Abstract
Purpose
To determine whether helium-3 diffusion MR can detect the changes in the lungs of healthy nonsmoking individuals who were regularly exposed to secondhand smoke.
Materials and Methods
Three groups were studied (Age: 59±9 years): 23 smokers, 37 exposure-to-secondhand-smoke subjects, and 29 control subjects. We measured helium-3 diffusion values at diffusion times from 0.23 to 1.97 seconds.
Results
One-Way ANOVA revealed that the mean area under the helium-3 diffusion curves (ADC AUC) of the smokers was significantly elevated compared to the controls and to the exposure-to-secondhand-smoke subjects (P < 0.001 both). No difference between the mean ADC AUC of the exposure-to-secondhand-smoke subjects and that of the controls was found (P=0.115). However, application of a receiver operator characteristic derived rule to classify subjects as either a “control” or a “smoker”, based on ADC AUC, revealed that 30% (11/37) of the exposure-to-secondhand subjects were classified as “smokers” indicating an elevation of the ADC AUC.
Conclusion
Using helium-3 diffusion MR, elevated ADC values were detected in 30% of nonsmoking healthy subjects who had been regularly exposed to secondhand smoke, supporting the concept that, in susceptible individuals, secondhand smoke causes mild lung damage.
Keywords: Helium-3 MRI, diffusion MRI, secondhand smoke, emphysema, ADC
INTRODUCTION
Exposure to secondhand tobacco smoke, which consists of smoke from burning tobacco in cigarettes, cigars or pipes and smoke exhaled by smokers, is an established cause of lung cancer and coronary artery disease in healthy nonsmoking individuals (1). A causal relationship between exposure to secondhand smoke and the development of chronic obstructive pulmonary disease (COPD) is less clear since the results from a variety of studies, some of which involved large study populations, are contradictory with some showing no effects and others finding minor changes (1–8).
Hyperpolarized helium-3 is a gaseous contrast agent for magnetic resonance imaging (MRI) that provides high-resolution images of the lung airspaces after the gas is inhaled (9–12). By using specific MR pulse-sequences, the diffusivity of the gas, which reflects the displacement of the helium-3 atoms due to random thermal motion, can be measured. In the airspaces of the lung, the diffusion of helium-3 atoms is restricted by the alveolar walls and associated structures. This restricted diffusion, denoted as the Apparent Diffusion Coefficient (ADC) (13), is relatively low in healthy subjects due to the uniformly small dimensions of the distal lung airspaces (14–17). When there is damage or destruction of the alveolar walls as happens in emphysema, the airspaces enlarge and as a result, the measured helium-3 diffusion increases leading to ADC values that are greater than those found in healthy individuals (14–16). Studies in animal models of emphysema have shown a strong correlation between the helium-3 ADC and the histological measurement of alveolar size (15, 18). Elevated ADC values have also been demonstrated in smokers who were otherwise healthy, had normal lung function, and had no other lung diseases. This suggests that hyperpolarized helium-3 diffusion MR may be highly sensitive to early structural changes in emphysema (19, 20). However, it is unknown whether helium-3 diffusion MR can detect similar changes in individuals who have never smoked themselves but have been regularly exposed to secondhand smoke for a considerable period of time.
The purpose of this study was to determine whether helium-3 diffusion MR can detect changes in the lungs of healthy nonsmoking individuals who were regularly exposed to secondhand smoke. Since only 20–35% of heavy smokers developed clinical COPD, we expect that only a portion of healthy non-smoking individuals with high secondhand smoke exposure will have elevated helium-3 diffusivity (21–26). The presence of such findings would support the concept that exposure to secondhand smoke in susceptible individuals leads to lung injury and possibly subclinical emphysema.
MATERIALS AND METHODS
Participants
Three groups of subjects were recruited from the local community by advertisement: Subjects who smoked actively or were prior smokers (smoker group), healthy subjects who had never smoked themselves but had regular exposure to secondhand smoke (exposure-to-secondhand-smoke group), and healthy subjects who had never smoked and only had occasional exposure to secondhand smoke (control group). The smoker group consisted of current and former smokers with a self-reported smoking history of at least 6 pack-years. Exclusion criteria for the exposure-to-secondhand-smoke and control groups were a personal history of active smoking, abnormal spirometry defined as a forced expiratory volume in the first second (FEV1) < 80% predicted or the portion of the forced vital capacity exhaled in the first second (FEV1/FVC) < 70%, a history of chronic lung disease, symptoms of asthma or other lung disease, allergies, or a history of pneumonia requiring hospitalization. Requirement for the exposure-to-secondhand-smoke subjects was that they had never smoked themselves but had lived for at least 10 years with a smoker who smoked in the home. Requirement for the controls was that they had never lived with a smoker, were never employed in an occupation with high secondhand smoke exposure, such as bartender or flight attendant, and never shared an office with a smoker. To minimize the influence of age, only subjects older than 40 years were recruited. A total of 89 subjects were enrolled in the study, including 23 in the smoker group, 37 in the exposure-to-secondhand-smoke group, and 29 in the control group. The smokers reported an average smoking history of 45 ± 27 pack years.
The study was performed under an institutional review board approved protocol, and informed written consent was obtained from all subjects. In addition, all imaging was performed under an FDA-approved investigational new drug application (IND # 57,866) for hyperpolarized helium-3 as an inhaled MR contrast agent.
