Abstract
Poor adherence to combined antiretroviral therapy (cART) has been shown to be a major determinant of virologic failure, emergence of drug resistant virus, disease progression, hospitalizations, mortality, and health care costs. While high adherence levels can be achieved in both resource-rich and resource-limited settings following initiation of cART, long-term adherence remains a challenge regardless of available resources. Barriers to optimal adherence may originate from individual (biological, socio-cultural, behavioral), pharmacological, and societal factors. Although patients and providers should continuously strive for maximum adherence to cART, there is accumulating evidence that each class of antiretroviral therapy has specific adherence-drug resistance relationship characteristics allowing certain regimens more flexibility than others. There is not a universally accepted measure for cART adherence, since each method has distinct advantages and disadvantages including cost, complexity, accuracy, precision, intrusiveness and bias. Development of a real-time cART adherence monitoring tool will enable the development of novel, pre-emptive adherence-improving strategies. The application of these strategies may ultimately prove to be the most cost-effective method to reduce morbidity and mortality for the individual and decrease the likelihood of HIV transmission and emergence of resistance in the community.
Keywords: HIV, antiretroviral therapy adherence, virologic failure, drug resistance, outcomes
1. INTRODUCTION
Medication adherence typically refers to the extent to which individuals take medications as prescribed. Optimal adherence to combination antiretroviral therapy (cART) can be defined based on the virologic (measured by HIV RNA viral load), immunologic (measured by CD4 T-cell count), and clinical outcomes of patients whose adherence was measured during longitudinal studies. For the currently out-moded unboosted protease inhibitor (PI)-based cART, Paterson et al. described the highest likelihood of treatment success in patients who take ≥95% of the medications prescribed by their physician [1]. In contrast, accumulating data shows that cART based on non-nucleoside reverse transcriptase inhibitors (NNRTIs) or boosted PIs have high rates of suppression at moderate levels of adherence (70-90%) [2-6].
It is now widely appreciated that adherence to antiretroviral therapy is the critical determinant of HIV treatment outcomes. Adherence to cART had been shown to be a major predictor of achieving adequate suppression of HIV replication [1-6], required to minimize the emergence of drug resistance (DR) [7], disease progression [8], and death [9-11]. Recently, there has been increasing discussion regarding the public health implications of antiretroviral therapy utilization and adherence as applied in ‘test and treat’ HIV prevention strategies [12]. Here, we will explore these principles in further detail. In addition, we will review levels of adherence to antiretroviral therapy in different populations, the association between adherence and the development of antiretroviral resistance mutations, the impact of adherence on the cost of medical care, and finally discuss the instruments used to measure adherence and their potential utility to impact HIV treatment outcomes in the future.
2. ADHERENCE AS MAJOR DETERMINANT OF HIV TREATMENT OUTCOMES: CLINICAL AND PUBLIC HEALTH
Some determinants of effective treatment of HIV infection include biology, behavior, and social or structural issues. A conceptual "pathway" model of successful HIV treatment determinants and outcomes illustrates the critical role of medication adherence Fig. (1). Biological determinants of adherence include predisposition to drug adverse effects (e.g., Abacavir hypersensitivity reaction and HLA B*5701) [13]. Behavioral determinants include issues such as maintenance of routines [14-15]. Social issues include stigma in the local community regarding HIV infection [16-17]. Structural issues relate to the health care system which includes the development and availability of potent medications, adoption of guidelines for correct prescribing of these drugs, and regular patient access to health care and medications [18-20]. Optimizing all of these factors will most likely lead to the highest levels of adherence possible.
Fig. (1).
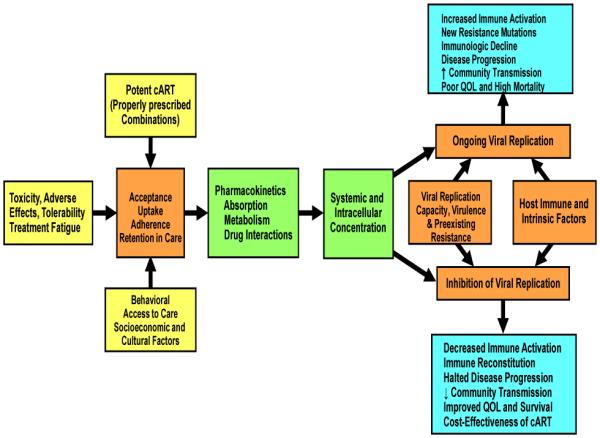
Determinants of HIV Treatment Success and Outcomes.
The pathway to adherence is threatened by the complexity of cART regimens. Fortunately, a South African patient initiating cART today is typically prescribed three individual tablets once a day: Tenofovir (TDF) 300mg + Lamivudine (3TC) 300 mg or Emtricitabine (FTC) 200 mg + Efavirenz (EFV) 600mg [21]. The pill burden can be simplified further by using medications that combine several drugs in a single pill as fixed-dose combinations (FDC). In other settings such as high-income countries, a one pill once a day combination of EFV + FTC + TDF in the same dosage as the individual tablets is commonly used [22].
On a once daily cART regimen, in order to remain >95% adherent to cART, a patient can miss no more than one out of the 30 doses per month. In addition, for maximal efficacy, specific cART doses must be taken at the prescribed time. When patients do not accurately adhere to their regimen schedule and instead take their drugs too late or too early or miss doses completely, blood concentrations can drop below the level necessary to fully suppress HIV, which may lead to the emergence of drug-resistance, disease progression and death, or rise to levels that are hazardous to the patient because of drug toxicity [23-24]. Unfortunately, how precisely the timing needs to be followed before virologic failure is not fully characterized and likely differs by HIV drug/ regimen [25]. A significant body of evidence has demonstrated the relationship between the presence of acquired HIVDR and AIDS/death outcomes [26-35]. In addition, data from CDC’s HIV Outpatient Study showed that patients who had resistance testing survived longer than those who did not. However, it is unlikely that testing per se was life-saving but served as an indicator of better care and better access to newer drugs [36].
Evidence from observational studies among heterosexual populations and men who have sex with men suggests that effective cART in highly adherent patients may greatly reduce sexual transmission of HIV from infected individuals to their sexual partners [37-38]. This concept has been explored in a modeling exercise that simulated the effects of a hypothetical “test-and-treat” strategy in which universal voluntary HIV testing is combined with immediate cART for infected persons regardless of their CD4 T-cell count. Based on these modeling data, and assuming sustained high levels of adherence with broad coverage and uptake, cART could reduce HIV transmission, and hence potentially curb the HIV epidemic by decreasing the incidence of HIV to less than one case per 1,000 people per year by 2016 [39-42]. However, achieving full access to cART and long-term ART adherence support for all at-risk populations may prove to be more difficult than any mathematical model could predict [40-41] and such approaches still raise concerns about individual rights, toxicity, drug resistance, financing. Also, as for all mathematical models, the voluntary “test-and-treat model is based on a number of assumptions that required to be validated by research. Indeed, feasibility studies are already underway in both developed and developing countries with preliminary results anticipated in the next few years.
