Abstract
Progress in understanding the genetic etiology of autism spectrum disorders (ASD) has fueled remarkable advances in our understanding of its potential neurobiological mechanisms. Yet, at the same time, these findings highlight extraordinary causal diversity and complexity at many levels ranging from molecules to circuits and emphasize the gaps in our current knowledge. Here we review current understanding of the genetic architecture of ASD and integrate genetic evidence, neuropathology and studies in model systems with how they inform mechanistic models of ASD pathophysiology. Despite the challenges, these advances provide a solid foundation for the development of rational, targeted molecular therapies.
Over the last few decades, technological and methodological advances in genetics and genomics have permitted the identification of mutations that are involved in thousands of rare Mendelian conditions and in the etiology of more common, complex diseases1–7 (http://www.ebi.ac.uk/gwas/). In this regard, genetic findings have played a major role in neurodevelopmental disorders, such as ASD, for which many contributing genes have been identified (Fig. 1), providing a platform for unraveling the causal chain of events that result in ASD.
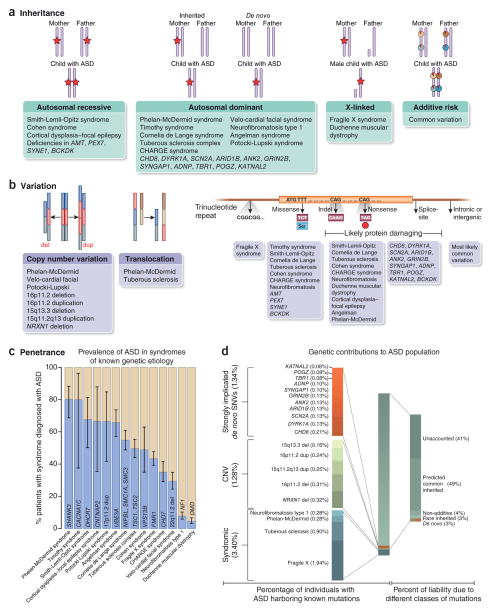
Figure 1.
Genetic architecture of autism spectrum disorders. (a) The inheritance patterns of syndromes with known genetic etiology and high incidence of autism, as well as that of genes recently identified to be associated with autism. The red stars indicate a causal allele and the red pie charts indicate a small proportion of risk. Most dominant disorders show de novo inheritance. Autosomal recessive, autosomal dominant and X-linked inheritance patterns best fit a major gene model, whereas a polygenic model is best represented by additive risk. (b) The types of genetic variation (left and middle) and the developmental disorders (right) associated with autism. Genes that have been associated with ASD are also indicated. (c) The penetrance of known syndromic mutations summarized from multiple studies. 95% binomial proportion confidence intervals, based on Wilson’s score interval, are shown. (d) The percentage of individuals with ASD harboring known mutations, as well as the percentage of liability from different classes of mutations (taken from ref. 57). The percentage variance in liability measures the contribution of a particular variant or class of variants relative to the population variance in a theoretical variable called liability. Liability is a continuous and normally distributed latent variable that represents each individual’s risk (both genetic and environmental) for developing a disease266. Notably, percentage variance in liability is directly dependent on the frequency of the variant and the effect size of the variant, and it is inversely dependent on the frequency of the disease in the population. References for this figure are found in Supplementary Table 1.
The challenges in understanding ASD are many, ranging from defining ASD’s heritable genetic components and understanding ASD risk more completely in individuals to determining whether the probable hundreds of different genetic forms of ASD might converge into a tractable set of targetable pathways for treatment8,9. Additionally, given ASD’s clinical and genetic heterogeneity, it is perhaps not surprising that no common macroscopic or microscopic neuropathology is recognized and that no specific brain region or cell type is uniquely implicated9–11 (Fig. 2). Nevertheless, the identification of veritable genetic risk factors provides a solid mechanistic grounding on which to base therapeutic development. Here we start by reviewing the clinical features of the syndrome and providing a broad overview of genetic findings. We then describe how mouse and in vitro human stem cell–based models can advance mechanistic understanding. Finally, we highlight the evidence for the most prevalent neurobiological models that attempt to bring together diverse genetic findings, molecular pathways and model systems to develop an evidence-based theoretical framework for understanding ASD.
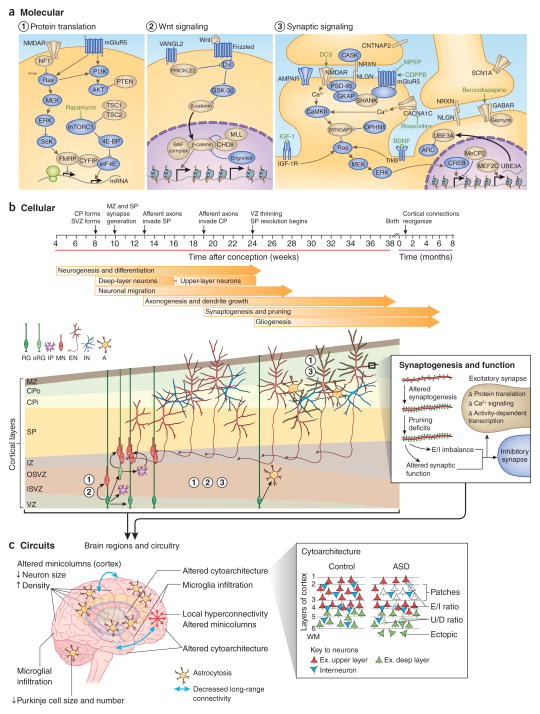
Figure 2.
Convergent neurobiological mechanisms in ASD. Normal brain development requires the generation and positioning of the correct number and type of cells, the growth and targeting of neuronal processes, and the formation of the precise number and type of synapses. (a) These events are regulated by molecular pathways in development. Genes within these pathways for which there is genetic evidence for a link to ASD18 (Fig. 1), including from our meta-analysis of SNVs and CNVs (compiled from refs. 43,44,73,74), are colored in gold. Chemical compounds that reverse behavioral or cellular ASD phenotypes in model systems are indicated in green font near their predicted site of action. (b) The cellular events leading to changes in the higher-order organization of the brain, including disruption of fetal cortical development and synaptic function. The cortical laminae are depicted from early fetal to neonatal stages (not to scale). The numbers indicate the molecular pathways important at each stage of development. (c) The widespread pathology10 and functional phenotypes observed in ASD, including altered brain growth trajectories, altered cortical cytoarchitecture (red triangles indicate excitatory upper layer neurons; green triangles are excitatory deep-layer neurons; blue triangles are interneurons; numbers indicate cortical layers; WM, white matter) and connectivity, may arise from combined deficits in neurogenesis, cell fate, neuronal migration and morphogenesis during fetal development and dysregulated synaptic function, possibly in combination with reactive microglia infiltration and astrocytosis. RG, radial glia; oRG, outer radial glia; IP, intermediate progenitor; MN, migrating neuron; EN, excitatory neuron; IN, interneuron; A, astrocyte; E/I, excitatory or inhibitory neuron; U/D, upper-layer or deep-layer neuron. MPEP, 2-methyl-6-(phenylethynyl)-pyridine; CDPPB, 3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide; DCS, D-cycloserine; IGF1, insulin-like growth factor 1. VZ, ventricular zone; ISVZ, inner subventricular zone; OSVZ, outer subventricular zone; IZ, intermediate zone; SP, subplate; CPi, inner cortical plate; CPo, outer cortical plate; MZ, marginal zone.
Clinical overview
The Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (ref. 12) defines ASD by deficits in two core domains—social interaction and communication, and repetitive, restrictive behaviors—with onset during early development. ASD unifies three previously separate but highly related diagnoses: autistic disorder, Asperger’s disorder and pervasive developmental disorder–not otherwise specified (PDD-NOS). Although prevalence estimates in the early 1990s were on the order of 1 in 1,000, they have consistently increased and are presently 1 in 68 children under 8 years of age in the USA13. There are substantial differences in ASD prevalence between the sexes (1 in 42 for males; 1 in 189 for females), suggesting mediation by as yet unknown biological factors14,15. ASD also occurs with frequent comorbidities, such as motor deficits (hypotonia, apraxia or motor delay), sleep abnormalities, gastrointestinal disturbances and epilepsy16. Sensory hyper- or hyposensitivity, previously listed as a frequent (~90%) comorbidity, is now integrated into the core diagnosis within the repetitive and restrictive domain. Another salient comorbidity is intellectual disability (ID), which is observed in ~35% of individuals with ASD and can markedly confound diagnostic instruments17,18. These comorbidities also present challenges in disease modeling, as they can complicate assessment of core ASD behaviors in animal models and overlap with phenotypes observed in other neuropsychiatric disorders, such as schizophrenia, attention deficit–hyper-activity disorder (ADHD) and obsessive-compulsive disorder (OCD).
Current therapeutic options are predominantly restricted to behavioral interventions, which can be highly successful in a subset of patients, and, as such, early intervention is warranted19,20. The only FDA-approved drugs, risperidone, which is effective in treating aggressive and repetitive behaviors, and aripiprazole, which reduces irritability, are not directed at the core social deficits. We have few ways to prognosticate and stratify patients for treatment at this point. We also do not have any clear biomarkers, although tracking eye movements21 and electroencephalogram (EEG) parameters22 have shown promise. The hope is that understanding the underlying genetic risk and neurobiologically anchored disease mechanisms in individual patients will fuel therapeutic development and patient selection for the most appropriate treatments.
Genetic architecture of ASD
We are just starting to elucidate the genetic architecture of ASD (Box 1). In Figure 1, we highlight the specific risk genes that have been identified, the types of mutations, the patterns of inheritance and the contribution of these genes to ASD. A key early insight into the genetic basis of ASD came from the recognition that dozens of rare medical genetic syndromes with diverse modes of inheritance have high penetrance (Box 1) for ASD23–26. Each of these known syndromes are rare, and none are found in more than 1% of patients with ASD; however, collectively they are estimated to be found in ~5% of the total population of individuals with ASD (Fig. 1).
BOX 1.
Glossary of genetic terms
- Genetic architecture
the relative contributions of different forms of genetic variation.
- Penetrance
the proportion of mutation carriers who also are diagnosed with the disease or carry a given phenotype.