Study Design
Each subject made one study visit during which gender, age, weight, height, and a medical history, including details on personal smoking and secondhand smoke exposure, were assessed. During the same visit, spirometry and hyperpolarized helium-3 diffusion MR were performed. Spirometry was performed seated (PB100, Puritan Bennett; Lenexa, KS) using the Knudson tables for predicted normal limits (27). Hyperpolarized helium-3 diffusion MR was performed using a 1.5-T commercial scanner (Magnetom Sonata, Siemens Medical Solutions, Malvern, PA) equipped with the multi-nuclear imaging package and a flexible, vest-shaped chest RF coil (Clinical MR Solutions, Brookfield, WI) tuned to the helium-3 resonance frequency.
The helium-3 gas was polarized by collisional spin exchange with an optically-pumped rubidium vapor using a commercial system (Model 9600 Helium Polarizer; Magnetic Imaging Technologies Inc., Durham, NC) (28); polarizations of 27% – 37% were achieved. Immediately prior to imaging, approximately 50 ml of hyperpolarized helium-3 gas was dispensed into a Tedlar bag (Jensen Inert Products, Coral Springs, FL), and medical grade N2 was added to yield a total volume that was approximately 1/3 of the subject’s forced vital capacity (FVC). The bag was transported to the scanner room where the gas mixture was inhaled through a small plastic straw by the study subject while positioned supine in the MR scanner. All subjects were instructed to exhale completely (e.g. to residual volume) immediately prior to inhaling helium-3 gas mixture.
Hyperpolarized helium-3 diffusion MR was performed during breath holding immediately following inhalation of the gas mixture. Since the optimal diffusion time for detecting smoking-related lung changes was unknown, a diffusion MR pulse sequence was used that obtained global (whole lung) data for 29 different diffusion times during a single breath hold lasting less than 10 seconds (29). The diffusion times were equally spaced with the gap of 62 ms between a minimum value of 0.23 s and a maximum value of 1.97 s. The key imaging parameters included: tag wavelength (the wavelength of the sinusoidal modulation for longitudinal magnetization), 10 mm; flip angle, 5°; readout bandwidth, 1500 Hz/pixel; and diffusion-sensitization direction, anterior-posterior. Additional details of the pulse sequence are described in Ref (29). Following the MR acquisition, the data were transferred from the MR scanner to a personal computer, and the ADC values were calculated at each diffusion time using an in-house program written in MATLAB (The Math Works, Inc, Natick, MA) (29).
Statistical Analysis
Demographics and Spirometry
Demographic categorical data were analyzed via the Pearson chi-squared test. Continuous scaled demographic and spirometry data were analyzed via One-way ANOVA.
Helium-3 ADC Analysis
We measured the ADC values at a series of diffusion times from 0.23 to 1.97 s. Rather than to compare the ADC values at a single diffusion time, we calculated the area under ADC time-profile curve (ADC AUC) (the integral of the ADC from 0.23 to 1.97 s) as a summary measure of the subject-specific ADC time-profile. A Principal Component Analysis of the ADC time-profile curve was used to determine whether the ADC AUC is a good summary measure, that is whether it captures most of the information in the ADC time-profile curve (30). After that, the ADC AUC data were analyzed via One-Way ANOVA and between-group comparisons of mean ADC AUC were conducted via the Welch t-test (31). A P ≤ 0.05 decision rule was utilized as the criterion for rejecting the null hypothesis of equal means.
Since only a fraction of heavy smokers (~20-35%) develop clinical COPD (21–26), we expect that at most a similar fraction of the exposure-to-secondhand-smoke subjects would have elevations in the measured ADC values and that the magnitude of the elevations in ADC would be much smaller than that found in the smokers. To detect a small change that occurs in only a fraction of the exposure-to-secondhand-smoke subjects, we used the ADC AUC data from the smoker and control group to develop an optimal classification rule for classifying a subject as a "control" or a "smoker" based on a receiver operator characteristic (ROC) analysis (32, 33). We then applied this classification rule to the exposure-to-secondhand-smoke group to determine the percentage of these subjects classified as a "smoker". To determine a plausible range for the 95% confidence interval of this percentage, we used the bootstrap method as described below (34).
Classification Rule Development
Let Yi be an indicator variable that equals the value 1 if subject i belongs to the smoker group and equals the value 0 if the subject i belongs to the control group. Let ADC AUCi denote the value for the integrated area under the ADC profile curve of subject i. Let denote the logistic regression predicted probability that subject i belongs to the “smoker group” based on ADC AUC of subject i (βo is the intercept from the linear regression equation while β1 is the regression coefficient). Let p denote a cutoff point in the unit interval [0, 1], such that if p̂i ≥ p subject i is classified as a "smoker" and as a "control" otherwise. Let poptimum be the value of the cutoff point p that minimizes the false classification error rate across all controls and smokers.
To find poptimum we first produce an empirical receiver operator characteristic curve of the relationship between the true positive (TP) and the false positive (FP) probabilities of classification based on a 0.001 incremental sequence of cutoff point values p in the unit interval [0, 1]. Then by utilizing the two parameter (i.e. R and E are fitting parameters to specify the functional form of the ROC curve as weighted exponential) exponential model of England: TP=R·FP[1/E]+(1-R)·[1-(1-FP)[E]] (33), we estimate TP as a smooth function of FP. The next step in finding poptimum is to find the optimum threshold point t on the exponential ROC curve (EROC) such that the false classification error rate is minimized. The final step in finding poptimum is to find the cutoff point p that produces a 2 × 2 cross classification table which matches the EROC optimum FP and FN error rates.
After poptimum is determined, the optimum classification rule was applied to the ADC AUC values of the exposure-to-secondhand-smoke subjects to determine what proportion of these individuals would be classified as a "smoker".
