Abstract
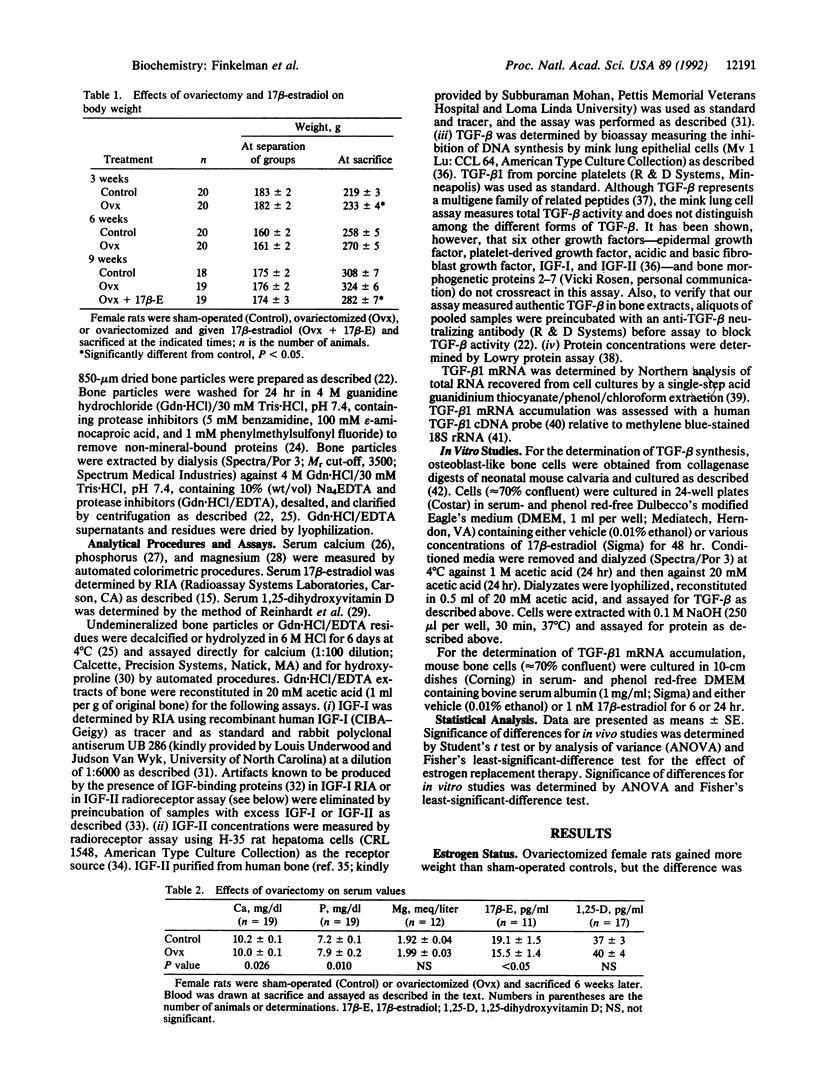
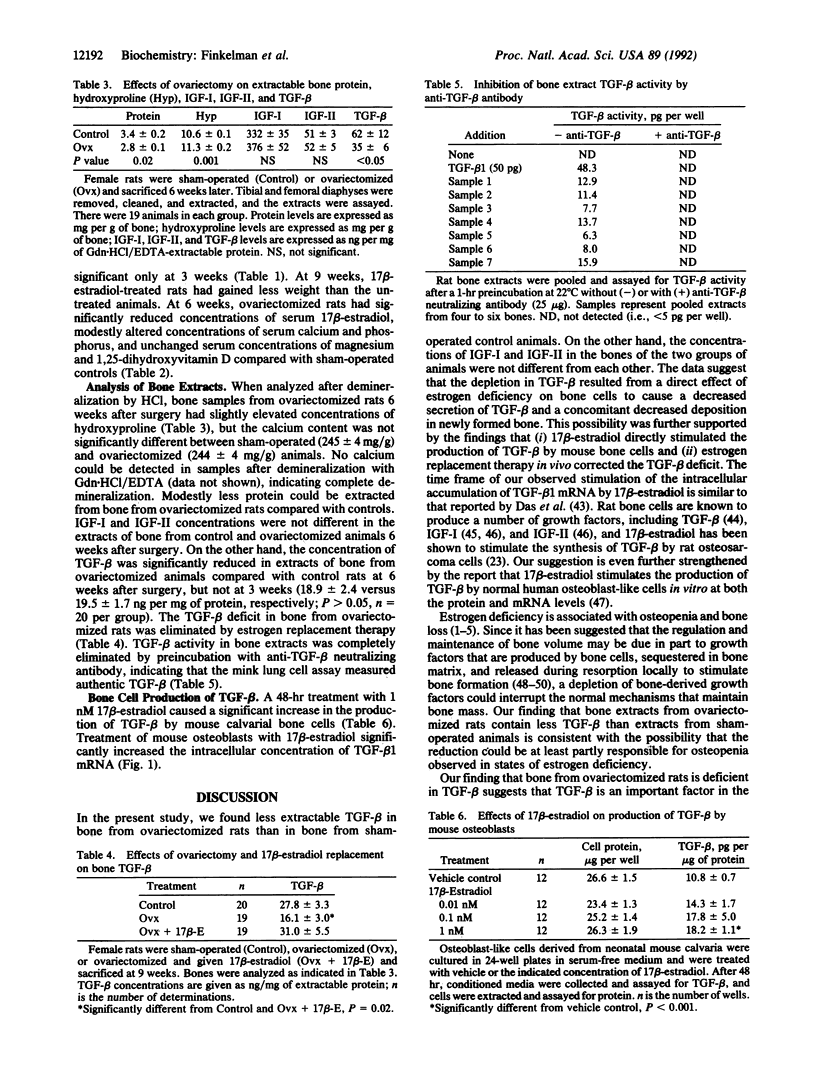
Previous work showed that production of transforming growth factor beta (TGF-beta) by osteoblast-like rat UMR 106 cells was increased by 17 beta-estradiol at physiological concentrations. To determine whether ovariectomy alters the concentration of TGF-beta in rat long bones, female Sprague-Dawley rats were either sham-operated (n = 19) or ovariectomized (n = 19), pair-fed a semisynthetic diet for 6 weeks, and sacrificed. Tibial and femoral diaphyses were removed and extracted by demineralization. Ovariectomy lowered serum estrogen; did not alter body weight, serum magnesium, or serum 1,25-dihydroxyvitamin D; and produced only modest differences in serum calcium and phosphate concentrations. Hydroxyproline was higher and extractable protein was lower in bones from ovariectomized rats than in bones from sham-operated rats; calcium content did not differ between the two groups of animals. Ovariectomy lowered the concentration of TGF-beta in bone but did not change the concentration of insulin-like growth factors I or II compared with values in bone from control animals. The reduction of bone TGF-beta was evident 6 weeks after surgery but not at 3 weeks. Treatment of ovariectomized rats with estrogen eliminated the TGF-beta deficit. To determine whether 17 beta-estradiol increased TGF-beta production by normal bone cells, mouse osteoblasts were treated for 2 days with 17 beta-estradiol. The production of TGF-beta was increased almost 2-fold by 1 nM 17 beta-estradiol, and short-term treatment stimulated the intracellular accumulation of TGF-beta 1 mRNA. We conclude that ovariectomy reduces deposition of TGF-beta in rat bone and that diminished skeletal TGF-beta could play a role in the pathogenesis of bone loss, fractures, and microfractures that occur in estrogen-deficient states. Our results support the possibility that estrogen and bone TGF-beta may be necessary for normal maintenance of the skeleton in female rats.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken J. M., Armstrong E., Anderson J. B. Osteoporosis after oophorectomy in the mature female rat and the effect of oestrogen and-or progestogen replacement therapy in its prevention. J Endocrinol. 1972 Oct;55(1):79–87. doi: 10.1677/joe.0.0550079. [DOI] [PubMed] [Google Scholar]
- Barbagallo M., Carbognani A., Palummeri E., Chiavarini M., Pedrazzoni M., Bracchi P. G., Passeri M. The comparative effect of ovarian hormone administration on bone mineral status in oophorectomized rats. Bone. 1989;10(2):113–116. doi: 10.1016/8756-3282(89)90008-2. [DOI] [PubMed] [Google Scholar]
- Beck L. S., Deguzman L., Lee W. P., Xu Y., McFatridge L. A., Gillett N. A., Amento E. P. Rapid publication. TGF-beta 1 induces bone closure of skull defects. J Bone Miner Res. 1991 Nov;6(11):1257–1265. doi: 10.1002/jbmr.5650061117. [DOI] [PubMed] [Google Scholar]
