Abstract
Background:
While progestin addition to estrogen mitigates endometrial cancer risk, the magnitude of the effect on incidence, specific endometrial cancer histologies, and endometrial cancer mortality remains unsettled. These issues were assessed by analyses after extended follow-up of the Women’s Health Initiative (WHI) randomized clinical trial evaluating continuous combined estrogen plus progestin use.
Methods:
The WHI enrolled 16 608 postmenopausal women into a randomly assigned, double-blind, placebo-controlled trial. Women age 50 to 79 years with intact uteri with normal endometrial biopsy at entry were randomly assigned to once-daily 0.625mg conjugated equine estrogen plus 2.5mg medroxyprogesterone acetate (n = 8506) as a single pill or matching placebo (n = 8102). Follow-up beyond the original trial completion date required reconsent, obtained from 12 788 (83%) of surviving participants. Analyses were by intent-to-treat. All statistical tests were two-sided.
Results:
After 5.6 years’ median intervention and 13 years’ median cumulative follow-up, there were fewer endometrial cancers in the combined hormone therapy compared with the placebo group (66 vs 95 case patients, yearly incidence, 0.06% vs 0.10%; hazard ratio [HR] = 0.65, 95% confidence interval [CI] = 0.48 to 0.89, P = .007). While there were somewhat fewer endometrial cancers during intervention (25 vs 30, respectively; HR = 0.77, 95% CI = 0.45 to 1.31), the difference became statistically significant postintervention (41 vs 65, respectively; HR = 0.59, 95% CI = 0.40 to 0.88, P = .008), but hazard ratios did not differ between phases (P difference = .46). There was a statistically nonsignificant reduction in deaths from endometrial cancer in the estrogen plus progestin group (5 vs 11 deaths, HR = 0.42, 95% CI = 0.15 to 1.22).
Conclusion:
In postmenopausal women, continuous combined estrogen plus progestin decreases endometrial cancer incidence.
Exogenous estrogen use increases endometrial cancer risk (1–3). While combined estrogen plus progestin use results in less endometrial hyperplasia, an endometrial cancer precursor, than does estrogen alone (4,5), the magnitude of estrogen plus progestin influence on estrogen-associated endometrial cancer risk has not been conclusively defined (6). While a few case-control studies describe increased endometrial cancer risk with long-duration combined hormone therapy (7,8), a recent meta-analysis of published cohort studies found a statistically significantly lower endometrial cancer risk with continuous combined estrogen plus progestin use (6). Nonetheless, several recent menopause society (9,10) and task force (11) updates do not describe continuous estrogen plus progestin use as reducing endometrial cancer. In addition, the influence of continuous combined hormone therapy on types of endometrial cancer and endometrial cancer mortality remains unsettled (12).
Against this background, in earlier reports from the Women’s Health Initiative (WHI) randomized, placebo-controlled trial evaluating continuous combined estrogen plus progestin fewer endometrial cancers were seen with combined hormone therapy use, but the differences were not statistically significant (13,14). Recently, with median cumulative follow-up of 13 years in this trial, estrogen plus progestin use was found to statistically significantly decrease endometrial cancer incidence (15). In the current report, we extend that finding by providing analyses on estrogen plus progestin effects on endometrial cancer type, findings in relevant clinical subgroups, and information on endometrial cancer–related mortality.
Methods
Study Design and Participants
Trial methodology has been described (16,17). Briefly, postmenopausal women age 50 to 79 years with an intact uterus were enrolled at 40 US clinical centers. Exclusions were prior breast cancer, anticipated survival of less than 3 years, and previous invasive cancer within 10 years. A three-month wash-out period was required for hormone therapy users at screening. Institutional review board approval was obtained at each clinical center, and all participants provided written, informed consent.
Women were randomly allocated to daily combined conjugated equine estrogens (0.625mg/day) plus medroxyprogesterone acetate (2.5mg/day) (n = 8506) as a single pill (Prempro; Wyeth-Ayerst, Collegeville, PA) or an identical-appearing placebo (n = 8102) using a computerized, permuted-block algorithm, stratified by age group and clinical center. Random assignment and double-blind study drug dispensing utilizing a secured database system were implemented by the WHI Clinical Coordinating Center (Seattle, WA). The trial protocol can be accessed at whi.org. This trial is registered with clinicaltrials.gov, number NCT000000611.
Data Collection
Baseline characteristics were collected using standardized questionnaires or by interview. Nonstudy drug use was accessed by interviewer-administered questionnaire serially. Study medication adherence was annually measured initially by pill counts and later by weighing return bottles. Health status was accessed twice yearly.
Endometrial Cancer
Endometrial cancer was one of the prospectively identified main study outcomes, which also included coronary heart disease (CHD), invasive breast cancer, stroke, pulmonary embolism, colorectal cancer, hip fracture, and a global index of these outcomes. Initial outcomes reports were confirmed locally by centrally trained physician adjudicators after medical record review. Final cancer adjudication was conducted at the Clinical Coordinating Center (17). Reasons for hysterectomy were prospectively collected.