The major issue facing the developing world is the scaling up of cART access. This depends on political will, local infrastructure and available resources. These aspects are important not just for delivering cART and providing health care, but in promoting and monitoring treatment adherence [43]. Required aspects of infrastructure include not only mechanisms to obtain and dispense drugs, but also to teach patients about adverse effects, adherence and lifestyle modifications to improve treatment effectiveness. There is an urgent need for the development and implementation of simplified, standardized treatment and monitoring algorithms that will facilitate roll-out and scale-up of programs and enable counseling and follow-up of patients.
3. LEVELS AND DETERMINANTS OF ANTIRETROVIRAL THERAPY ADHERENCE IN DEVELOPED COMPARED WITH DEVELOPING COUNTRIES
Historically, there has been an expectation of poor antiretroviral therapy adherence in poverty-affected regions of the world as expressed by selected high-level international agency decision-making bodies. These opinions arguably contributed to the delay in cART roll-out in resource-limited settings [44-46]. Furthermore, until Mills and colleagues conducted a meta-analysis to assess adherence in Africa, comprehensive assessments of levels of cART adherence were limited or based on anecdotes and personal observation. In this meta-analysis, reported levels of adherence were measured in a variety of ways, including pill-counts, pharmacy refill data, Medication Event Monitoring System (MEMS), and self-report [47]. On average, 77% of African patients (95% Confidence Interval [CI]: 68-85%) met the standard definition of good adherence (≥80%) compared to 55% (95% CI: 49-62%) of North American patients. These findings have encouraged international assistance regarding improved access to cART and were referred to by former U.S. President Bill Clinton at the 2006 IAS Conference as the “nail in the coffin” on discrimination regarding drug access [48].
Given the individual and public health benefits associated with adherence to cART, there is a need for a greater understanding of actual adherence rates within specific populations. Moreover, it is vital to examine reasons for poor adherence and possible motivators to improve adherence so as to inform the design of adherence-improving interventions. A review of the literature shows that prevalent factors associated with poor treatment adherence in resource-rich and limited settings include untreated depression, active substance abuse, poor insight into disease and treatment, being an adolescent or young adult, higher pill burden and more frequent dosing, and forgetfulness [49]. In addition, the following risk factors for non-adherence were more prevalent in sub-Saharan Africa: cost or structural barriers such as pharmacy stock-outs or lack of transportation means to the health facility for cART refills [50-51]; food insecurity [52]; non-disclosure to a loved one or fear of being stigmatized [53]. Studies report that the majority of patients accessing cART have disclosed their HIV status to family or friends and that those who have not disclosed appear to have worse outcomes [53]. Patients who do not disclose their infection to their intimate partner or household may have frequent treatment interruptions due to the fact that tablets must be hidden and not taken in the presence of others. Encouraging voluntary HIV status disclosure in a community with access to ART may result in improved uptake of voluntary counseling and testing (VCT) and may help decrease stigma and improve adherence. Of note, very few interventions have been designed that have successfully demonstrated improved adherence, and most have been limited to North American settings [54] Development of effective, culturally-sensitive cART adherence interventions in developed and developing world settings is an important area of ongoing and future research [54-55].
4. RELATIONSHIP BETWEEN ANTIRETROVIRAL THERAPY ADHERENCE, DRUG CLASS AND DRUG RESISTANCE
The phenomenon of HIV drug resistance continues to receive major clinical and public health attention because resistant HIV not only threatens the patient in which it develops but can be transmitted to others [56]. Widespread resistance has the potential to undermine our ability to fight the HIV/AIDS pandemic by rendering first-line treatments ineffective. This is especially important in the developing world where second-line and salvage regimens are either extremely expensive, unavailable, or both [57].
HIV drug resistance (HIVDR) can be classified as acquired (secondary) or transmitted (primary). Acquired HIVDR occurs when mutations develop to drugs in individuals who have received ARV, often because of poor adherence, treatment interruptions, inadequate drug concentrations, or use of sub-optimal drug combinations leading to treatment failure. Transmitted HIVDR occurs in the context of HIV-infected individuals who have never received ART, and occurs when individuals are newly infected with a drug-resistant virus. If virus replication is not suppressed by medication, millions of viruses harboring different mutations arise each day owing to the high error rate of the reverse transcription process and the lack of genetic proofreading. Although many of these mutations have a detrimental effect on viral survival, occasionally, a mutation results in an altered viral protein which renders viral replication less susceptible to inhibition by a particular antiretroviral drug. In the presence of drug, cART inhibits replication of the wild-type strain, but mutant viral strains with reduced susceptibility continue to replicate and become the predominant circulating viral populations in the individual Fig. (2).
Fig. (2).
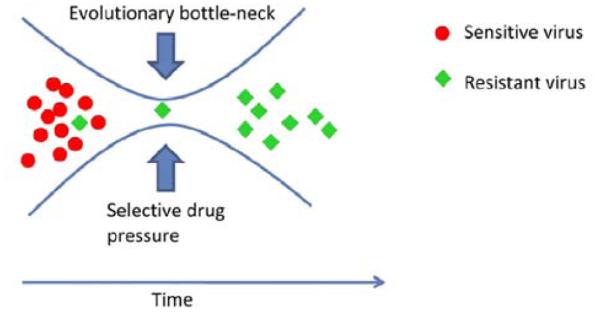
Emergence of Resistant Viral Population due To Selective Drug Pressure.
It is grossly apparent that non-adherence to medication invariably breeds drug resistance. However, the relationship between adherence and resistance is much more complex and follow an inverted “U-shaped” curve. At the highest levels of adherence, the threat of resistance is lowest because mutations cannot occur without replication. However, at lower levels of adherence the complex interplay between potency, viral fitness after mutation, the genetic barrier to resistance of cART components, and the other regimen components determine whether or not resistant virus will be circulating Fig. (3). Even relatively small degrees of non-adherence are thought to substantially increase resistance.
Fig. (3).
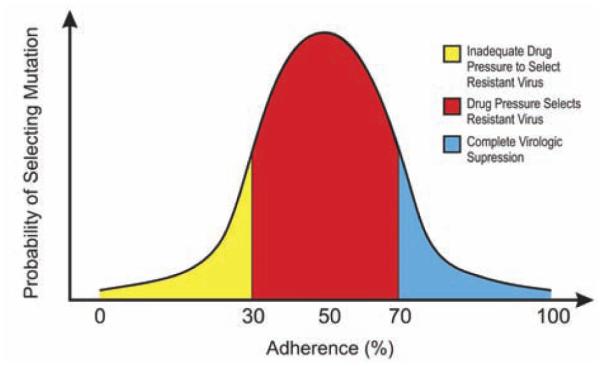
Inverted “U-Shaped” Hypothetical Curve of the Relationship Between Probability of Drug Resistance and HIV Treatment Adherence Levels.