- Bentz H., Nathan R. M., Rosen D. M., Armstrong R. M., Thompson A. Y., Segarini P. R., Mathews M. C., Dasch J. R., Piez K. A., Seyedin S. M. Purification and characterization of a unique osteoinductive factor from bovine bone. J Biol Chem. 1989 Dec 5;264(34):20805–20810. [PubMed] [Google Scholar]
- Canalis E., McCarthy T., Centrella M. Growth factors and the regulation of bone remodeling. J Clin Invest. 1988 Feb;81(2):277–281. doi: 10.1172/JCI113318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centrella M., Canalis E. Isolation of EGF-dependent transforming growth factor (TGF beta-like) activity from culture medium conditioned by fetal rat calvariae. J Bone Miner Res. 1987 Feb;2(1):29–36. doi: 10.1002/jbmr.5650020106. [DOI] [PubMed] [Google Scholar]
- Cesnjaj M., Stavljenić A., Vukicević S. Decreased osteoinductive potential of bone matrix from ovariectomized rats. Acta Orthop Scand. 1991 Oct;62(5):471–475. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Civitelli R., Agnusdei D., Nardi P., Zacchei F., Avioli L. V., Gennari C. Effects of one-year treatment with estrogens on bone mass, intestinal calcium absorption, and 25-hydroxyvitamin D-1 alpha-hydroxylase reserve in postmenopausal osteoporosis. Calcif Tissue Int. 1988 Feb;42(2):77–86. doi: 10.1007/BF02556338. [DOI] [PubMed] [Google Scholar]
- Connerty H. V., Briggs A. R. Determination of serum calcium by means of orthocresolphthalein complexone. Am J Clin Pathol. 1966 Mar;45(3):290–296. doi: 10.1093/ajcp/45.3.290. [DOI] [PubMed] [Google Scholar]
- Daly J. A., Ertingshausen G. Direct method for determining inorganic phosphate in serum with the "CentrifiChem". Clin Chem. 1972 Mar;18(3):263–265. [PubMed] [Google Scholar]
- Das S. K., Flanders K. C., Andrews G. K., Dey S. K. Expression of transforming growth factor-beta isoforms (beta 2 and beta 3) in the mouse uterus: analysis of the periimplantation period and effects of ovarian steroids. Endocrinology. 1992 Jun;130(6):3459–3466. doi: 10.1210/endo.130.6.1375903. [DOI] [PubMed] [Google Scholar]
- Daughaday W. H., Kapadia M., Mariz I. Serum somatomedin binding proteins: physiologic significance and interference in radioligand assay. J Lab Clin Med. 1987 Mar;109(3):355–363. [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Eaton D. H., Bell J. R., Assoian R. K., Roberts A. B., Sporn M. B., Goeddel D. V. Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells. Nature. 1985 Aug 22;316(6030):701–705. doi: 10.1038/316701a0. [DOI] [PubMed] [Google Scholar]
- Ettinger B., Genant H. K., Cann C. E. Long-term estrogen replacement therapy prevents bone loss and fractures. Ann Intern Med. 1985 Mar;102(3):319–324. doi: 10.7326/0003-4819-102-3-319. [DOI] [PubMed] [Google Scholar]