Clinical Trial Course
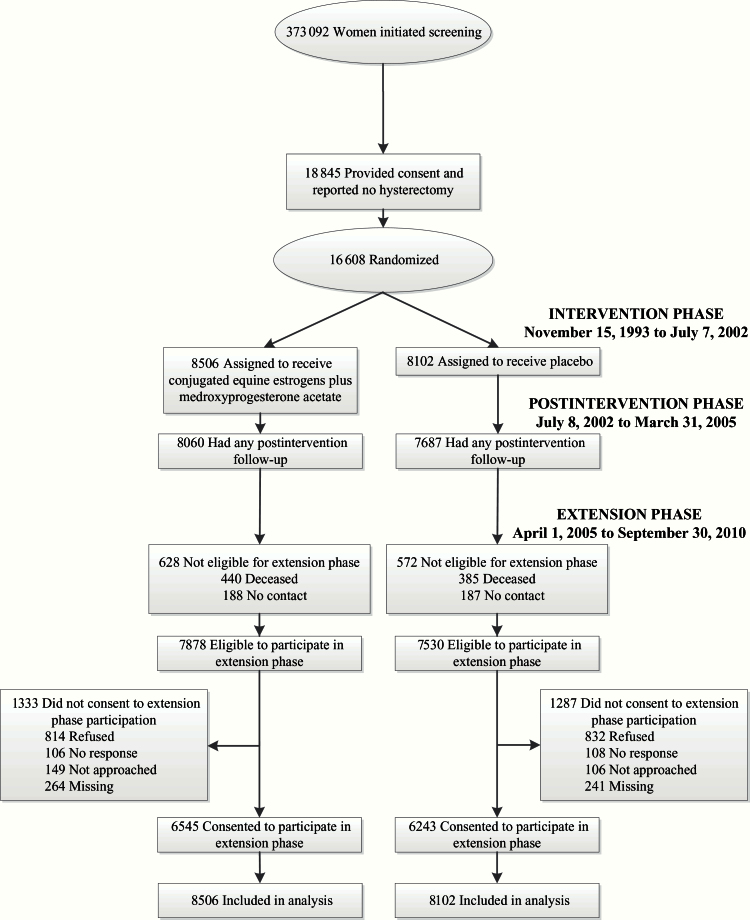
After 5.6 years of median follow-up, the intervention was stopped when overall risks exceeded benefits and participants were instructed to discontinue study drug on July 8, 2002 (16). Protocol-defined follow-up continued through March 31, 2005, the prospectively determined trial completion date. Follow-up after that date required reconsent, obtained from 12 788 (83%) of 15 408 surviving participants. The participant flow is described in a CONSORT diagram (Figure 1) (18). Deaths were documented by death certificate and medical record review by centrally trained physician adjudicators. Additionally, linkage to the National Death Index linkage was conducted on December 31, 2008.
Figure 1.
Participant flow diagram of the Women’s Health Initiative randomized trial of continuous combined estrogen plus progestin through extended follow-up. Estrogen indicates conjugated equine estrogens, and progestin indicates medroxyprogesterone acetate. The intervention phase ran from November 15, 1993 to July 7, 2002. The postintervention phase began July 8, 2002, the day after participants were instructed to stop study medication use (conjugated equine estrogens plus medroxyprogesterone acetate or placebo), and continued through the original trial completion date (March 31, 2005). The extension phase began on April 1, 2005 and includes follow-up for participants who reconsented (83% of those eligible) through September 30, 2010. During this period, 2.8% dropped out.
Endometrial Evaluation
For safety, endometrial biopsies were required prior to study entry. Biopsy reports of endometrial cancer and complex adenomatous hyperplasia, with or without atypia, precluded study entry. Women with simple hyperplasia were not eligible but could subsequently enter with resolution of histologic abnormality. A cohort of 5% of participants was randomly identified to undergo scheduled biopsies at three years of follow-up. Biopsies were performed by WHI clinical staff (13) and read by pathologists blinded to random assignment. During follow-up, other endometrial biopsies and vaginal ultrasounds were performed for medical indications. Women with persistent or heavy bleeding were evaluated by clinical center gynecologists. If biopsy was indicated, gynecologist unblinding was permitted. Diagnosis of endometrial cancer and complex or adenomatous hyperplasia, with or without atypia, required discontinuation of study medications. For simple hyperplasia, placebo group participants had study medication discontinued and received referral to their health care providers for further management. Estrogen plus progestin group participants with simple hyperplasia were continued on study medication with additional 20mg/day of medroxyprogesterone acetate with biopsies repeated in three to six months.
Statistical Analysis
The target sample size of 15 125 was based primarily on projected coronary heart disease benefit. Results for endometrial cancer incidence and deaths directly attributed to endometrial cancer were assessed with time-to-event methods based on the intent-to-treat principle, which included all 16 608 randomly assigned participants. Cancer incidence rate comparisons, presented as hazard ratios (HRs) and 95% confidence intervals (CIs) from Cox proportional hazard models, were stratified by age and randomization groups in the WHI dietary trial, and proportionality was verified with the Grambsch and Therneau’s test (19).
Analyses were conducted for three time periods: the intervention phase from random assignment through July 7, 2002, the postintervention phase beginning July 8, 2002, and for cumulative results (through September 30, 2010). Participants were included in postintervention phase analyses if they were alive, in follow-up, and had no prior endometrial cancer as of July 8, 2002. Censoring was defined by the earliest date of death and last follow-up date.
Analyses of cumulative results begin at random assignment with censoring as above and included all randomly assigned participants. The heterogeneity of hazard ratios across endometrial cancer subtypes was assessed with competing risk methods (19). In analyses examining deaths from endometrial cancer, women were censored at last contact date.
Exploratory interactions were examined for eleven baseline characteristics potentially related to endometrial cancer risk (20) within the primary Cox model, expanded to include the designated baseline factors, random assignment, and interaction term(s). At most, one interaction was expected to be statistically significant at the .05 level by chance alone.
When initiated in 1993, the trial originally included random assignment to an estrogen alone arm. When clinical trial results indicated estrogen alone increased endometrial epithelial proliferation (4), that arm was dropped and the 331 women in the estrogen alone group were added to the combined therapy group.
All analyses were conducted using SAS version 9.3 (SAS Institute Inc., Cary, NC). Figures were created using R 2.15 (R Foundation for Statistical Computing, Vienna, Austria). All P values are two-sided, and P values of .05 or less were regarded as statistically significant.
Results
Participant characteristics in the two randomly assigned groups were closely comparable in both the initial 16 608 randomly assigned participants (16,21) and the 12 788 participants reconsenting for extended follow-up with no statistically significant differences (Table 1). Reconsent rates were similar between randomly assigned groups, even when considered by baseline characteristics, with the exception that obese women in the placebo group had slightly lower reconsent rates.
Table 1.
