SUMMARY
BACKGROUND: In accordance with the existing hypothesis, the application of an endobronchial valve (EbV) leads to selective curative atelectasis of the affected part of the lung, contributing to early closure of cavities.
OBJECTIVE: To assess the effect of EbV treatment on the course of tuberculosis (TB).
METHODS: We compared the efficacy of EbV treatment and complex second-line treatment in treating patients with destructive pulmonary multidrug-resistant TB (MDR-TB). Bacteriological conversion and closure of cavities were selected as criteria to assess the effectiveness of EbV application. A total of 102 patients with destructive MDR-TB were enrolled into the study and randomly divided into two groups: 49 patients had an EbV installed (intervention group) and 53 patients received complex second-line treatment (control group). Complex chemotherapy was administered to both groups throughout the study period.
RESULTS: The cure rate in the short- and long-term follow-up periods in the intervention group was shown to be much higher, 95.9% by bacteriological conversion and 67.3% by cavity closure. On comparison with the control group, this was respectively 37.7% and 20.7% (P < 0.0001).
CONCLUSIONS: The application of EbV treatment can significantly improve the effectiveness of second-line chemotherapy regimens in MDR-TB patients.
Keywords: non-pharmacological treatment of TB, EbV, anti-tuberculosis treatment, bronchoscopy
RESUME
CADRE : En accord avec l'hypothèse existante, la pose d'une valve endobronchique (EbV) aboutit à une atélectasie sélective et curative de la partie affectée du poumon, ce qui contribute à une fermeture rapide des cavernes.
OBJECTIF : Evaluer l'effet du traitement par EbV sur l'évolution de la tuberculose (TB).
MÉTHODES : Nous avons comparé l'efficacité du traitement par EbV dans le cadre du traitement de patients atteints de TB multirésistante (TB-MDR) pulmonaire destructive par rapport à un traitement complexe de deuxième ligne. La conversion bactériologique et la fermeture des cavernes ont été les critères sélectionnés pour évaluer l'efficacité de la mise en œuvre de l'EbV. Au total, 102 patients atteints de TB-MDR destructive ontété enrôlés dans l'étude ; ils ont été répartis de manière aléatoire en deux groupes : 49 patients ont eu une EbV (le groupe d'intervention) et 53 patients ont reçu un traitement complexe de deuxième ligne (le groupe témoin). Une chimiothérapie complexe a été administrée aux deux groupes tout au long de la période d'étude.
RÉSULTATS : Le taux de guérison pendant les périodes de suivi à court terme et à long terme dans le groupe d'intervention a été beaucoup plus élevé, comme l'a démontré la conversion bactériologique de 95,9% et le taux de fermeture des cavités de 67,3% comparéà 37,7% et 20,7% dans le groupe témoin (P < 0,0001).
CONCLUSION : La mise en œuvre d'un traitement par EbV peut significativement améliorer l'efficacité d'un protocole de chimiothérapie de deuxième ligne pour les patients atteints de TB-MDR.
RESUMEN
MARCO DE REFERENCIA: Según las hipótesis existentes, la colocación de una válvula endobronquial (EbV) da lugar a una atelectasia curativa selectiva de la parte afectada del pulmón, lo cual facilita el colapso temprano de las cavernas durante el tratamiento de la tuberculosis (TB).
OBJETIVO: Evaluar el efecto la utilización de una EbV durante el tratamiento de la TB.
MÉTODOS: La eficacia de la utilización de la EbV durante el difícil tratamiento de los pacientes con diagnóstico de TB pulmonar multirresistente (TB-MDR) destructiva se comparó con el tratamiento complejo con medicamentos de segunda línea. Se escogieron como criterios de eficacia de la EbV, la conversión bacteriológica y el colapso de las cavernas. Participaron en el estudio 102 pacientes con TB-MDR destructiva, quienes de manera aleatoria se repartieron en dos grupos, a saber: un grupo experimental conformado por 49 pacientes en quienes se instaló una EbV y un grupo testigo conformado por 53 pacientes que recibieron un tratamiento complejo con antituberculosos de segunda línea. El tratamiento con medicamentos antituberculosos se administró a ambos grupos durante todo el período del estudio.
RESULTADOS: Se observó una tasa de curación mucho más alta en el grupo experimental durante el seguimiento a corto plazo y a largo plazo, según la conversión bacteriológica de 95,9% y el colapso de cavernas de 67,3%, en comparación con el grupo testigo (37,7% y 20,7%, respectivamente; P < 0,0001).