The complexity of this relationship is in part determined by the relative fitness of resistant compared with wild type virus. Most mutant strains are less fit than the wild-type strains, which means a certain level of selection pressure is required before the mutant strains are able to out-compete the wild-type strains in order to predominate. As a result, low levels of adherence do not impose the necessary selection pressure on the viral population to favor the resistant less fit strains than the wild-type fitter strains and thus emergence of resistance is less likely. Of course low adherence is not advisable since it allows wild-type virus to replicate unchecked, leading to disease progression. This inverted ‘U-shaped’ relationship leaves moderately adherent patients at the greatest risk for the development of resistance [58].
Work by Bangsberg and others [59-63] has found that the relationship between adherence and drug resistance differs depending on the antiretroviral drug class Fig. (4). Indeed, they have shown that NNRTI-based drug regimens are more likely to produce resistance than PI-based because of several factors. The high potency and long half-life of NNRTIs may lead to better virologic suppression at moderate adherence levels, but paradoxically lead to the development of antiretroviral drug resistance during a treatment interruption of triple therapy containing NRTIs and NNRTI lasting more than 48 hours [64]. During a treatment interruption, the comparatively short half-lives of the NRTIs in the regimen lead to prolonged NNRTI monotherapy. The low genetic barrier for resistance of NNRTIs allows resistant virus to accumulate rapidly. Also, NNRTIs inhibit RT allosterically, by binding to an area outside the active (substrate binding) site, thus NNRTI resistance mutations have little effect on RT’s overall function and hence little impact on viral fitness.
Fig. (4).
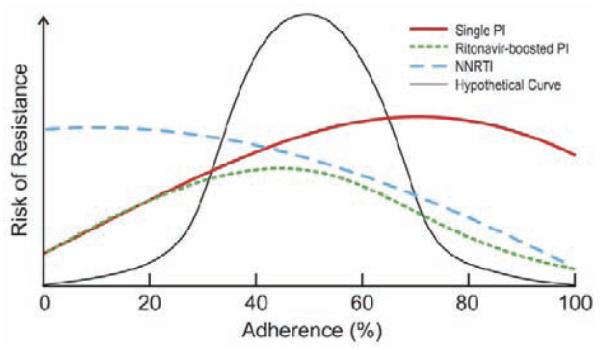
Probability of Resistance by Adherence Level and Class of Antiretroviral Therapy Regimen (adapted from reference 60, with permission from Oxford University Press, June 30, 2010).
A study in Kampala, Uganda [20], of patients purchasing generic fixed-dose NNRTI-based cART found that 65% had a treatment interruption of greater than 48 hours as evaluated by electronic adherence monitoring, and these treatment interruptions accounted for 90% of missed doses. Eight of the 62 (13%) participants who had treatment interruptions experienced treatment failure due to selection of drug resistant virus, compared to none of the 33 participants without treatment interruptions greater than 48 hours. Importantly, there was also a significant decrease in virologic suppression rates, from 80% to 50%, for patients with ≥95% adherence versus <95% adherence, respectively. As a consequence, the threat of resistance to first-generation NNRTIs is highest at low levels of adherence, rather than at moderate adherence, because even the lowest concentrations of these NNRTIs create enough selection pressure to affect HIV. Protease inhibitors (PIs) and most nucleoside reverse transcriptase inhibitors (NRTIs) require multiple mutations, each of which alter enzyme function and could make the virus less fit [65]. These drugs also have more rapid clearance. It is therefore not surprising that NNRTI resistance is seen more often than PI or NRTI resistance. Reported mutations to NNRTIs, NRTIs, PIs, Entry and Integrase Strand Transfer Inhibitors are updated regularly by the International AIDS Society (IAS)-USA Drug Resistance Group [66] and can be accessed at IAS-USA’s website [67].
5. NON-ADHERENCE TO ANTIRETROVIRAL THERAPY AND ITS IMPACT ON HEALTH CARE COSTS
Nachega et al. [68] found that greater adherence to cART was associated with lower monthly health costs in a private managed care program in South Africa, mainly due to reduced hospital admissions. In contrast, in a U.S. study by Gardner et al. [69], better adherence to cART was associated with higher direct medical costs despite lower rates of hospitalization in more adherent individuals. The differences in the relative contribution of cART costs to overall costs probably accounts for the apparent discrepancy among the studies conducted in South-Africa (where the cART cost component accounted only for 9% of total costs) and in the U.S. (where ART costs represent 60% of total costs). This hypothesis is supported by the fact that overall health care utilization was lower in both the South African and U.S. populations in the setting of better adherence. Other possible explanations include different study methodology, variations in the epidemiology of HIV infection, variations in CD4 T-cell count at initiation of antiretroviral therapy, structural dissimilarities between health care systems, and differences in health resources consumption. A retrospective cohort analysis within the Adherence sub-study of the Italian Cohort Naïve Antiretroviral (AdICoNA) found that mean annual hospital expenses were significantly higher for non-adherent compared to adherent patients (417 1250 Euro vs. 192 670 Euro; p<0.01). Hence, in this cohort, mostly at early stages of HIV infection and followed-up in Europe, savings in cART costs were associated with non-adherence and offset by the increased risk of hospitalization and the rise in inpatient costs [70].
Antiretroviral therapy is cost-effective in resource-limited and resource-rich settings [71-73]. The data discussed above showed that in resource-limited settings, where cART comprises a smaller proportion of overall health care costs, excellent adherence to antiretroviral therapy is cost saving [68]. In resource rich settings, better adherence decreases health care utilization, but is not cost saving [69]. However, it is important to note that no formal cost-effectiveness analyses of adherence to antiretroviral therapy have been completed to date. When cost-utility and cost savings that are unmeasurable in system-level studies are taken into account, it is very likely that adherence to antiretroviral therapy will be cost-effective in all settings. Indeed, in United States, the cost-effectiveness of a weekly home visit nursing intervention on antiretroviral adherence (for as much as $1,000 per person) using data from a randomized controlled clinical trial as input to a computer-based state transition model of HIV disease, showed that the incremental cost-effectiveness ratio was $14,100 per quality-adjusted life year gained compared with standard care. Therefore, adherence interventions with modest effectiveness are likely to provide long-term survival benefit to patients and to be cost-effective compared with other uses of HIV care funds [74].
