70, P < .001 vs negative lymph nodes), and African American race/ethnicity (HR = 1.28, 95% CI = 1.12 to 1.46, P < .001 vs white). Those who were not exposed to aromatase inhibitors had increased risk of subsequent breast cancer (HR = 1.33, 95% CI = 1.16 to 1.53, P < .001) (data not shown).
Nested Case-Control Analysis
To test the robustness of our main findings, we examined the subset of women in the nested case-control study (250 case patients and 250 matched control patients). This analysis also accounted for a number of reproductive covariates (menopause, parity, depression, vasomotor symptoms, family history of breast cancer, history of dense breast, BMI, and lifestyle variables (smoking and alcohol use history) that could only be ascertained from paper medical charts. In the nested analysis, the hazard ratios of subsequent breast cancer and use of tamoxifen concurrently with paroxetine were similar to the hazard ratios of the full cohort. Hence, the addition of the eight covariates did not improve model’s goodness of fit (P = .30) (data not shown).
Discussion
In a study of 16 887 breast cancer survivors followed a maximum of 14 years, we found no statistically increased risk of subsequent breast cancer associated with concomitant antidepressant and tamoxifen use. This was true regardless of type of antidepressant used, including paroxetine and other SSRIs. We used comprehensive pharmacy and electronic medical record databases and were unlikely to miss either antidepressant use or breast cancer outcomes. Adjustments for additional reproductive and lifestyle confounders in the nested case-control analysis did not affect our results. Even if hazard ratios had been statistically significant, the point estimate of the absolute effect would be relatively small for a common amount of overlap for tamoxifen and antidepressant use.
Prior in vitro studies argue that CYP2D6 inhibitors, such as the SSRI antidepressant paroxetine, should not be coprescribed with tamoxifen. However, laboratory-based studies have limited applicability to clinical decision-making (7–10), and two small epidemiologic studies demonstrated mixed results (12–14). Based on our clinical study, our results do not support routine pharmacogenetic testing for CYP2D6. Kelley and colleagues determined that breast cancer mortality was associated with concomitant tamoxifen and paroxetine use; however, their study lacked stage data and, therefore, might have included patients with late-stage disease, which might have overestimated the association (15).
Our study has several key advantages. Patient follow-up was long-term. We captured day-by-day medication use, examined medications as time-dependent variables to address survivor bias and drug switching, and considered a comprehensive list of covariates. We confirmed the majority of the outcomes via chart reviews while remaining outcomes were identified through the tumor registry or a robust computerized algorithm (16). The racially diverse cohort and numbers of outcomes (nearly 3000) and those exposed to antidepressants (nearly 8500) all enhance the clinical inference.
Certain limitations must be considered. Although we could not capture the indication for antidepressants, confounding by indication is unlikely to have affected our main effect estimates as the results of the nested case-control study (in which we captured several key covariates) provided similar results. It is possible that women prescribed antidepressants were at lower risk of subsequent breast cancer because they were more proactive in seeking care. This could bias our findings towards a nonassociation. However, to address this concern, we accounted for cancer stage at initial diagnosis, treatments, comorbidities, and other health care utilization factors. Importantly, the eligibility criteria included women who had pharmacy benefits, underwent breast-conserving surgery, and were estrogen-positive or progesterone-positive. These strategies ensured similar risk profiles and minimized confounding. We acknowledge missing values for some tumor characteristics; however, the overall fractions of missing data were small and equally distributed between antidepressant users and nonusers. We also could not examine the SSRI venlafaxine specifically because the fraction of only users was so small (<1%). The short median duration of tamoxifen and its overlap days with antidepressants might have contributed to the lack of an association. Finally, although we adjusted for other CYP2D6 inhibitors, future larger studies can determine if concomitant use of any CYP2D6 inhibitor with tamoxifen is associated with recurrence.
Although postmenopausal women are increasingly being treated with aromatase inhibitors as first-line therapy, tamoxifen is a mainstay for premenopausal women and, therefore, the clinical question about the safety of tamoxifen and antidepressant use remains. Using one of the most complete pharmacy databases of insured patients, our data does not suggest statistically significantly increased risk of subsequent breast cancer in women who concurrently used tamoxifen and antidepressants, including paroxetine. Information about the safety of using antidepressants concurrently with tamoxifen will help improve the quality of life of breast cancer survivors given that thousands of survivors struggle with depression, sleep disturbance, and vasomotor symptoms while on tamoxifen treatment (1).
