Abstract
Obesity is strongly associated with metabolic syndrome, a combination of risk factors that predisposes to development of the cardiometabolic diseases: atherosclerotic cardiovascular disease and type 2 diabetes mellitus. Prevention of metabolic syndrome requires novel interventions to address this health challenge. The objective of this study was to identify candidate molecules for the prevention and treatment of insulin resistance and atherosclerosis, conditions that underlie type 2 diabetes mellitus and cardiovascular disease, respectively. We used an unbiased bioinformatics approach to identify molecules that are upregulated in both conditions by combining murine and human data from a microarray experiment and meta-analyses. We obtained a pool of 8 genes that were upregulated in all the databases analyzed. This included well-known and novel molecules involved in the pathophysiology of type 2 diabetes mellitus and cardiovascular disease. Notably, matrix metalloproteinase 12 (MMP12) was highly ranked in all analyses and was therefore chosen for further investigation. Analyses of visceral and subcutaneous white adipose tissue from obese compared with lean mice and humans convincingly confirmed the upregulation of MMP12 in obesity at the mRNA, protein and activity levels. In conclusion, by using this unbiased approach, an interesting pool of candidate molecules was identified, all of which have potential as targets in the treatment and prevention of cardiometabolic diseases.
INTRODUCTION
Metabolic syndrome is a multifactorial condition, which includes insulin resistance, visceral obesity, hypertension and atherogenic dyslipidemia, conferring a markedly elevated risk for type 2 diabetes mellitus and atherosclerotic cardiovascular disease, collectively called cardiometabolic diseases. (1,2) According to data from the World Health Organization, cardiovascular diseases are the leading cause of death globally. In 2012, 17.5 million people died from cardiovascular diseases, representing 31% of all global deaths. Also in 2012, an estimated 1.5 million deaths were directly caused by diabetes, and in 2014 the global prevalence of diabetes was estimated to be 9% among adults ages 18 years and older. (3) In terms of both human health and increasing health care expenses related to cardiovascular disease and type 2 diabetes, no society can afford to ignore the rise of cardiometabolic diseases, and new therapeutic strategies are urgently needed. (4)
Research has identified chronic low-grade inflammation induced by obesity as a common mechanism that is causally involved in obesity-related insulin resistance and atherosclerosis, precursors for type 2 diabetes and cardiovascular disease, respectively. (5,6) This raises the possibility of treatment strategies by neutralization, inactivation or elimination of key factors implicated in the development of chronic inflammation. Active immunotherapeutic approaches against self-antigen molecules have recently been clinically tested for the treatment of noncommunicable diseases such as Alzheimer’s, hypertension and chronic inflammatory and autoimmune diseases. (7–9) Immunotherapy based on active immunization against pathogenetically crucial molecules may offer a tool to treat these diseases, with advantages such as high specificity compared with small molecules and long-lasting efficacy at overall limited cost.
During the past decade, a number of genome-wide association studies have revealed 40 loci consistently associated with susceptibility to type 2 diabetes and have rapidly expanded the knowledge of the genetic architecture of this disease. (10–13) However, the genes located in or near these loci do not fully elucidate the specific molecular mechanisms underlying the development of type 2 diabetes. The aim of this study was to identify candidate molecules that could be targeted for the prevention and treatment of cardiometabolic disease. An unbiased bioinformatics approach was used to identify genes from different published databases related to cardiometabolic disease and mouse models, thereby analyzing the main tissues involved in the development of type 2 diabetes and cardiovascular disease by microarrays, combining published (14) as well as newly obtained original data. The differentially expressed and upregulated genes present in all these studies were selected, resulting in a list of genes that included known as well as novel candidate molecules for the treatment of cardiometabolic disease.
Using this approach, a pool of 8 candidate molecules with the potential to be targeted by immunotherapy or other specific blockade was obtained. After a thorough evaluation of the literature, matrix metalloproteinase 12 (MMP12) was selected as the best candidate for further investigation. Subsequent evaluation of MMP12 at the mRNA and protein levels and an increased caseinolytic activity corresponding with the MMP12 molecular weight in adipose tissue in murine and human obesity confirms the validity of the selection process. Hence, these data provide a highly valuable basis for identification of novel drug targets in the prevention and treatment of cardiovascular disease and type 2 diabetes.
MATERIALS AND METHODS
Animals and Diets
For the microarray experiments, we used an insulin-resistance/atherosclerosis mouse model established in our lab (15) and a well-established model for diet- induced obesity. Male wild type (WT) and LDL receptor–deficient mice (Ldlr-/-), both on a C57BL/6 J background, were purchased from Charles River Laboratories. At 9 wks of age, WT and Ldlr-/- mice were placed for up to 20 wks on a high-fat diet (HFD) containing 60% kcal% fat (D12492; Research Diets Inc.), and a sucrose-enriched high-fat diet (HFSC) consisting of 58 kcal% fat (primarily lard) and 28 kcal% carbohydrates (with 17.5 kcal% from sucrose; D09071704, Research Diets Inc.), respectively. For the microarray validation, WT animals on a C57BL/6J background were used. At 9 wks of age, they were placed for up to 14 wks on HFD. Normal chow diet containing 4 kcal% fat (V1126-000, Ssnif) or low-fat diet containing 10 kcal% fat (D12450B; Research Diets Inc.) was used as control diet (CD) in each study. Animals were anesthetized with ketamine/xylazin and euthanized by cervical dislocation. After the animals were euthanized, the target tissues were collected. Gonadal white adipose tissue (GWAT), subcutaneous white adipose tissue (SWAT) and whole aortae were immediately snap frozen in liquid nitrogen. All mice were housed in a specific pathogen-free facility with a 12 h light/dark cycle. Mice had free access to food and water. The protocol was approved by the local ethics committee for animal studies, and the Austrian Federal Ministry for Science and Research complied fully with the guidelines on accommodation and care of animals formulated by the European convention for the protection of vertebrate animals used for experimental and other scientific purposes. (16)
Human Samples
Omental white adipose tissue (OWAT) collected from the omentum major as a commonly investigated source for visceral, intra-abdominal adipose tissue and abdominal SWAT were obtained from lean controls (body mass index [BMI] < 30 kg/m2) matched for age and sex with severely obese (BMI > 40 kg/m2) nondiabetic patients who were scheduled for either elective abdominal surgery or elective laparoscopic bariatric surgery. (17) Adipose tissue samples were taken from similar locations in all patients. Patients underwent bariatric surgery at the Medical University of Vienna. Elective abdominal surgery in the lean control group was performed at the Medical University of Vienna or at the Göttlicher Heiland Hospital in Vienna. Patients were included if they were between 18 and 75 years of age. Criteria for exclusion were the presence of any infectious, inflammatory, neoplastic or systemic disease; diabetes (excluded by fasting plasma glucose or the use of antidiabetic drugs) or other uncontrolled endocrine disease; and the presence of any serious acute or chronic illness within the last 2 wks prior to inclusion. Diagnosis of diabetes as an exclusion criterion was performed by measuring either fasting plasma glucose, HbA1c or 2h glucose in an oral glucose tolerance test. Pregnancy and lactation were also exclusion criteria. Current use of antibiotics, anti-inflammatories or anti-obesity drugs led to exclusion from the study. Samples were collected between 2008 and 2011. Anthropometric measurements were performed shortly before surgery. Blood sampling was performed a few days prior to bariatric surgery, or immediately before surgery in the case of elective abdominal surgery. The study was performed in accordance with the Helsinki Declaration of 1975 as revised in 1983 and with Good Clinical Practice guidelines and was approved by the Ethics Committee of the Medical University of Vienna and Göttlicher Heiland Hospital (EK Nr. 963/2009, EK Nr. 488/2006 and E10-N01-01). All subjects provided written informed consent.