6. MEASURING ANTIRETROVIRAL THERAPY ADHERENCE
Although adherence is of critical clinical importance, few studies have empirically studied adherence measurement tools [75-78]. Common methods of adherence measurement include pill counts, pharmacy record reviews, self-report measures, electronic monitoring and therapeutic drug monitoring (TDM) [79-80]. To date, there is not a universally accepted tool to measure cART adherence, since each method has distinct advantages and disadvantages including cost, complexity, accuracy, intrusiveness and bias (Table 1). Oyugi et al. [81] evaluated different adherence measurement tools including patient self-report, pill count and MEMS caps, which is an electronic monitoring system. In this pilot study they found excellent agreement between the three measurement tools in a Ugandan HIV cohort with documented high levels of cART adherence. In contrast, Gill et al. [82] found large discrepancies among estimated adherence for different methods when reviewing several cohorts in resource-limited settings. They constructed a relative hierarchy of adherence measurement methods, and reported that physician assessment and self-report were the least accurate, pill counts were intermediate, and electronic monitoring provided the most accurate surrogate of cART adherence. This is similar to the conclusions from a U.S. study which determined that MEMS underestimated adherence while pill counts and patient self-report overestimated adherence [79].
Table 1.
Antiretroviral Therapy Adherence Monitoring Tools: Accuracy, Advantages and Disadvantages
| ART Adherence Monitoring Tool |
Validity/Reliability | Detection of Ad- herence Patterns |
Real-Time Monitoring |
Setting of Use | Cost |
|---|---|---|---|---|---|
| Self-Report | Specific, Very Insensitive. Poor Reliability |
Yes | No | Clinical Practice & Research |
Low |
| Pharmacy Refill or Claim | Specific, Fairly Sensitive & Reliable |
No | Could be | Clinical Practice & Research |
Low |
| Announced or Unannounced Pill Count |
Fairly Specific, Fairly Sensi- tive, Fair Reliability |
No | No | Clinical Practice & Research |
Low |
| Electronic Pill-Container Caps (MEMS caps) |
Specific, Too Sensitive & High Reliability |
Yes | No | Research | High |
| Electronic Web-Enabled Pill Box (Med-eMonitor; Wise Pill; etc.) |
Specific, Too Sensitive | Yes | Yes | Research | High |
| Directly Observed or Admin- istered Therapy (DOT/DAART) |
Specific, Sensitive and Reli- able |
N/A | Yes | Vulnerable Popula- tions & Research |
Labor- Intensive |
| Monitoring of Antiretroviral Drug Concentration (Blood, Urine, Hair, etc.) |
Specific, Sensitive, Reliable | Yes | Yes | Research | High |
As there is currently no gold standard objective measure of adherence in a real-life setting, studies of adherence typically rely on limited assessment of criterion-related forms of validity such as predictive or concurrent validity. That is, in most cases, validation of an adherence measure is based on how strongly the measure is associated with virologic or other laboratory and clinical outcomes or how well a particular measure compares to other adherence measures [80]. While the ability of measures to predict virologic outcomes is important, it is problematic when the measure is not in fact assessing behavior. For example, if an individual fails therapy due to acquisition of resistant virus, interventions to improve adherence behavior will not be relevant. In addition, some have argued that virologic failure is an inadequate indicator of non-adherence, as several other factors (e.g., viral load level at initiation of cART, potency of the therapy prescribed, individual differences in absorption, and drug interactions) also influence virologic outcomes.
Self-report or pharmacy refills are the most commonly used cART adherence measurement tools in resource-limited settings. These tools can be useful over and above their utility in alerting providers to the need for adherence intervention. Indeed, Bisson et al. [83] reported that pharmacy refill-based adherence levels outperformed CD4 T-cell count changes as a predictor of virologic failure in the first year following cART initiation for patients in Cape Town, South Africa, enrolled in the private sector Aid for AIDS disease management program. In light of this success, there is a need for of the practical implementation of novel, feasible, and cost-effective adherence monitoring tools capable of capturing real-time adherence behavior, which are useful in both resource-limited and resource-rich settings and useful for routine application in clinical care and research studies [77]. Further development of these tools for real-world real-time adherence assessment, linked with interventions to improve adherence when necessary, require further study and will be critically important to the long-term successful utilization of currently available antiretroviral medications.
7. SUMMARY
Adherence to antiretroviral treatment plays a crucial role in the success or failure of therapy for HIV infection. Many barriers to adherence exist and many are shared by developed and developing world settings. No single method for measurement or estimation of adherence has been widely accepted. Further, the relation between adherence and treatment outcomes is complex and likely varies by drug and maybe even by individual. Identifying a simple and affordable method for accurately measuring adherence will facilitate the development of effective, pre-emptive adherence interventions. If applied widely, these interventions will likely prove to be cost-effective in reducing morbidity and mortality when treating large populations in both resource-rich and resource-limited settings.
ACKNOWLEDGEMENT
Funding Sources: The United States National Institutes for Allergy and Infectious Disease (NIAID-NIH), Division of AIDS (DAIDS): K23 AI 068582-01(JBN); and The European Developing Countries Clinical Trial Partnership (EDCTP) Senior Fellowship Award: TA-08-40200-021 (JBN); the National Institute for Allergy and Infectious Disease: T32 AI007438-16 and L30 AI080268-02 (SYH); the NIAID-NIH K01-AI067063 (EMG); the NIMH-NIH R34 MH083592-01A1 (EJM); P30 AI45008 and Agency for Health Research and Quality (AHRQ) U18 HS016946 (RG).