Funding
This study was supported by National Institutes of Health/National Cancer Institute R01CA093772 (to RH), “Antidepressant and Breast Cancer Pharmacoepidemiology,” with additional support from the California Breast Cancer Research Program 14IB-0086 (to RH).
Supplementary Material
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; nor the decision to submit the manuscript for publication.
References
- 1. Mom CH, Buijs C, Willemse PH, et al. Hot flushes in breast cancer patients. Crit Rev Oncol Hematol. 2006;57 (1):63–77. [DOI] [PubMed] [Google Scholar]
- 2. Sestak I, Kealy R, Edwards R, et al. Influence of hormone replacement therapy on tamoxifen-induced vasomotor symptoms. J Clin Oncol. 2006;24 (24):3991–3996. [DOI] [PubMed] [Google Scholar]
- 3. Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;(32):57–71. [DOI] [PubMed] [Google Scholar]
- 4. Reich M, Lesur A, Perdrizet-Chevallier C. Depression, quality of life and breast cancer: a review of the literature. Breast Cancer Res Treat. 2008;110 (1):9–17. [DOI] [PubMed] [Google Scholar]
- 5. Jin Y, Desta Z, Stearns V, et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst. 2005;97 (1):30–39. [DOI] [PubMed] [Google Scholar]
- 6. Stearns V, Johnson MD, Rae JM, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95 (23):1758–1764. [DOI] [PubMed] [Google Scholar]
- 7. Goetz MP, Rae JM, Suman VJ, et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J Clin Oncol. 2005;23 (36):9312–9318. [DOI] [PubMed] [Google Scholar]
- 8. Goetz MP, Knox SK, Suman VJ, et al. The impact of cytochrome P450 2D6 metabolism in women receiving adjuvant tamoxifen. Breast Cancer Res Treat. 2007;101 (1):113–121. [DOI] [PubMed] [Google Scholar]
- 9. Holmes FA, Liticker JD. Pharmacogenomics of Tamoxifen in a Nutshell--And Who Broke the Nutcracker? J Oncol Pract. 2005;1 (4):155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. FDA Executive Summary. http://www.fda.gov/ohrms/dockets/ac/06/briefing/2006-4248B1-01-FDA-Tamoxifen%20Background%20Summary%20Final.pdf Accessed January 2, 2008.
- 11. Dusetzina SB, Alexander GC, Freedman RA, et al. Trends in co-prescribing of antidepressants and tamoxifen among women with breast cancer, 2004–2010. Breast Cancer Res Treat. 2013;137 (1):285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nowell SA, Ahn J, Rae JM, et al. Association of genetic variation in tamoxifen-metabolizing enzymes with overall survival and recurrence of disease in breast cancer patients. Breast Cancer Res Treat. 2005;91 (3):249–258. [DOI] [PubMed] [Google Scholar]
- 13. Wegman P, Elingarami S, Carstensen J, et al. Genetic variants of CYP3A5, CYP2D6, SULT1A1, UGT2B15 and tamoxifen response in postmenopausal patients with breast cancer. Breast Cancer Res Treat. 2007;9 (1):R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chubak J, Buist DS, Boudreau DM, et al. Breast cancer recurrence risk in relation to antidepressant after diagnosis. Breast Cancer Res Treat. 2008;112 (1):123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kelly CM, Juurlink DN, Gomes T, et al. Selective serotonin reuptake inhibitors and breast cancer mortality in women receiving tamoxifen: a population based cohort study. BMJ. 2010;340:c693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17 (6):1471–1474. [DOI] [PubMed] [Google Scholar]
- 17. Haque R, Shi J, Schottinger JE, et al. A hybrid approach to identify subsequent breast cancer using pathology and automated health information data. Med Care. 2015;53 (4):380–385. [DOI] [PubMed] [Google Scholar]
- 18. Krieger N, Waterman P, Lemieux K, et al. On the wrong side of the tracts? Evaluating the accuracy of geocoding in public health research. Am J Public Health. 2001;91 (7):1114–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tabachnick BG, Fidell LS. Using Multivariate Statistics. 5th ed Pearson Education Inc, 2007. [Google Scholar]
- 20. Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed John Wiley & Son’s Inc, 2000. [Google Scholar]
- 21. Sikka R, Xia F, Aubert RE. Estimating medication persistency using administrative claims data. Am J Manag Care. 2005;11 (7):449–457. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.