HOMA-IR was calculated by the formula: fasting insulin (microU/L) × fasting glucose (nmol/L)/22.5.
The anthropometric and laboratory measurements determined in these 2 cohorts are described in detail in Supplementary Table S1.
Microarrays
RNA was isolated for gene expression microarray analyses at the exon level (GeneChip Mouse Exon 2.0 ST Array, Affymetrix). To isolate RNA, the frozen tissue samples were homogenized in TRIzol® reagent (Invitrogen/Life Technologies) and processed according to the manufacturer’s instructions. Total RNA (1 μg) was then used for GeneChip analysis, individual samples were used in GWAT preparation and 3 samples were pooled and used for aorta preparations. Terminal-labeled cDNA, hybridization to genome-wide mouse gene 2.0 ST GeneChips and scanning of the arrays were carried out according to the manufacturer’s instructions (Affymetrix). The data discussed in this publication have been deposited in the National Center for Biotechnology Information’s Gene Expression Omnibus (Stulnig 1732748090 et al., 2016) and are accessible through GEO Series accession number GSE76811 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc = GSE76811).
Real-time Quantitative PCR
Total RNA (1 μg) from adipose tissue was transcribed into cDNA using Superscript II and random hexamer primers (Invitrogen). Gene expression of murine MMP12 (Mm00500554_m1) and human MMP12 (Hs00899662_m1) was analyzed in duplicate by quantitative real-time RT-PCR on an ABI Step One Plus cycler using assays-on-demand kits (TaqMan® Gene Expression Assay, Life Technologies) and normalized to murine (Ubc, Mm01198158_m1) or human ubiquitin C mRNA (UBC, Hs00824723_m1), respectively. (18) The comparative threshold cycle (CT) method was used to calculate relative expression. (19)
Western Blot and Zymography
We homogenized 100mg of adipose tissue in RIPA buffer (0.05M Tris/HCl pH 8, 1mM EDTA, 0.5mM EGTA, 1% NP-40, 25% sodium deoxycholate, 0.1% SDS, 5% glycerol, 0.15M NaCl, 0.05M NaF, 1mM sodium orthovanadate and 1 × complete-protease inhibitor cocktail [Roche]) by Polytron (PT 3100, Kinematica AG) and homogenates were clarified by centrifugation at 12,000 × g for 30 min. Equal amounts of protein were separated by SDS-PAGE. Gels were transferred to PVDF membrane (Millipore, IPVH 30470) and incubated with anti-MMP12 rabbit monoclonal antibody (Abcam, Ab52897) or anti β-actin mouse monoclonal antibody (Sigma, A1978). Western blotting was developed by BM Chemiluminescence blotting substrate (Roche) and signals were detected with Fusion FxVilberLourman (Peqlab). Full-length and cleaved MM1732748007 P12 was quantified by densitometry (ImageJ® Software) and normalized to β-actin.
Proteolytic activity was determined by casein zymography. Extracts were prepared by adipose tissue homogenization in 20mM Tris/HCl pH 8,137mM NaCl, 10% glycerol, 1% Triton X-100 and 2mM PMSF, followed by the procedure described for western blot. Equal amounts of protein were subjected to nonreducing SDS-PAGE using 10% gels containing 0.25% skim milk. Electrophoresis gels were washed 3 times, for 10 min each time, with zymogram renaturation buffer (Bio-Rad, 161-0765) and then incubated for 48 hrs at 37°C with zymogram development buffer (Bio-Rad, 161-0766). Next, the gels were stained with Coomassie brilliant blue R-250 (Sigma) and de-stained with distilled water. Caseinolytic bands were evident as de-stained bands against the dark background.
Statistical Methods
Human study baseline characteristics
Data are expressed as means ± SEM. Testing for normality was performed using Shapiro-Wilk test and differences between groups were analyzed by unpaired 2-tailed Student t test. Statistical significance was set at p <0.05.
Microarrays
Output primary row data were analyzed with Expression Console software (Affymetrix), from which the signal per probe was obtained and the fold change calculated. Differences between groups were analyzed by unpaired 2-tailed Student t test. Adjustment for multiple testing was done by Benjamini-Hochberg- correction. Statistical significance was set at p <0.05.