Baseline characteristics of those that consented to additional follow-up in the estrogen plus progestin trial (n = 12 788)
| Characteristic | Estrogen plus progestin (n = 6545) |
Placebo (n = 6243) |
P* |
|---|---|---|---|
| No. (%) | No. (%) | ||
| Age, y | |||
| 50–54 | 846 (12.9) | 767 (12.3) | .72 |
| 55–59 | 1420 (21.7) | 1361 (21.8) | |
| 60–69 | 3019 (46.1) | 2887 (46.2) | |
| 70–79 | 1260 (19.3) | 1228 (19.7) | |
| Years since menopause | |||
| <5 | 1071 (18.0) | 988 (17.0) | .36 |
| 5-<15 | 2466 (41.5) | 2448 (42.2) | |
| ≥15 | 2410 (40.5) | 2370 (40.8) | |
| Race/ethnicity | |||
| White | 5616 (85.8) | 5357 (85.8) | .97 |
| Black | 406 (6.2) | 401 (6.4) | |
| Hispanic | 291 (4.4) | 261 (4.2) | |
| American Indian | 16 (0.2) | 14 (0.2) | |
| Asian/Pacific Islander | 132 (2.0) | 128 (2.1) | |
| Unknown | 84 (1.3) | 82 (1.3) | |
| >High school/GED | 4932 (75.8) | 4646 (74.9) | .29 |
| BMI, kg/m2 | |||
| <25 | 1998 (30.7) | 1949 (31.4) | .16 |
| 25-<30 | 2278 (35.0) | 2215 (35.7) | |
| 30-<35 | 1396 (21.4) | 1250 (20.2) | |
| 35-<40 | 593 (9.1) | 523 (8.4) | |
| ≥40 | 251 (3.9) | 265 (4.3) | |
| Baseline vasomotor symptoms | |||
| None | 3935 (60.6) | 3784 (61.2) | .59 |
| Mild | 1738 (26.8) | 1660 (26.8) | |
| Moderate or severe | 816 (12.6) | 741 (12.0) | |
| Smoking status | |||
| Never | 3288 (50.7) | 3139 (50.9) | .94 |
| Past | 2597 (40.0) | 2452 (39.8) | |
| Current | 600 (9.3) | 577 (9.4) | |
| Treated diabetes (pills or shots) | 244 (3.7) | 222 (3.6) | .60 |
| Hypertensive (self-report or high blood pressure) | 2496 (41.4) | 2406 (40.6) | .34 |
| Episodes/wk of mod/strenuous recreational physical activity ≥20 min | |||
| No activity | 1063 (17.9) | 985 (16.8) | .07 |
| Some activity | 2525 (42.6) | 2500 (42.7) | |
| 2 -<4 episodes/wk | 972 (16.4) | 920 (15.7) | |
| ≥4 episodes/wk | 1363 (23.0) | 1454 (24.8) | |
| Unopposed estrogen use ever | 682 (10.4) | 645 (10.3) | .87 |
| Estrogen + progesterone use ever | 1215 (18.6) | 1131 (18.1) | .51 |
* Chi-squared test of association.
In intent-to-treat analyses, data were available for a median follow-up of 5.6 years (interquartile range [IQR] = 4.8–6.5) from random assignment until termination of study medicine intervention and a median follow-up of 8.2 years (IQR = 6.6–8.2) postintervention and 13.2 years (IQR = 10.5–14.2) of cumulative median follow-up.
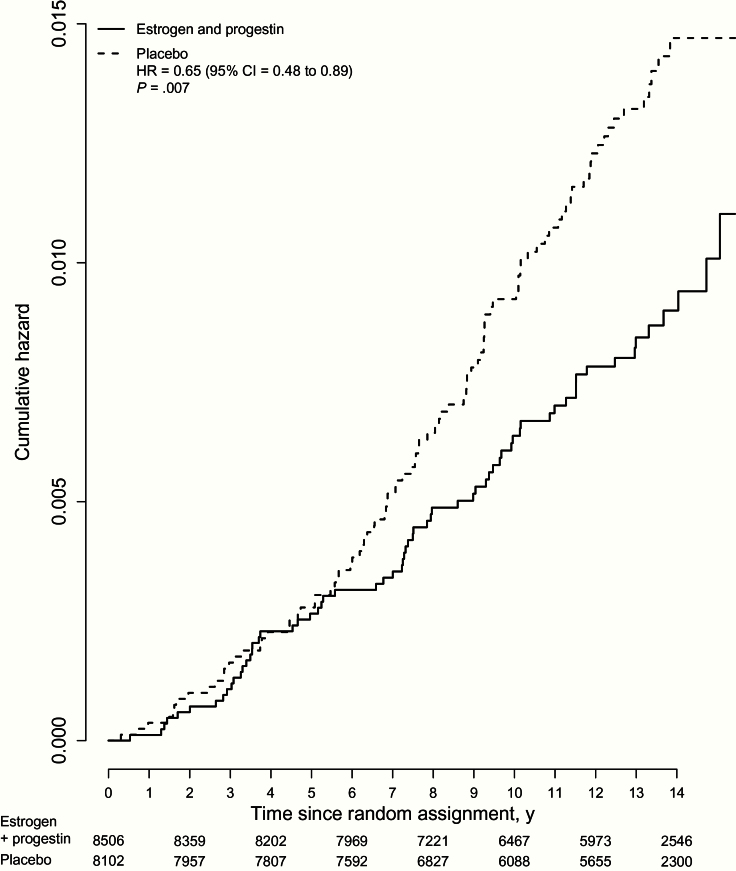
Over cumulative follow-up, continuous combined estrogen plus progestin use decreased endometrial cancer incidence (66 case patients, 0.06% per year) compared with placebo (95 case patients, 0.10% per year, HR = 0.65, 95% CI = 0.48 to 0.89, P = .007) (Figure 2). During intervention, fewer endometrial cancers were diagnosed in the estrogen plus progestin group, but the difference was not statistically significant (25 vs 30 case patients, HR = 0.77, 95% CI = 0.45 to 1.31, P = .33). Similar findings were apparent through the protocol defined completion date (March 31, 2005) (HR = 0.73, 95% CI = 0.49 to 1.09). A statistically significant difference in endometrial cancer incidence between randomly assigned groups emerged postintervention (41 case patients, 0.08% per year vs 65 case patients, 0.13% per year, respectively [HR = 0.59, 95% CI = 0.40 to 0.88, P = .008]), but intervention and postintervention phase hazard ratios were not different (P difference = .46).
Figure 2.