- WES
Whole-exome sequencing—reading only the genetic sequence that encodes for proteins in an organism.
- WGS
Whole-genome sequencing—reading the entirety of the genetic code of an organism.
- De novo mutation
A mutation that is present in the offspring but that was not inherited from either parent.
- SNV
Single-nucleotide variant—a rare (<1%) or common single-bp change in the genome.
- CNV
Copy-number variation—deletion or duplication of large genomic regions leading to changes in the number of copies of the genetic elements encoded within those regions.
- Polygenic model
a model that describes the genetic risk factors of a disease as many inherited variants, each of which contributes a small, additive risk for developing a disease.
- Oligogenic model
a model that describes the genetic risk factors of a disease as a few variants, each of which contributes a large risk for developing a disease.
- Major gene model
a model that describes the genetic risk factors of a disease as due to genetic variants, each of which contributes a large risk for developing a disease. One major gene mutation is typically considered sufficient to cause a disease in an individual.
- SNP
Single-nucleotide polymorphism—a single bp change that is common (>1%) in the population.
- Simplex family
a family in which only one individual is affected with a disease.
- Multiplex family
a family in which multiple individuals are affected with a disease.
Family and twin studies show that ASD is highly heritable27–32, and this has spurred genome-wide analyses of genetic variants using microarrays, whole-exome sequencing (WES) and, more recently, whole-genome sequencing (WGS) (Box 1). However, the most successful efforts in gene discovery so far have identified rare protein- disrupting genetic variants in the affected child that are not found in the healthy parents, which represent new, or de novo, copy-number variants (CNVs)33–41 or single–base pair mutations (single-nucleotide variants; SNVs) that have arisen in the germline18,42–49 (Box 1). These studies have shown that there is an overall enrichment in ‘likely gene-disrupting’ mutations (LGDs; nonsense, frameshift and splice site mutations that often result in production of truncated proteins) in individuals with ASD as compared to their healthy relatives or to other unaffected individuals. Furthermore, these studies have identified individual rare de novo mutations that show strong evidence for their involvement in ASD, including mutations in chromodomain helicase DNA binding protein 8 (CHD8) and dual-specificity tyrosine phosphorylation–regulated kinase 1A (DYRK1A), and a deletion or duplication of chromosome 16 (16p11.2) that encompass a ~600-kb region containing dozens of genes (Fig. 1 and Supplementary Table 1). Because the ASD-linked mutations are rare, comparing the difference in variant frequency at an individual gene in individuals with ASD versus the variant frequency in control subjects, which is a typical case-control design, while correcting for genome-wide comparisons of mutation rate has not yet reached statistical significance for the association of a mutation in individuals with ASD at any individual gene within current sample sizes. In general, current estimates of the significance of an association between a mutation and ASD are based on comparing the frequency of the observed mutations in patients to the expected rate at which null mutations would occur in that gene47,50,51. This has resulted in somewhat of a moving target for genetic significance estimates, as significance depends on the number of variants found in cases and controls, as well as the overall number of cases and controls. WES is not only allowing the discovery of rare de novo heterozygous mutations, but it has also identified rare recessive mutations that are inherited in consanguineous families52–55.
Genetic models
Several models have been presented to explain the observed familial aggregation patterns and recent genetic findings (Fig. 1). Polygenic risk models (Box 1) assume that there are many inherited variants contributing to ASD, each with a small effect that, in combination with environmental factors, result in an individual crossing a risk threshold to develop the disease56–59. In contrast, major gene models (Box 1) assume that either one highly penetrant rare mutation or a limited number of moderately to highly penetrant mutations (oligogenic) are sufficient to cause ASD60,61. An important instance of the major gene model is a unified theory of ASD inheritance and occurrence62,63 that groups families into two types: low risk and high risk. In the more prevalent low-risk group, children develop ASD due to de novo mutations62. Female children of low-risk families with de novo mutations are ‘protected’ by an as yet unidentified mechanism and are less likely to develop ASD. In high-risk families, unaffected mothers transmit mutations that have reduced penetrance in females in a dominant manner to multiple, predominantly male, affected children. The polygenic and major gene models are not mutually exclusive, and both have support in the literature, but the extent of their contribution to ASD is a subject of debate57,63.
Polygenic models are supported by multiple lines of evidence. Firstly, recurrence of ASD in families implies a strong inherited genetic component28,29. Secondly, the first-degree relatives of children with ASD show related phenotypes more than in the general population, such as social and behavioral differences64–66. Finally, heritability estimates using single-nucleotide polymorphism (SNP) (Box 1) data demonstrate that commonly inherited genetic variants (minor allele frequency > 0.05) or variants that are tagged by common genetic variants collectively explain a large proportion of the variance in susceptibility to ASD57,67,68, as in many other common, complex disorders67,69. However, a weakness of the polygenic model is that statistically significant and replicable commonly inherited variants have not yet been identified for ASD70. This is probably due to the limited statistical power using current sample sizes and study designs6,71,72.
The unified major gene model62,63 is supported by the significant increase in damaging de novo mutations found in subjects with ASD as compared to their unaffected siblings44,49. Further support for this model is seen in the phenomenon that there are more inherited SNVs that disrupt protein function in conserved genes transmitted from the mother to individuals with ASD than in unaffected siblings73. However, all pathogenic CNVs considered as a group have not yet shown evidence for enriched maternal transmission to probands74.
There are findings that are inconsistent with a major gene model. For example, if de novo mutations comprise the majority of genetic risk, then one expects monozygotic (MZ) twins (who share both germline de novo and inherited genetic risk factors) to be concordant for ASD more than twice as frequently as dizygotic (DZ) twins, who share on average only half of their inherited genetic risk factors. Evidence for this was demonstrated by early twin studies. However, larger, more recent studies show a DZ concordance rate that is higher than expected for de novo mutations that comprise the major contribution to ASD risk (reviewed in refs. 32,49). Similarly, if most cases of ASD were due to de novo mutations in the parental germline, then risk in relatives would be very low50. However, familial risk implies a strong inherited genetic component, consistent with the polygenic model described above29. Furthermore, estimates of the influence of de novo mutation on risk for ASD have been derived mostly from a sample of simplex families (Box 1), which may inflate the contribution of de novo mutations40,44,45,47,49. Estimates of the heritability of ASD on the basis of the amount of common genetic variation (as measured from SNPs) shared in unrelated affected individuals57,67,68 demonstrate that the collective commonly inherited genetic variants can explain a large proportion of the variance in predisposition of a population to ASD, supporting the polygenic model. It is also recognized that some predictions of a major gene model have not yet been rigorously tested; the model predicts dominant maternal transmission of highly penetrant alleles, but estimating the contribution of dominance effects from family or population data is difficult to separate from the contribution of interaction between different loci29,75.
After comparison of the currently available data for genetic association with ASD, the data fit a model in which the largest component of genetic risk derives from common genetic variants of an additive effect with a smaller, although clearly important, contribution from de novo and rare inherited variation57 (Fig. 1). This is relevant because presumptions about genetic architecture have important implications for future study design, nosology and treatment. For example, if de novo mutations provide the major contribution to ASD risk, then we should focus genetic discovery efforts in simplex families with parents (and unaffected siblings when available). On the other hand, common variation is most efficiently detected with large case-control association studies, whereas heritable rare-variant detection is best served by studying larger multiplex families76 (Box 1).
With regards to nosology, highly penetrant variants may be very useful for defining subtypes of ASD77. For example, LGDs in CHD8 and DYRK1A have been found in individuals with ID and ASD but as yet have never been observed in unaffected individuals, indicating high penetrance of these mutations43,44. Patients with these rare mutations were then extensively phenotyped to identify distinct syndromic subtypes78–80. In contrast, individual common variants indicate only a small risk for disease and cannot be used for diagnosis. It is possible that the aggregate risk from common variants can be used as a part of the classification tools for diagnosis and subtype definition within and across disorders after confounders, such as population structure, are properly taken into account81–85.
Finally, the complexity of therapy development is probably proportional to the number of targetable biological pathways in the population, not the number of genetic insults identified. Although the polygenic model predicts a large number of loci to be associated with ASD, drug development may be simplified if convergent targetable pathways are identified. Conversely, if many different pathways are associated with ASD, then many different treatments may be necessary to alleviate the burden of disease across the population. Current evidence from known mutations does suggest significant convergence in the pathways in which the mutations are found, but the full extent of convergence will be better defined after further clarification of the genetic landscape8,18,86–89. Notwithstanding these challenges, the identification of genetic variants provides a clear causal foothold into the underlying biology of ASD18,90.
Neurobiological models and mechanisms of ASD
Genetic advances have fueled the generation and characterization of numerous mouse models of ASD (Table 1 and Supplementary Table 2), the major strengths and limitations of which are summarized in Box 2 (refs. 91,92). A variety of established assays are now considered standard assessments for ASD-related behavioral phenotypes93 (Box 2). It is notable that the majority of mouse models for monogenic forms of ASD have social impairment or repetitive behaviors; however, these core features of ASD and additional comorbidities, such as motor dysfunction, hyper-activity and anxiety, vary widely (Table 1 and Supplementary Table 2). Despite their potential limitations (Box 2), mouse models have been valuable in translating genetic findings and have provided evidence for shared molecular pathways and phenotypes in ASD. Primate models or invertebrates have not yet been widely used to model ASD, but they are complementary to mouse models92. Nonhuman primates are expected to more closely model complex behavior and higher cortical functions, whereas zebrafish and invertebrates offer efficient, higher-throughput genetic manipulation92,94,95.
Table 1.
