Abstract
A 21% prostate cancer (PCa) mortality reduction was observed in the European Randomized Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. A direct correlation between stage shift and changes in PCa-mortality would support earlier detection through screening as the main reason for this reduction. In this study we empirically estimate how changes in the risk of being diagnosed with (advanced) PCa are related to the changes in PCa death in the ERSPC using a meta-regression approach. In total 81% and 89% of the changes in PCa mortality could be explained by changes in PCa incidence. Although this analysis cannot show direct causal relations, results support the hypothesis that PSA screening reduced PCa mortality by detecting cancer at an earlier stage while still curable. These findings do however not open the way to unrestricted PSA based screening for PCa. A balance between harm and benefit needs to be found.
PSA-based prostate cancer (PCa) screening results in a reduction in disease specific mortality but also generates harm due to false positives, overdiagnosis and overtreatment [1]. Hence, the key question is whether the observed mortality reduction can, at the least, be largely attributed to earlier detection (stage shift) or e.g. is predominantly the result of improvements in management of the disease. If the latter would be true, screening should be abandoned since it would mainly cause harm.
Most PCa deaths occur in men with advanced disease, diagnosed when already disseminated to other organs. The mechanism of screening is based on the assumption that potentially life-threatening PCa’s are detected at an early stage, before they metastasize. This implies that screening is expected to reduce the incidence of advanced cancers. In particular, in a randomized setting like ERSPC, a reduction of advanced PCa at time of diagnosis in the screening (S) arm as compared to the control (C) arm should reflect the impact of screening activities alone.
Previously published data from the ERSPC show a stage shift in favor of screening. There is a 24.4% reduction in high risk and M+ Pca at time of diagnosis together with a 21% PCa mortality reduction at 13 years of follow-up [1]. As mentioned before, differences in treatment could also affect the main outcome [2]. Detailed analyses on treatment patterns between study arms within ERSPC and more recently within ERSPC section Rotterdam showed that there are differences. Men diagnosed with organ confined PCa in the C- arm of the ERSPC trial are more likely to receive radiotherapy (as compared to radical prostatectomy). In addition, in ERSPC Rotterdam it was shown that if radiotherapy was chosen as the initial therapy, PCa patients in the C-arm received superior treatment (higher dosages often in combination with hormonal therapy). This being the result of progressive insight into treatment of PCa combined with, on average, a later detection in the C-arm [3,4].
The purpose of the current study was to empirically estimate how changes in the risk of being diagnosed with (advanced) PCa are related to changes in PCa death in ERSPC using a meta-regression approach as previously applied in breast cancer screening trials [5].
All PCa cases diagnosed (complete data until Dec 31, 2010) in the S and C arm of the ERSPC (men aged 50-74 yrs, N= 181,999 from 7 centers) were divided into 4 risk groups (low:T1-2 and Gleason <=6, intermediate:T1-2 and Gleason 7 or T3 and Gleason <=7, high:T1-3 and Gleason >=8 or T4), metastatic: M+ or PSA >100ng/ml). Missing data on T-stage, Gleason, PSA and M-stage (7% of all values) were imputed. Subsequently we regressed the natural logarithm of the rate ratio (number of events per person years: ln(RR)) of PCa incidence per risk group and ERSPC center on the ln(RR) of PCa mortality per risk group and center. Each risk group was weighted by the inverse variance of the ln(RR) of PCa mortality. The regression line was forced through zero, i.e. assuming that the rate ratio for PCa mortality between the study arms would be 1.0 if the rate ratio of PCa incidence at diagnosis between the study arms was also 1.0.
These analyses were conducted for all four risk groups combined and the high and M+ risk group ( i.e. advanced PCa) separately and included sensitivity analyses in the age group 55-69 years ( pre-defined core age group of ERSPC [1]) and using the D’Amico risk group classification with a separate group for M+ disease at diagnosis.