Finally, a plausible range of values (95% confidence interval) for the true percentage of exposed-to-secondhand-smoke subjects who would be classified as a "smoker" based on their ADC AUC was derived via the bootstrap resampling methods of Efron and Tibshirani (34).
Bootstrap 95% Confidence Interval Construction
The resampling methods of Efron and Tibshirani were utilized to derive the bootstrap sampling distribution for the percentage of exposed-to-secondhand-smoke subjects in the target population who would be classified via the optimum threshold classification rule as a "smoker" based on their ADC AUC values (34). At each of 10,000 bootstrap sampling repetitions, ADC AUC measurements were randomly selected with replacement from the set of all ADC AUC measurements of the exposure-to-secondhand-smoke group. Each ADC AUC measurement in the bootstrap sample was then inserted into the original logistic regression equation (i.e. the equation that was optimize to predict the log-odds of being a smoker based on the ADC AUC measurements of the controls and the smokers) and the prediction (p̂i) for the probability of being a smoker was calculated. Utilizing the individual p̂i, as well as the optimum threshold cutoff probability (poptimum), each of the selected ADC AUC measurements in the random sample was classified as either a "control" or a "smoker". Based on the classifications generated by the 10,000 bootstrap samples, we estimated the bootstrap frequency distribution, and the bootstrap cumulative distribution function for the percentage of exposed-to-secondhand-smoke subjects in the study population who would be classified via the optimum threshold classification rule as a "smoker" based on their ADC AUC measurements. The cumulative distribution function was then used to determine the lower and the upper limits of the bootstrap 95% confidence interval.
Statistical Software
The aforementioned analyses were conducted with the software of SAS version 9.2 (SAS Institute Inc., Cary, NC) and Spotfire Splus 8.1 (TIBCO Inc., Palo Alto, CA).
RESULTS
Demographics and Spirometry
There were no significant differences in age, gender, height, weight or Body Mass Index (BMI) between the three groups, Table 1. Either FEV1% predicted or FEV1/FVC was abnormal in 14 (61%) of 23 smokers, and both were normal in the remaining 9 smokers. Of the 37 exposure-to-secondhand-smoke subjects, 34 (92%) had been exposed for at least 10 years at home during their childhood and 19 (56%) of these also had lived with one or more smokers for 5 or more years during adulthood. Only one (3%) was exposed only as an adult.
Table 1.
Demographic and spirometric data for all subjects.
| Variable | Control (n=29) |
Exposure-to- secondhand- smoke (n=37) |
Smoker (n=23) |
P value | ||
|---|---|---|---|---|---|---|
| Ho2 | Ho3 Pairwise Comparison |
|||||
| Gender | 12M, 17F | 9M, 28F | 7M, 16F | 0.331 | ||
| Age (yrs)1 | 57.3±9.0 | 58.9±8.3 | 61.0±8.3 | 0.324 | ||
| Height (cm)1 | 168.4±8.1 | 164.4±8.6 | 167.6±8.4 | 0.135 | ||
| Weight (kg)1 | 78.4±12.8 | 74.5±14.9 | 70.4±16.6 | 0.159 | ||
| BMI1 | 27.5±3.2 | 27.5±4.9 | 25.0±5.6 | 0.148 | ||
| FEV1%predicted1 | 98.7±9.1 | 98.6±11.7 | 77.0±25.1 | <0.001 | Cont vs. ESS Cont vs. Smok6 ESS vs. Smok |
0.981 <0.001 <0.001 |
| FEV1/FVC1 | 78.5±4.1 | 77.7±4.3 | 64.2±15.2 | <0.001 | Cont vs. ESS Cont vs. Smok ESS vs Smok |
0.436 <0.001 <0.001 |
Values are mean±SD (standard deviation).
Ho: Null Hypothesis: Categorical: Homogenous proportions. Continuous: All means equal.
Ho: Null Hypothesis: Equal means.
Cont: Control.
ESS: Exposure-to-secondhand-smoke.
Smok: Smoker.
For the exposure-to-secondhand-smoke and the control groups, the FEV1% predicted and FEV1/FVC were normal for all subjects, and there were no significant differences in spirometry indices between these two groups (P > 0.436), Table 1.