REFERENCES
- [1].Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, Wagener MM, Singh N. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann. Intern. Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- [2].Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin. Infect. Dis. 2006;43:939–941. doi: 10.1086/507526. [DOI] [PubMed] [Google Scholar]
- [3].Maggiolo F, Ravasio L, Ripamonti D, Gregis G, Quinzan G, Arici C, Airoldi M, Suter F. Similar adherence rates favor different virologic outcomes for patients treated with nonnucleoside analogues or protease inhibitors. Clin. Infect. Dis. 2005;40:158–163. doi: 10.1086/426595. [DOI] [PubMed] [Google Scholar]
- [4].Nachega JB, Hislop M, Dowdy DW, Chaisson RE, Regensberg L, Maartens G. Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Ann. Intern. Med. 2007;146:564–573. doi: 10.7326/0003-4819-146-8-200704170-00007. [DOI] [PubMed] [Google Scholar]
- [5].Shuter J, Sarlo JA, Kanmaz TJ, Rode RA, Zingman BS. HIV-infected patients receiving lopinavir/ritonavir-based antiretroviral therapy achieve high rates of virologic suppression despite adherence rates less than 95% J. Acquir. Immune Defic. Syndr. 2007;45:4–8. doi: 10.1097/QAI.0b013e318050d8c2. [DOI] [PubMed] [Google Scholar]
- [6].Martin M, Del Cacho E, Codina C, Tuset M, De Lazzari E, Mallolas J, Miro JM, Gatell JM, Ribas J. Relationship between adherence level, type of the antiretroviral regimen, and plasma HIV type 1 RNA viral load: a prospective cohort study. AIDS Res. Hum. Retroviruses. 2008;24:1263–1268. doi: 10.1089/aid.2008.0141. [DOI] [PubMed] [Google Scholar]
- [7].Harrigan PR, Hogg RS, Dong WW, Yip B, Wynhoven B, Woodward J, Brumme CJ, Brumme ZL, Mo T, Alexander CS, Montaner JS. Predictors of HIV drug-resistance mutations in a large antiretroviral-naive cohort initiating triple antiretroviral therapy. J. Infect. Dis. 2005;191:339–347. doi: 10.1086/427192. [DOI] [PubMed] [Google Scholar]
- [8].Bangsberg DR, Perry S, Charlebois ED, Clark RA, Roberston M, Zolopa AR, Moss A. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15:1181–1183. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- [9].Hogg RS, Heath K, Bangsberg D, Yip B, Press N, O'Shaughnessy MV, Montaner JS. Intermittent use of triple-combination therapy is predictive of mortality at baseline and after 1 year of follow-up. AIDS. 2002;16:1051–1058. doi: 10.1097/00002030-200205030-00012. [DOI] [PubMed] [Google Scholar]
- [10].Wood E, Hogg RS, Yip B, Moore D, Harrigan PR, Montaner JS. Impact of baseline viral load and adherence on survival of HIV-infected adults with baseline CD4 cell counts > or = 200 cells/microl. AIDS. 2006;20:1117–1123. doi: 10.1097/01.aids.0000226951.49353.ed. [DOI] [PubMed] [Google Scholar]
- [11].Nachega JB, Hislop M, Dowdy DW, Lo M, Omer SB, Regensberg L, Chaisson RE, Maartens G. Adherence to highly active antiretroviral therapy assessed by pharmacy claims predicts survival in HIV-infected South African adults. J. Acquir. Immune Defic. Syndr. 2006;43:78–84. doi: 10.1097/01.qai.0000225015.43266.46. [DOI] [PubMed] [Google Scholar]
- [12].Montaner JSG. Memorial Lecture: Treatment Adherence as Prevention; IAPAC and NIMH/NIH 5th International Conference on HIV Treatment Adherence; Miami. May 23-25, 2010. Available from: http://www.iapac.org/AdherenceConference/Downloads-ADC10/2-Memorial_Lecture-Treatment_as_Prevention-Julio_ SG_Montaner.pdf [Accessed on: 17th Jan 2011] [Google Scholar]
- [13].Mallal S, Phillips E, Carosi G, Molina JM, Workman C, Tomazic J, Jagel-Guedes E, Rugina S, Kozyrev O, Cid JF, Hay P, Nolan D, Hughes S, Hughes A, Ryan S, Fitch N, Thorborn D, Benbow A, PREDICT-1 Study Team HLA-B*5701 screening for hypersensitivity to abacavir. N. Engl. J. Med. 2008;358:568–579. doi: 10.1056/NEJMoa0706135. [DOI] [PubMed] [Google Scholar]
- [14].Barfod TS, Sorensen HT, Nielsen H, Rodkjaer L, Obel N. 'Simply forgot' is the most frequently stated reason for missed doses of HAART irrespective of degree of adherence. HIV. Med. 2006;7:285–290. doi: 10.1111/j.1468-1293.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- [15].Ryan GW, Wagner GJ. Pill taking 'routinization': a critical factor to understanding episodic medication adherence. AIDS Care. 2003;15:795–806. doi: 10.1080/09540120310001618649. [DOI] [PubMed] [Google Scholar]
- [16].Sayles JN, Wong MD, Kinsler JJ, Martins D, Cunningham WE. The association of stigma with self-reported access to medical care and antiretroviral therapy adherence in persons living with HIV/AIDS. J. Gen. Intern. Med. 2009;24:1101–1108. doi: 10.1007/s11606-009-1068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Makoae LN, Portillo CJ, Uys LR, Dlamini PS, Greeff M, Chirwa M, Kohi TW, Naidoo J, Mullan J, Wantland D, Durrheim K, Holzemer WL. The impact of taking or not taking ARVs on HIV stigma as reported by persons living with HIV infection in five African countries. AIDS Care. 2009;21:1357–1362. doi: 10.1080/09540120902862576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Krusi A, Wood E, Montaner J, Kerr T. Social and structural determinants of HAART access and adherence among injection drug users. Int. J. Drug Policy. 2010;21:4–9. doi: 10.1016/j.drugpo.2009.08.003. [DOI] [PubMed] [Google Scholar]
- [19].Kagee A, Remien RH, Berkman A, Hoffman S, Campos L, Swartz L. Structural barriers to ART adherence in Southern Africa: Challenges and potential ways forward. Glob. Public. Health. 2010:1–15. doi: 10.1080/17441691003796387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Oyugi JH, Byakika-Tusiime J, Ragland K, Laeyendecker O, Mugerwa R, Kityo C, Mugyenyi P, Quinn TC, Bangsberg DR. Treatment interruptions predict resistance in HIV-positive individuals purchasing fixed-dose combination antiretroviral therapy in Kampala, Uganda. AIDS. 2007;21:965–971. doi: 10.1097/QAD.0b013e32802e6bfa. [DOI] [PubMed] [Google Scholar]
- [21].South African National Department of Health Clinical Guidelines for the Management of HIV/AIDS in Adults and Adolescents. 2010 Available from: http://www.hiv911.org.za/wp-content/uploads/ 2010/04/2010-Adult-ART-Guidelines.pdf [Accessed on: 17th Jan 2011]