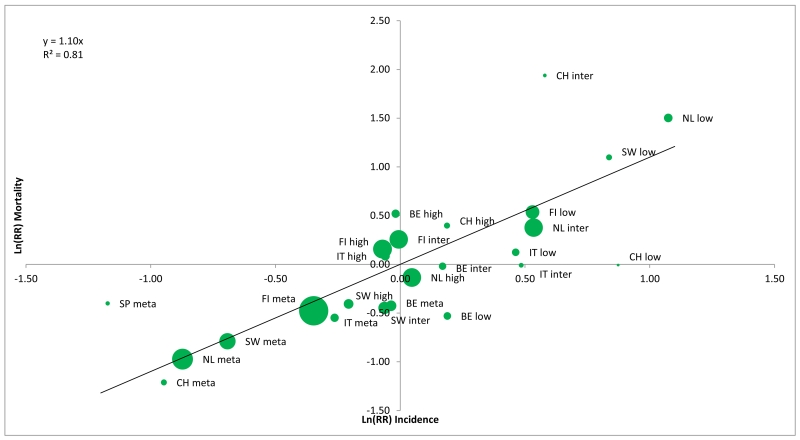
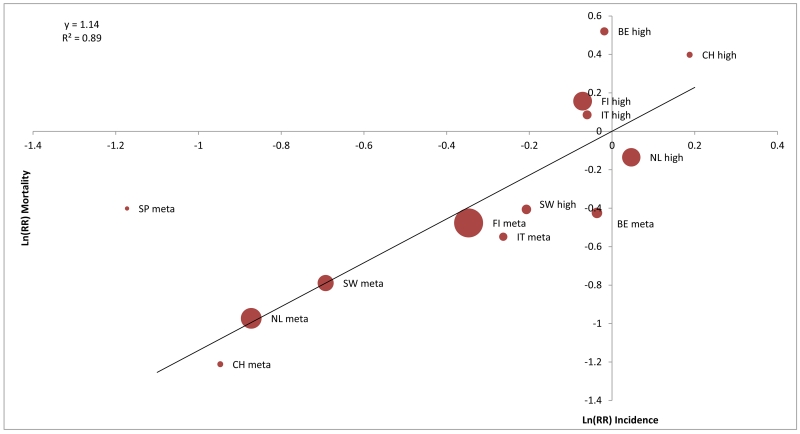
In total 8,839 men were diagnosed with PCa and 494 died of PCa in the S-arm, and 7,309 men were diagnosed and 699 died of PCa in the C-arm. The rate ratios of PCa incidence and mortality per risk group and ERSPC center is given in table 1. The slope of the regression line of the ln(RR) incidence on ln(RR) mortality for all risk groups (figure 1, panel A) and for the high and metastatic risk groups (figure 1 panel B) were 1.10 (95%CI 0.89-1.32) and 1.14 (95%CI 0.89-1.39) respectively, indicating a near direct correlation between changes in PCa incidence and PCa mortality. The associated R2 statistics, representing the variability in PCa mortality explained by changes in PCa incidence were 0.81 and 0.89 res. ( figure 1) which can be translated to 81% and 89% of the changes in PCa mortality between the study arms to be explained by differences in incidence (stage shift).
Table 1.
Rate ratio (RR*) of prostate cancer incidence and mortality per risk group and center.
| low | intermediate | high | metastatic | total | ||
|---|---|---|---|---|---|---|
| NL | RR incidence | 2.93 | 1.71 | 1.05 | 0.42 | 1.98 |
| RR mortality | 4.49 | 1.46 | 0.87 | 0.38 | 0.83 | |
| BE | RR incidence | 1.21 | 1.18 | 0.98 | 0.96 | 1.16 |
| RR mortality | 0.59 | 0.98 | 1.68 | 0.65 | 0.86 | |
| SW | RR incidence | 2.31 | 0.94 | 0.81 | 0.50 | 1.55 |
| RR mortality | 3.00 | 0.64 | 0.67 | 0.45 | 0.61 | |
| FI | RR incidence | 1.70 | 0.99 | 0.93 | 0.71 | 1.27 |
| RR mortality | 1.71 | 1.29 | 1.17 | 0.62 | 0.90 | |
| IT | RR incidence | 1.59 | 1.62 | 0.94 | 0.77 | 1.38 |
| RR mortality | 1.13 | 0.99 | 1.09 | 0.58 | 0.90 | |
| SP | RR incidence | 2.69 | 1.41 | 1.38 | 0.31 | 1.76 |
| RR mortality | - | - | - | 0.67 | 1.00 | |
| CH | RR incidence | 2.39 | 1.78 | 1.21 | 0.39 | 1.99 |
| RR mortality | 0.99 | 6.95 | 1.49 | 0.30 | 1.05 | |
| Total | RR incidence | 2.04 | 1.18 | 0.96 | 0.58 | 1.49 |
| RR mortality | 1.84 | 1.26 | 1.07 | 0.51 | 0.84 |
NL=the Netherlands, BE=Belgium, SW=Sweden, FI=Finland, IT=Italy, SP=Spain, CH=Switzerland.