Kaplan-Meier estimates of cumulative hazards of endometrial cancer in the Women’s Health Initiative randomized trial of continuous combined estrogen plus progestin with the intent-to-treat principle. Hazard ratio (estrogen plus progestin vs placebo) with 95% confidence intervals and P values is from Cox regression models stratified by age and random assignment in the dietary modification trial. Estrogen indicates conjugated equine estrogens, and progesterone indicates medroxyprogesterone acetate. All statistical tests were two-sided. CI = confidence interval; HR = hazard ratio.
To address the potential imbalance of reconsent for obese participants between randomly assigned groups, analyses stratified by BMI group had little effect on postintervention incidence (HR = 0.59, 95% CI = 0.40 to 0.87, P = .008). Inclusion of three endometrial sarcoma cases (1 spindle cell sarcoma in the placebo group and 2 stromal sarcomas in the estrogen plus progestin group) yielded a cumulative hazard ratio of 0.67 (95% CI = 0.49 to 0.91, P = .01), as previously reported (15). Endometrial cancer incidence results were somewhat greater in analyses adjusted for adherence censoring events that occurred six months after consuming less than 80% of study pills or starting non-protocol hormone therapy (HR = 0.49, 95% CI = 0.30 to 0.80, P = .004).
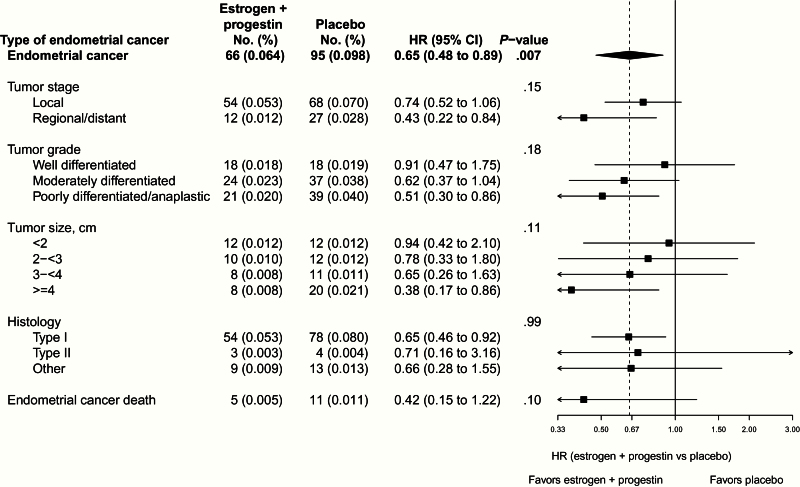
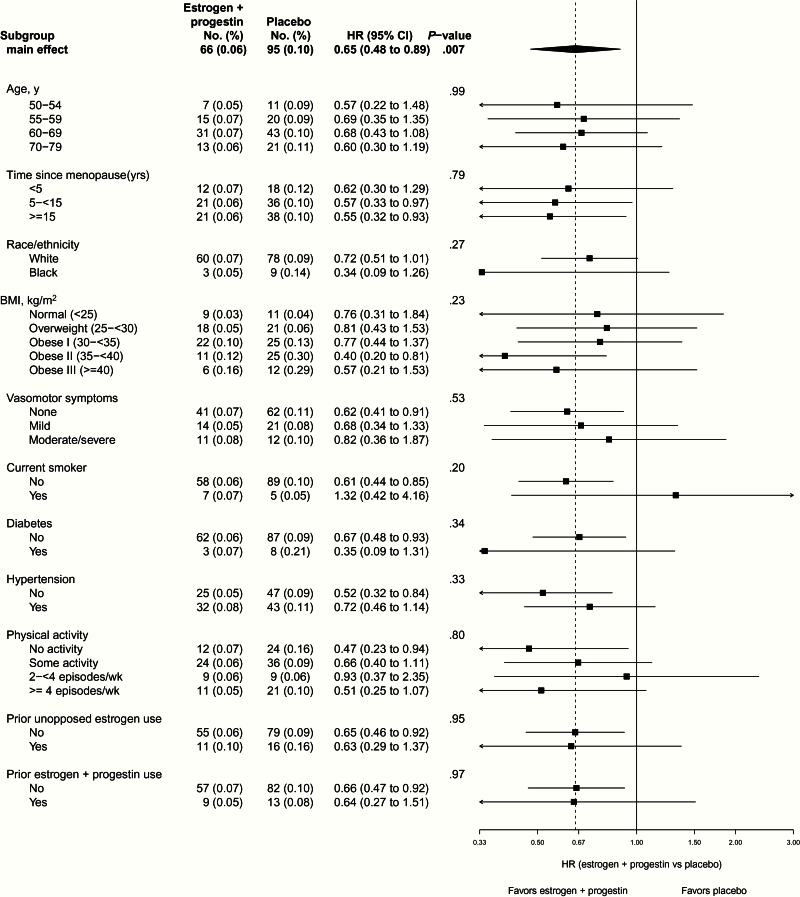
In terms of tumor characteristics, there was no evidence of a differential estrogen plus progestin effect by grade or stage (Figure 3). In intent-to-treat analysis over the entire study period, there was a statistically nonsignificant reduction in deaths from endometrial cancer (5 vs 11 deaths, HR = 0.42, 95% CI = 0.15 to 1.22, P = .10), but the number of events was insufficient for definitive assessment. None of the 11 subgroup interactions examined were statistically significant (P > .20), suggesting a similar influence of estrogen plus progestin on endometrial cancer risk generally, including in women in the highest BMI categories and in cases with type II histology (Figure 4).
Figure 3.
Cumulative number of events (annual percentages) and hazard ratios (95% confidence intervals) for incidence of endometrial cancer, tumor types, and deaths from endometrial cancer in the Women’s Health Initiative randomized trial of continuous combined estrogen plus progestin by random assignment. Tumor size was estimated in accordance with the Surveillance, Epidemiology, and End Results 1988, 2nd edition rules for coding cancers with size measured as the length along the longest axis. Tumors were classified as type I or type II by methods previously described by Setiawan in the Endometrial Cancer Consortium (12). Briefly, endometrioid carcinoma, adenocarcinoma NOS, and adenocarcinoma with squamous differentiation were classified as type I, serous/papillary serous and mixed cell adenocarcinoma as type II, and remaining tumors classified as other. Hazard ratios, 95% confidence intervals, and P values are from Cox proportional hazards models stratified according to age and random assignment in the dietary modification trial. Dotted line represents overall hazard ratio for endometrial cancer incidence attributed to estrogen plus progestin. P value corresponds to a score x2 test from a competing risks analysis that tested whether hazard ratios differed between tumor types. Estrogen indicates conjugated equine estrogens, and progesterone indicates medroxyprogesterone acetate. Number of events may not add up because of missing endometrial cancer type. All statistical tests were two-sided. CI = confidence interval; HR = hazard ratio.