Meta-analysis
To perform the cardiovascular disease meta-analysis, published microarrays from both human and murine atherosclerotic samples were collected from gene expression data sets (NCBI Gene Expression Omnibus; keywords: atheroscl*, vascular tissue, coronary, carotid, aorta) (Table 1 and Supplementary Tables 2 and 3). Data were transformed by log2 if not already done, and normalized by conducting a quantile-normalization (Bioconductor Package Limma, function normalize Quantiles). (20) In each experiment, probes were removed if no gene symbol could be assigned. If more than one Entrez-ID could be assigned to a probe, 1 record per gene ID was added to the data set. If more than 1 probe mapped to the same Entrez-ID, we summarized the data by calculating the mean.
Table 1.
Overview of the study design.
| Type of study | Species | Disease (model) | Tissue | Processing method | N° of samples | N° of genes/ probe sets |
|---|---|---|---|---|---|---|
| Microarray | Mus musculus C57BL/6 | Cardiovascular disease | Atherosclerotic tissue (aorta) | Expression ConsoleTM Software (Benjamini-Hochberg correction) | 3/3 Ldlr-/- (HSFC/CD) | 26,995 |
| Microarray | Mus musculus C57BL/6 | Obesity and type 2 diabetes | Gonadal White adipose tissue | Expression ConsoleTM Software (Benjamini-Hochberg-correction) | 6/6 WT (HFD/CD) | 26,995 |
| Meta-analysis | Homo sapiens Mus musculus | Cardiovascular disease | Atherosclerotic tissue (coronary, carotid and aorta vasculatory tissue) | Bioconductor package MetaMA and Mixed Model (Benjamini-Hochberg-correction) | 452 | 8,101 |
| Meta-analysis | Homo sapiens Mus musculus Rattus norvegicus | Type 2 diabetes | Adipose tissue, liver, muscle, pancreatic islets, kidney and hypothalamus | Genome-wide association study (eGWAS) (Benjamini-Hochberg-correction) | 1,175 | 24,898 |
For statistical analysis, only genes that were analyzed in all 10 experiments were considered (number of genes = 8,108). For each array, the original data were transformed first to gene ranks, and then the uniformly distributed gene ranks were transformed to the standard normal distribution by computing the standard normal quantiles of the gene ranks divided by the number of genes plus 1. A mixed model based on the transformed data was then calculated for each gene separately, with group (case versus control) as independent variable and experiment number as random factor (R-package nlme, R-function lme). (21) To correct for multiple testing, Benjamini-Hochberg correction was applied. Adjusted p values <0.05 were considered statistically significant. The statistical analyses were carried out with R 3.0.1 and Bioconductor 2.12. In addition, a published type 2 diabetes meta-analysis was included in this study (14) (Table 1). In total, 24,898 genes were analyzed. The raw p values of the published data were adjusted for multiple testing by Benjamini-Hochberg correction to obtain a list of top genes dysregulated in adipose tissue microarrays to combine with the cardiovascular disease meta-analysis and both microarray databases as described in the results section.
Western blot, zymography and real-time PCR quantification
Data are expressed as means ± SEM. Differences between groups were analyzed by unpaired 2-tailed Student t test. Statistical significance was set at p <0.05.
RESULTS
Screening and Selection of a Short List of Potential New Targets
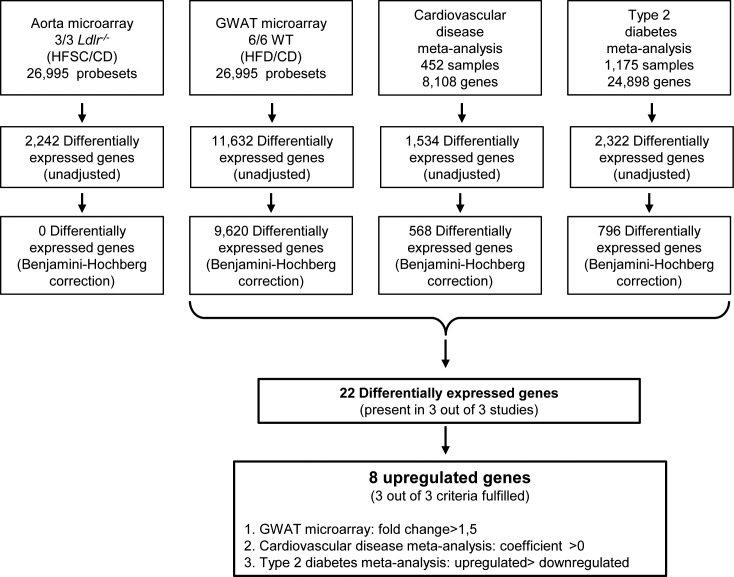
In the search for molecules that could be targeted for treatment and/or prevention of cardiometabolic disease, the present study was performed using data from 2 microarrays and 2 meta-analyses (Table 1). The microarrays were performed with aortae and GWAT of mouse models of atherosclerosis and obesity. The cardiovascular disease meta-analysis included microarrays from mouse and human atherosclerotic samples, and the type 2 diabetes meta- analysis was previously performed with metabolic tissue from mouse, rat and human samples (14) (Table 1). Microarrays were evaluated for upregulation of gene expression caused by HFD and HFSC as compared with CD in GWAT from WT mice and aortae from Ldlr-/- mice, respectively. From 26,995 probe sets, 2,242 were found unadjusted significantly dysregulated in the aorta microarray, but they did not remain significant after Benjamini-Hochberg correction. By contrast, in the GWAT microarray experiment, the number of probe sets with a significance <0.05 was 11,632 before and 9,620 after Benjamini-Hochberg correction. For the cardiovascular disease meta-analysis, 1,534 out of 8,101 genes with an unadjusted p value <0.05 were found; of these, 568 genes remained significantly dysregulated after Benjamini-Hochberg correction, considering 2-sample (case/control) and 1-sample (case only) experiments. To also include genes implicated in the pathogenesis of type 2 diabetes, primary data from a previously published gene expression–based genome-wide association study were taken into account as well. (14) This study included 130 case-control microarrays, 1,175 samples obtained from adipose tissue, liver, muscle, pancreatic islets, kidney and hypothalamus. Of the 24,898 genes analyzed in this study, 2,322 were unadjusted differentially expressed genes, and after Benjamini-Hochberg correction, a list of 796 differentially expressed genes was obtained. When combining differentially expressed genes with an adjusted p value <0.05 from the 3 studies, namely GWAT, cardiovascular disease meta-analysis and type 2 diabetes meta-analysis, 22 genes were significantly differentially expressed in all 3 studies. The aorta microarray experiment was not considered for gene selection, as no differentially expressed genes with an adjusted p <0.05 were found. Out of these 22 genes, only upregulated genes were considered due to possible blockage studies. A gene was considered upregulated if there was a fold change ≥1.5 in the GWAT microarray study, a coefficient >0 from the mixed model in the cardiovascular disease meta-analysis and more microarrays upregulated than downregulated in the type 2 diabetes meta-analysis (Figure 1). Finally, a short list of 8 genes that were upregulated in all the studies was obtained to be considered for further investigations (Table 2), with Mmp12, Trem2 and Mpeg1 at the top of the list with the most striking p values.