Helium-3 ADC Analysis
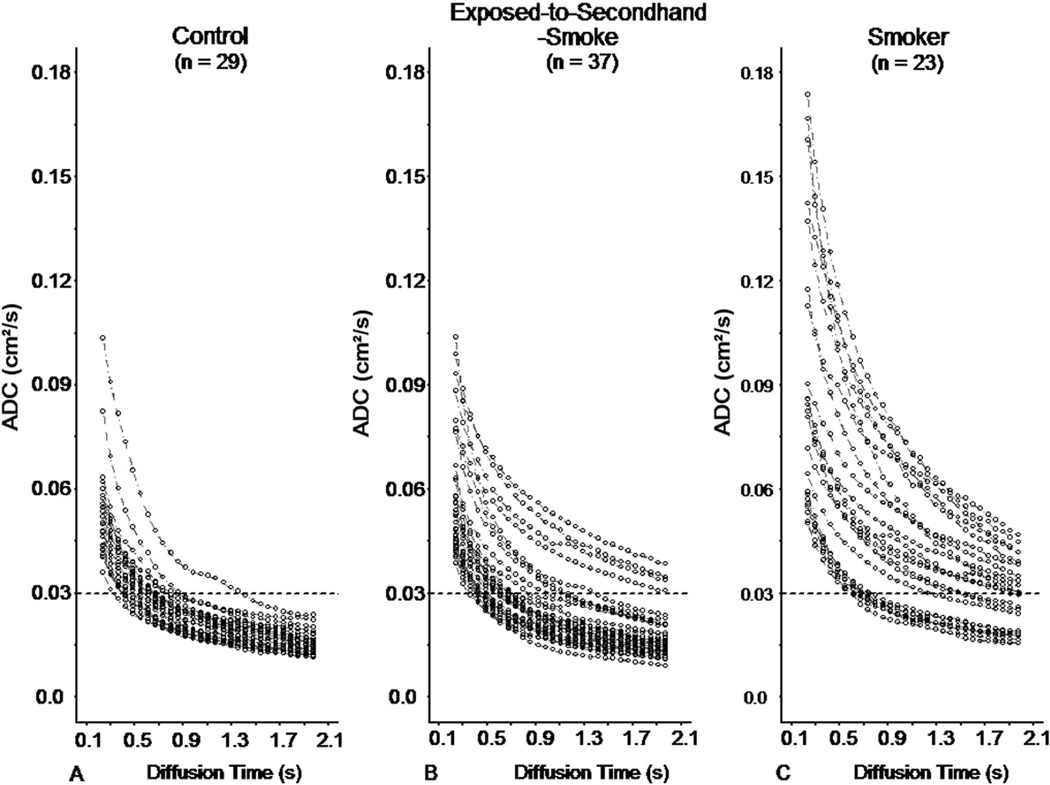
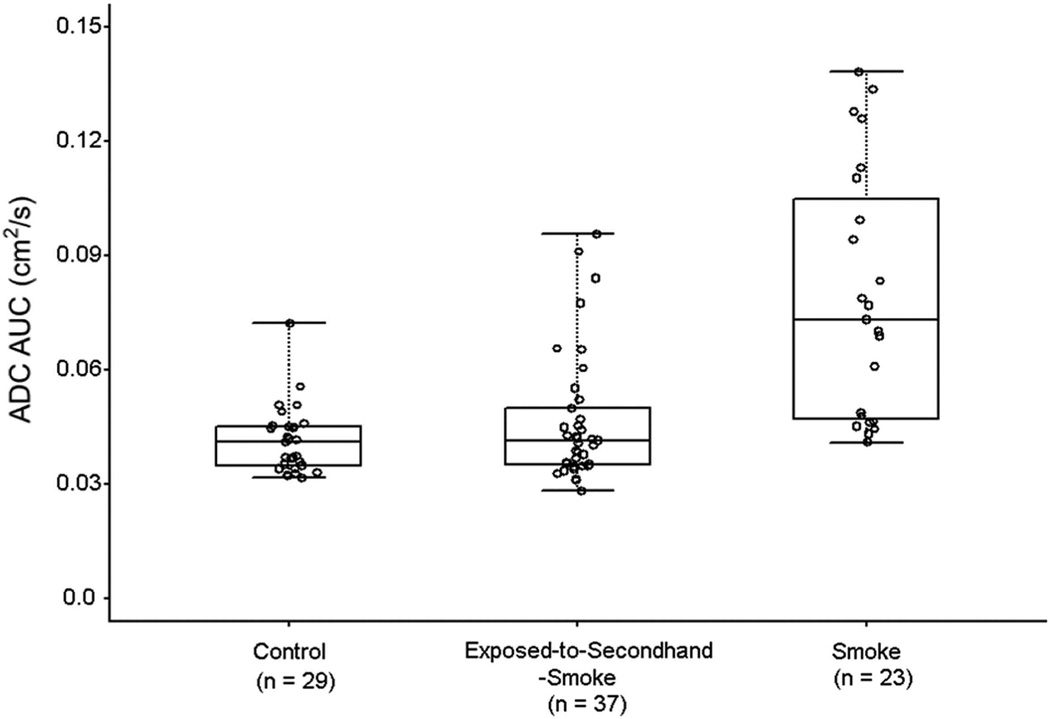
The ADC values as a function of the diffusion time for the controls, exposure-to-secondhand-smoke subjects, and smokers are displayed in Figure 1. A Principal Components Analysis showed that 97.1% of the variations in the ADC measurements were explained by the first principal component score and a subsequent linear regression analysis showed that over 99.8% of the variations in the first principal component score were explained by the ADC AUC, proving that ADC AUC is a good summary measure of the ADC time-profile (30). The corresponding measurements for ADC AUC for each group are displayed in Figure 2. The corresponding ADC AUC are 0.04 ± 0.01, 0.05 ± 0.02 and 0.08 ± 0.03 (cm2 [0.23 s – 1.97 s]), respectively for the controls, exposure-to-secondhand-smoke subjects and smokers.
Figure 1.
ADC values as a function of diffusion time for the three groups over the range of diffusion times from 0.23 to 1.97 s. (a) Control; (b) Exposure-to-secondhand-smoke; and (c) smoker. Horizontal dotted lines are lines of reference.
Figure 2.
Empirical (observed data) distributions for ADC AUC, displayed as box-whisker plots, with the lower whisker defining the minimum value of ADC AUC, the upper whisker defining the maximum value, the box defining the inter-quartile range between the values at the 25th and 75th percentiles of the distribution, and the horizontal line extending through the box defining the location of median of the distribution. There is considerable overlap of values but in both the Exposed-to-Secondhand-Smoke group and the Smoker group, there are more outliers.