Figure 4.
Cumulative number of events (annualized percentages) and hazard ratios (HRs [95% confidence intervals] for endometrial cancer incidence) in the Women’s Health Initiative randomized trial of continuous combined estrogen plus progestin by baseline characteristics and random assignment. Hazard ratios, 95% confidence intervals, and P values are from Cox proportional hazards models stratified according to age and random assignment in the dietary modification trial. Dotted line represents overall hazard ratio for endometrial cancer incidence attributed to estrogen plus progestin. P value is from a one degree-of-freedom score x2 test of the interaction between the given subgroup and random assignment. Estrogen indicates conjugated equine estrogens, and progestin indicates medroxyprogesterone acetate. Number of events may not add up because of missing baseline data. All statistical tests were two-sided. BMI = body mass index; CI = confidence interval; HR = hazard ratio.
There were four endometrial cancers diagnosed in the 331 women originally randomly assigned to estrogen alone, seven in the 573 women randomly assigned to combined hormones, and four in the 522 women randomly assigned to placebo during the same period. Removing these case patients did not change the results (55 vs 91 case patients, HR = 0.59, 95% CI = 0.43 to 0.83, P = .002), nor did only excluding women originally randomly assigned to estrogen alone (62 vs 95 case patients, HR = 0.64, 95% CI = 0.47 to 0.88, P = .006).
Overall, there were more hysterectomies in the combined hormone therapy group (510 [0.51% per year] vs 450 [0.48% per year], HR = 1.08, 95% CI = 0.95 to 1.23, P = .23), but the difference was not statistically significant. In addition, very few (2.2%) were performed for atypical hyperplasia (Table 2), and the sensitivity analysis that censored women at the time of hysterectomy did not change the endometrial cancer results (HR = 0.65, 95% CI = 0.48 to 0.90, P = .008).
Table 2.
Reason for hysterectomy by randomization groups in the WHI randomized trial of estrogen plus progestin
| Hysterectomy (total) | Estrogen plus progestin No. (%) | Placebo No. (%) |
|---|---|---|
| Total | 510 | 450 |
| Cancer | 108 (21.2) | 121 (26.9) |
| Atypical hyperplasia | 11 (2.2) | 10 (2.2) |
| Bleeding | 31 (6.1) | 17 (3.8) |
| Fibroids (myomas) | 50 (9.8) | 19 (4.2) |
| Endometriosis | 1 (0.2) | 2 (0.4) |
| Descensus (prolapse) | 236 (46.3) | 210 (46.7) |
| Other | 73 (14.3) | 71 (15.8) |
The expected strong association between BMI and endometrial cancer risk was seen among all participants (HR = 1.0 [reference], HR = 1.71, 95% CI = 1.99 to 2.93, HR = 3.58, 95% CI = 2.11 to 6.07, HR = 6.66, 95% CI = 3.82 to 11.61, and HR = 7.40, 95% CI = 3.86 to 14.16 for BMI <25, 25<30, 30<35, 35<40, and ≥40kg/m2, respectively) in analyses that added covariates of age (linear) and race/ethnicity in the core model.
Discussion
Continuous combined estrogen plus progestin use for 5.6 years in postmenopausal women with normal endometrial biopsy at therapy initiation resulted in a statistically significant reduction in endometrial cancer incidence, with the difference becoming statistically significant during longer-term postintervention follow-up. Subgroup analyses suggest a favorable effect of estrogen plus progestin on endometrial cancer risk generally, including in women in the highest BMI groups.
This WHI study is the first randomly assigned, double-blind, placebo-controlled trial to demonstrate that endometrial cancer incidence is statistically significantly lower in women taking continuous combined estrogen plus progestin compared with placebo. Similar findings were reported from the much smaller, randomized Heart and Estrogen/progestin Replacement Study (HERS 2), where fewer endometrial cancers were diagnosed in the estrogen plus progestin group (2 vs 8 case patients, HR = 0.25, 95% CI = 0.01 to 1.18, P = .48) (22). The progestin effect in reducing, rather than just mitigating, the adverse influence of exogenous estrogen on endometrial cancer risk suggests additional progestin effects on endometrial cancer risk related to endogenous estrogen exposure as well.
While case-control studies of continuous combined hormone therapy and endometrial cancer provide somewhat mixed results (23,24), the current trial findings support results from most cohort studies. Continuous combined hormone therapy has been associated with a statistically significantly lower endometrial cancer incidence in three (3,25,26) of four (27) cohort analyses with a meta-analysis demonstrating an overall lower endometrial cancer risk (6), often with greatest affect among obese women (3,26,27). In the current trial, while no interaction between BMI and combined hormone therapy was seen, risk estimates were somewhat suggestive of a larger effect in obese women. One difference from such observational study findings was the identification of several years’ lag preceding separation of the incidence rates between randomly assigned groups in the WHI trial, likely the result of the entry requirement for normal endometrial histology, precluding entry of women with existing proliferative endometrial epithelium.
Consideration of the progestin dose, schedule, and duration can explain most study differences. Sequential regimens with fewer days of progestin exposure are less effective in reducing endometrial cancer risk. In a meta-analysis of cohort studies (6), estrogen plus sequential progestin use for fewer than 10 days per month was associated with higher endometrial cancer risk (odds ratio [OR] = 1.32, 95% CI = 1.06 to 1.65), while progestin sequential use for 10 to 14 days per month was neutral for risk (OR = 1.05, 95% CI = 0.84 to 1.30). In addition, while estrogen regimens that include micronized progesterone have been less strongly associated with breast cancer risk in some studies (28,29), they also appear to provide limited or no protection against endometrial cancer (HR = 2.42 95% CI = 1.53 to 3.83) (25,30) despite mitigating short term, estrogen-associated endometrial proliferation (4). These findings suggest that short-term endometrial proliferation change may not be a reliable surrogate for endometrial cancer risk. In the current study, histologic findings did not differ between randomly assigned groups in the 5% subgroup having protocol determined repeat endometrial biopsies at year 3 (13).