Mouse models of ASD
| Molecular function | Mouse model | Social interaction |
Social communi- cation |
Repetitive behavior |
Other symptoms | Molecular, cellular and circuit phenotypes |
Treatment |
|---|---|---|---|---|---|---|---|
| Multiple | dup 15q11-q13 (refs. 267,268) | Impaired | ↓Calls | Behavioral inflexibility | NA | Altered serotonergic signaling, ↑spine dynamics | NA |
|
| |||||||
| Transcriptional regulator | Tbr1 HT | Impaired | Impaired STFP | Behavioral inflexibility | CTA defects, learning deficits | Axonal projection defects in amygdala ↓NMDAR function |
DCS (adult)170 clioquinol (A)269 |
|
| |||||||
| Translational regulator | Fmr1 KO255 | Impaired | ↓Calls | Hand flapping | PPI of startle, audiogenic seizure, learning deficits | ↑mGluR function, immature protrusion | MPEP (A)233,237 |
| Learning impairment | PI3K signaling, ↑spine density, impaired AMPAR-mediated synaptic plasticity | 5-HT and DA compound (A)270,271 | |||||
| Audiogenic seizure | Hypersensitivity to ERK1/2 pathway activation, ↑protein synthesis | SL327 (A)272 | |||||
| ↓Calls | NA | ↑Fetal or early postnatal GABA and Cl−, abnormal EEG | Bumetanide, oxytocin (P13-15)251 | ||||
| Tsc1 HT, Tsc1Cb KO | Impaired | ↑Calls | Grooming, behavioral inflexibility | Ataxia | Cerebellar deficits | Rapamycin (P7)183 | |
| Tsc2 HT | Impaired | ↑Calls | Increased marble burying | Lethality, learning deficits | Brain enlargement, hyperactive mTOR signaling, autophagy deficiency | Rapamycin (A)144,240 | |
| Pten cKO | Impaired | NA | NA | Learning deficits, seizure, anxiety | Macrocephaly, cellular hypertrophy, PI3K pathway hyperactivation | Rapamycin (4–6 weeks)114,184 | |
|
| |||||||
| Neuron-glia interaction, K+ channel clustering | Cntnap2 KO | Impaired | ↓Calls | Grooming | Seizure, hyperactivity | ↓Interneuron number, abnormal neuronal migration | Risperidone (A)109 |
| Impaired | Grooming | Hyperactivity | ↓Oxytocin neurons | Oxytocin (P7–P21; A)249 | |||
|
| |||||||
| Na+ channel | Scn1a KO | Impaired | NA | Grooming | Seizure, learning impairment | ↓GABAergic interneuron firing | Clonazepam (A)239 |
|
| |||||||
| Synaptic adhesion molecule | Nrxn1a KO273,274 | Impaired, ↑ aggression in males | NA | Grooming | Anxiety, sensory-gating deficits, motor learning | ↓Glutamatergic trans. and synaptic density | NA |
| Nlgn3 R451C KI275,276 | Impaired | ↑Calls | NA | Enhanced learning | Context-dependent impaired glutamatergic and GABAergic trans | NA | |
| Nlgn3 KO277 | Impaired | ↓Calls | Normal behavioral flexibility, Stereotyped motor routine | Hyperactivity | ↓Brain volume, cerebellar deficit | NA | |
| ↓GABAergic trans. in D1-MSN in NAc | NLGN3 expression in D1-MSN211 | ||||||
| Nlgn4 KO278 | ↓Impaired, aggression | ↓Calls | Normal | NA | ↓Brain volume | NA | |
|
| |||||||
| Synaptic scaffolding molecule | Shank2 exon7 KO279 | Impaired | ↓Calls, pattern change | Grooming | Hyperactivity, anxiety | ↑NMDAR function, ↑LTP | NA |
| Shank2 exons 6–7 KO | Impaired | ↓Calls | Jumping | Hyperactivity, anxiety | ↓NMDAR function, ↓LTP and LTD | CDPPB, DCS (A)232 clioquinol (A)269 |
|
| Shank3B KO210 | Impaired | NA | Grooming | Anxiety | Striatal dysfunction | NA | |
| Shank3 exons 4–9 KO280 | Impaired | Pattern change | Grooming | Learning deficits | ↓Activity-dependent AMPAR distribution and LTP | NA | |
| Shank3 HT281 | Impaired | ↓Calls | NA | Motor coordination | ↓Glutamatergic trans. by presynaptic mechanism, ↓LTP | IGF1 (P13–P28)177 | |
| Shank3+/ΔC (ref. 282) | Impaired | NA | Grooming | NA | ↓NMDAR function, Rac1, PAK, cofilin signaling defects, | TAT–p-cofilin peptide (A) | |
| Impaired | Grooming | F-actin dysregulation in PFC | CA-Rac1 (A) | ||||
Selected mouse models of ASD organized by the molecular function of the mutated gene. Phenotypes rescued by tested therapeutic or gene re-expression strategies are in bold, whereas non-rescued phenotypes are italicized. Non-bold or non-italicized phenotypes were not tested in rescue experiments. Genetic evidence for each modeled variant in ASD, if available, can be found in the listed reference. Up and down arrows signify an increase or decrease in the measured phenotype, respectively. STFP, social transmission of food preference; CTA, conditioned taste aversion; PPI, prepulse inhibition; A, adult; DCS, D-cycloserine; MPEP, 2-methyl-6-(phenylethynyl)-pyridine; PI3K, phosphoinositide 3-kinase; 5-HT, 5-hydroxytryptamine (serotonin); DA, dopamine; SL327, α-[amino[(4-aminophenyl) thio]methylene]-2-(trifluoromethyl)benzeneacetonitrile; P, postnatal day; EEG, electroencephalogram; D1-MSN, D1-dopamine receptor–expressing medium spiny neuron; NAc, nucleus accumbens; LTP, long-term potentiation; LTD, long-term depression; trans., transmission; CDPPB, 3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl) benzamide; IGF1, insulin-like growth factor 1; TAT-p-cofilin, HIV TAT domain conjugated with phospho-cofilin; CA-Rac1, constitutively active Rac1; PFC, prefrontal cortex; KO, knockout; cKO, conditional knockout; KI, knock-in; HT, heterozygous.
BOX 2. Modeling ASD with mice.
Mouse models provide an experimental platform for studying ASD at multiple levels, including molecular, cellular, circuit and behavioral analyses, and offer one of the few systems in which behavioral abnormalities and reversal by potential therapeutics can be tested before translating them to humans. A variety of established assays are now considered standard assessments for ASD-related behavioral phenotypes in mouse models93. The juvenile social interaction and three-chamber test, in which the time spent with a conspecific versus a novel object is measured, are widely used in both juvenile and adult mice to assess social interaction and social novelty recognition and is presumed to test behavioral correlates of social deficits in humans. The ultrasonic vocalization (USV) test measures the frequency and properties of vocal communication in multiple settings, including separation of pups from dams or adult males interacting with estrous females. Motor stereotypies, such as repetitive grooming, jumping and digging, are assessed by the marble-burying assay or home-cage behavioral analysis. Human correlates for restricted patterns of behavior and perseverance are tested by the alternating T-maze and reversal learning in the Morris water maze.
One serious challenge in neuropsychiatric disease is that the circuitry underlying social behaviors in most model systems or their human parallels are unknown. Moreover, many relevant phenotypes in humans are assessed via patient report, whereas internal states can only be inferred from outward behavior in animal models. Although these comorbidities are also observed in individuals with ASD, they confound interpretation. Sociability can be impaired by sensory dysfunction, fear and anxiety, learning deficits and abnormal locomotor activity, and similar cross-modal effects can influence repetitive behavior. Therefore, it is important to perform a full battery of behavioral tests, rather than to evaluate only core ASD-associated behaviors. Rigorous assessment of construct validity (whether a model recapitulates the genetic variant or mechanism of disease as in individuals with ASD) and convergent validity (whether application of more than one test in a given domain yields a high correlation among tasks) is critical to interpreting animal model behavioral data91.
| Advantages and limitations of mouse models
| |
|---|---|
| Advantages | Limitations |
|
|
In parallel, advances in stem cell biology in the past decade make it possible to generate and study human neurons and their development92. Differentiation into functional neurons that may model phenotypes of ASD has been shown to be possible from human embryonic stem cells (hESCs), human induced pluripotent stem cells (hiPSCs) and primary human neural progenitor cells (phNPCs) (Table 2 and Supplementary Table 3), although limitations exist (Box 3). Together, human neural stem cell models suggest that abnormalities in neurogenesis, cell fate, neuronal morphogenesis and synaptic function contribute to the pathogenesis of ASD (Table 2 and Supplementary Table 3). Transcriptomic studies in these models point to dysregulation in specific molecular processes that may be driving pathogenesis, including chromatin modifications, RNA-splicing, Wnt signaling and Ca2+ signaling96–99. A small number of drugs, including insulin-like growth factor 1 (IGF1) and roscovitine, have been used to reverse phenotypes associated with Rett syndrome, Timothy syndrome and Phelan-McDermid syndrome (PMDS) in hiPSC models99–102 (Table 2). Although promising, human in vitro studies using neurons derived from stem cells have focused on syndromic ASD variants, and thus, modeling of idiopathic ASD103 and ASD-associated de novo variants will be crucial to obtain a comprehensive picture of phenotypic overlap and potential, convergent disease mechanisms. A further challenge to in vitro studies is that it is not currently certain what the most relevant cellular and physiological ASD phenotypes are that need to be modeled in vitro.
Table 2.