CONCLUSIÓN: La colocación de una EbV durante el tratamiento mejora de manera significativa la eficacia de los regímenes con medicamentos antituberculosos de segunda línea en los pacientes con TB-MDR.
DATA REGARDING THE APPLICATION of an endobronchial valve (EbV) in treating various pulmonary diseases have recently been published in the medical literature. This drug-free, minimally invasive method is used to reduce lung volume in patients with pulmonary emphysema,1–3 to treat pneumothorax in patients with bronchopleural fistulas and/or in postoperative patients,4–9 and to treat spontaneous pneumothorax of different aetiologies.10 In Russia, EbV has been used to treat patients with various forms of destructive pulmonary tuberculosis (PTB).11,12 Despite the widespread application of this method, very little is known about its efficacy and safety.
The objective of the present study is to study the effect of EbV as an intervention among patients with destructive pulmonary multidrug-resistant TB (MDR-TB).
MATERIALS AND METHODS
We conducted an open, comparative, interventional randomised clinical trial to evaluate the safety and efficacy of the EbV (MedLung®, Barnaul, Russian Federation) to treat MDR-TB. The study was conducted in the Novosibirsk Tuberculosis Research Institute, Novosibirsk, the Russian Federation, from January 2008 to December 2014.
Patients with similar clinical, laboratory and radiological results were randomised into two study groups using Statistica 6.0 (Dell Software, Austin, TX, USA) (Figure 1). All patients had documented destructive MDR-TB, and had received appropriate second-line anti-tuberculosis treatment in accordance with their drug resistance pattern for more than 12 months before being enrolled into the study; this included patients with treatment failure. All patients were smear-positive and had not demonstrated bacteriological conversion for the previous 6 months. Chemotherapy for all patients included a combination of at least five first- or second-line anti-tuberculosis drugs according to individual drug resistance patterns. Drug combinations mainly comprised pyrazinamide, ethambutol, capreomycin or aminoglycosides, fluoroquinolones (ofloxacin [OFX], levofloxacin [LVX] or moxifloxacin [MFX]), cycloserine (CS) and para-aminosalicylic acid (PAS). Chemotherapy regimens were comparable between the two groups.
Figure 1.
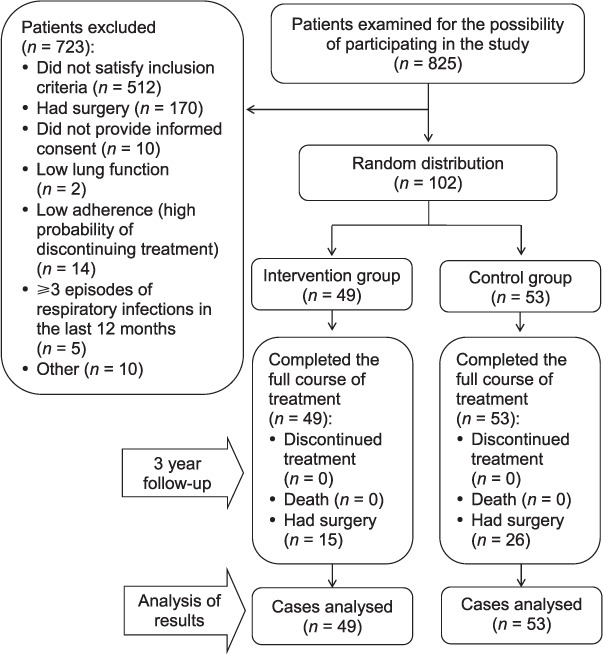
Inclusion of patients in the study.
Study inclusion criteria were as follows: 1) clinically, radiologically and bacteriologically confirmed destructive MDR-TB of the upper lobes of lungs after treatment for 12–24 months; 2) smear-positive MDR-TB patients with no bacteriological conversion for the last 6 months; and 3) signed informed consent (the patient read, understood and signed the informed consent form).
Study exclusion criteria were as follows: 1) refusal to provide informed consent; 2) respiratory infections requiring antibiotic treatment occurring more than three times a year; and 3) significant purulent endobronchial secretion in the site of the hypothetical EbV installation.
A total of 102 patients were included in the study. Sample size was determined based on the results of previous studies,10–12 study power (β-10%) and the high risk of loss to follow-up (low treatment adherence). The intervention group included 49 patients with destructive MDR-TB who received an EbV in addition to complex second-line treatment. The control group included 53 patients who were administered appropriate conservative complex anti-tuberculosis treatment without EbV.