- [22].United States Department of Health and Human Services (DHHS) Guidelines for the Use of Antiretroviral Agents in HIV-infected Adults and Adolescents. 2009 Dec 1; Available from: http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf [Accessed on: 17th Jan 2011]
- [23].Woods SP, Dawson MS, Weber E, Gibson S, Grant I, Atkinson JH, HIV NEUROBEHAVIORAL RESEARCH CENTER GROUP Timing is everything: antiretroviral nonadherence is associated with impairment in time-based prospective memory. J. Int. Neuropsychol. Soc. 2009;15:42–52. doi: 10.1017/S1355617708090012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Liu H, Miller LG, Golin CE, Hays RD, Wu T, Wenger NS, Kaplan AH. Repeated measures analyses of dose timing of antiretroviral medication and its relationship to HIV virologic outcomes. Stat. Med. 2007;26:991–1007. doi: 10.1002/sim.2592. [DOI] [PubMed] [Google Scholar]
- [25].Gross R, Bilker WB, Wang H, Chapman J. How long is the window of opportunity between adherence failure and virologic failure on efavirenz-based HAART? HIV. Clin. Trials. 2008;9:202–206. doi: 10.1310/hct0903-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cozzi-Lepri A, Phillips AN, Clotet B, Mocroft A, Ruiz L, Kirk O, Lazzarin A, Wiercinska-Drapalo A, Karlsson A, Lundgren JD, EuroSIDA Study Group Detection of HIV drug resistance during antiretroviral treatment and clinical progression in a large European cohort study. AIDS. 2008;22:2187–2198. doi: 10.1097/QAD.0b013e328310e04f. [DOI] [PubMed] [Google Scholar]
- [27].Zaccarelli M, Tozzi V, Lorenzini P, Trotta MP, Forbici F, Visco-Comandini U, Gori C, Narciso P, Perno CF, Antinori A. Collaborative Group for Clinical Use of HIV Genotype Resistance Test (GRT) at National Institute for Infectious Diseases Lazzaro Spallanzani Multiple drug class-wide resistance associated with poorer survival after treatment failure in a cohort of HIV-infected patients. AIDS. 2005;19:1081–1089. doi: 10.1097/01.aids.0000174455.01369.ad. [DOI] [PubMed] [Google Scholar]
- [28].Di Giambenedetto S, Colafigli M, Pinnetti C, Bacarelli A, Cingolani A, Tamburrini E, Cauda R, de Luca A. Genotypic resistance profile and clinical progression of treatment-experienced HIV type 1-infected patients with virological failure. AIDS Res. Hum. Retroviruses. 2008;24:149–154. doi: 10.1089/aid.2007.0070. [DOI] [PubMed] [Google Scholar]
- [29].Ormaasen V, Sandvik L, Asjo B, Holberg-Petersen M, Gaarder PI, Bruun JN. An algorithm-based genotypic resistance score is associated with clinical outcome in HIV-1-infected adults on antiretroviral therapy. HIV Med. 2004;5:400–406. doi: 10.1111/j.1468-1293.2004.00244.x. [DOI] [PubMed] [Google Scholar]
- [30].Napravnick S, Keys J, Stalzer B, Eron JJ. Extensive HIV-1 antiretroviral drug class resistance is associated with inferior virological and clinical outcomes [abstract 59] Antivir. Ther. 2007;12(Suppl. 1):S68. [Google Scholar]
- [31].Lohse N, Jorgensen LB, Kronborg G, Moller A, Kvinesdal B, Sorensen HT, Obel N, Gerstoft J, Danish HIV Cohort Study Genotypic drug resistance and long-term mortality in patients with triple-class antiretroviral drug failure. Antivir Ther. 2007;12:909–917. [PubMed] [Google Scholar]
- [32].Lucas GM, Gallant JE, Moore RD. Relationship between drug resistance and HIV-1 disease progression or death in patients undergoing resistance testing. AIDS. 2004;18:1539–1548. doi: 10.1097/01.aids.0000131339.68666.1a. [DOI] [PubMed] [Google Scholar]
- [33].Lucas GM. Antiretroviral adherence, drug resistance, viral fitness and HIV disease progression: a tangled web is woven. J. Antimicrob. Chemother. 2005;55:413–416. doi: 10.1093/jac/dki042. [DOI] [PubMed] [Google Scholar]
- [34].Hogg RS, Bangsberg DR, Lima VD, Alexander C, Bonner S, Yip B, Wood E, Dong WW, Montaner JS, Harrigan PR. Emergence of drug resistance is associated with an increased risk of death among patients first starting HAART. PLoS Med. 2006;3:e356. doi: 10.1371/journal.pmed.0030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kozal MJ, Hullsiek KH, Leduc R, Novak RM, MacArthur RD, Lawrence J, Baxter JD. Terry beirn community programs for clinical research on AIDS (CPCRA) prevalence and impact of HIV-1 protease codon 33 mutations and polymorphisms in treatment-naive and treatment-experienced patients. Antivir Ther. 2006;11:457–463. [PubMed] [Google Scholar]
- [36].Palella FJ, Jr, Armon C, Buchacz K, Cole SR, Chmiel JS, Novak RM, Wood K, Moorman AC, Brooks JT, HOPS (HIV Outpatient Study) Investigators The association of HIV susceptibility testing with survival among HIV-infected patients receiving antiretroviral therapy: a cohort study. Ann. Intern. Med. 2009;151:73–84. doi: 10.7326/0003-4819-151-2-200907210-00003. [DOI] [PubMed] [Google Scholar]
- [37].Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, Meehan MO, Lutalo T, Gray RH. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N. Engl. J. Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- [38].Donnell D, Baeten JM, Kiarie J, Thomas KK, Stevens W, Cohen CR, McIntyre J, Lingappa JR, Celum C, Partners in Prevention HSV/HIV Transmission Study Team Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375:2092–2098. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Montaner JS, Hogg R, Wood E, Kerr T, Tyndall M, Levy AR, Harrigan PR. The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet. 2006;368:531–536. doi: 10.1016/S0140-6736(06)69162-9. [DOI] [PubMed] [Google Scholar]
- [40].Lima VD, Johnston K, Hogg RS, Levy AR, Harrigan PR, Anema A, Montaner JS. Expanded access to highly active antiretroviral therapy: a potentially powerful strategy to curb the growth of the HIV epidemic. J. Infect. Dis. 2008;198:59–67. doi: 10.1086/588673. [DOI] [PubMed] [Google Scholar]