- Farley J. R., Tarbaux N., Murphy L. A., Masuda T., Baylink D. J. In vitro evidence that bone formation may be coupled to resorption by release of mitogen(s) from resorbing bone. Metabolism. 1987 Apr;36(4):314–321. doi: 10.1016/0026-0495(87)90200-9. [DOI] [PubMed] [Google Scholar]
- Finkelman R. D., Linkhart T. A., Mohan S., Lau K. H., Baylink D. J., Bell N. H. Vitamin D deficiency causes a selective reduction in deposition of transforming growth factor beta in rat bone: possible mechanism for impaired osteoinduction. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3657–3660. doi: 10.1073/pnas.88.9.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman R. D., Mohan S., Jennings J. C., Taylor A. K., Jepsen S., Baylink D. J. Quantitation of growth factors IGF-I, SGF/IGF-II, and TGF-beta in human dentin. J Bone Miner Res. 1990 Jul;5(7):717–723. doi: 10.1002/jbmr.5650050708. [DOI] [PubMed] [Google Scholar]
- GRANT R. A. ESTIMATION OF HYDROXYPROLINE BY THE AUTOANALYSER. J Clin Pathol. 1964 Nov;17:685–686. doi: 10.1136/jcp.17.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray T. K., Lipes B., Linkhart T., Mohan S., Baylink D. Transforming growth factor beta mediates the estrogen induced inhibition of UMR106 cell growth. Connect Tissue Res. 1989;20(1-4):23–32. doi: 10.3109/03008208909023871. [DOI] [PubMed] [Google Scholar]
- Gray T. K., Mohan S., Linkhart T. A., Baylink D. J. Estradiol stimulates in vitro the secretion of insulin-like growth factors by the clonal osteoblastic cell line, UMR106. Biochem Biophys Res Commun. 1989 Jan 31;158(2):407–412. doi: 10.1016/s0006-291x(89)80062-2. [DOI] [PubMed] [Google Scholar]
- Jennings J. C., Mohan S., Linkhart T. A., Widstrom R., Baylink D. J. Comparison of the biological actions of TGF beta-1 and TGF beta-2: differential activity in endothelial cells. J Cell Physiol. 1988 Oct;137(1):167–172. doi: 10.1002/jcp.1041370120. [DOI] [PubMed] [Google Scholar]
- Joyce M. E., Roberts A. B., Sporn M. B., Bolander M. E. Transforming growth factor-beta and the initiation of chondrogenesis and osteogenesis in the rat femur. J Cell Biol. 1990 Jun;110(6):2195–2207. doi: 10.1083/jcb.110.6.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce M. E., Terek R. M., Jingushi S., Bolander M. E. Role of transforming growth factor-beta in fracture repair. Ann N Y Acad Sci. 1990;593:107–123. doi: 10.1111/j.1749-6632.1990.tb16104.x. [DOI] [PubMed] [Google Scholar]
- Kalu D. N. Evaluation of the pathogenesis of skeletal changes in ovariectomized rats. Endocrinology. 1984 Aug;115(2):507–512. doi: 10.1210/endo-115-2-507. [DOI] [PubMed] [Google Scholar]
- Kiel D. P., Felson D. T., Anderson J. J., Wilson P. W., Moskowitz M. A. Hip fracture and the use of estrogens in postmenopausal women. The Framingham Study. N Engl J Med. 1987 Nov 5;317(19):1169–1174. doi: 10.1056/NEJM198711053171901. [DOI] [PubMed] [Google Scholar]