Figure 1.
Screening and selection of a short list of potential new targets. Diagram bounding several stages to identify common genes in cardiovascular disease and obesity models. Differentially expressed genes from cardiovascular disease meta-analysis and type 2 diabetes and adipose tissue microarrays were combined to identify common genes upregulated in all 3 studies. First, common genes from all 3 databases were identified (22 genes) to proceed with identification of the upregulated genes in 3 out of 3 studies (8 genes). The aorta microarray was not included in the selection procedure, as no differentially expressed genes were found after Benjamini-Hochberg correction.
Table 2.
List of the eight candidate genes ordered by the expression in the GWAT microarray selected as potential new targets.
| Gene name | Gene symbol | Microarray (Fold change) | Meta-analysis | ||
|---|---|---|---|---|---|
| AortaA Ldlr-/- (HFSC) versus Ldlr-/- (CD) | GWAT WT (HFD) versus WT (CD) | Cardiovascular disease pVal | Type 2 diabetes pVal | ||
| Macrophage metalloelastase | MMP12/Mmp12 | 11.2 | 16.9 | 0.00232 | 0.00077 |
| Triggering receptor expressed on myeloid cells 2 | TREM2/Trem2 | 2.5 | 13.3 | 0.00067 | 0.01094 |
| Macrophage-expressed gene 1 protein | MPEG1/Mpeg1 | 4.4 | 7.8 | 0.00097 | 0.00018 |
| G protein-coupled receptor 137B | GPR137B/Gpr137b | 2.0 | 5.7 | 0.03752 | 0.00298 |
| Spleen tyrosine kinase | SYK/Syk | 1.5 | 3.0 | 0.01389 | 0.00163 |
| v-mafmusculoaponeurotic fibrosarcoma oncogene family, protein B | MAFB/Mafb | - | 2.7 | 0.00034 | 0.04552 |
| Phospholipid transfer protein | PLTP/Pltp | 1.4 | 2.3 | 0.04339 | 0.00107 |
| Ribonucleoside-diphosphate reductase subunit M2 | RRM2/Rrm2 | - | 1.8 | 0.04549 | 0.00726 |
The aorta microarray experiment was not considered for gene selection, as no differentially expressed genes with an adjusted p < 0.05 was found.
Selection of MMP12 for Validation
A pool of interesting genes potentially involved in type 2 diabetes and cardiovascular disease development was defined (Table 2). All of these genes were thoroughly investigated in terms of published data concerning the protein encoded by each of them as well as gene expression profiles. In Supplementary Table S4, fold changes of an individual patient case/control microarray study (GSE28829) in the cardiovascular disease meta-analysis are reported. We considered the physiological importance of each gene in obesity, type 2 diabetes and/or cardiovascular disease models and knowledge of putative functions in inflammatory and/or metabolic processes as a basis for the development of further in vitro experiments. Mmp12 had a top position on the list, ordered by expression in the GWAT microarray, as well as in the other data sets. In addition, MMP12 is known to be involved in adipose tissue expansion, insulin sensitivity and the expression of inducible nitric oxide synthase in angiogenesis during adipose tissue expansion, (22,23) and is positively associated with adipose tissue inflammation. (24) Notably, MMP12 is also related to the acceleration of atherosclerosis. (25) Hence, MMP12 appears to be related not only to metabolic disease, but also to cardiovascular disease. However, there is virtually no information on the regulation of MMP12 in human subjects. (22) Based on this investigation, we finally selected Mmp12 for further validation as a candidate molecule. Moreover, Mmp12 was the highest ranked gene from the GWAT microarray (Supplementary Table 5), which also included secreted phosphoprotein 1/osteopontin (Spp1/OPN). Osteopontin has a well-described role in obesity-induced insulin resistance (26, 27) and atherosclerosis (28) and has already been investigated as a treatment target for cardiometabolic disease, indicating the validity of our approach. OPN is cleaved with MMP12 (29) (Supplementary Figure 1), indicating a potential amplification of osteopontin action by simultaneous upregulation of MMP12.
Mmp12 is upregulated in adipose tissue from diet-induced obese animals and obese patients.
To validate data from the in silico study and the microarray experiment, Mmp12 mRNA expression levels were examined in samples used for GWAT microarray (Figure 2A) and samples from a separate experiment evaluating diet-induced obesity (Figure 2B). Additionally, OWAT and SWAT from lean and obese human subjects were examined (Figure 2C). As shown in Figure 2, Mmp12 was significantly upregulated in adipose tissue of all samples of obese adipose tissue studied.
Figure 2.