RR incidence = rate ratio calculated as number of PCa detected / person years in the screening arm divided by the number of PCa detected / person years in the control arm.
RR mortality = rate ratio calculated as number of PCa deaths / person years in the screening arm divided by the number of PCa deaths / person years in the control arm.
Figure 1.
Panel A: the natural logarithm of the rate ratio (S/C) of the prostate cancer incidence versus the natural logarithm of the rate ratio (S/C) of prostate cancer mortality: All risk groups
Panel B: the natural logarithm of the rate ratio (S/C) of the prostate cancer incidence versus the natural logarithm of the rate ratio (S/C) of prostate cancer mortality: high risk and metastatic risk groups.
Sensitivity analysis restricted to the core age group showed similar results with a slope of 1.15 for all risk groups and 1.28 for the high and metastatic risk groups and R2 values of 0.78 and 0.91 res. A sensitivity analysis using the D’Amico risk groups (all ages) showed a slope of 1.15 for all risk groups and 1.12 for high risk and metastatic disease with associating R2 values of 0.83 and 0.85 respectively.
Although this analysis cannot show direct causal relations, results support the hypothesis that PSA screening reduces PCa mortality by detecting cancer at an earlier stage while still curable. It must be noted however that early detection is no guarantee for cure. This is reflected in the RR of PCa mortality in the low and intermediate risk group where although screening led to earlier diagnosis, cancer was still detected too late in some of these men. PCa death in the S-arm was increased in these risk groups due to enrichment by men at higher risk as compared to the C-arm.
The results of the current analysis are in line with a similar analysis based on ERSPC Rotterdam data where it was found that 94% of the changes in PCa mortality could be explained by changes in PCa incidence [6]. In addition, a recent analysis of PCa incidence by risk category at diagnosis across the study arms in ERSPC showed a reduction in metastatic disease at diagnosis in the screening arm, preceding mortality reduction by almost 3 year confirming that this decrease in metastatic disease at diagnosis is the major determinant of the PCa mortality reduction in ERSPC [7].
These findings do however not open the way to unrestricted PSA based screening for PCa. A balance between harm and benefit needs to be found where it must be realized that the severity of harm depends on multiple factors such as e.g. the intensity of testing and age to start and stop. Looking at the ample data available related to PCa screening it is obvious that there is no one-size-fits-all approach, indeed, not even a one-size-fits-most approach. PCa requires an individual approach to control harm and maintain benefit [8]. Unfortunately, despite recommendations on individualized PSA based screening in well informed men to avoid misuse leading to excessive harm [9], daily clinical practice shows the opposite. Men that will never benefit are tested repeatedly; conversely men that might benefit are not tested at all [10]. Neither scenario is the way to go; they lead to an unfavorable harm-benefit ratio. It is obvious that only by stopping misuse of the PSA test, we can prevent the loss of a screening test that has the potential to bring benefit to many men.
Acknowledgements
Most funding for ERSPC was obtained from national cancer research funding agencies, European funding in the form of Framework programmes, some private sponsors, and an unconditional grant of the former Beckman/Hybritech company. Dr. Leonard Bokhorst and Dr. M.J.Roobol’s work on this paper was supported by the Prostate Cancer Research Foundation Rotterdam (SWOP).Dr. Sigrid Carlsson’s work on this paper was supported in part by a Cancer Center Support Grant from the National Cancer Institute made to Memorial Sloan Kettering Cancer Center (P30 CA008748). Dr. Carlsson is also supported by a post-doctoral research grant from AFA Insurance.
Footnotes
Conflicts of Interest: M. Kwiatkowski reports personal fees from Astellas (advisory board), Myriad Genetics (lecture and consulting fees), Roche (lecture fee), Janssen (advisory board/congress support), outside the submitted work. Dr. S.V. Carlsson reports travel support from Sanofi-Aventis, outside the submitted work. Dr. S. Moss reports grants from the prostate cancer research foundation (SWOP), during the conduct of the study. Dr. Auvinen reports personal fees from Epid research, personal fees from MSD, outside the submitted work. Dr. Roobol is advisory board member for OPKO health.