The current endometrial cancer findings highlight the completely different influences estrogen plus progestin and estrogen alone have on endometrial cancer and breast cancer (31,32). In the WHI randomly assigned trial, estrogen plus progestin statistically significantly increased breast cancer incidence (18,21) and, in the current report, reduced endometrial cancer incidence. In contrast, in the separate WHI randomized trial in women with prior hysterectomy, estrogen alone statistically significantly reduced breast cancer incidence (29,30). Further, in observational studies, estrogen alone use is associated with statistically significantly higher endometrial cancer risk (3,25).
As previously described (13,33), participation in the combined hormone therapy compared with the placebo group was associated with more frequent endometrial bleeding (13,33) and more total endometrial biopsies (33% vs 6%, P < .001) (13) during active intervention. Despite this more active surveillance, fewer endometrial cancers were diagnosed in the estrogen plus progestin group. In addition, cumulative hysterectomy rates were not statistically significantly higher in the combined hormone therapy group, hysterectomies were rarely performed for proliferative histologies (in only 2.2%), and the sensitivity analysis adjusting for hysterectomy did not change the endometrial cancer results. Thus, the hysterectomy findings cannot explain the lower endometrial cancer incidence in the combined estrogen plus progestin groups.
This trial assessed one hormone regimen; consequently, the results cannot be extrapolated to other hormone regimens. Whether regimens incorporating lower progestin dosage have similar endometrial cancer influence is unknown. While profound differences in pharmacology and in molecular actions between various progestins are described (34), clear differences in the clinic for endometrial cancer risk have only emerged for micronized progesterone use (25).
After the initial WHI report in 2002 (16), combined hormone therapy use rapidly decreased in the United States (35), followed by an endometrial cancer increase, recently attributed to the hormone therapy decrease (36). However, rising obesity rates during the same period also could be a factor. Previously, a decrease in breast cancer incidence during the same period was also attributed to the hormone therapy decrease (37,38,39), a suggestion supported by the preponderance of subsequent reports (39,40,41). While the directions of changes are opposite, the magnitude of the relative influence on endometrial cancer and breast cancer incidence attributed to the hormone therapy decrease are similar. Consequently, because breast cancers are five times more common than endometrial cancers, a net absolute decrease in cancer incidence likely occurred after the 2002 WHI report (16), considering both endometrial and breast cancer.
A recent publication has updated the WHI hormone therapy trial findings (15), which concluded that the estrogen plus progestin effect in decreasing endometrial cancers does not substantially alter the risk and benefit balance of continuous combined hormone therapy use or the recommendation against its use for chronic disease reduction in postmenopausal women (15).
It is unclear why estrogen and progestin have different influences on epithelial proliferation in the endometrium and the breast. Classically, estrogen drives endometrial epithelial proliferation and progesterone inhibits proliferation and causes cell differentiation (42,43,44). While estrogen also usually stimulates breast epithelium (45) and inhibits apoptosis (46), preclinical findings indicate that, after estrogen deprivation, estrogen can act as an apoptosis inducer (47,48). In contrast to endometrial findings, progestins stimulate mammary epithelial proliferation (49) and stem cell expansion (50). While differential stromal tissue effects (with substantially more stromal tissue found in the endometrium than in the breast) have been proposed as potential mediator of these differences (51), full discussion of these complex processes is beyond the present report scope.
As progestins can decrease endometrial epithelial proliferation (52), progestin use has been proposed as a potential chemoprevention strategy (53). In this regard, the substantial reduction in endometrial cancers seen with estrogen plus progestin in the current trial in those with high BMIs who are at increased endometrial cancer risk (54,55) may be of relevance. However, more information regarding the overall risks and benefits of progestin alone use is needed before the prevention question is pursued.
Study strengths include the randomly assigned, double-blind, placebo-controlled design with endometrial cancer as a prospectively identified outcome, the large sample size, endometrial biopsy screening to exclude pre-existing endometrial pathology, long postintervention follow-up, the extremely low rate of postintervention hormone therapy use (56), and high-quality outcome assessment. Limitations include the modest number of endometrial cancers observed and absence of cancer treatment information. Finally, as high-risk women based on endometrial biopsy findings were excluded from trial entry, results in a general population could differ.
In summary, in postmenopausal women, continuous combined estrogen plus progestin use reduces endometrial cancer incidence by 35%. The favorable effects were not limited to any particular histologic subtype and were seen generally, including in women in the highest BMI categories.
Funding
The Women’s Health Initiative (WHI) program is reported by the National Heart, Lung, and Blood Institute (NHLBI), National Institute of Health, and Department of Health and Human Services through contracts N01WH22110, 24152, 32100–2, 32105–6, 32108–9, 32111–13, 32115, 32118–2119, 32122, 42107–26, 42129–32, and 44221.
Acknowledgments
Acknowledgments: We acknowledge the dedicated efforts of investigators and staff at the Women’s Health Initiative (WHI) clinical centers, the WHI Clinical Coordinating Center, and the National Heart, Lung, and Blood program office (listing available at http://www.whi.org). We also recognize the WHI participants for their extraordinary commitment to the WHI program.
The WHI Project Office at the US National Heart, Lung, and Blood Institute reviewed and approved the final manuscript but had no other role in the preparation of this report.
Contributors: RTC wrote the analysis proposal and initial draft of the report. He accepts full responsibility for the overall content of the report. All authors contributed to the interpretation of data and the approval of the final report. GLA and AKA undertook the statistical analysis. RTC, GLA, JM, CT, GS, MS, and JW collected the data and obtained study funding.