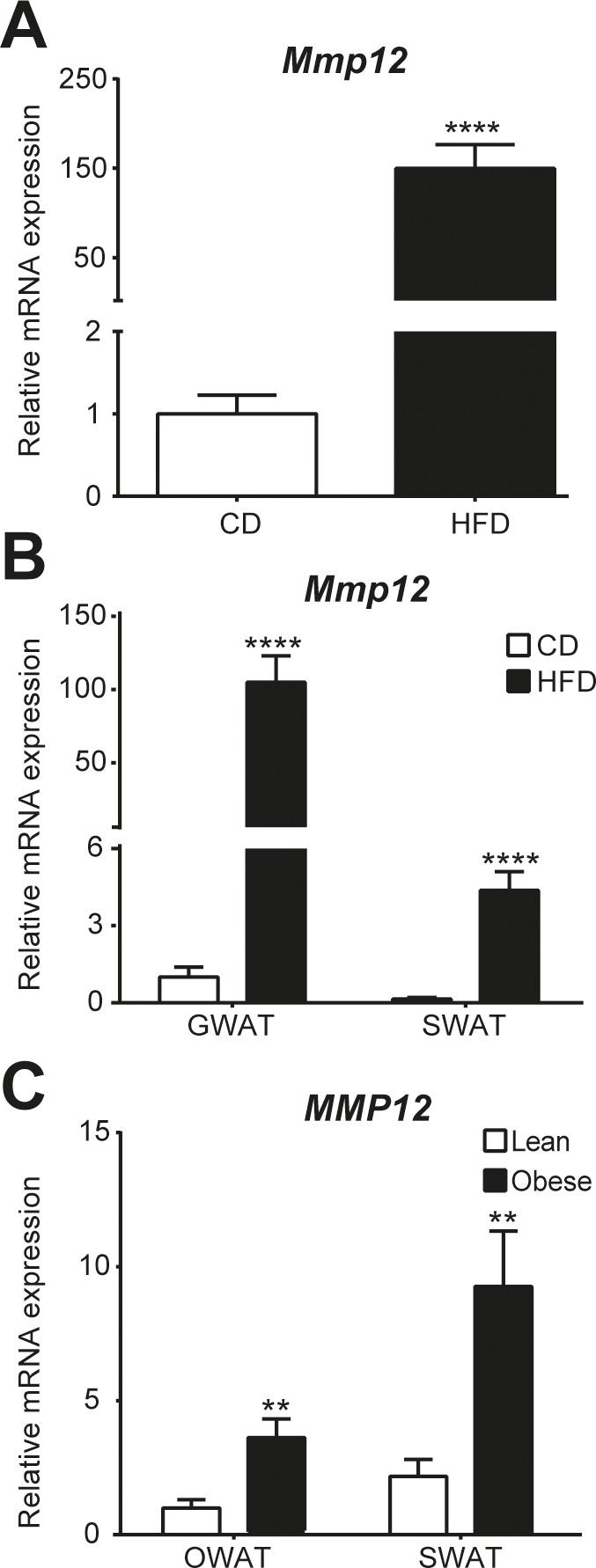
Gene expression of Mmp12/MMP12 in obese mouse/human adipose tissue. Total RNA was extracted from GWAT and SWAT from mice (A and B) and humans (C), respectively. (A) Mmp12 expression in GWAT of WT mice fed HFD or CD from the microarray experiment was analyzed by RT-PCR with mice on CD being set to 1; n = 12 mice of each condition; (B) Mmp12 expression in GWAT and SWAT of WT mice fed HFD or CD was analyzed by RT-PCR in a separate experiment with mice on CD being set to 1; n = 15 mice of each condition; (C) MMP12 expression in OWAT and SWAT from obese and lean subjects, OWAT of lean human was set to 1; n = 12 of each group. Results are expressed as mean ± SEM. * p < 0.05; ** p < 0.01; *** p < 0.001 and **** p < 0.0001 compared with CD (A, B) or lean (C), respectively.
MMP12 protein expression is increased and an elevated caseinolytic activity corresponding with the active MMP12 molecular weight is detected in obesity.
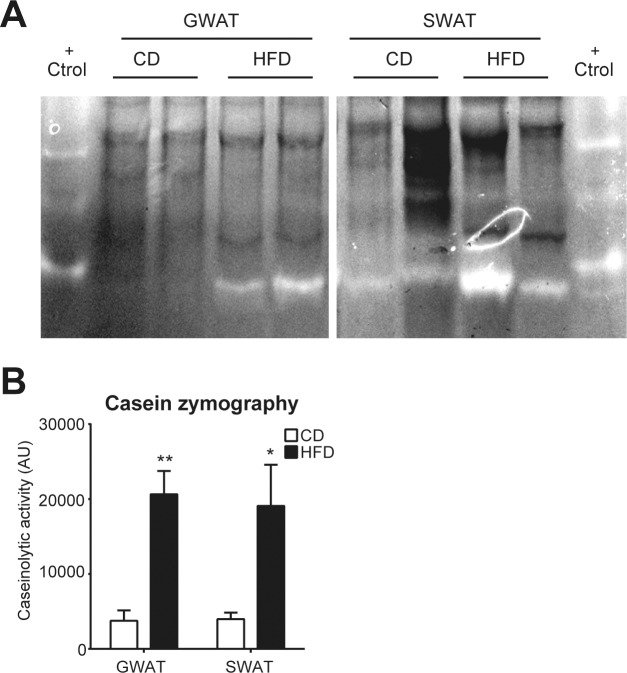
To evaluate Mmp12 expression also on a protein level, full-length (56 kDa) and active-form (46 kDa) MMP12 in GWAT and SWAT were analyzed by western blot. The sum of full-length and active MMP12 was significantly upregulated by 2.31-fold in GWAT (p = 0.04) and 3.06-fold in SWAT (p = 0.002) from obese compared with lean mice (Figures 3A and B). This was due to higher expression of the 46 kDa active form of MMP12, which was significantly increased in SWAT of obese animals (3.46-fold; p = 0.002), with a strong trend being evident also in GWAT depots (2.18-fold; p = 0.09) (Figures 3A and C), which did not have statistical significance due to a single extreme value. Casein zymography was performed to investigate whether differences in protein expression correlated with differences in activity levels. Strikingly, an elevated caseinolytic activity corresponding to the molecular weight of the active form of MMP12 was detected in the samples from obese animals (Figures 4A and B).
Figure 3.