As expected, the One-Way ANOVA analysis indicated that the mean of the ADC AUC differed between the controls and the smokers (0.04 ± 0.01 vs. 0.08 ± 0.03, P < 0.001). Similarly, the One-Way ANOVA analysis indicated that the mean of the ADC AUC differed between the exposure-to-secondhand-smoke subjects and smokers (0.05 ± 0.02 vs. 0.08 ± 0.03, P < 0.001). Not surprisingly, the One-Way ANOVA analysis failed to indicate that the mean of ADC AUC differed between the controls and exposure-to-secondhand-smoke subjects (0.04 ± 0.01 vs. 0.05 ± 0.02, P = 0.115)
Classification Rule Performance
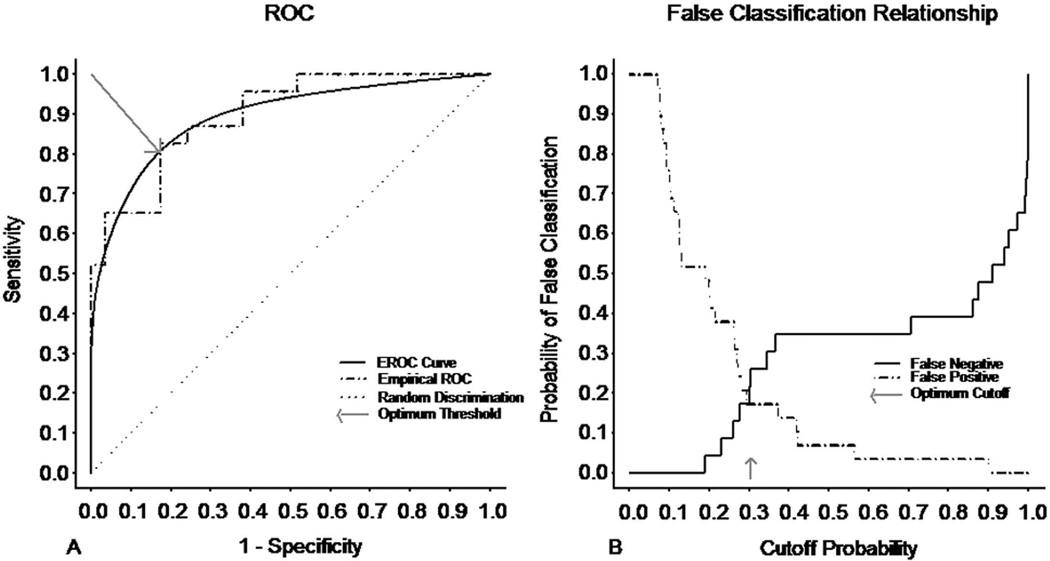
The ROC and EROC curves that were generated from the predictions of the logistic regression model, in which the ADC AUC measurements from the controls and smokers were utilized as the predictor of smoking status, are displayed in the Figure 3 (32, 33). At the optimum threshold cutoff probability, the specificity of the classification rule was 0.83, and the sensitivity of the classification rule was also 0.83. Out of the 23 smokers, 4 were misclassified as controls (false negative (FN) error rate = 17%), while out of the 29 controls, 5 were misclassified as smokers (false positive (FP) error rate = 17%). Area under the EROC was 0.90, Table 2.
Figure 3.
(a) Empirical ROC and exponential ROC (EROC) and (b) false classification relationship as a function of the cutoff probability.
Table 2.
Classification of controls and smokers at the optimum threshold cutoff probability (poptimum)
| Predicted | |||||
| Actual | Smoker | Control | n | ||
| Smoker | 19 | 4 | 23 | ||
| Control | 5 | 24 | 29 | ||
| 54 | |||||
| Optimal Threshold Cutoff Probability Poptimum |
Specificity† | Sensitivity‡ | False Positive (FP) Error Rate+ |
False Negative (FN) Error Rate* |
ROC§ |
| 0.301 | 0.828 | 0.826 | 0.172 | 0.174 | 0.90 |
the conditional probability that the classification rule classified the subject as a member of the control group when the subject was actually a member of the control group.
the condition probability that the classification rule classified the subject as a member of the smoking group when the subject was actually a member of the smoking group.
the conditional probability that the classification rule classified the subject as a member of the smoking group when the subject was actually a member of the control group.
the conditional probability that the classification rule classified the subject as a member of the control group when the subject was actually a member of the smoking group.
the integrated area under the receiver operator curve (ROC): with range [0.5 (denoting random classification) to 1.0 (denoting perfect classification)].
Classification of the Exposed-to-Secondhand-Smoke Subjects
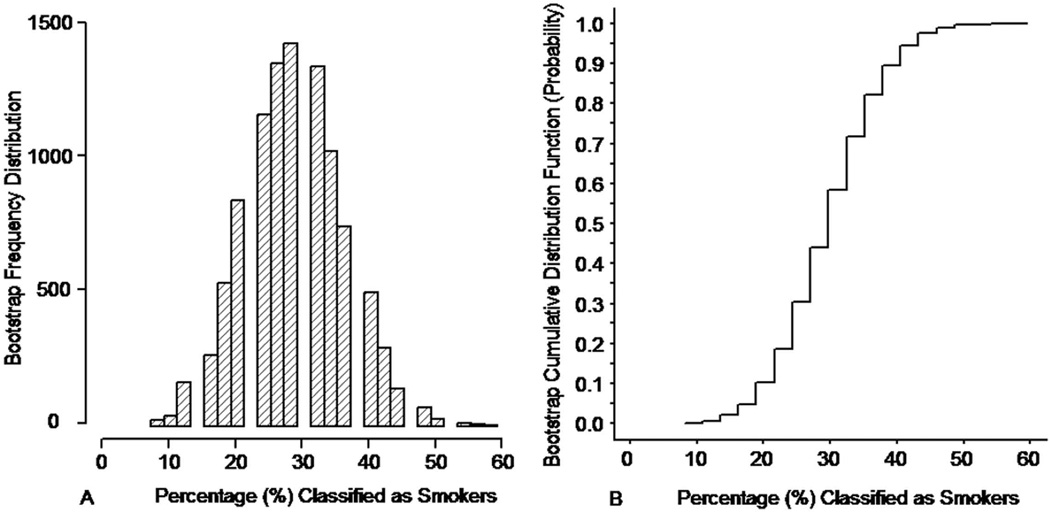
Applying the optimum threshold classification rule to the ADC AUC data of the 37 exposure-to-secondhand-smoke subjects resulted in 11 out the 37 subjects (30%) being classified a "smoker". Based on the bootstrap cumulative sampling distribution function as in Figure 4b, the 95% confidence interval for the percentage of exposure-to-secondhand-smoke subjects who would be classified “smokers” via the optimum classification rule extends from 16% to 46%.