The primary endpoint of the study was bacteriological conversion and cavity closure within 365 days after EbV installation. The primary safety endpoint consisted in reducing the number of complications observed in patients within 365 days of the procedure.
EbV (MedLung, certified in Russia and Europe) was installed in the appropriate lobe bronchus corresponding to the primary cavitation and to the related destructive pathology. Fissure integrity was assessed by manual marking by an experienced radiologist on computed tomography (CT) scan. Indication for EbV installation was progressive destructive MDR-TB with no bacteriological conversion in the previous 6 months. Indications for EbV withdrawal were stable bacteriological conversion (⩾3 months), complications caused by EbV, absence of any effect of EbV for 3 months or preparation for surgical treatment.
The EbV is a hollow cylinder made from medically inert rubber composite material (Figure 2). The valve's inner bore is round and has a smooth surface, with a nylon strut at one side and a falling petal valve on the other, lockable due to significant external pressure and its own elastic properties. Two thirds of the valve's outer surface consists of thin plate radial petals, securing the device within the bronchus (Appendix). The choice of valve size depends on the diameter of the draining bronchus where it is to be placed (main, lobar, segmental, subsegmental); it should exceed the diameter of the bronchus by 1.2–1.5-fold. The requisite EbV size was determined after visual assessment of the bronchial tree by comparing its diameter with that of the bronchoscope. We also used endobronchial forceps of different types and sizes to assess bronchus diameter, measuring the distance between the fully open jaws. The valve allowed for unidirectional outflow of air and sputum during expiration and coughing.
Figure 2.

Endobronchial valve structure: 1 = hollow cylinder; 2 = valve inner bore; 3 = strut for holding; 4 = radial petals for fixing the valve in the bronchus; 5 = falling petal valve.
For patients in both groups, evaluation of treatment efficacy was conducted synchronously, in accordance with the order of the Russian Ministry of Health (order #109 dated 21 March 2003) and World Health Organization (WHO) recommendations.13 A cured case was defined as a patient who had completed treatment per national recommendations with no evidence of failure and three or more consecutive negative cultures taken at least 30 days apart after the intensive phase of treatment. Long-term results were evaluated 3 years after EbV removal in the intervention group, or 3 years after the patient's discharge from hospital in the control group. Long-term treatment results were evaluated based on criteria for clinical cure, progression of TB disease or death.
Statistical analysis was carried out using Statistica 6.0 and Statistical Package for the Social Sciences 18.0 software (IBM Corp, Armonk, NY, USA). We calculated the average, standard deviation or standard error. Under normal distribution (the Kolmogorov-Smirnov test), the statistical significance of differences (P values) was determined using Pearson's χ2 test, Mann-Whitney U-test and Wilcoxon paired test. If entered into a 2×2 table, Fisher's exact test was used to obtain values with a P value of <5. Differences were considered statistically significant at P < 0.05.
RESULTS
The proportion of male patients was 36.3%. This was not due to deliberate selection, but because of the overall trend in hospital admissions, which was reflected in the randomised distribution. The largest age group was 21–30 years (n = 76, 74.5%). The average age of patients enrolled in the study was 29.8 ± 11.0 years (age 18–64 years).
Rare cough, which resolved without further intervention, was observed in 12 patients in the intervention group (24.5%). Four (8.2%) patients experienced dry cough, dyspnoea on exertion and intermittent elevations of temperature (up to subfebrile readings). These patients required additional symptomatic drug therapy. Exacerbation of chronic obstructive pulmonary disease requiring bronchodilator therapy occurred in 4 (8.2%) patients. All of these symptoms resolved within 3 weeks after EbV installation.
During application of the EbV, special attention was paid to the speed of bacteriological conversion and the effect of EbV on radiographic changes. It is important to highlight the high percentage of patients with bacteriological conversion in the intervention group—47 (95.9%) within the first 3 months of study enrolment compared to only 20 (37.7%) in the control group (P < 0.0001). Bacteriological conversion in the control group no doubt occurred due to the directly observed chemotherapy provided at the Novosibirsk Tuberculosis Research Institute.