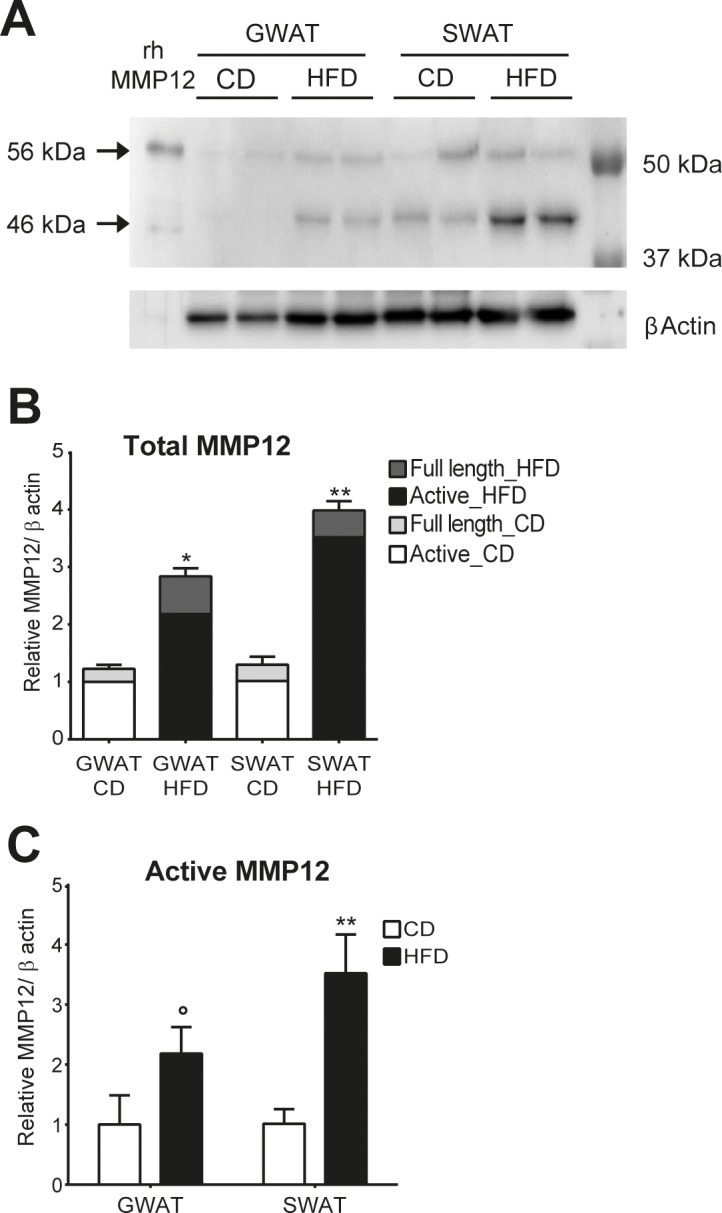
Identification of MMP12 protein expression in white adipose tissue from diet induced obese mice. Protein extracts from GWAT and SWAT from 23-wks old mice after 14 wks on HFD or CD, respectively, was analyzed by Western blot and zymography for MMP12. β-actin was used as a loading control in Western blot. (A) Representative image of 4 Western blots (n = 8 mice per condition) are shown. (B) Quantification of total MMP12 protein expression in GWAT and SWAT on CD and HFD. (C) Quantification of active MMP12 protein expression in GWAT and SWAT on CD and HFD. Results are expressed as mean ± SEM.°p = 0.09; * p < 0.05; ** p < 0.01.
Figure 4.
Caseinolytic activity in white adipose tissue from diet-induced obese mice. (A) Representative image of 2 zymography (n = 4 mice per condition) are shown. (B) Quantification of caseinolytic activity by arbitrary units (AU) in GWAT and SWAT on CD and HFD. Results are expressed as mean ± SEM. * p < 0.05; ** p < 0.01.
DISCUSSION
Metabolic syndrome is a multifactorial disorder that comprises insulin resistance, obesity, hypertension and dyslipidemia and results in an increased incidence of cardiometabolic disease. Preventive and therapeutic strategies to reduce the cardiometabolic risk associated with metabolic syndrome have been developed for both health and economic reasons, but often in the absence of defined molecular targets or even a solid understanding of the underlying pathogenesis. (30) Thus, developing novel strategies and investigate new molecules as potential candidates for future treatments is among the main efforts in current biomedical research.
In this study, an unbiased bioinformatic approach was carried out to identify candidate target molecules for the prevention and treatment of cardiometabolic disease, combining published data and new results from a murine microarray experiment and a meta-analysis. The screening strategy established in the present study combined microarray data with meta-analysis, applying a range of tools that facilitated the merging of information from expression profiles between different published and original data sets. In meta-analyses, there will inevitably be heterogeneity in terms of gene expression and cellular properties in the samples included, and this leads to significant variations that reduce the statistical power. However, we considered meta-analyses to be appropriate for this study, as they provide a robust basis for identifying common dysregulated molecules in the multifactorial conditions associated with cardiometabolic disease while considering inflammation as a crucial process involved in the development of type 2 diabetes and cardiovascular disease.
Upregulated genes present in all the considered studies were preselected, resulting in a list of 8 genes, including well-known as well as novel candidate molecules for treatment of cardiometabolic disease (Figure 1 and Table 2). Due to its unbiased nature, this list of genes is probably very useful for researchers looking for candidate molecules for simultaneous targeting of obesity-related cardiovascular and metabolic disease. Nevertheless, it has to be kept in mind as a limitation of this study that the upregulation of these genes is not necessarily causally associated with the pathophysiology of cardiometabolic disease, but may also point to counteracting mechanisms.