Human in vitro models of ASD
| Syndrome | Key gene | Modeled mutation | Cell model | Brain region or cell type | Cell maturity | Cellular phenotype(s) | Suggested mechanism | Rescue or treatment |
|---|---|---|---|---|---|---|---|---|
| ASD98 | CHD8 | CHD8 loss of function (shRNA) | iPSC | Not defined | NPC | Dysregulated expression of genes related to neuronal development (RNA-seq) | CHD8 regulates different sets of genes associated with ASD by direct and indirect mechanisms | NA |
| PMDS102 | SHANK3 | 22q11.3 | iPSC | Forebrain neurons | 3 weeks, synapses, electrical activity (APs, sEPSC, sIPSCs) | ↓neuron production, = resting membrane potential, capacitance and APs, ↑input resistance, ↓amplitude and frequency of sEPSCs, ↓AMPA and NMDA EPSC amplitude, = IPSC amplitude and frequency, ↓AMPA and NMDA receptors. (WB), ↓currents from focal AMPA or NMDA application, but not GABA, ↓excitatory synapse density, ↓SHANK3 expression | Reduced SHANK3 expression leads to excitatory synapse dysfunction. IGF1 promotes synapse maturation | SHANK3 expression: sEPSC amplitude and frequency, evoked AMPA (all cells) and AMPA + NMDA (partial) EPSC; IGF1: excitatory synapse density, sEPSC amplitude and frequency, evoked EPSC amplitude (AMPA or NMDA), focal AMPA application (partial), focal NMDA application (full), input resistance |
| Rett100 | MECP2 | T158M, Q244X, R306C, 1155del32; MECP2-specific shRNA, MECP2 expression | iPSC | Not defined | NPC–8 weeks, synapses, dendritic spines, electrical activity (APs, sEPSCs, sIPSCs) | = cell death, = GABAergic neuron number, = cell cycle progression, ↓soma size, ↓dendritic spine density, ↓synapse number, ↓activity (Ca2+ imaging), ↓sEPSC and sIPSC frequency and amplitude | Dysregulation in MeCP2 expression | MECP2 expression: synapse number. IGF1: synapse number. Gentamycin: MeCP2 levels and synapse number |
| Timothy99 | CACNA1C | 1216G->A | iPSC | Cortical neurons (lower 85%/upper 15%) | NPC–45 d, electrical activity (APs) | = NPC proliferation, = neuron generation, = NPC migration, = AP amplitude or threshold, = resting membrane potential, input resistance or capacitance, wider AP at midpoint, ↑depolarization-induced Ca2+ rise, ↓in lower-layer neurons, ↑in upper-layer neurons, altered callosal/subcortical projection neuron production, ↑in TH+ neurons and catecholamine secretion | Decreased CACNA1C inactivation leads to Ca2+ signaling dysregulation, dysregulated gene expression and increased catecholamine synthesis in a subpopulation of cortical neurons | Roscovitine: proportion of TH+ neurons |
| Non-syndromic103 | Unknown | NA | iPSC 3D organoid | Dorsal forebrain neurons | Rosette–50 d, synapses, electrical activity (APs, sEPSCs) | = neuron generation, = AP threshold; upregulation of neural cell fate, axon guidance, synaptic and GABAergic genes, downregulation of non-neuronal genes, ↓ cell cycle length, ↑MAP2 density, ↑inhibitory synapse density, ↑GABAergic progenitor and neuron production | Altered cell fate leading to increased production of GABAergic progenitors and neurons caused by FOXG1 upregulation | FOXG1-specific shRNA: DLX1, DLX2 and GAD1 expression, GABAergic progenitor and neuron production |
Selected human in vitro syndromic and idiopathic models of ASD point to dysregulation in gene expression, neurogenesis and cell fate, and synaptic function in ASD. Rescued phenotypes are listed under the specific treatment or genetic manipulation. Upward and downward arrows signify an increase or decrease in the measured phenotype, respectively. Equal sign signifies no change in that phenotype. iPSC, induced pluripotent stem cells; NPC neural progenitor cell; PMDS, Phelan-McDermid syndrome; AP, action potential; EPSC, excitatory postsynaptic current; IPSC, inhibitory postsynaptic current; sEPSC, spontaneous excitatory postsynaptic current; sIPSC, spontaneous inhibitory postsynaptic current; IGF1, insulin-like growth factor 1; WB, western blot. References for each model are included in the first column.
BOX 3. Human in vitro models of ASD.
The need for human models of brain development and function is supported by a growing list of differences between rodents and humans212,287, a poor track record in drug development92 and the scarcity of human tissue, especially representing the period of disease onset10. Human neural stem cells overcome the species barrier, provide a high-throughput experimental platform for drug discovery and phenotypic screening, and could potentially be used in isogenic cell-based therapies. To gain meaningful insights into disease mechanisms, the differentiation of neural progenitors into specific neuronal subtypes of sufficient maturity is necessary116,288; however, cell identity and maturity have not been consistently assessed in human neural stem cell models of ASD (Table 2 and Supplementary Table 3), with all but a few studies using markers to rigorously define the populations of the cells under study99,101–103,289,290. Human stem cell–derived neurons are immature, approaching fetal stages of development even after prolonged in vitro culture, thus limiting their potential to model synaptogenesis and synaptic function130,291–294. Unbiased genome-wide frameworks can be applied130 to measure the extent to which in vitro models recapitulate in vivo development and to assess their neuroanatomical identity and maturity. Thus, this and other studies295 point to the validity of using neural stem cells to model early stages of human corticogenesis that are predicted to be dysregulated in ASD88,296. Advances in 3D organoid culture systems, organotypic slice culture and cell engraftment into rodent models may provide avenues for studying cortical lamination, circuit connectivity and more mature stages, including synaptic function290,297–300.
| Advantages and limitations of human in vitro models
| |
|---|---|
| Advantages | Limitations |
|
|
Several molecular or cellular mechanisms of ASD pathophysiology have multiple lines of supporting evidence from studies in humans or model systems (Table 3). Most of these mechanisms are individually quite broad and considerable work is needed to refine these models to targetable molecular pathways. Furthermore, these mechanisms are not entirely distinct. Indeed, the same genes or molecular pathways contribute to several of these processes at different points during development (Fig. 2), and it is not always clear how early developmental dysfunction relates to later events. An important caveat is that we only have a limited knowledge of the specific genetic contributions to autism susceptibility, and study designs to date have presumably identified the low-hanging fruit or specific types of mutations (for example, de novo mutations with large effects), which may paint a skewed picture of the underlying biology. With these factors in mind we discuss the evidence for each, in turn, below.
Table 3.
Evidence for distinct neurobiological mechanisms in ASD
| Brain region and mechanism | Supporting evidence | Caveats and limitations | Treatment potential |
|---|---|---|---|
| Neocortex | |||
| Brain overgrowth |
|
|
|
| Altered cortical cytoarchitecture (neuron size, number, positioning and/or orientation) |
|
|
|
| Neuronal morphogenesis |
|
|
|
| Synaptogenesis | |||
| Synaptic dysfunction E/I imbalance |
|
|
|
| Cerebellum | |||
| Purkinje cell (PC) loss and dysfunction |
|
|
|
| Widespread | |||
| Neuron-glia signaling |
|
|
|
Neuropathological and neuroimaging findings discussed here were recently reviewed10. See the section titled ‘Neurobiological models and mechanisms of ASD’ for additional references and detailed information on the genetic evidence and the function of specific genes involved in each biological process. hiPSC, human induced pluripotent stem cells; MRI, magnetic resonance imaging; PET, positron emission tomography; FXS, fragile X syndrome; IGF1, insulin-like growth factor 1; PC, Purkinje cell.
Altered fetal cortical development
Both human genetic evidence and post-mortem studies indicate that ASD can be caused by dysregulation of fetal cortical development10,87–89. Neuropathological studies, albeit with small cohorts (fewer than 36 individuals per study), have identified a number of cortical abnormalities in individuals with ASD that may be caused by errors in cortical development—including decreased neuron size, increased neuron number, ectopic cells, misoriented pyramidal neurons, irregular lamination, reduction in white matter tracks and dendritic abnormalities10 (Fig. 2). A few small studies consistently report narrower and more densely packed cortical minicolumns (a basic processing unit of cortical circuits)104. Recently, patches of cortical cells lacking specific laminar markers were observed in a large proportion of ASD cases within a small cohort105, although the neurodevelopmental process that this abnormality corresponds to is unknown. Furthermore, smaller brain size at birth followed by overgrowth during childhood is a widely reported phenotype for individuals with ASD106, although its anatomical or cellular basis also remains undefined.
Studies in model systems have established a role for genetic mutations associated with major syndromic forms of ASD—including mutations in fragile X mental retardation 1 (FMR1), tuberous sclerosis 1 and 2 (TSC1 and TSC2), phosphatase and tensin homolog (PTEN), contactin associated protein-like 2 (CNTNAP2) and chromodomain helicase DNA binding protein 7 (CHD7)—in fetal brain development107–111. A number of these syndromes are caused by loss-of-function variants in multiple genes that may converge in the mechanistic target of rapamycin (mTOR) pathway, which regulates cell proliferation, growth and neuronal morphogenesis (Fig. 2)112,113. Indeed, PTEN mutations cause fore-brain macrocephaly in both mice and humans, consistent with defects in corticogenesis51,114.
Similarly, ASD-associated genetic variants are enriched in genes involved in the Wnt pathway115 (Fig. 2), a regulator of the balance between radial glia self-renewal and neuronal differentiation, as well as dorsoventral patterning in the brain116,117. In mice, modulation of the Wnt pathway results in altered cortical neuronal production and ASD-like social deficits118–121. Moreover, many of the ASD-related rare de novo mutations that are predicted to disrupt gene function are in genes also thought to regulate or have cross-talk with canonical Wnt signaling and that are involved in chromatin modification and regulation of gene expression, such as CHD8, T-box brain 1 (TBR1), and members of the Brg1-associated factors (BAF) and mixed-lineage leukemia (MLL) complexes122–129 (Fig. 2). Recent work indicates that these genes are highly coexpressed in the human fetal brain during the period of neurogenesis (4–24 weeks after conception)88,130 and are expressed in both neural progenitors and newly born neurons131, again implicating altered fetal cortical development, as mutations in these genes are expected to affect cortical development.
The CNS function of the majority of these chromatin-modifying genes and transcriptional regulators is mostly unknown, with the exception of the BAF complex, a multi-subunit chromatin-remodeling complex that regulates neurogenesis and neuronal morphogenesis127. The ASD candidate gene SMARCC2, which encodes a subunit of the SWI/SNF chromatin-remodeling complex, was shown to regulate cortical thickness by modulating neurogenesis132. In addition, TBR1 has a role in cortical deep layer neuron generation, and mice lacking Tbr1 have impaired callosal and thalamocortical axon projections128. Knockdown of CHD8 expression in human neural progenitors causes downregulation of genes governing cell adhesion, neuronal differentiation and axon guidance, the Wnt pathway and genes related to chromatin modification that are enriched in de novo ASD variants98,133. Moreover, individuals with LGD mutations in CHD8 have macrocephaly, consistent with its reported function as a regulator of Wnt signaling, which is known to regulate brain size via regulation of neurogenesis80,134.