Serial control X-ray examination revealed that the majority of the patients (27, 55.1%) had hypoventilation in the blocked area, while 13 (26.5%) had complete atelectasis. However, hypoventilation was not observed after EbV placement in nine (18.4%) patients. Despite the absence of any signs of hypoventilation, two patients had cavity closure. EbV was ineffective in 7 patients; in 5 of these an increase in cavity size was observed without growth of the infiltrative component, TB progression was observed in 1 patient, and 1 person had no dynamic changes.
Cavity closures were recorded in 33 (67.3%) cases in the intervention group and in 11 (20.7%) cases in the control group (relative risk [RR] 2.72, 95% confidence interval (CI) 2.3–3.14) (Figure 3). The duration of EbV occlusion in the intervention group was 201.6 ± 14.77 days.
Figure 3.

Study results: efficacy and 3 years follow-up. EbV = endobronchial valve; MTB = bacteriological conversion; MTB+= biological specimen is positive on smear microscopy or culture; CV = closure of cavities; CV+= persistence of cavities; TB = tuberculosis.
Cavity closures within the first 3 months of treatment occurred in most patients in the intervention group (27, 55.1%) vs. only two (3.8%) patients in the control group (P < 0.0001). Cavity closures in the intervention group occurred within 113.7 ± 23.5 days vs. 134.4 ± 13.0 days in the control group (P = 0.003). The speed of cavity closure did not correlate with cavity size: in the intervention group the Spearman correlation coefficient was 0.154 (P = 0.3) vs. 0.07 in the control group (P = 0.68).
In those patients with halted TB progression, reduction in cavity size and partial infiltrate resorption following EbV intervention, the condition of three (37.5%) patients stabilised and a positive trend in the progression of TB disease was noted, while five (62.5%) had severe pulmonary fibrotic changes, with fibrous strands extending to the pleura and cavities developing in the fibrous walls. These patients were prepared for surgery, and partial lung resection was performed (Figures 1 and 3). Residual changes in a blocked bronchus were assessed after EbV removal. Immediately after EbV removal, proliferation of granulation at the site of the contact between the valve body and the bronchial wall was observed in all 49 patients.
According to the National Clinical Recommendations13 and WHO recommendations,14 indications for surgical treatment in patients of both groups were as follows: 1) irreversible TB progression despite adequate anti-tuberculosis chemotherapy; 2) localised forms of cavitary TB with continuous Mycobacterium tuberculosis excretion confirmed on bacteriological examination and drug susceptibility testing after 4–6 months of supervised anti-tuberculosis chemotherapy; 3) MDR-TB characterised by failure of anti-tuberculosis chemotherapy; and 4) complications of the TB process, including MDR/XDR-TB (extensively drug-resistant TB).
Long-term improvement in treatment results occurred in 77 (75.5%) patients: 41 (83.7%) in the intervention group and 36 (67.9%) in the control group. The follow-up period lasted 3 years (Figure 3). A total of 42 (54.5%) MDR-TB patients from both groups were cured: 33 (80.5%) in the intervention group and 9 (25.0%) in the control group (RR 3.44, 95%CI 2.79–4.08). Relapse was observed in 5 (6.5%) patients: 2 (4.9%) in the intervention group and 3 (8.3%) in the control group (P = 0.43). We defined relapse according to the WHO definitions,15 as a patient who completed chemotherapy but who was once again diagnosed with TB.
We also analysed patients with TB progression during long-term (3-year) follow-up. TB progression was observed in 30 (38.9%) patients: 6 (14.6%) in the intervention group and 24 (66.7%) in the control group (RR 3.72, 95%CI 3.01–4.43).
DISCUSSION
This study presents the results of EbV intervention among patients with destructive MDR-TB. The study follow-up period was 36 months. Our study shows that EbV intervention in patients with PTB is safe and potentially reversible, and is associated with a low complication rate; no intervention-associated mortality was observed in the study. The use of this procedure contributes to stabilisation and improvement of the PTB process. As the study is only an initial description of EbV intervention in PTB patients, further research and large randomised controlled trials are needed to evaluate the safety and efficacy of the procedure. The demographic profile of the study participants matched that of Russian TB patients in general, allowing for a degree of generalisability of these study results, and further highlighting the unfavourable epidemiological situation in Russia,16,17 as these patients essentially represent a stable bacterial vector of infection.