- [41].Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- [42].Lima VD, Hogg RS, Montaner JS. Expanding HAART treatment to all currently eligible individuals under the 2008 IAS-USA Guidelines in British Columbia, Canada. PLoS One. 2010;5:e10991. doi: 10.1371/journal.pone.0010991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].de Bruin M, Viechtbauer W, Schaalma HP, Kok G, Abraham C, Hospers HJ. Standard care impact on effects of highly active antiretroviral therapy adherence interventions: A meta-analysis of randomized controlled trials. Arch. Intern. Med. 2010;170:240–250. doi: 10.1001/archinternmed.2009.536. [DOI] [PubMed] [Google Scholar]
- [44].Stevens W, Kaye S, Corrah T. Antiretroviral therapy in Africa. BMJ. 2004;328:280–282. doi: 10.1136/bmj.328.7434.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hogg R, Cahn P, Katabira ET, Lange J, Samuel NM, O'Shaughnessy M, Vella S, Wainberg MA, Montaner J. Time to act: global apathy towards HIV/AIDS is a crime against humanity. Lancet. 2002;360:1710–1711. doi: 10.1016/s0140-6736(02)11722-3. [DOI] [PubMed] [Google Scholar]
- [46].Harries AD, Nyangulu DS, Hargreaves NJ, Kaluwa O, Salaniponi FM. Preventing antiretroviral anarchy in sub-Saharan Africa. Lancet. 2001;358:410–414. doi: 10.1016/s0140-6736(01)05551-9. [DOI] [PubMed] [Google Scholar]
- [47].Mills EJ, Nachega JB, Buchan I, Orbinski J, Attaran A, Singh S, Rachlis B, Wu P, Cooper C, Thabane L, Wilson K, Guyatt GH, Bangsberg DR. Adherence to antiretroviral therapy in sub-Saharan Africa and North America: a meta-analysis. JAMA. 2006;296:679–690. doi: 10.1001/jama.296.6.679. [DOI] [PubMed] [Google Scholar]
- [48].Anonymous Voice of America [online]; Bill Clinton Addresses International AIDS Conference; Aug 16, 2006. http://www.voanews.com/english/news/a-13-africa.html [Accessed on: 17th Jan 2011] [Google Scholar]
- [49].Mills EJ, Nachega JB, Bangsberg DR, Singh S, Rachlis B, Wu P, Wilson K, Buchan I, Gill CJ, Cooper C. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med. 2006;3:e438. doi: 10.1371/journal.pmed.0030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Crane JT, Kawuma A, Oyugi JH, Byakika JT, Moss A, Bourgois P, Bangsberg DR. The price of adherence: qualitative findings from HIV positive individuals purchasing fixed-dose combination generic HIV antiretroviral therapy in Kampala, Uganda. AIDS. Behav. 2006;10:437–442. doi: 10.1007/s10461-006-9080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Weiser S, Wolfe W, Bangsberg D, Thior I, Gilbert P, Makhema J, Kebaabetswe P, Dickenson D, Mompati K, Essex M, Marlink R. Barriers to antiretroviral adherence for patients living with HIV infection and AIDS in Botswana. J. Acquir. Immune Defic. Syndr. 2003;34:281–288. doi: 10.1097/00126334-200311010-00004. [DOI] [PubMed] [Google Scholar]
- [52].Weiser SD, Tuller DM, Frongillo EA, Senkungu J, Mukiibi N, Bangsberg DR. Food insecurity as a barrier to sustained antiretroviral therapy adherence in Uganda. PLoS One. 2010;5:e10340. doi: 10.1371/journal.pone.0010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Nachega JB, Stein DM, Lehman DA, Hlatshwayo D, Mothopeng R, Chaisson RE, Karstaedt AS. Adherence to antiretroviral therapy in HIV-infected adults in Soweto, South Africa. AIDS Res. Hum. Retroviruses. 2004;20:1053–1056. doi: 10.1089/aid.2004.20.1053. [DOI] [PubMed] [Google Scholar]
- [54].Simoni JM, Pearson CR, Pantalone DW, Marks G, Crepaz N. Efficacy of interventions in improving highly active antiretroviral therapy adherence and HIV-1 RNA viral load. A meta-analytic review of randomized controlled trials. J. Acquir. Immune Defic. Syndr. 2006;43(Suppl 1):S23–35. doi: 10.1097/01.qai.0000248342.05438.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Amico KR, Harman JJ, Johnson BT. Efficacy of antiretroviral therapy adherence interventions: a research synthesis of trials, 1996 to 2004. J. Acquir. Immune Defic. Syndr. 2006;41:285–297. doi: 10.1097/01.qai.0000197870.99196.ea. [DOI] [PubMed] [Google Scholar]
- [56].Little SJ, Holte S, Routy JP, Daar ES, Markowitz M, Collier AC, Koup RA, Mellors JW, Connick E, Conway B, Kilby M, Wang L, Whitcomb JM, Hellmann NS, Richman DD. Antiretroviral-drug resistance among patients recently infected with HIV. N. Engl. J. Med. 2002;347:385–394. doi: 10.1056/NEJMoa013552. [DOI] [PubMed] [Google Scholar]
- [57].Mills EJ, Nachega JB. A wake-up call for global access to salvage HIV drug regimens. Lancet. 2007;370:1885–1887. doi: 10.1016/S0140-6736(07)61790-5. [DOI] [PubMed] [Google Scholar]
- [58].Braithwaite RS, Shechter S, Roberts MS, Schaefer A, Bangsberg DR, Harrigan PR, Justice AC. Explaining variability in the relationship between antiretroviral adherence and HIV mutation accumulation. J. Antimicrob. Chemother. 2006;58:1036–1043. doi: 10.1093/jac/dkl386. [DOI] [PubMed] [Google Scholar]
- [59].Gardner EM, Burman WJ, Steiner JF, Anderson PL, Bangsberg DR. Antiretroviral medication adherence and the development of class-specific antiretroviral resistance. AIDS. 2009;23:1035–1046. doi: 10.1097/QAD.0b013e32832ba8ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Bangsberg DR, Moss AR, Deeks SG. Paradoxes of adherence and drug resistance to HIV antiretroviral therapy. J. Antimicrob. Chemother. 2004;53:696–699. doi: 10.1093/jac/dkh162. [DOI] [PubMed] [Google Scholar]
- [61].Bangsberg DR, Acosta EP, Gupta R, Guzman D, Riley ED, Harrigan PR, Parkin N, Deeks SG. Adherence-resistance relationships for protease and non-nucleoside reverse transcriptase inhibitors explained by virological fitness. AIDS. 2006;20:223–231. doi: 10.1097/01.aids.0000199825.34241.49. [DOI] [PubMed] [Google Scholar]