Figure 4.
(a) Bootstrap sampling frequency distribution and (b) bootstrap sampling cumulative distribution for the percentage of the exposed-to-secondhand smoke individuals in the target population who would be classified “smokers” based on their ADC AUC values and the optimum threshold classification rule. The bootstrap frequency distribution and the bootstrap cumulative distribution function were generated based on 10,000 bootstrap random samples of size 37 (i.e. sampling with replacement) drawn from the sample of 37 exposed to second hand smoke subjects.
DISCUSSION
Two important observations were made in this study. First, the ADC AUC values of the smokers were significantly elevated compared to those of the non-smoking healthy subjects. This is not surprising considering that the ADC is sensitive to detect increases in the size and connections of the distal lung airspaces and can thus detect the early structural changes of emphysema that occur in subjects who have smoked for a considerable time (14–16). The second and more striking observation was the ADC AUC was also found to be elevated/abnormal in 30% of the exposure-to-secondhand-smoke subjects (16% – 46%, 95% confidence interval). Although these elevations were not as large as those found in the smokers, the findings indicate that there were changes in the microstructure of the lungs of some of the healthy non-smoking subjects who had been exposed for a prolonged period to secondhand smoke.
Although it has long been postulated that exposure to secondhand smoke damages the lung and may lead to COPD, this has been difficult to prove (1–8, 35). In most studies spirometry or pulmonary function tests were used as the endpoint and such approaches are inherently insensitive because a considerable fraction of the lung must be damaged before changes in lung function are detectable (36, 37). Our findings suggest that hyperpolarized helium-3 diffusion MR is a more sensitive test than spirometry for detecting the early alterations of the lung structure that may be associated to prolonged exposure to secondhand smoke. Further, that the percentage of subjects with elevated ADC from secondhand smoke exposure is similar to the percentage of heavy smokers who develop clinical COPD suggests that there may a subgroup of the population that is particularly susceptible to the deleterious effects of cigarette smoke (26).
The vast majority of subjects in our exposure-to-secondhand-smoke group were exposed during childhood, and a number of these also had substantial additional exposure as an adult. Unfortunately, due to the relatively low number of subjects, we were not able to determine whether there were any differences in ADC between those exposed only in childhood compared to those with exposure only as an adult. As a result, we were not able to assess whether prolonged exposure to secondhand smoke during childhood is potentially more damaging than inhalation as an adult, but future studies using hyperpolarized helium-3 diffusion MR might address this issue.
Alveolar destruction induced by cigarette smoke is not the only process that has been associated with an increase in helium-3 ADC values. In a recent study involving subjects with difficult-to-treat asthma and no history of active or prior smoking, ADC values were elevated compared to healthy subjects (38). It is uncertain what these alterations represented in this group of patients, particularly since asthma is not primarily an alveolar-destructing disease such as emphysema; possibly the changes were related to airway remodeling after chronic inflammation. However, in this study exposure to secondhand smoke was not assessed, and therefore it is uncertain whether tobacco smoke had any effect on these findings. Nevertheless, other airway diseases may also lead to increases in helium-3 diffusion detectable with diffusion MR. Thus, the results of our current study involving healthy subjects with extensive exposure to secondhand smoke should be interpreted as indicative of lung injury but it is unclear whether this is subclinical emphysema, related to an airway process, or another form of lung injury. To better understand the types of structural changes underlying our observations, smoking animal models could be used to determine whether early-stage emphysema can be detected with our method.
To our knowledge there has only been one prior study investigating the effects of secondhand smoke using hyperpolarized helium-3 diffusion MR (39). In this study 13 subjects with such exposure had elevated ADC values compared to 11 never smokers with normal values. However, the study is limited because of the small number of subjects, the unmatched population age, and the criteria for the intensity and duration of secondhand smoke exposure were not defined.
A limitation of our method of measuring the ADC globally over the entire lung was that it did not provide information about regional differences in ADC values, and thus we could not determine which areas of the lung were most affected. Wang et al. previously developed an MRI pulse sequence that provides regional maps of the ADC values at a specific diffusion time. And this technique was found to be more discriminatory than other studies (29, 38). It could potentially increase our understanding of the local effects of secondhand smoke in the lung by using this technique.
In conclusion, 30% of non-smoking healthy subjects who had prolonged exposure to secondhand smoke, and who had normal lung function and no other lung disease had abnormalities in helium-3 diffusion that were similar to those found in smokers, albeit to a lesser degree. These abnormalities likely reflect lung injury from exposure to secondhand smoke, although it is uncertain whether these abnormalities represent subclinical emphysema. Hyperpolarized helium-3 diffusion MR appears to be a sensitive test for assessing the potential harmful effects of secondhand smoke in the lung.