The treatment of such patients presents many challenges, including both patients' motivation to adhere to and comply with treatment protocols and the problem of tolerability of anti-tuberculosis drugs. Side effects commonly lead to treatment interruption and incomplete therapy.18
In Russia, two kinds of surgical procedures are used for the treatment of such patients: partial lung resection and lung volume reduction with bone block. These operative methods invariably present some risk to the patient, and patients suffer cosmetic defects in the postoperative period.19 A significant number of patients consequently refuse surgical management. Minimally invasive and/or drug-free treatment methods are therefore being investigated to explore options for complementary therapy methods to improve treatment outcomes.
In this study, EbV was installed in the lobe bronchus in which the destructive process was occurring. Although no complications were observed at the time of the procedure, a number of late complications led to EbV removal. Complications were noted in 14% of the patients in the intervention group. We independently analysed possible causes of the complications and concluded that most of them could have been prevented. Ten per cent of patients experienced an increase in cavity size and radiological evidence of an air-fluid level. This may be attributed to compromised drainage of the bronchus in which the EbV was placed, with simultaneous formation of interbronchial anastomoses working on a valve principle. It was not possible to prevent this by manual marking of fissure integrity on the CT scan, and the problem was not resolved during bronchoscopy. After EbV installation, there were initially no radiological signs of atelectasis in these patients. Based on this experience, we recommend that the EbV be removed in the absence of radiological signs of atelectasis or hypoventilation.
One patient experienced excessive proliferation of granulation, which completely filled the bronchus lumen. It was noted before and during EbV installation that the lumen of the bronchus was narrower and longer than in the rest of the bronchi. The EbV was installed deep in the bronchus, and all valve parts were completely immersed in the bronchus lumen. The abundant proliferation of granulation in this patient could be attributed to permanent irritation and possible mucosal damage by the distal portion of the EbV device. In one study patient, it was not possible to withdraw the EbV using an endoscope; planned resection was therefore performed.
In conclusion, EbV interventions can lead to reversible changes in respiratory function, and complications are rare and reversible. EbV installation leads to faster bacteriological conversion and cavity closure in MDR-TB patients; we may therefore assume that it can significantly improve the efficacy of anti-tuberculosis treatment. We believe that further research should focus on the mechanisms by which EbV promotes regression of the TB process. Randomised controlled clinical trials will provide further characterisation of the advantages and additional clinical aspects of employing EbV as an adjunct treatment for patients with cavernous TB.
APPENDIX
Procedure of EbV installation
EbV installation was carried out under local anaesthesia combined with medical sedation of the patient in the presence of an anaesthetist. The average procedure lasted approximately 35 min (Figures A.1 A and B and A.2 A and B).
After valve installation, the endoscope was introduced into the bronchial tree in the appropriate area. The valve was then fixed using biopsy forceps (bronchoscopy) on the strut and secured to the lumen of the bronchus with visual confirmation. The radial plate petals of the valve model allowed for tight fixation of the valve in the bronchial lumen. While holding the valve in the bronchus, the bronchoscope was then withdrawn from the valve (Figure A.3 A–C).
No complications occurred during the procedure. In the time immediately following EbV fixation (2 h), the valve migrated into the lower divisions of the bronchial tree in three patients. This complication was believed to have been due to an incorrect assessment of the diameter of the bronchus. This complication was remedied using a repeat procedure with the correct valve size.
Figure A.1.

Valve installation using an endoscope. This image can be viewed online in colour at http://www.ingentaconnect.com/content/iuatld/ijtld/2016/00000020/00000011/art00021
Figure A.2.

Bronchoscope with the endobronchial valve.
Figure A.3.

Endobronchial valve installation: A) removal of the valve from the bronchoscope using forceps; B) valve removal from the distal endoscope (endophoto); C) endoscope withdrawal from bronchial valve (endophoto). This image can be viewed online in colour at http://www.ingentaconnect.com/content/iuatld/ijtld/2016/00000020/00000011/art00021
Footnotes
Conflicts of interest: AL has a close family member who is a chief executive officer in MedLung, which produces endobronchial valves. AL is the rights holder of the Russian patent and European certification for the endobronchial valve. There are no other potential conflicts.