Synaptic dysfunction
Mutations in genes encoding excitatory and inhibitory synaptic cell-adhesion molecules (including neurexins43,73,74,135,136 and neuroligins137), excitatory synaptic scaffolding molecules (including the SH3 and multiple ankyrin-repeat domain (SHANK) proteins43,74,138,139), the excitatory glutamatergic receptor GRIN2B43,44,73 and inhibitory GABAergic receptor (GABAR) subunits43,73,140, and exonic deletions in the gene encoding the inhibitory synaptic scaffolding molecule gephyrin (GPHN)43,141, are associated with ASD in multiple unbiased and targeted sequencing studies. Neurotransmitter release regulators including the synaptotagmins43,45,47 and synapsins43,142,143 also harbor mutations, but so far there is less statistical support for their involvement in ASD.
Genetic evidence for synaptic dysfunction is also supported by neuropathological studies, providing suggestive evidence of increased spine density144,145, abnormalities in inhibitory function (such as reduced GABARs in the cortex and hippocampus146–149), abnormal mRNA expression of glutamate decarboxylase (GAD1 and GAD2) in the cortex and cerebellum150–153, and downregulation of interneuron markers (such as parvalbumin (PVALB) and somatostatin (SST)) in post-mortem brains89. Impaired glutamatergic and GABAergic transmission, as has been reported in several mouse models of ASD, can result in ASD-like behaviors that can be alleviated by modulators of AMPA receptor (AMPAR), NMDA receptor (NMDAR) and GABAAR (Table 1). Similarly, human neurons derived from individuals with PMDS, a syndromic form of ASD associated with deletions of SHANK3, have deficits in excitatory (AMPAR- and NMDAR-mediated) transmission102. Together, these studies provide evidence that dysregulation in synaptogenesis and synaptic transmission154 has a role in ASD (Fig. 2 and Tables 1 and 2).
The observation of defects in both glutamatergic and GABAergic synaptic function has led to the hypothesis that alterations in the excitatory/inhibitory (E/I) balance contribute to ASD. Consistent with this, mouse models with altered synaptic transmission or plasticity (activity-dependent changes in synaptic strength usually related to learning and memory155,156) that are outside the normal range in either direction exhibit social dysfunction. Indeed, directly increasing the E/I ratio in the medial prefrontal cortex (mPFC) of the brain, using optogenetic stimulation, led to impaired social interactions in mice157.
However, E/I imbalance is a broad concept that is frequently observed in a wide variety of brain disorders including epilepsy, Alzheimer’s disease and schizophrenia158–160; therefore, the contribution of E/I imbalance to ASD pathophysiology requires considerable refinement. Identifying spatiotemporal dynamics of synaptic dysfunction in multiple ASD model systems may help to delineate this question—for example, whether there is a critical period for an E/I imbalance that mediates ASD-associated behavior, or whether the E/I imbalance in ASD is circuit specific. Furthermore, an E/I imbalance may arise not only from changes in synaptic physiology but also from altered cell fate that can lead to abnormal proportions of inhibitory and excitatory cells, as evidenced by recent findings in human in vitro models103 (Table 2 and Fig. 2). This further highlights how early developmental abnormalities may have repercussions later on. It is also important to note that E/I imbalance studies have mainly been carried out in animal models, hence a detailed evaluation of when, where and how an E/I shift contributes to the ASD phenotypes in humans is warranted161–163.
Activity-dependent transcription and translation
In neurons, gene transcription and protein translation are dynamically regulated by neuronal activity, creating spatially or contextually restricted gene expression within subcellular compartments164,165. Disruptions in activity- dependent transcriptional regulators or their targets are associated with ASD. These include mutations in methyl-CpG–binding protein 2 (MeCP2)166 and calcium channel, voltage-dependent L-type, alpha 1C subunit (CACNA1C)167; de novo mutations in the neuronal activity-induced transcription factor myocyte enhancer factor 2C (MEF2C)168; abnormal imprinting and microdeletion of MEF2-regulated ubiquitin-protein ligase E3A (UBE3A) (which causes Angelman syndrome169) or duplication (dup) 15q11-q13 syndrome (which also encompasses UBE3A); and de novo mutations in TBR1, whose product is required for activity-dependent Grin2b expression170,171. Studies with iPSCs derived from individuals with Timothy syndrome demonstrate that CACNA1C regulates a network of genes involved in synaptic function96,99. Moreover, targets of Mef2 (such as activity-regulated cytoskeleton- associated protein, Arc172, and brain-derived neurotrophic factor, BDNF173), Mecp2 (such as BDNF174) and Tbr1 (such as Grin2b171) have established roles in synaptic transmission and plasticity, thus providing a point of mechanistic convergence between distinct genetic etiologies of ASD (Fig. 2). IGF1 treatment rescued core ASD behaviors that are present in untreated Mecp2y/− (refs. 175,176) and Shank3+/− mice177, as well as synaptic defects in iPSCs derived from subjects with PMDS102, presumably via cross-talk with activity-dependent signaling pathways175,178 (Fig. 2).
Mutations in TSC1 and TSC2, which encode canonical components of the mTOR pathway113, support dysregulation of neuronal translation in individuals with ASD179. Other ASD risk loci, including FMR1, which encodes fragile X mental retardation protein (FMRP) that also regulates neuronal translation180, and dup15q11-q13, which contains the FMRP interactor and translational repressor cytoplasmic FMR1-interacting protein 1 (CYFIP1)181,182, also suggest convergence on neuronal translational regulation (Figs. 1 and 2). Consistent with this, mice with disruptions in mTOR signaling or translation initiation have core ASD behaviors144,183–187 (Table 1), possibly through the modulation of translation of neuroligin 2 (Nlgn2), neurexin 1 (Nrxn1) and Shank3 through Fmrp188, a point of molecular convergence between synaptic function and translational regulation. Further, metabotropic glutamate receptor (mGluR) activation modulates FMRP-mediated translational inhibition and FMRP modulates AMPAR trafficking and mGluR- mediated LTD189, emphasizing the cross-talk between synaptic plasticity and translational control.
Activity-dependent transcription and translation also regulate synaptic pruning and stability190,191. Increased dendritic spine density in the temporal lobe of individuals with ASD has been reported, although the cohorts have been very small144,145. Mef2 and Fmr1 cooperatively modulate synapse elimination192, and Mef2 has a crucial role in mGluR5-mediated synapse elimination by stimulating the expression and dendritic translation of the Mef2 target gene Arc193. Spine-elimination deficits were also observed in Tsc2+/− mice, which were restored by treatment of the mice with the mTOR inhibitor rapamycin144.
Many genes known to be associated with an increased risk for ASD (which we refer to as ASD risk genes) are also predicted to be transcriptionally co-regulated by MEF2A, MEF2C and SATB homeobox 1 (SATB1), and to be translationally regulated via FMRP, further implicating activity-dependent gene regulation as a potential convergent mechanism in ASD pathogenesis88. How changes in synapse dynamics that are under the control of ASD risk genes lead to the specific behavioral deficits observed in model systems and humans with ASD is a critical area of investigation. One can speculate that even small changes in synaptic function and timing will preferentially disrupt the connectivity of higher-order association areas that mediate social behavior, which include the frontal-parietal, frontal-temporal and frontal-striatal circuits11,194. Identification of the spatiotemporal dynamics of transcriptional and translational regulation and the subsequent changes in micro- and macro-circuit connectivity will be necessary to link synaptic dysfunction to complex behavioral traits in individuals with ASD.
Altered neural circuitry
Human cognitive neuroscience and neuroimaging in ASD have been extensively reviewed elsewhere195–197. Neuroimaging and neuropathology studies in humans suggest that changes occur both in resting state network activity and alterations in macro-circuit connectivity within the cortex and in corticostriatal circuits198–203. The only study using systematic imaging phenotyping in ASD mouse models highlighted the parieto-temporal lobe, the cerebellar cortex, the frontal lobe, the hypothalamus and the striatum as the most affected regions, which were shared across many of the 26 models examined204; however, not all mouse models showed structural phenotypes, including mice lacking Cntnap2, which had normal gross anatomy.
The brain circuitry underlying social behavior in mice is not yet well defined. In addition to frontal circuits, cerebellar function has also been implicated in social behavior. Cell type–specific knockout of Tsc1 in cerebellar Purkinje cells was sufficient to elicit core ASD-like behavior in mice183, providing experimental evidence that cerebellar dysfunction can lead to ASD-like social deficits in mice. Systemic analysis of cerebellar function in five ASD mouse models has identified defects in cerebellum-dependent learning, although the functional implication of cerebellar dysfunction in core ASD behaviors remains to be identified205. In fact, developmental injury in cerebellar circuitry may increase ASD risk 36-fold, whereas adult injury does not lead to social dysfunction206, suggesting that the cerebellum may not be the direct neural correlate of social behavior, but that instead cerebellar injury during early development may lead to a cascade of long-term deficits in cerebellar-associated targets, leading to the core behavioral deficits observed in ASD. Therefore, comprehensive mapping of developmental circuit formation will be essential to finding the neural correlates of social behavior.
The amygdala is another candidate region that may be affected in ASD because of its role in modulating fear and social behavior202,207,208. Tbr1+/− mice have defective amygdala axonal projections and neuronal activation. Notably, direct infusion of D-cycloserine, a partial agonist of NMDAR, to basolateral amygdala restored social deficits of Tbr1+/− mice170, even though Tbr1 has an established role in deep-layer neuron generation and cortical lamination.
In contrast to social behavior, the neuroanatomical substrate for repetitive behavior is better understood in both mice and humans. Several lines of evidence suggest that striatal dysfunction is a neural substrate for repetitive behavior and motor routine learning in mice and in humans209. Mice lacking Shank3b showed striatal dysfunction, including striatal hypertrophy, and reduced corticostriatal excitatory synaptic transmission along with repetitive behavior210. Mice lacking Nlgn3 display stereotyped motor routines that are dependent on inhibitory transmission by D1-dopamine receptor–expressing medium spiny neuron (D1-MSNs) in the nucleus accumbens211.
How these mouse phenotypes relate to human circuits is not well understood, and because many of the implicated brain regions in humans, such as the frontal and temporal lobes, have undergone massive changes during primate evolution212, additional comparative studies, which may involve primate models in addition to mouse models, are needed to relate neuroanatomical traits to the candidate brain circuits implicated in ASD pathogenesis. Moreover, given the developmental stage–related manifestations of ASD, the temporal trajectory of neural circuitry abnormalities and the developmental disconnectivity in ASD warrants study. Recent advances in connectomics213,214 and optogenetics215,216 may help in delineating the functional neural correlates for core ASD-associated behaviors, thus providing therapeutic opportunities.