ACKNOWLEDGEMENTS
The authors thank John M. Christopher, RT(R)(MR), Doris A. Harding, RN and Joanne C. Gersbach, RN for valuable assistance with the MR experiments and scheduling of subjects.
Grant Support:
This research was supported by a Clinical Innovator Award from the Flight Attendant Medical Research Institute, grant numbers R01HL105586 and R01HL079077 from the National Heart, Lung and Blood Institute, and Siemens Medical Solutions. The content is solely the responsibility of the authors and does not necessarily represent the official reviews of the National Heart, Lung and Blood Institute or the National Institute of Health.
References
- 1.The health consquence of involuntary exposure to tobacco smoke: a report of the Surgeon General. Atlanta, GA: U.S. Dept. of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006. [Google Scholar]
- 2.Robbins AS, Abbey DE, Lebowitz MD. Passive smoking and chronic respiratory disease symptoms in non-smoking adults. Int J Epidemiol. 1993;22(5):809–817. doi: 10.1093/ije/22.5.809. [DOI] [PubMed] [Google Scholar]
- 3.Leuenberger P, Schwartz J, Ackermann-Liebrich U, et al. Passive smoking exposure in adults and chronic respiratory symptoms (SAPALDIA Study). Swiss Study on Air Pollution and Lung Diseases in Adults, SAPALDIA Team. Am J Respir Crit Care Med. 1994;150(5 pt 1):1222–1228. doi: 10.1164/ajrccm.150.5.7952544. [DOI] [PubMed] [Google Scholar]
- 4.Dayal HH, Khuder S, Sharrar R, Trieff N. Passive smoking in obstructive respiratory disease in an industrialized urban population. Environ Res. 1994;65(2):161–171. doi: 10.1006/enrs.1994.1029. [DOI] [PubMed] [Google Scholar]
- 5.Forastiere F, Mallone S, Lo Presti E, et al. Characteristics of nonsmoking women exposed to spouses who smoke: epidemiologic study on environment and health in women from four Italian areas. Environ Health Perspect. 2000;108(12):1171–1177. doi: 10.1289/ehp.001081171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalandidi A, Trichopoulos D, Hatzakis A, Tzannes S, Saracci R. Passive smoking and chronic obstructive lung disease. Lancet. 1987;2(8751):1325–1326. doi: 10.1016/s0140-6736(87)91210-4. [DOI] [PubMed] [Google Scholar]
- 7.Sandler DP, Comstock GW, J HK, Shore DL. Deaths from all causes in non-smokers who lived with smokers. Am J Public Health. 1989;79(2):163–167. doi: 10.2105/ajph.79.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trédaniel J, Boffetta P, Saracci R, Hirsch A. Exposure to environmental tobacco smoke and adult non-neoplastic respiratory diseases. Eur Respir J. 1994;7(10):173–185. doi: 10.1183/09031936.94.07010173. [DOI] [PubMed] [Google Scholar]
- 9.de Lange EE, Mugler JP, 3rd, Brookeman JR, et al. Lung air spaces: MR imaging evaluation with hyperpolarized 3He gas. Radiology. 1999;210(3):851–857. doi: 10.1148/radiology.210.3.r99fe08851. [DOI] [PubMed] [Google Scholar]
- 10.Johnson GA, Cates G, Chen XJ, et al. Dynamics of magnetization in hyperpolarized gas MRI of the lung. Magn Reson Med. 1997;38(1):66–71. doi: 10.1002/mrm.1910380111. [DOI] [PubMed] [Google Scholar]
- 11.Kauczor H, Surkau R, Roberts T. MRI using hyperpolarized noble gases. Eur Radiol. 1998;8(5):820–827. doi: 10.1007/s003300050479. [DOI] [PubMed] [Google Scholar]
- 12.MacFall JR, Charles HC, Black RD, et al. Human lung air spaces: potential for MR imaging with hyperpolarized He-3. Radiology. 1996;200(2):553–558. doi: 10.1148/radiology.200.2.8685356. [DOI] [PubMed] [Google Scholar]
- 13.Caramori G, Fabbri M, Paioli D, et al. Asthma is not a common cause of severe chronic respiratory failure in non-smokers: ALOT study. Monaldi Arch Chest Dis. 2005;63(2):84–87. doi: 10.4081/monaldi.2005.643. [DOI] [PubMed] [Google Scholar]
- 14.Salerno M, de Lange EE, Altes TA, Truwit JD, Brookeman JR, Mugler JP., 3rd Emphysema: hyperpolarized helium 3 diffusion MR imaging of the lungs compared with spirometric indexes--initial experience. Radiology. 2002;222(1):252–260. doi: 10.1148/radiol.2221001834. [DOI] [PubMed] [Google Scholar]
- 15.Chen XJ, Hedlund LW, Moller HE, Chawla MS, Maronpot RR, Johnson GA. Detection of emphysema in rat lungs by using magnetic resonance measurements of 3He diffusion. Proc Natl Acad Sci U S A. 2000;97(21):11478–11481. doi: 10.1073/pnas.97.21.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saam BT, Yablonskiy DA, Kodibagkar VD, et al. MR imaging of diffusion of (3)He gas in healthy and diseased lungs. Magn Reson Med. 2000;44(2):174–179. doi: 10.1002/1522-2594(200008)44:2<174::aid-mrm2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Yablonskiy DA, Sukstanskii AL, Leawoods JC, et al. Quantitative in vivo assessment of lung microstructure at the alveolar level with hyperpolarized 3He diffusion MRI. Proc Natl Acad Sci U S A. 2002;99(5):3111–3116. doi: 10.1073/pnas.052594699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mata JF, Altes TA, Cai J, et al. Evaluation of emphysema severity and progression in a rabbit model: comparison of hyperpolarized 3He and 129Xe diffusion MRI with lung morphometry. J Appl Physiol. 2007;102(3):1273–1280. doi: 10.1152/japplphysiol.00418.2006. [DOI] [PubMed] [Google Scholar]