Women’s Health Initiative Investigations: Program Office: Jacques Roscoe, Shari Ludlum, Dale Burden, Joan McGowan, Leslie Ford, and Nancy Geller (National Heart, Lung, and Blood Institute, Bethesda, MD); Clinical Coordinating Center: Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg (Fred Hutchinson Cancer Research Center, Seattle, WA); Investigators and Academic Centers: JoAnn E. Manson (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA); Barbara V. Howard (MedStar Health Research Institute/Howard University, Washington, DC); Marcia L. Stefanick (Stanford Prevention Research Center, Stanford, CA); Rebecca Jackson (The Ohio State University, Columbus, OH); Cynthia A. Thomson (University of Arizona, Tucson/Phoenix, AZ); Jean Wactawski-Wende (University at Buffalo, Buffalo, NY); Marian Limacher (University of Florida, Gainesville/Jacksonville, FL); Robert Wallace (University of Iowa, Iowa City/Davenport, IA); Rowan T. Chlebowski (Los Angeles BioMedical Research Institute at Harbor-UCLA Medical Center, Torrance, CA); Lewis Kuller (University of Pittsburgh, Pittsburgh, PA); Sally Shumaker (Wake Forest University School of Medicine, Winston-Salem, NC).
Women’s Health Initiative Memory Study: Sally Shumaker (Wake Forest University School of Medicine, Winston-Salem, NC).
Conflict of interest: RTC has received speaker’s fees and honorarium from Novartis and honorarium for advisory boards and consulting for Novartis, Astra-Zeneca, Pfizer, and Amgen. No other author has conflicts to declare.
References
- 1. Smith DC, Prentice R, Thompson DJ, Herrmann WL. Association of exogenous estrogen and endometrial carcinoma. N Engl J Med. 1975;293 (93):1164–1167. [DOI] [PubMed] [Google Scholar]
- 2. Ziel HK, Finkle WD. Increased risk of endometrial carcinoma among users of conjugated estrogens. N Engl J Med. 1975;293 (23):1167–1171. [DOI] [PubMed] [Google Scholar]
- 3. Beral V, Bull D, Reeves G, et al. Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2005;365(9470):1543–1551. [DOI] [PubMed] [Google Scholar]
- 4. The Writing Group for the PEPI Trial. Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Intervention (PEPI) Trial. JAMA. 1996;275 (5):370–375. [DOI] [PubMed] [Google Scholar]
- 5. Speroff L, Rowan J, Symons J, et al. The comparative effect on bone density, endometrium and lipids of continuous hormones as replacement therapy (CHART study). JAMA. 1996;170 (17):1213–1223. [PubMed] [Google Scholar]
- 6. Briton BA, Felix AS. Menopausal hormone therapy and risk of endometrial cancer. J Steroid Biochem Mol Biol. 2014;142:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lacey JV, Brinton LA, Lubin JH, et al. Endometrial carcinoma risks among menopausal estrogen plus progestin and unopposed estrogen users in a cohort of postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2005;14 (7):1724–1731. [DOI] [PubMed] [Google Scholar]
- 8. Razavi P, Pike MC, Horn-Ross PL, et al. Long-term postmenopausal hormone therapy and endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2010;19 (2):475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schmidt P. The 2012 hormone therapy position statement of the North American Menopausal Society. Menopause. 2012;19 (3):257–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Villers TJ, Gass MLS, Haines CJ, et al. Global consensus statement on menopausal hormone therapy. Climacteric. 2013;16 (2):203–204. [DOI] [PubMed] [Google Scholar]
- 11. Nelson HD, Walker M, Zakher B, Mitchell J. Menopausal hormone therapy for the primary prevention of chronic conditions: a systematic review to update the U.S. Preventive Services Task Force recommendations. Ann Intern Med. 2012;157 (2):104–113. [DOI] [PubMed] [Google Scholar]
- 12. Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol. 2013;31 (20):2607–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anderson GL, Judd HL, Kaunitz AM, et al. Effects of estrogen plus progestin on gynecologic cancers and associated diagnostic procedures. The Women’s Health Initiative randomized trial. JAMA. 2003;290 (13):1739–1748. [DOI] [PubMed] [Google Scholar]
- 14. Heiss G, Wallace R, Anderson GL, et al. Health risks and benefits 3 years after stopping randomized treatment with estrogen and progestin. JAMA. 2008;299 (9):1036–1045. [DOI] [PubMed] [Google Scholar]
- 15. Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended post-stopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;319 (13):1353–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. JAMA. 2002;288 (3):321–333. [DOI] [PubMed] [Google Scholar]
- 17. Anderson GL, Manson J, Wallace R, et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13(9 Suppl):S5–S17. [DOI] [PubMed] [Google Scholar]
- 18. Chlebowski RT, Anderson GL, Gass M, et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304 (15):1684–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grambsch TM, Therneau P. Modeling Survival Data: Extending the Cox Model. Statistics for Biology and Health; 2000. [Google Scholar]
- 20. Cramer DW. The epidemiology of endometrial and ovarian cancer. Hematol Oncol Clin North Am. 2012;26 (1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative randomized trial. JAMA. 2003;289 (24):3243–3253. [DOI] [PubMed] [Google Scholar]
- 22. Hulley S, Furberg C, Barrett-Connor E, et al. Non-cardiovascular disease outcomes during 6.8 years of hormone therapy. JAMA. 2002;288 (1):58–66. [DOI] [PubMed] [Google Scholar]
- 23. Phipps AI, Doherty JA, Voigt LY, et al. Long-term use of continuous-combined estrogen-progestin hormone therapy and risk of endometrial cancer. Cancer Causes Control. 2011;22 (12):1639–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Strom BL, Schinnar R, Weber AL, et al. Case-control study of postmenopausal hormone replacement therapy and endometrial cancer. Am J Epidemiol. 2006;164:775–786. [DOI] [PubMed] [Google Scholar]
- 25. Allen NE, Tsilidis KK, Key TJ, et al. Menopausal hormone therapy and risk of endometrial carcinoma among postmenopausal women. European Prospective Investigation into Cancer and Nutrition. Am J Epidemiol. 2010;172 (12):1394–1403. [DOI] [PubMed] [Google Scholar]