Dysregulated neuron-glia signaling and neuroinflammation
Another consistently reported observation in brains from individuals with ASD is the presence of activated microglia and astrocytosis in multiple brain regions (Fig. 2). Neuropathological and positron emission tomography (PET) imaging studies have identified microglial infiltration and activation in the frontal, prefrontal, cingulate, frontoinsular and visual cortices and in the cerebellum217–220. Astrocytosis has been observed in the frontal, parietal, cingulate and temporal cortices and in the cerebellum220–222. Alongside these observations, transcriptomic studies in post-mortem brains consistently find that genes enriched in activated microglia and astrocytes are upregulated in the cortex of brains from individuals with idiopathic ASD, and to a lesser extent in the cerebellum89,223. No studies have identified causal genetic variants associated with ASD in microglia- or astrocyte-specific genes, which combined with transcriptomic and genetic evidence suggests that this process is most likely a reaction or a secondary process coupled to underlying synaptic dysfunction89. However, the lack of primary genetic evidence for association with ASD does not reduce the value of these microglial or astrocyte pathways as potential therapeutic windows to explore. Notably, knockdown of chemokine (C-X3-C motif) receptor 1 (CX3CR1), which is not mutated in individuals with ASD, leads to a reduction in microglia, deficits in synaptic pruning and ASD-like behavioral and functional connectivity defects224,225. Thus, synaptic dysfunction in individuals with ASD could also arise from dysregulated synaptic pruning and homeostasis that is promoted by a vicious cycle of microglial and astrocyte upregulation. Because astrocytes and microglia regulate synaptic development and pruning226–228, this may provide another opportunity for therapeutic development. Mouse models have not been rigorously assessed for microglial activation, but given the observations in human brain, this should be done.
Therapeutic strategies
Currently, the following major molecular pathways have been primarily targeted in model systems to evaluate novel therapeutic strategies. The E/I imbalance hypothesis highlights glutamatergic and GABAergic receptor modulators as potential therapeutic strategies; roscovitine, mGluR5 antagonists and agonists, NMDAR agonists and GABAAR agonists have shown varying degrees of preclinical efficacy in mouse models, including the alleviation of social deficits or repetitive behavior (Table 1 and Supplementary Table 2)170,229–239. Translational inhibition by eukaryotic translation initiation factor (eIF) 4E and eIF4G interaction inhibitor (4EGI-1) and rapamycin have been effective in alleviating behavioral and neuronal phenotypes in models with perturbations in the mTOR pathway144,183–186,240–242. Transcriptional modulation through phosphatidylinositol 3-kinase (PI3K) and Ras signaling by IGF1 and BDNF also showed efficacy by rescuing physiological and behavioral abnormalities in some models, including in Mecp2-null mice175–177,243,244. Treatment with clenbuterol and fingolimod also alleviated behavioral deficits in Mecp2-null mice by increasing levels of BDNF and IGF1 (refs. 245,246). Treatment with oxytocin, a neuropeptide involved in the modulation of various aspects of social behavior247,248, ameliorated ASD-like social deficits in several mouse models249–251 and has been implicated in improving information transfer by modulating inhibitory transmission252. In addition to these genetic models, striking evidence for a role of the gut microbiome in brain development and function has been demonstrated. Oral treatment of mice with Bacteroides fragilis restored social behavioral abnormalities in maternal immune activation (MIA) models253, but the direct relevance to various forms of human ASD has not yet been established.
The lack of consistency in experimental findings between different laboratories and the genetic background effects of different mouse strains are important issues that should be addressed to increase the therapeutic utility of animal models254–256. Even when mouse models suggest a potential mechanism or therapeutic avenue, caution should be exercised in translating animal studies to humans. The efficacy of mGluR5 antagonists and oxytocin, which showed promise in mouse models, is uncertain following clinical trials in humans257–260. It is unknown whether these failures are due to inadequate trial design and outcome measures, the lack of appropriate target engagement or simply choosing the wrong target261. It should be noted that the outcome measurements used in clinical trials are often not the same as those in animal models. This emphasizes the importance of understanding the factors that lead to variable results. Social behavior is particularly vexing in this regard, as its environmental context and the state of the subject are so important, both in humans and in model organisms. In addition to genetic background effects, how the animals are housed and treated, when they are tested and who the examiner is can have profound effects on the outcome262. The development of objective scoring systems, as well as the identification of measurable biomarkers and endophenotypes (for example, EEG, magnetic resonance imaging (MRI) scans and molecular profiles) will help in more accurate cross-species assessment of the therapeutic effects.
Conclusions and future directions
Genetic evidence frames ASD not as a single disease but as a number of etiologically distinct conditions with diverse pathophysiological mechanisms that lead to similar behavioral manifestations. This is supported by mouse models, which demonstrate that multiple mechanisms can lead to parallel outward social deficits. At the same time, the convergence of risk variants in molecular pathways and the identification of common transcriptomic signatures in brains from subjects with ASD argue for an unexpected degree of convergence at the molecular and cellular levels. Genetic findings now provide a firm causal foundation on which to understand the relationship of molecular pathways with cellular and circuit dysfunction, and ultimately with behavior. Furthermore, although little is known about the mechanisms of increased male prevalence, genetic models provide a route for identifying and testing the role of potential female protective factors, which could provide new therapeutic opportunities14,263. Finally, evidence for several models based on maternal environmental factors, such as infection and the gut microbiome, are also growing253, but their definitive causal role in ASD and their relationship to genetic risk factors warrant further definition.
Work in animal models and in human cell lines supports some of the observations in post-mortem human brains and in clinical studies; yet we are far from parsimonious explanations. Microglial activation is variably observed in brains from individuals with ASD, along with a deficit in synaptic pruning. Conversely, a mouse model with a reduction in microglia displays deficits in synaptic pruning225. Understanding the role of glial cells in synaptic homeostasis and their adaptive or maladaptive roles in brains from subjects with ASD are thus important goals with significant therapeutic implications.
Interdisciplinary approaches combining genetics, functional genomics, experimental modeling and ultimately their integration into cohesive biological models, in line with those that are developing in cancer, may help drive therapeutic innovations264. The road ahead necessitates advances in each of these approaches. We must gauge where to put our resources for genetic discovery. Lessons from other common neuropsychiatric disorders suggest that large cohorts (>50,000 subjects) are needed to identify predicted common variants6. The success of identifying de novo variants should not occlude the efforts of identifying inherited variants. Shared resources, such as single-cell transcriptomes and expression quantitative trait loci (eQTL) studies at relevant epochs, will provide critical support to molecular pathway analysis that can bridge genetics, animal model and human studies.
Translating mechanistic understanding that is derived from model systems faces many challenges. One of them is understanding the optimal timing for treatment249. Model systems based on neurodevelopmental syndrome genes demonstrate the ability to reverse certain deficits in adults, providing important hope that treatment long after birth can be efficacious. Yet, given the developmental roles of many ASD risk genes, we must acknowledge that there may be critical periods for certain treatment modalities249,265. Another important avenue is developing human biomarkers that are robust and, optimally, have parallels in animal models. These are challenging problems to be faced with, but they underscore the extraordinary recent progress in defining both the causes and mechanisms of ASD and a number of plausible routes toward developing more effective treatments.
Acknowledgments
We thank members of the Geschwind laboratory for helpful discussions and critical reading of the manuscript. This work was supported by US National Institutes of Health (NIH) grants 5R37 MH060233 (D.H.G.), 5R01 MH094714 (D.H.G.) and K99MH102357 (J.L.S.), the California Institute for Regenerative Medicine (CIRM)–Broad Stem Cell Research Center (BSCRC) training grant TG2-01169 (L.d.l.T.-U.) and the Glenn–American Federation for Aging Research (AFAR) Postdoctoral Fellowship Program for Translational Research on Aging award 20145357 (H.W.).