- 19.Fain SB, Panth SR, Evans MD, et al. Early emphysematous changes in asymptomatic smokers: detection with 3He MR imaging. Radiology. 2006;239(3):875–883. doi: 10.1148/radiol.2393050111. [DOI] [PubMed] [Google Scholar]
- 20.Swift AJ, Wild JM, Fichele S, et al. Emphysematous changes and normal variation in smokers and COPD patients using diffusion 3He MRI. Eur J Radiol. 2005;54(3):352–358. doi: 10.1016/j.ejrad.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Lundback B, Lindberg A, Lindstrom M, et al. Not 15 but 50% of smokers develop COPD?--Report from the Obstructive Lung Disease in Northern Sweden Studies. Respir Med. 2003;97(2):115–122. doi: 10.1053/rmed.2003.1446. [DOI] [PubMed] [Google Scholar]
- 22.Barnes PJ, Kleinert S. COPD--a neglected disease. Lancet. 2004;364(9434):564–565. doi: 10.1016/S0140-6736(04)16866-9. [DOI] [PubMed] [Google Scholar]
- 23.Lindberg A, Jonsson A-C, Ronmark E, Lundgren R, Larsson L-G, Lundback B. Ten-Year Cumulative Incidence of COPD and Risk Factors for Incident Disease in a Symptomatic Cohort. Chest. 2005;127(5):1544–1552. doi: 10.1378/chest.127.5.1544. [DOI] [PubMed] [Google Scholar]
- 24.Bartal M. COPD and tobacco smoke. Monaldi Arch Chest Dis. 2005;63(4):213–225. doi: 10.4081/monaldi.2005.623. [DOI] [PubMed] [Google Scholar]
- 25.Lokke A, Lange P, Scharling H, Fabricius P, Vestbo J. Developing COPD: a 25 year follow up study of the general population. Thorax. 2006;61(11):935–939. doi: 10.1136/thx.2006.062802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rennard SI, Vestbo J. COPD: the dangerous underestimate of 15% Lancet. 2006;367(9518):1216–1219. doi: 10.1016/S0140-6736(06)68516-4. [DOI] [PubMed] [Google Scholar]
- 27.Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127(6):725–734. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- 28.Walker TG, Happer W. Spin-exchange optical pumping of noble-gas nuclei. Rev Mod Phys. 1997;69(2):629–642. [Google Scholar]
- 29.Wang C, Miller GW, Altes TA, de Lange EE, Cates GD, Jr, Mugler JP., 3rd Time dependence of 3He diffusion in the human lung: measurement in the long-time regime using stimulated echoes. Magn Reson Med. 2006;56(2):296–309. doi: 10.1002/mrm.20944. [DOI] [PubMed] [Google Scholar]
- 30.Johnson RA, DW W. Applied Multivariate Statistical Analysis. 5th Edition. Saddle River, NJ: Prentice Hall Upper; 2002. [Google Scholar]
- 31.Zar JH. Biostatistical analysis. 4th Edition. Upper Saddle River, New Jersey: Prentice Hall; 1999. [Google Scholar]
- 32.Obuchewski NA. Fundamentals of clinical research for radiologists: ROC analysis. AJR. 2005;184:364–372. [Google Scholar]
- 33.England WL. An exponential model used for optimum threshold selection on ROC curves. Med Decis Making. 1998;8:120–131. doi: 10.1177/0272989X8800800208. [DOI] [PubMed] [Google Scholar]
- 34.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. London: Chapman & Hall; 2005. [Google Scholar]
- 35.Lee PN, Chamberlain J, Alderson MR. Relationship of passive smoking to risk of lung cancer and other smoking-associated diseases. Br J Cancer. 1986;54(1):97–105. doi: 10.1038/bjc.1986.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Enright PL, Crapo RO. Controversies in the use of Spirometry for early recognition and Diagnosis of Chronic Obstructive Pulmonary Disease in Cigarette smokers. Clin Chest Med. 2000;21(4):645–652. doi: 10.1016/s0272-5231(05)70174-x. [DOI] [PubMed] [Google Scholar]
- 37.Webb WR. Radiology of obstructive pulmonary disease. AJR. 1997;169(3):637–647. doi: 10.2214/ajr.169.3.9275869. [DOI] [PubMed] [Google Scholar]
- 38.Wang C, Altes TA, Mugler JP, 3rd, et al. Assessment of the lung microstructure in patients with asthma using hyperpolarized 3He diffusion MRI at two time scales: Comparison with healthy subjects and patients with COPD. J Magn Reson Imaging. 2008;28(1):80–88. doi: 10.1002/jmri.21408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waters B, Owers-Bradley J, Silverman M. Acinar structure in symptom-free adults by Helium-3 magnetic resonance. Am J Respir Crit Care Med. 2006;173(8):847–851. doi: 10.1164/rccm.200411-1595OC. [DOI] [PubMed] [Google Scholar]