To select an individual gene for further evaluation, these 8 genes were deeply evaluated in a systematic manner, with an emphasis on secreted molecules with the potential to act as immunological targets, which led to the selection of Mmp12 as a highly interesting molecule. We also identified several other genes from the matrix metalloproteinase (MMP) family that were upregulated in the considered studies (Supplementary Table 6). Tissue MMPs are multidomain and zinc-dependent endopeptidases with a common molecular structure that consists of an aminoterminal propeptide, a catalytic domain and a regulatory carboxyterminal end. (31) After secretion by a variety of cells, activation of MMPs occurs in the extracellular matrix by tissue or bacterial proteinases activated through cleavage of the propeptide domain. (32,33) Despite these 3 well-conserved domains, there are differences described between MMP family members due to the presence or absence of additional domains, which can result in specific enzyme characteristics like inhibitor binding or substrate specificity. (34) Moreover, MMP activities can be regulated at 3 different levels: gene transcription, proenzyme activation and endogenous tissue inhibitors of MMPs, which directly inhibit their activity when bound to the catalytic domain through to a disulfide bond. (35,36) Mmp12 was one of the genes with the highest regulation in all the included studies (Table 2), mirroring the published data regarding its regulation and expression in adipose tissue. (22,37,38)
To validate the results from the unbiased in silico approach, the gene and protein expression profiles of Mmp12 in different depots of adipose tissue were analyzed by RT-PCR and western blot, respectively. As reported in previous studies (39) and in agreement with the microarray and meta-analysis results, Mmp12 was significantly upregulated in diet-induced obesity in mice (Figures 2A and B). In addition, we show here for the first time to our knowledge a significant upregulation of MMP12 in both SWAT and OWAT from obese patients (Figure 2C), a higher expression of MMP12 total protein and active form in SWAT as well as a clear trend in GWAT from obese mice in comparison with their respective controls (Figures 3A, B and C). The upregulation of Mmp12 is in accordance with previous findings demonstrating that MMP12 is mainly secreted by macrophages in adipose tissue that accumulate in obesity. (40) This fact, together with reported data indicating a less pronounced macrophage infiltration and activation in human in comparison with murine adipose tissue in obesity, (41) could explain the somewhat lower, but still significant, extent of MMP12 upregulation in humans.
MMP12 can degrade different constituents of the extracellular matrix, playing an essential role in the extracellular matrix remodeling process. (31,42) Notably, casein zymography has revealed increased caseinolytic activity in both investigated fat pads from obese mice, potentially attributed to the differential expression of MMP12 active fragment (Figures 4A and B). Hence, our data suggest that MMP12, as well as its enzymatic activity, is present in significantly increased quantities in obese adipose tissue, as previously shown for atherosclerosis, (25) extending published data on adipose tissue homeostasis. (22) Elastin is one of the major substrates of active MMP12, but it was recently shown that remodeling of the elastin network during obesity is independent of MMP12. (43) Hence, the functional impact of active MMP12 in cardiometabolic disease appears to involve other substrates. MMP12 is able to cleave other molecules, including OPN (Supplementary Figure S1). By promoting OPN cleavage, MMP12 can be directly related to the proinflammatory and proatherogenic functions of activated OPN, (26,28) independent of extracellular matrix remodeling.
MMPs are secreted proteins that have been associated with a variety of physiological processes and diseases, which made them identifiable as highly interesting targets. In the past, various blockage studies with a broad spectrum of inhibitors, mainly associated with cancer treatment, have been carried out with equivocal results, questioning the role of MMPs as potential targets in these indications. However, recently these trials have been judged differently. (44) A substantial body of evidence in the literature identifies MMP12 as a potential candidate for the treatment of cardiometabolic diseases. However, different signaling pathways and beneficial physiological functions where it is involved should be taken into account and investigated more deeply when considering MMP12 as a potential targetable molecule.
With respect to the possible involvement of MMP12 in the development of type 2 diabetes, MmP12-/- mice following high-fat feeding exhibited improved insulin sensitivity in spite of obesity, in line with our selection approach. (22) However, other studies have failed to induce persistent obesity and insulin sensitivity, (24,43) maybe due to variations in genetic background, housing conditions or microbiome. Hence, further studies will be needed to elucidate the pathophysiological role of MMP12 in obesity and its cross-talk with OPN.
CONCLUSION
In conclusion, we describe here a stepwise and multimethodological screening study with the combination of new experimental data and published meta-analyses to screen a large number of studies and identify 8 molecules potentially involved in the development and progression of type 2 diabetes and cardiovascular disease. The relevant data obtained in this study, in combination with other published research, identify MMP12 as an interesting candidate molecule for the prevention and treatment of cardiometabolic disease, as well as a valuable list of other novel candidates to be evaluated in future studies.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Federal Ministry of Economy, Family and Youth and the National Foundation for Research, Technology and Development (to T.M. Stulnig). The authors acknowledge A Pellicoro (University of Edinburgh/Medical Research Council Queen’s Medical Research Institute, UK) for technical assistance, and A Jürets, B Wanko and C Trevella for their support during submission of this manuscript.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
Cite this article as: Amor M, et al.. (2016) Identification of matrix metalloproteinase-12 as a candidate molecule for prevention and treatment of cardiometabolic disease. Mol. Med. 22:487–96.