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Supplementary Material
References
- 1.Altshuler D, Daly MJ, Lander ES. Genetic mapping in human disease. Science. 2008;322:881–888. doi: 10.1126/science.1156409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilissen C, et al. Genome sequencing identifies major causes of severe intellectual disability. Nature. 2014;511:344–347. doi: 10.1038/nature13394. [DOI] [PubMed] [Google Scholar]
- 3.Lee H, et al. Clinical exome sequencing for genetic identification of rare Mendelian disorders. J Am Med Assoc. 2014;312:1880–1887. doi: 10.1001/jama.2014.14604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Psychiatric GWAS Consortium Bipolar Disorder Working Group. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near. ODZ4 Nat Genet. 2011;43:977–983. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambert JC, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45:1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hindorff LA, et al. Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci USA. 2009;106:9362–9367. doi: 10.1073/pnas.0903103106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geschwind DH. Autism: many genes, common pathways? Cell. 2008;135:391–395. doi: 10.1016/j.cell.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geschwind DH. Genetics of autism spectrum disorders. Trends Cogn Sci. 2011;15:409–416. doi: 10.1016/j.tics.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen JA, Peñagarikano O, Belgard TG, Swarup V, Geschwind DH. The emerging picture of autism spectrum disorder: genetics and pathology. Annu Rev Pathol. 2015;10:111–144. doi: 10.1146/annurev-pathol-012414-040405. [DOI] [PubMed] [Google Scholar]
- 11.Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 12.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5. American Psychiatric Association; Arlington, Viginia, USA: 2013. [Google Scholar]
- 13.Autism and Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators. Prevalence of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63:1–21. [PubMed] [Google Scholar]
- 14.Werling DM, Geschwind DH. Understanding sex bias in autism spectrum disorder. Proc Natl Acad Sci USA. 2013;110:4868–4869. doi: 10.1073/pnas.1301602110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson EB, Lichtenstein P, Anckarsäter H, Happé F, Ronald A. Examining and interpreting the female protective effect against autistic behavior. Proc Natl Acad Sci USA. 2013;110:5258–5262. doi: 10.1073/pnas.1211070110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geschwind DH. Advances in autism. Annu Rev Med. 2009;60:367–380. doi: 10.1146/annurev.med.60.053107.121225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volkmar FR, McPartland JC. From Kanner to DSM-5: autism as an evolving diagnostic concept. Annu Rev Clin Psychol. 2014;10:193–212. doi: 10.1146/annurev-clinpsy-032813-153710. [DOI] [PubMed] [Google Scholar]
- 18.Geschwind DH, State MW. Gene hunting in autism spectrum disorder: on the path to precision medicine. Lancet Neurol. 2015;14:1109–1120. doi: 10.1016/S1474-4422(15)00044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogers SJ, et al. Autism treatment in the first year of life: a pilot study of infant start, a parent-implemented intervention for symptomatic infants. J Autism Dev Disord. 2014;44:2981–2995. doi: 10.1007/s10803-014-2202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasari C, Shire S, Factor R, McCracken C. Psychosocial treatments for individuals with autism spectrum disorder across the lifespan: new developments and underlying mechanisms. Curr Psychiatry Rep. 2014;16:512. doi: 10.1007/s11920-014-0512-6. [DOI] [PubMed] [Google Scholar]
- 21.Jones W, Klin A. Attention to eyes is present but in decline in 2- to 6-month-old infants later diagnosed with autism. Nature. 2013;504:427–431. doi: 10.1038/nature12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeste SS, Frohlich J, Loo SK. Electrophysiological biomarkers of diagnosis and outcome in neurodevelopmental disorders. Curr Opin Neurol. 2015;28:110–116. doi: 10.1097/WCO.0000000000000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zafeiriou DI, Ververi A, Dafoulis V, Kalyva E, Vargiami E. Autism spectrum disorders: the quest for genetic syndromes. Am J Med Genet B Neuropsychiatr Genet. 2013;162B:327–366. doi: 10.1002/ajmg.b.32152. [DOI] [PubMed] [Google Scholar]
- 24.Miles JH. Autism spectrum disorders—a genetics review. Genet Med. 2011;13:278–294. doi: 10.1097/GIM.0b013e3181ff67ba. [DOI] [PubMed] [Google Scholar]
- 25.Betancur C. Etiological heterogeneity in autism spectrum disorders: more than 100 genetic and genomic disorders and still counting. Brain Res. 2011;1380:42–77. doi: 10.1016/j.brainres.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 26.Gillberg C, Coleman M. The Biology of the Autistic Syndromes. Mac Keith Press; London: 1992. [Google Scholar]
- 27.Folstein S, Rutter M. Genetic influences and infantile autism. Nature. 1977;265:726–728. doi: 10.1038/265726a0. [DOI] [PubMed] [Google Scholar]
- 28.Hallmayer J, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68:1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandin S, et al. The familial risk of autism. J Am Med Assoc. 2014;311:1770–1777. doi: 10.1001/jama.2014.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bailey A, et al. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med. 1995;25:63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- 31.Lichtenstein P, Carlström E, Råstam M, Gillberg C, Anckarsäter H. The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. Am J Psychiatry. 2010;167:1357–1363. doi: 10.1176/appi.ajp.2010.10020223. [DOI] [PubMed] [Google Scholar]
- 32.Ronald A, Hoekstra RA. Autism spectrum disorders and autistic traits: a decade of new twin studies. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:255–274. doi: 10.1002/ajmg.b.31159. [DOI] [PubMed] [Google Scholar]
- 33.Sebat J, et al. Strong association of de novo copy-number mutations with autism. Science. 2007;316:445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szatmari P, et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39:319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marshall CR, et al. Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet. 2008;82:477–488. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bucan M, et al. Genome-wide analyses of exonic copy-number variants in a family-based study point to novel autism susceptibility genes. PLoS Genet. 2009;5:e1000536. doi: 10.1371/journal.pgen.1000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glessner JT, et al. Autism genome-wide copy-number variation reveals ubiquitin and neuronal genes. Nature. 2009;459:569–573. doi: 10.1038/nature07953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Itsara A, et al. De novo rates and selection of large copy-number variation. Genome Res. 2010;20:1469–1481. doi: 10.1101/gr.107680.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinto D, et al. Functional impact of global, rare copy-number variation in autism spectrum disorders. Nature. 2010;466:368–372. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levy D, et al. Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron. 2011;70:886–897. doi: 10.1016/j.neuron.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 41.Griswold AJ, et al. Evaluation of copy-number variations reveals novel candidate genes in autism spectrum disorder–associated pathways. Hum Mol Genet. 2012;21:3513–3523. doi: 10.1093/hmg/dds164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neale BM, et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 2012;485:242–245. doi: 10.1038/nature11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Rubeis S, et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014;515:209–215. doi: 10.1038/nature13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iossifov I, et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515:216–221. doi: 10.1038/nature13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iossifov I, et al. De novo gene disruptions in children on the autistic spectrum. Neuron. 2012;74:285–299. doi: 10.1016/j.neuron.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Roak BJ, et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012;485:246–250. doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanders SJ, et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012;485:237–241. doi: 10.1038/nature10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilman SR, et al. Rare de novo variants associated with autism implicate a large functional network of genes involved in formation and function of synapses. Neuron. 2011;70:898–907. doi: 10.1016/j.neuron.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanders SJ, et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron. 2011;70:863–885. doi: 10.1016/j.neuron.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gratten J, Visscher PM, Mowry BJ, Wray NR. Interpreting the role of de novo protein-coding mutations in neuropsychiatric disease. Nat Genet. 2013;45:234–238. doi: 10.1038/ng.2555. [DOI] [PubMed] [Google Scholar]
- 51.O’Roak BJ, et al. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science. 2012;338:1619–1622. doi: 10.1126/science.1227764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morrow EM, et al. Identifying autism loci and genes by tracing recent shared ancestry. Science. 2008;321:218–223. doi: 10.1126/science.1157657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strauss KA, et al. Recessive symptomatic focal epilepsy and mutant contactin-associated protein-like 2. N Engl J Med. 2006;354:1370–1377. doi: 10.1056/NEJMoa052773. [DOI] [PubMed] [Google Scholar]
- 54.Lim ET, et al. Rare complete knockouts in humans: population distribution and significant role in autism spectrum disorders. Neuron. 2013;77:235–242. doi: 10.1016/j.neuron.2012.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu TW, et al. Using whole-exome sequencing to identify inherited causes of autism. Neuron. 2013;77:259–273. doi: 10.1016/j.neuron.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee SH, et al. Estimating the proportion of variation in susceptibility to schizophrenia, captured by common SNPs. Nat Genet. 2012;44:247–250. doi: 10.1038/ng.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gaugler T, et al. Most genetic risk for autism resides with common variation. Nat Genet. 2014;46:881–885. doi: 10.1038/ng.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bulik-Sullivan BK, et al. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47:291–295. doi: 10.1038/ng.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wray NR, Visscher PM. Quantitative genetics of disease traits. J Anim Breed Genet. 2015;132:198–203. doi: 10.1111/jbg.12153. [DOI] [PubMed] [Google Scholar]
- 60.O’Roak BJ, et al. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat Genet. 2011;43:585–589. doi: 10.1038/ng.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schaaf CP, et al. Oligogenic heterozygosity in individuals with high-functioning autism spectrum disorders. Hum Mol Genet. 2011;20:3366–3375. doi: 10.1093/hmg/ddr243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao X, et al. A unified genetic theory for sporadic and inherited autism. Proc Natl Acad Sci USA. 2007;104:12831–12836. doi: 10.1073/pnas.0705803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ronemus M, Iossifov I, Levy D, Wigler M. The role of de novo mutations in the genetics of autism spectrum disorders. Nat Rev Genet. 2014;15:133–141. doi: 10.1038/nrg3585. [DOI] [PubMed] [Google Scholar]