Contributor Information
Leonard P. Bokhorst, Department of Urology, Erasmus University Medical Center, Rotterdam, The Netherlands
Marco Zappa, Unit of Clinical and Descriptive Epidemiology, ISPO, Florence, Italy.
Sigrid V. Carlsson, Department of Surgery (Urology Service), Memorial Sloan Kettering Cancer Center, New York, USA” and “Institute of Clinical Sciences, Sahlgrenska Academy at Gothenburg University, Gothenburg, Sweden
Maciej Kwiatkowski, Department of Urology, Kantonsspital Aarau,Aarau, Switzerland.
Louis Denis, Oncology Centre Antwerp, Antwerp, Belgium.
Alvaro Paez, Department of Urology, Hospital Universitario de Fuenlabrada, Madrid, Spain.
Jonas Hugosson, Department of Urology, Sahlgrenska Academy at Goteborg University,Goteborg, Sweden.
Sue M. Moss, Centre for Cancer Prevention, Queen Mary University of London, London, UK
Anssi Auvinen, School of Health Sciences, University of Tampere, Tampere, Finland.
Monique J. Roobol, Department of Urology, Erasmus University Medical Center, Rotterdam, the Netherlands
References
- 1.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014 Dec 6;384(9959):2027–35. doi: 10.1016/S0140-6736(14)60525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry MJ. Screening for prostate cancer--the controversy that refuses to die. N Engl J Med. 2009 Mar 26;360(13):1351–4. doi: 10.1056/NEJMe0901166. [DOI] [PubMed] [Google Scholar]
- 3.Wolters T, Roobol MJ, Steyerberg EW, et al. The effect of study arm on prostate cancer treatment in the large screening trial ERSPC. Int J Cancer. 2010 May 15;126(10):2387–93. doi: 10.1002/ijc.24870. [DOI] [PubMed] [Google Scholar]
- 4.Bokhorst LP, Kranse R, Venderbos LD, et al. Differences in Treatment and Outcome After Treatment with Curative Intent in the Screening and Control Arms of the ERSPC Rotterdam. Eur Urol. 2015 Aug 68;(2):179–82. doi: 10.1016/j.eururo.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Autier P, Héry C, Haukka J, Boniol M, Byrnes G. Advanced breast cancer and breast cancer mortality in randomized controlled trials on mammography screening. J Clin Oncol. 2009 Dec 10;27(35):5919–23. doi: 10.1200/JCO.2009.22.7041. [DOI] [PubMed] [Google Scholar]
- 6.Bokhorst LP, Venderbos LD, Schröder FH, Bangma CH, Steyerberg EW, Roobol MJ. Do Treatment Differences between Arms Affect the Main Outcome of ERSPC Rotterdam? J Urol. 2015 Aug 194;(2):336–42. doi: 10.1016/j.juro.2015.02.045. [DOI] [PubMed] [Google Scholar]
- 7.Buzzoni C, Auvinen A, Roobol MJ, et al. Metastatic Prostate Cancer Incidence and Prostate-specific Antigen Testing: New Insights from the European Randomized Study of Screening for Prostate Cancer. Eur Urol. 2015 Nov;68(5):885–90. doi: 10.1016/j.eururo.2015.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roobol MJ, Carlsson SV. Risk stratification in prostate cancer screening. Nat Rev Urol. 2013 Jan;10(1):38–48. doi: 10.1038/nrurol.2012.225. doi: 10.1038/nrurol.2012.225. Epub 2012 Dec 18. Review. Erratum in: Nat Rev Urol. 2013 May;10(5):248. [DOI] [PubMed] [Google Scholar]
- 9.EAU Guidelines on prostate Cancer. Mottet N, et al. [assessed at February 7, 2016];2015 http://uroweb.org/wp-content/uploads/09-Prostate-Cancer_LR.pdf.
- 10.Roobol M. Perspective: Enforce the clinical guidelines. Nature. 2015 Dec 17;528(7582):S123. doi: 10.1038/528S123a. [DOI] [PubMed] [Google Scholar]