- [62].Gardner EM, Hullsiek KH, Telzak EE, Sharma S, Peng G, Burman WJ, MacArthur RD, Chesney M, Friedland G, Mannheimer SB. Terry Beirn Community Programs for Clinical Research on AIDS and the International Network for Strategic Initiatives in Global HIV Trials Antiretroviral medication adherence and class-specific resistance in a large prospective clinical trial. AIDS. 2010;24:395–403. doi: 10.1097/qad.0b013e328335cd8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Parienti JJ, Ragland K, Lucht F, de la Blanchardiere A, Dargere S, Yazdanpanah Y, Dutheil JJ, Perre P, Verdon R, Bangsberg DR, ESPOIR and REACH study groups Average adherence to boosted protease inhibitor therapy, rather than the pattern of missed doses, as a predictor of HIV RNA replication. Clin. Infect. Dis. 2010;50:1192–1197. doi: 10.1086/651419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Taylor S, Allen S, Fidler S, White D, Gibbons S, Fox J, Clarke J, Weber J, Cane P, Wade A, Smit E, Back D. Stop Study: after discontinuation of Efavirenz, plasma concentrations may persist for 2 weeks or longer; 11th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA, USA. Feb 8-11, 2004. (WedOralAb#131) 131 - Abstract (11th CROI). Available from: http://www.retroconference.org/2004/cd/Abstract/131.htm [Accessed on: 17th Jan 2011] [Google Scholar]
- [65].Clotet B. Strategies for overcoming resistance in HIV-1 infected patients receiving HAART. AIDS. Rev. 2004;6:123–130. [PubMed] [Google Scholar]
- [66].Johnson VA, Brun-Vezinet F, Clotet B, Gunthard HF, Kuritzkes DR, Pillay D, Schapiro JM, Richman DD. Update of the drug resistance mutations in HIV-1: December 2009. Top. HIV. Med. 2009;17:138–145. [PubMed] [Google Scholar]
- [67].International AIDS Society-USA - Drug Resistance Mutations. http://www.iasusa.org/resistance_mutations/index.html [Accessed on: 17th Jan 2011]
- [68].Nachega JB, Leisegang R, Bishai D, Nguyen H, Hislop M, Cleary S, Regensberg L, Maartens G. Association of antiretroviral therapy adherence and health care costs. Ann. Intern. Med. 2010;152:18–25. doi: 10.7326/0003-4819-152-1-201001050-00006. [DOI] [PubMed] [Google Scholar]
- [69].Gardner EM, Maravi ME, Rietmeijer C, Davidson AJ, Burman WJ. The association of adherence to antiretroviral therapy with healthcare utilization and costs for medical care. Appl. Health. Econ. Health. Policy. 2008;6:145–155. doi: 10.1007/bf03256129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ammassari A, Trotta P, d’arminio M, Antinori A. Non-Adherence to Antiretroviral Therapy Impact Health Care Costs Worldwide. (Letter) Ann. Intern. Med. 2011 In Press. [Google Scholar]
- [71].Freedberg KA, Losina E, Weinstein MC, Paltiel AD, Cohen CJ, Seage GR, Craven DE, Zhang H, Kimmel AD, Goldie SJ. The cost effectiveness of combination antiretroviral therapy for HIV disease. N. Engl. J. Med. 2001;344:824–831. doi: 10.1056/NEJM200103153441108. [DOI] [PubMed] [Google Scholar]
- [72].Cleary SM, McIntyre D, Boulle AM. The cost-effectiveness of antiretroviral treatment in Khayelitsha, South Africa--a primary data analysis. Cost. Eff. Resour. Alloc. 2006;4:20. doi: 10.1186/1478-7547-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Goldie SJ, Yazdanpanah Y, Losina E, Weinstein MC, Anglaret X, Walensky RP, Hsu HE, Kimmel A, Holmes C, Kaplan JE, Freedberg KA. Cost-effectiveness of HIV treatment in resource-poor settings--the case of Cote d'Ivoire. N. Engl. J. Med. 2006;355:1141–1153. doi: 10.1056/NEJMsa060247. [DOI] [PubMed] [Google Scholar]
- [74].Freedberg KA, Hirschhorn LR, Schackman BR, Wolf LL, Martin LA, Weinstein MC, Goldin S, Paltiel AD, Katz C, Goldie SJ, Losina E. Cost-effectiveness of an intervention to improve adherence to antiretroviral therapy in HIV-infected patients. J. Acquir. Immune Defic. Syndr. 2006;43(Suppl 1):S113–118. doi: 10.1097/01.qai.0000248334.52072.25. [DOI] [PubMed] [Google Scholar]
- [75].Nachega JB, Mills EJ, Schechter M. Antiretroviral therapy adherence and retention in care in middle-income and low-income countries: current status of knowledge and research priorities. Curr. Opin. HIV. AIDS. 2010;5:70–77. doi: 10.1097/COH.0b013e328333ad61. [DOI] [PubMed] [Google Scholar]
- [76].Miller LG, Hays RD. Measuring adherence to antiretroviral medications in clinical trials. HIV. Clin. Trials. 2000;1:36–46. doi: 10.1310/enxw-95pb-5ngw-1f40. [DOI] [PubMed] [Google Scholar]
- [77].Bangsberg DR. Monitoring adherence to HIV antiretroviral therapy in routine clinical practice: The past, the present, and the future. AIDS. Behav. 2006;10:249–251. doi: 10.1007/s10461-006-9121-7. [DOI] [PubMed] [Google Scholar]
- [78].Nieuwkerk PT, Oort FJ. Self-reported adherence to antiretroviral therapy for HIV-1 infection and virologic treatment response: a meta-analysis. J. Acquir. Immune Defic. Syndr. 2005;38:445–448. doi: 10.1097/01.qai.0000147522.34369.12. [DOI] [PubMed] [Google Scholar]
- [79].Liu H, Golin CE, Miller LG, Hays RD, Beck CK, Sanandaji S, Christian J, Maldonado T, Duran D, Kaplan AH, Wenger NS. A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann. Intern. Med. 2001;134:968–977. doi: 10.7326/0003-4819-134-10-200105150-00011. [DOI] [PubMed] [Google Scholar]
- [80].Hugen PW, Langebeek N, Burger DM, Zomer B, van Leusen R, Schuurman R, Koopmans PP, Hekster YA. Assessment of adherence to HIV protease inhibitors: comparison and combination of various methods, including MEMS (electronic monitoring), patient and nurse report, and therapeutic drug monitoring. J. Acquir. Immune Defic. Syndr. 2002;30:324–334. doi: 10.1097/00126334-200207010-00009. [DOI] [PubMed] [Google Scholar]
- [81].Oyugi JH, Byakika-Tusiime J, Charlebois ED, Kityo C, Mugerwa R, Mugyenyi P, Bangsberg DR. Multiple validated measures of adherence indicate high levels of adherence to generic HIV antiretroviral therapy in a resource-limited setting. J. Acquir. Immune Defic. Syndr. 2004;36:1100–1102. doi: 10.1097/00126334-200408150-00014. [DOI] [PubMed] [Google Scholar]
- [82].Gill CJ, Hamer DH, Simon JL, Thea DM, Sabin LL. No room for complacency about adherence to antiretroviral therapy in sub-Saharan Africa. AIDS. 2005;19:1243–1249. doi: 10.1097/01.aids.0000180094.04652.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Bisson GP, Gross R, Bellamy S, Chittams J, Hislop M, Regensberg L, Frank I, Maartens G, Nachega JB. Pharmacy refill adherence compared with CD4 count changes for monitoring HIV-infected adults on antiretroviral therapy. PLoS Med. 2008;5:e109. doi: 10.1371/journal.pmed.0050109. [DOI] [PMC free article] [PubMed] [Google Scholar]