References
- 1.Stratakos G, Emmanouil P, Gasparini S. Novel modalities and agents in bronchoscopic lung volume reduction. Curr Drug Targets. 2013;14:253–261. doi: 10.2174/1389450111314020010. [DOI] [PubMed] [Google Scholar]
- 2.Shah P L, Slebos D J, Cardoso P F et al. Bronchoscopic lung-volume reduction with Exhale airway stents for emphysema (EASE trial): randomised, sham-controlled, multicentre trial. Lancet. 2011;378:997–1005. doi: 10.1016/S0140-6736(11)61050-7. EASE trial study group. [DOI] [PubMed] [Google Scholar]
- 3.Kotecha S, Westall G P, Holsworth L et al. Long-term outcomes from bronchoscopic lung volume reduction using a bronchial prosthesis. Respirology. 2011;16:167–173. doi: 10.1111/j.1440-1843.2010.01896.x. [DOI] [PubMed] [Google Scholar]
- 4.Abu-Hijleh M, Blundin M. Emergency use of an endobronchial one-way valve in the management of severe air leak and massive subcutaneous emphysema. Lung. 2010;188:253–257. doi: 10.1007/s00408-009-9204-0. [DOI] [PubMed] [Google Scholar]
- 5.Schweigert M, Kraus D, Ficker J H, Stein H J. Closure of persisting air leaks in patients with severe pleural empyema—use of endoscopic one-way endobronchial valve. Eur J Cardiothorac Surg. 2011;39:401–403. doi: 10.1016/j.ejcts.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Giddings O, Kuhn J, Akulian J. Endobronchial valve placement for the treatment of bronchopleural fistula: a review of the current literature. Curr Opin Pulm Med. 2014;20:347–351. doi: 10.1097/MCP.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 7.Hodges A M, Gillham M J, Lewis C A. Bedside placement of an endobronchial valve to aid invasive ventilation and weaning from extracorporeal membrane oxygenation. Crit Care Resusc. 2015;17:219–222. [PubMed] [Google Scholar]
- 8.Toma T P, Todrys K W, Amon J J. Reduction of persistent air leak with endoscopic valve implants. Thorax. 2007;62:829–832. doi: 10.1136/thx.2005.044537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wood D E, Cerfolio R J, Gonzalez X, Springmeyer S C. Bronchoscopic management of prolonged air leak. Clin Chest Med. 2010;31:127–133. doi: 10.1016/j.ccm.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Tseimakh Ye, Levin A, Zimonin P et al. Endobronchial valve in a complex treatment of a spontaneous pneumothorax. European Respiratory Society Annual Congress, Barcelona, Spain, 18–22 September 2010. (Abstract 2181) Eur Respir J. 2009;34(Suppl):390. [Google Scholar]
- 11.Krasnov D V, Grishchenko N G, Beschetnyy T G et al. The use of valve stem bronchus blockade in patients with advanced fibro-cavernous pulmonary tuberculosis after osteoplastic thoracoplasty. Tuberc Lung Dis. 2010;9:8–13. [Russian] [Google Scholar]
- 12.Yaichnikov V P. Application of endobronchial valve in treatment of patients with infiltrative destructive pulmonary tuberculosis. PhD dissertation. Barnaul, Russian Federation: Altai State Medical University Publications, 2011. [Russian]
- 13.National Association of TB Specialists, Association of Thoracic Surgery of Russia. National clinical guidelines on the use of surgical techniques in the treatment of pulmonary tuberculosis. Moscow, Russian Federation: Association of Thoracic Surgery of Russia; 2013. [Russian] [Google Scholar]
- 14.World Health Organization. The role of surgery in the treatment of pulmonary TB and multidrug and extensively drug resistant TB. Geneva, Switzerland: WHO; 2014. [Google Scholar]
- 15.World Health Organization. Definitions and reporting framework for tuberculosis—2013 revision. Geneva, Switzerland: WHO; 2013. WHO/HTM/TB/2013.2. [Google Scholar]
- 16.Shilova M V. The epidemiological situation of tuberculosis in the Russian Federation to the beginning of 2009. Probl Tuberk Bolezn Legk. 2010;5:14–21. [Russian] [Google Scholar]
- 17.Shilova M V. Tuberculosis hospitals in Russia: requirements, prospects for development. Probl Tuberk Bolezn Legk. 2009;5:9–15. [Russian] [PubMed] [Google Scholar]
- 18.Woith W, Volchenkov G, Larson J. Barriers and motivators affecting tuberculosis infection control practices of Russian health care workers. Int J Tuberc Lung Dis. 2012;16:1092–1096. doi: 10.5588/ijtld.10.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perelman M I, Strelzov V P. Surgery for pulmonary tuberculosis. World J Surg. 1997;21:457–467. doi: 10.1007/pl00012270. [DOI] [PubMed] [Google Scholar]