- 26. Trabert B, Wentzensen N, Yang HP, et al. Is estrogen plus progestin menopausal hormone therapy safe with respect to endometrial cancer risk? Int J Cancer. 2013;132 (2):417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karageorgi S, Hankinson SE, Kraft P, De Vivo I. Reproductive factors and post-menopausal hormone use in relation to endometrial cancer risk in the Nurses’ Health Study cohort 1976–2004. Int J Cancer. 2010;126 (1):208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bakken K, Fournier A, Lund E, et al. Menopausal hormone therapy and breast cancer risk: impact of different treatments. The European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2011;128 (1):144–156. [DOI] [PubMed] [Google Scholar]
- 29. Fournier A, Berrino F, Clavel-Chapelon F. Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N-EPIC cohort study. Breast Cancer Res Treat. 2008;107 (1):103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fournier A, Dossus L, Mesrine S, et al. Risks of endometrial cancer associated with different hormone replacement therapies in the E3N cohort, 1992–2008. Am J Epidemiol. 2014; In press. [DOI] [PubMed] [Google Scholar]
- 31. Lacroix AZ, Chlebowski RT, Manson JE, et al. Health risks and benefits 5 years after stopping randomized treatment with conjugated equine estrogens in postmenopausal women with prior hysterectomy. JAMA. 2011;305 (13):1305–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Anderson GL, Chlebowski RT, Aragaki A, et al. Estrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy extended follow-up of the Women’s Health Initiative randomized trial. Lancet Oncol. 2012;13 (5):476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barnabei VM, Cochrane BB, Aragaki AK, et al. Menopausal symptoms and treatment-related effects of estrogen and progestin in the Women’s Health Initiative. Obstet Gynecol. 2005;105(5 Pt 1):1063–1073. [DOI] [PubMed] [Google Scholar]
- 34. Stanczyk FZ, Hapgood JP, Winer S, Mishell DR. Progestogens used in postmenopausal hormone therapy: Differences in their pharmacological properties, intracellular actions, and clinical effects. Endocrine Rev. 2012;34 (2):171–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hersh AL, Stefanick ML, Stafford RS. National use of menopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291 (1):47–53. [DOI] [PubMed] [Google Scholar]
- 36. Wartko P, Sherman ME, Yang HP, Felix AS, Brinton LA, Trabert B. Recent changes in endometrial cancer trends among menopausal-age US women. Cancer Epidemiol. 2013;37 (4):374–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Glass AG, Lacey JV, Jr, Carreon JD, Hoover RN. Breast cancer incidence, 1980–2006: combined roles of menopausal hormone therapy, screening mammography, and estrogen receptor status. J Natl Cancer Inst. 2007;99 (15):1152–1161. [DOI] [PubMed] [Google Scholar]
- 38. Ravdin PM, Cronin KA, Howlander N, et al. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med. 2007;356 (16):1670–1674. [DOI] [PubMed] [Google Scholar]
- 39. Chlebowski RT, Keller LH, Prentice RL, et al. Breast cancer after estrogen plus progestin use in postmenopausal women. New Eng J Med. 2009;360 (6):573–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sprague BL, Trentham-Dietz A, Remington PL. The contribution of postmenopausal hormone use cessation to the declining incidence of breast cancer. Cancer Causes Control. 2011;22 (1):125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zbuk K, Anand SS. Declining incidence of breast cancer after decreased use of hormone-replacement therapy: magnitude and time lags in different countries. J Epidemiol Community Health. 2012; 66 (1):1–7. [DOI] [PubMed] [Google Scholar]
- 42. Ito K, Utsunomiya H, Yaegasshi N, Sasano H. Biological roles of estrogen and progesterone in human endometrial carcinoma-new developments in potential endocrine therapy for endometrial cancer. Endocr J. 2007;54 (5):667–679. [DOI] [PubMed] [Google Scholar]
- 43. Yang S, Thiel KW, Leslie KK. Progesterone: the ultimate endometrial tumor suppressor. Trends Endocrinol Metabl. 2011;22 (4):145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kesterson JP, Fanning J. Fertility-sparing treatment of endometrial cancer: options, outcomes and pitfalls. J Gynecol Oncol. 2012;23 (2):120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354 (3):270–282. [DOI] [PubMed] [Google Scholar]
- 46. Lewis-Wambi JS, Jordan VC. Estrogen regulation of apoptosis: how can one hormone stimulate and inhibit? Breast Cancer Res. 2009;11 (3):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yao K, Lee ED, Bentrem DJ, et al. Antitumor action of physiological estradiol on tamoxifen-stimulated breast tumors grown in athymic mice. Clin Cancer Res. 2000;6 (5):2028–2036. [PubMed] [Google Scholar]
- 48. Jordan VC, Ford LG. Paradoxical clinical effects of estrogen on breast cancer risk: a “new” biology of estrogen-induced apoptosis. Cancer Prev Res. 2011;4 (5):633–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hofseth LJ, Raafat AM, Osuch JR, Pathak DR, Slomski CA, Haslam SZ. Hormone replacement therapy with estrogen or estrogen plus medroxyprogesterone acetate is associated with increased epithelial proliferation in the normal postmenopausal breast. J Clin Endocrinol Metab. 1999;84 (12):4559–4568. [DOI] [PubMed] [Google Scholar]
- 50. Joshi PA, Jackson HW, Beristain AG, et al. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465(7299):803–807. [DOI] [PubMed] [Google Scholar]
- 51. Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev. 2013;34 (1):130–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wheeler DT, Bristow RE, Kurman RJ. Histologic alterations in endometrial hyperplasia and well-differentiated carcinoma treated with progestins. Am J Surg Pathol. 2007;31 (7):988–980. [DOI] [PubMed] [Google Scholar]
- 53. Carlson MJ, Thiel KW, Yang S, Leslie KK. Catch it before it kills: progesterone, obesity, and the prevention of endometrial cancer. Discov Med. 2012;14 (76):215–222. [PMC free article] [PubMed] [Google Scholar]
- 54. Crosbie EJ, Zwhalen M, Kitchener HC, Egger M, Renehan AG. Body mass index, hormone replacement therapy, and endometrial cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;19 (12):3119–3130. [DOI] [PubMed] [Google Scholar]
- 55. Nagle CM, Marguart L, Bain CJ, et al. Impact of weight change and weight cycling on risk of different subtypes of endometrial cancer. Eur J Cancer. 2013;49 (12):2717–2726. [DOI] [PubMed] [Google Scholar]
- 56. Ockene JK, Barad DH, Cochrane BB, et al. Symptom experience after discontinuing use of estrogen plus progestin. JAMA. 2005;294 (2):183–193. [DOI] [PubMed] [Google Scholar]