- Krett N. L., Heaton J. H., Gelehrter T. D. Mediation of insulin-like growth factor actions by the insulin receptor in H-35 rat hepatoma cells. Endocrinology. 1987 Jan;120(1):401–408. doi: 10.1210/endo-120-1-401. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linde A., Bhown M., Butler W. T. Noncollagenous proteins of dentin. A re-examination of proteins from rat incisor dentin utilizing techniques to avoid artifacts. J Biol Chem. 1980 Jun 25;255(12):5931–5942. [PubMed] [Google Scholar]
- Lindgren J. U., Lindholm T. S. Effect of 1-alpha-hydroxyvitamin D3 on osteoporosis in rats induced by oophorectomy. Calcif Tissue Int. 1979 Apr 17;27(2):161–164. doi: 10.1007/BF02441179. [DOI] [PubMed] [Google Scholar]
- Lindsay R., Aitken J. M., Hart D. M., Purdie D. The effect of ovarian sex steroids on bone mineral status in the oöphorectomized rat and in the human. Postgrad Med J. 1978;54 (Suppl 2):50–58. [PubMed] [Google Scholar]
- Lindsay R., Hart D. M., Aitken J. M., MacDonald E. B., Anderson J. B., Clarke A. C. Long-term prevention of postmenopausal osteoporosis by oestrogen. Evidence for an increased bone mass after delayed onset of oestrogen treatment. Lancet. 1976 May 15;1(7968):1038–1041. doi: 10.1016/s0140-6736(76)92217-0. [DOI] [PubMed] [Google Scholar]
- Linkhart T. A., Jennings J. C., Mohan S., Wakley G. K., Baylink D. J. Characterization of mitogenic activities extracted from bovine bone matrix. Bone. 1986;7(6):479–487. doi: 10.1016/8756-3282(86)90007-4. [DOI] [PubMed] [Google Scholar]
- Mackie E. J., Trechsel U. Stimulation of bone formation in vivo by transforming growth factor-beta: remodeling of woven bone and lack of inhibition by indomethacin. Bone. 1990;11(4):295–300. doi: 10.1016/8756-3282(90)90083-b. [DOI] [PubMed] [Google Scholar]
- Mohan S., Bautista C. M., Herring S. J., Linkhart T. A., Baylink D. J. Development of valid methods to measure insulin-like growth factors-I and -II in bone cell-conditioned medium. Endocrinology. 1990 May;126(5):2534–2542. doi: 10.1210/endo-126-5-2534. [DOI] [PubMed] [Google Scholar]
- Mohan S., Jennings J. C., Linkhart T. A., Baylink D. J. Isolation and purification of a low-molecular-weight skeletal growth factor from human bones. Biochim Biophys Acta. 1986 Nov 19;884(2):234–242. doi: 10.1016/0304-4165(86)90168-6. [DOI] [PubMed] [Google Scholar]
- Mohan S., Jennings J. C., Linkhart T. A., Baylink D. J. Primary structure of human skeletal growth factor: homology with human insulin-like growth factor-II. Biochim Biophys Acta. 1988 Jul 14;966(1):44–55. doi: 10.1016/0304-4165(88)90127-4. [DOI] [PubMed] [Google Scholar]
- Noda M., Camilliere J. J. In vivo stimulation of bone formation by transforming growth factor-beta. Endocrinology. 1989 Jun;124(6):2991–2994. doi: 10.1210/endo-124-6-2991. [DOI] [PubMed] [Google Scholar]
- Oursler M. J., Cortese C., Keeting P., Anderson M. A., Bonde S. K., Riggs B. L., Spelsberg T. C. Modulation of transforming growth factor-beta production in normal human osteoblast-like cells by 17 beta-estradiol and parathyroid hormone. Endocrinology. 1991 Dec;129(6):3313–3320. doi: 10.1210/endo-129-6-3313. [DOI] [PubMed] [Google Scholar]