References
- Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med. 1997;14 Suppl 5:S1–85. [PubMed] [Google Scholar]
- Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–7. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- [WHO] World Health Organization. Geneva: World Health Organization; 2014. Global status report on noncommunicable diseases 2014; p. 298. [Google Scholar]
- Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world—a growing challenge. N Engl J Med. 2007;356:213–15. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- O'Rourke RW. Inflammation in obesity-related diseases. Surgery. 2009;145:255–9. doi: 10.1016/j.surg.2008.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–14. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- Rohn TA, Bachmann MF. Vaccines against non-communicable diseases. Curr Opin Immunol. 2010;22:391–6. doi: 10.1016/j.coi.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Hawkes CA, McLaurin J. Immunotherapy as treatment for Alzheimer’s disease. Expert Rev Neurother. 2007;7:1535–48. doi: 10.1586/14737175.7.11.1535. [DOI] [PubMed] [Google Scholar]
- Peakman M, Dayan CM. Antigen-specific immunotherapy for autoimmune disease: fighting fire with fire? Immunology. 2001;104:361–6. doi: 10.1046/j.1365-2567.2001.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena R, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–6. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- Scott LJ, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–5. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek R, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–5. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- Wellcome Trust Case Control. Genome-wide association study of 14, 000 cases of seven common diseases and 3, 000 shared controls. Nature. 2007;447:661–78. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama K, et al. Expression-based genome-wide association study links the receptor CD44 in adipose tissue with type 2 diabetes. Proc Natl Acad Sci U S A. 2012;109:7049–54. doi: 10.1073/pnas.1114513109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhofer A, et al. An accelerated mouse model for atherosclerosis and adipose tissue inflammation. Cardiovasc Diabetol. 2014;13:23–23. doi: 10.1186/1475-2840-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council of Europe. European convention for the protection of vertebrate animals used for experimental and other scientific purposes. 1986 [Google Scholar]
- Itariu BK, et al. Long-chain n-3 PUFAs reduce adipose tissue and systemic inflammation in severely obese nondiabetic patients: a randomized controlled trial. Am J Clin Nutr. 2012;96:1137–49. doi: 10.3945/ajcn.112.037432. [DOI] [PubMed] [Google Scholar]
- Bustin SA, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–22. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2–ΔΔCT Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Gentleman R, Carey V, Huber W, Irizarry R, Dudoit S. Bioinformatics and Computational Biology Solutions Using R and Bioconductor. New York: Springer Verlag; 2006. p. 2005. [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D. Linear and nonlinear mixed effects models. R package version 3. 2007:57. [Google Scholar]
- Lee JT, et al. Macrophage metalloelastase (MMP12) regulates adipose tissue expansion, insulin sensitivity, and expression of inducible nitric oxide synthase. Endocrinology. 2014;155:3409–20. doi: 10.1210/en.2014-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansbury BE, Hill BG. Regulation of obesity and insulin resistance by nitric oxide. 1732748056. Free R Biol Med. 2014;73:383–99. doi: 10.1016/j.freeradbiomed.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauters D, Van Hul M, Lijnen HR. Macrophage elastase (MMP-12) in expanding murine adipose tissue. Biochim Biophys Acta. 2013;4:4. doi: 10.1016/j.bbagen.2012.12.024. [DOI] [PubMed] [Google Scholar]
- Yamada S, et al. Matrix metalloproteinase 12 accelerates the initiation of atherosclerosis and stimulates the progression of fatty streaks to fibrous plaques in transgenic rabbits. Am J Pathol. 2008;172:1419–29. doi: 10.2353/ajpath.2008.070604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer FW, et al. Neutralization of osteopontin inhibits obesity-induced inflammation and insulin resistance. Diabetes. 2010;59:935–46. doi: 10.2337/db09-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomiyama T, et al. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J Clin Invest. 2007;117:2877–88. doi: 10.1172/JCI31986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoda K, et al. Osteopontin transgenic mice fed a high-cholesterol diet develop early fatty-streak lesions. Circulation. 2003;107:679–81. doi: 10.1161/01.cir.0000055739.13639.d7. [DOI] [PubMed] [Google Scholar]
- Goncalves DaSilva A, Liaw L, Yong VW. Cleavage of osteopontin by matrix metalloproteinase-12 modulates experimental autoimmune encephalomyelitis disease in C57BL/6 mice. Am J Pathol. 2010;177:1448–58. doi: 10.2353/ajpath.2010.091081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller DE. New drug targets for type 2 diabetes and the metabolic syndrome. Nature. 2001;414:821–7. doi: 10.1038/414821a. [DOI] [PubMed] [Google Scholar]
- Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. 1732748058. Cardiovasc Res. 2006;69:562–73. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Nagase H, Enghild JJ, Suzuki K, Salvesen G. Stepwise activation mechanisms of the precursor of matrix metalloproteinase 3 (stromelysin) by proteinases and (4-aminophenyl) mercuric acetate. Biochemistry. 1990;29:5783–9. doi: 10.1021/bi00476a020. [DOI] [PubMed] [Google Scholar]
- Tallant C, Marrero A, Gomis-Rüth FX. Matrix metalloproteinases: fold and function of their catalytic domains. BBA-Mol Cell Res. 2010;1803:20–28. doi: 10.1016/j.bbamcr.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Overall CM. Molecular determinants of metalloproteinase substrate specificity. Mol Biotechnol. 2002;22:51–86. doi: 10.1385/MB:22:1:051. [DOI] [PubMed] [Google Scholar]
- Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;7:1–2. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem. 2003;253:269–85. doi: 10.1023/a:1026028303196. [DOI] [PubMed] [Google Scholar]
- Chavey C, et al. Matrix metalloproteinases are differentially expressed in adipose tissue during obesity and modulate adipocyte differentiation. J Biol Chem. 2003;278:11888–96. doi: 10.1074/jbc.M209196200. [DOI] [PubMed] [Google Scholar]
- Maquoi E, Munaut C, Colige A, Collen D, Lijnen HR. Modulation of adipose tissue expression of murine matrix metalloproteinases and their tissue inhibitors with obesity. Diabetes. 2002;51:1093–101. doi: 10.2337/diabetes.51.4.1093. [DOI] [PubMed] [Google Scholar]
- de Meijer VE, Sverdlov DY, Le HD, Popov Y, Puder M. Tissue-specific differences in inflammatory infiltrate and matrix metalloproteinase expression in adipose tissue and liver of mice with diet-induced obesity. Hepatol Res. 2012;42:601–10. doi: 10.1111/j.1872-034X.2011.00960.x. [DOI] [PubMed] [Google Scholar]
- Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. 1732748060 J1732748060 Clin1732748060 Invest. 2011;121:2111–17. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeyda M, et al. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes. 2007;31:1420–28. doi: 10.1038/sj.ijo.0803632. 1732748060. [DOI] [PubMed] [Google Scholar]
- Massova I, Kotra LP, Fridman R, Mobashery S. Matrix metalloproteinases: structures, evolution, and diversification. Faseb J. 1998;12:1075–95. [PubMed] [Google Scholar]
- Martinez-Santibanez G, et al. Obesity-induced remodeling of the adipose tissue elastin network is independent of the metalloelastase MMP-12. Adipocyte. 2015;4:264–72. doi: 10.1080/21623945.2015.1027848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbroucke RE, Libert C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat Rev Drug Discov. 2014;13:904–27. doi: 10.1038/nrd4390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.