- Reinhardt T. A., Horst R. L., Orf J. W., Hollis B. W. A microassay for 1,25-dihydroxyvitamin D not requiring high performance liquid chromatography: application to clinical studies. J Clin Endocrinol Metab. 1984 Jan;58(1):91–98. doi: 10.1210/jcem-58-1-91. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B. Transforming growth factor beta. Adv Cancer Res. 1988;51:107–145. [PubMed] [Google Scholar]
- Saville P. D. Changes in skeletal mass and fragility with castration in the rat; a model of osteoporosis. J Am Geriatr Soc. 1969 Feb;17(2):155–166. doi: 10.1111/j.1532-5415.1969.tb03169.x. [DOI] [PubMed] [Google Scholar]
- Stracke H., Schulz A., Moeller D., Rossol S., Schatz H. Effect of growth hormone on osteoblasts and demonstration of somatomedin-C/IGF I in bone organ culture. Acta Endocrinol (Copenh) 1984 Sep;107(1):16–24. doi: 10.1530/acta.0.1070016. [DOI] [PubMed] [Google Scholar]
- Strong D. D., Beachler A. L., Wergedal J. E., Linkhart T. A. Insulinlike growth factor II and transforming growth factor beta regulate collagen expression in human osteoblastlike cells in vitro. J Bone Miner Res. 1991 Jan;6(1):15–23. doi: 10.1002/jbmr.5650060105. [DOI] [PubMed] [Google Scholar]
- Turner R. T., Farley J., Vandersteenhoven J. J., Epstein S., Bell N. H., Baylink D. J. Demonstration of reduced mitogenic and osteoinductive activities in demineralized allogeneic bone matrix from vitamin D-deficient rats. J Clin Invest. 1988 Jul;82(1):212–217. doi: 10.1172/JCI113573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R. T., Vandersteenhoven J. J., Bell N. H. The effects of ovariectomy and 17 beta-estradiol on cortical bone histomorphometry in growing rats. J Bone Miner Res. 1987 Apr;2(2):115–122. doi: 10.1002/jbmr.5650020206. [DOI] [PubMed] [Google Scholar]
- Turner R. T., Wakley G. K., Hannon K. S., Bell N. H. Tamoxifen inhibits osteoclast-mediated resorption of trabecular bone in ovarian hormone-deficient rats. Endocrinology. 1988 Mar;122(3):1146–1150. doi: 10.1210/endo-122-3-1146. [DOI] [PubMed] [Google Scholar]
- Turner R. T., Wakley G. K., Hannon K. S., Bell N. H. Tamoxifen prevents the skeletal effects of ovarian hormone deficiency in rats. J Bone Miner Res. 1987 Oct;2(5):449–456. doi: 10.1002/jbmr.5650020513. [DOI] [PubMed] [Google Scholar]
- Wronski T. J., Lowry P. L., Walsh C. C., Ignaszewski L. A. Skeletal alterations in ovariectomized rats. Calcif Tissue Int. 1985 May;37(3):324–328. doi: 10.1007/BF02554882. [DOI] [PubMed] [Google Scholar]
- Wronski T. J., Walsh C. C., Ignaszewski L. A. Histologic evidence for osteopenia and increased bone turnover in ovariectomized rats. Bone. 1986;7(2):119–123. doi: 10.1016/8756-3282(86)90683-6. [DOI] [PubMed] [Google Scholar]