- 64.Virkud YV, Todd RD, Abbacchi AM, Zhang Y, Constantino JN. Familial aggregation of quantitative autistic traits in multiplex versus simplex autism. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:328–334. doi: 10.1002/ajmg.b.30810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lyall K, et al. Parental social responsiveness and risk of autism spectrum disorder in offspring. JAMA Psychiatry. 2014;71:936–942. doi: 10.1001/jamapsychiatry.2014.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lowe JK, Werling DM, Constantino JN, Cantor RM, Geschwind DH. Social responsiveness, an autism endophenotype: genome-wide significant linkage to two regions on chromosome 8. Am J Psychiatry. 2015;172:266–275. doi: 10.1176/appi.ajp.2014.14050576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee SH, et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45:984–994. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klei L, et al. Common genetic variants, acting additively, are a major source of risk for autism. Mol Autism. 2012;3:9. doi: 10.1186/2040-2392-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maier R, et al. Joint analysis of psychiatric disorders increases accuracy of risk prediction for schizophrenia, bipolar disorder and major depressive disorder. Am J Hum Genet. 2015;96:283–294. doi: 10.1016/j.ajhg.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anney R, et al. Individual common variants exert weak effects on the risk for autism spectrum disorders. Hum Mol Genet. 2012;21:4781–4792. doi: 10.1093/hmg/dds301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gratten J, Wray NR, Keller MC, Visscher PM. Large-scale genomics unveils the genetic architecture of psychiatric disorders. Nat Neurosci. 2014;17:782–790. doi: 10.1038/nn.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Geschwind DH, Flint J. Genetics and genomics of psychiatric disease. Science. 2015;349:1489–1494. doi: 10.1126/science.aaa8954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krumm N, et al. Excess of rare, inherited truncating mutations in autism. Nat Genet. 2015;47:582–588. doi: 10.1038/ng.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pinto D, et al. Convergence of genes and cellular pathways dysregulated in autism spectrum disorders. Am J Hum Genet. 2014;94:677–694. doi: 10.1016/j.ajhg.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vinkhuyzen AA, Wray NR, Yang J, Goddard ME, Visscher PM. Estimation and partition of heritability in human populations using whole-genome analysis methods. Annu Rev Genet. 2013;47:75–95. doi: 10.1146/annurev-genet-111212-133258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sham PC, Purcell SM. Statistical power and significance testing in large-scale genetic studies. Nat Rev Genet. 2014;15:335–346. doi: 10.1038/nrg3706. [DOI] [PubMed] [Google Scholar]
- 77.Stessman HA, Bernier R, Eichler EE. A genotype-first approach to defining the subtypes of a complex disease. Cell. 2014;156:872–877. doi: 10.1016/j.cell.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ji J, et al. DYRK1A haploinsufficiency causes a new recognizable syndrome with microcephaly, intellectual disability, speech impairment and distinct facies. Eur J Hum Genet. 2015;23:1473–1481. doi: 10.1038/ejhg.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bronicki LM, et al. Ten new cases further delineate the syndromic intellectual disability phenotype caused by mutations in DYRK1A. Eur J Hum Genet. 2015;23:1482–1487. doi: 10.1038/ejhg.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bernier R, et al. Disruptive CHD8 mutations define a subtype of autism early in development. Cell. 2014;158:263–276. doi: 10.1016/j.cell.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jablensky A. Schizophrenia or schizophrenias? The challenge of genetic parsing of a complex disorder. Am J Psychiatry. 2015;172:105–107. doi: 10.1176/appi.ajp.2014.14111452. [DOI] [PubMed] [Google Scholar]
- 82.Skafidas E, et al. Predicting the diagnosis of autism spectrum disorder using gene pathway analysis. Mol Psychiatry. 2014;19:504–510. doi: 10.1038/mp.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arnedo J, et al. Uncovering the hidden risk architecture of the schizophrenias: confirmation in three independent genome-wide association studies. Am J Psychiatry. 2015;172:139–153. doi: 10.1176/appi.ajp.2014.14040435. [DOI] [PubMed] [Google Scholar]
- 84.Robinson EB, et al. Response to ‘Predicting the diagnosis of autism spectrum disorder using gene pathway analysis’. Mol Psychiatry. 2014;19:859–861. doi: 10.1038/mp.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Belgard TG, Jankovic I, Lowe JK, Geschwind DH. Population structure confounds autism genetic classifier. Mol Psychiatry. 2014;19:405–407. doi: 10.1038/mp.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Noh HJ, et al. Network topologies and convergent etiologies arising from deletions and duplications observed in individuals with autism. PLoS Genet. 2013;9:e1003523. doi: 10.1371/journal.pgen.1003523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Willsey AJ, et al. Coexpression networks implicate human midfetal deep cortical projection neurons in the pathogenesis of autism. Cell. 2013;155:997–1007. doi: 10.1016/j.cell.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Parikshak NN, et al. Integrative functional genomic analyses implicate specific molecular pathways and circuits in autism. Cell. 2013;155:1008–1021. doi: 10.1016/j.cell.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Voineagu I, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474:380–384. doi: 10.1038/nature10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pankevich DE, Wizemann TM, Altevogt BM. Improving the Utility and Translation of Animal Models for Nervous System Disorders: Workshop Summary. The National Academies Press; Washington, D.C: 2013. [PubMed] [Google Scholar]
- 92.Dolmetsch R, Geschwind DH. The human brain in a dish: the promise of iPSC-derived neurons. Cell. 2011;145:831–834. doi: 10.1016/j.cell.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Silverman JL, Yang M, Lord C, Crawley JN. Behavioral phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Watson KK, Platt ML. Of mice and monkeys: using nonhuman primate models to bridge mouse- and human-based investigations of autism spectrum disorders. J Neurodev Disord. 2012;4:21. doi: 10.1186/1866-1955-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McCammon JM, Sive H. Addressing the genetics of human mental health disorders in model organisms. Annu Rev Genomics Hum Genet. 2015;16:173–197. doi: 10.1146/annurev-genom-090314-050048. [DOI] [PubMed] [Google Scholar]
- 96.Tian Y, et al. Alteration in basal and depolarization-induced transcriptional network in iPSC-derived neurons from Timothy syndrome. Genome Med. 2014;6:75. doi: 10.1186/s13073-014-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fogel BL, et al. RBFOX1 regulates both splicing and transcriptional networks in human neuronal development. Hum Mol Genet. 2012;21:4171–4186. doi: 10.1093/hmg/dds240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sugathan A, et al. CHD8 regulates neurodevelopmental pathways associated with autism spectrum disorder in neural progenitors. Proc Natl Acad Sci USA. 2014;111:E4468–E4477. doi: 10.1073/pnas.1405266111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Paşca SP, et al. Using iPSC-derived neurons to uncover cellular phenotypes associated with Timothy syndrome. Nat Med. 2011;17:1657–1662. doi: 10.1038/nm.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Marchetto MC, et al. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell. 2010;143:527–539. doi: 10.1016/j.cell.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li Y, et al. Global transcriptional and translational repression in human embryonic stem cell–derived Rett syndrome neurons. Cell Stem Cell. 2013;13:446–458. doi: 10.1016/j.stem.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shcheglovitov A, et al. SHANK3 and IGF1 restore synaptic deficits in neurons from 22q13 deletion syndrome patients. Nature. 2013;503:267–271. doi: 10.1038/nature12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mariani J, et al. FOXG1-dependent dysregulation of GABA-glutamate neuron differentiation in autism spectrum disorders. Cell. 2015;162:375–390. doi: 10.1016/j.cell.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Casanova MF, et al. Minicolumnar abnormalities in autism. Acta Neuropathol. 2006;112:287–303. doi: 10.1007/s00401-006-0085-5. [DOI] [PubMed] [Google Scholar]
- 105.Stoner R, et al. Patches of disorganization in the neocortex of children with autism. N Engl J Med. 2014;370:1209–1219. doi: 10.1056/NEJMoa1307491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Redcay E, Courchesne E. When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biol Psychiatry. 2005;58:1–9. doi: 10.1016/j.biopsych.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 107.Magri L, et al. Sustained activation of mTOR pathway in embryonic neural stem cells leads to development of tuberous sclerosis complex–associated lesions. Cell Stem Cell. 2011;9:447–462. doi: 10.1016/j.stem.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 108.La Fata G, et al. FMRP regulates multipolar to bipolar transition affecting neuronal migration and cortical circuitry. Nat Neurosci. 2014;17:1693–1700. doi: 10.1038/nn.3870. [DOI] [PubMed] [Google Scholar]
- 109.Peñagarikano O, et al. Absence of Cntnap2 leads to epilepsy, neuronal migration abnormalities and core autism-related deficits. Cell. 2011;147:235–246. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Feng W, et al. The chromatin remodeler CHD7 regulates adult neurogenesis via activation of SoxC transcription factors. Cell Stem Cell. 2013;13:62–72. doi: 10.1016/j.stem.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 111.Zhou J, et al. Tsc1-mutant neural stem and progenitor cells exhibit migration deficits and give rise to subependymal lesions in the lateral ventricle. Genes Dev. 2011;25:1595–1600. doi: 10.1101/gad.16750211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 113.Lipton JO, Sahin M. The neurology of mTOR. Neuron. 2014;84:275–291. doi: 10.1016/j.neuron.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kwon CH, et al. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Krumm N, O’Roak BJ, Shendure J, Eichler EE. A de novo convergence of autism genetics and molecular neuroscience. Trends Neurosci. 2014;37:95–105. doi: 10.1016/j.tins.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hansen DV, Rubenstein JL, Kriegstein AR. Deriving excitatory neurons of the neocortex from pluripotent stem cells. Neuron. 2011;70:645–660. doi: 10.1016/j.neuron.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tiberi L, Vanderhaeghen P, van den Ameele J. Cortical neurogenesis and morphogens: diversity of cues, sources and functions. Curr Opin Cell Biol. 2012;24:269–276. doi: 10.1016/j.ceb.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 118.Lijam N, et al. Social interaction and sensorimotor-gating abnormalities in mice lacking Dvl1. Cell. 1997;90:895–905. doi: 10.1016/s0092-8674(00)80354-2. [DOI] [PubMed] [Google Scholar]
- 119.Sowers LP, et al. Disruption of the noncanonical Wnt gene PRICKLE2 leads to autism-like behaviors with evidence for hippocampal synaptic dysfunction. Mol Psychiatry. 2013;18:1077–1089. doi: 10.1038/mp.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fang WQ, et al. Overproduction of upper-layer neurons in the neocortex leads to autism-like features in mice. Cell Reports. 2014;9:1635–1643. doi: 10.1016/j.celrep.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 121.Long JM, LaPorte P, Paylor R, Wynshaw-Boris A. Expanded characterization of the social-interaction abnormalities in mice lacking Dvl1. Genes Brain Behav. 2004;3:51–62. doi: 10.1046/j.1601-183x.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 122.Bedogni F, et al. Tbr1 regulates regional and laminar identity of postmitotic neurons in developing neocortex. Proc Natl Acad Sci USA. 2010;107:13129–13134. doi: 10.1073/pnas.1002285107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sakamoto I, et al. A novel β-catenin–binding protein inhibits β-catenin–dependent Tcf activation and axis formation. J Biol Chem. 2000;275:32871–32878. doi: 10.1074/jbc.M004089200. [DOI] [PubMed] [Google Scholar]
- 124.Schuettengruber B, Martinez AM, Iovino N, Cavalli G. Trithorax group proteins: switching genes on and keeping them active. Nat Rev Mol Cell Biol. 2011;12:799–814. doi: 10.1038/nrm3230. [DOI] [PubMed] [Google Scholar]
- 125.Nozawa RS, et al. Human POGZ modulates dissociation of HP1-α from mitotic chromosome arms through Aurora B activation. Nat Cell Biol. 2010;12:719–727. doi: 10.1038/ncb2075. [DOI] [PubMed] [Google Scholar]
- 126.Chen T, Dent SY. Chromatin modifiers and remodelers: regulators of cellular differentiation. Nat Rev Genet. 2014;15:93–106. doi: 10.1038/nrg3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ronan JL, Wu W, Crabtree GR. From neural development to cognition: unexpected roles for chromatin. Nat Rev Genet. 2013;14:347–359. doi: 10.1038/nrg3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hevner RF, et al. Tbr1 regulates differentiation of the preplate and layer 6. Neuron. 2001;29:353–366. doi: 10.1016/s0896-6273(01)00211-2. [DOI] [PubMed] [Google Scholar]
- 129.Sierra J, Yoshida T, Joazeiro CA, Jones KA. The APC tumor suppressor counteracts β-catenin activation and H3K4 methylation at Wnt target genes. Genes Dev. 2006;20:586–600. doi: 10.1101/gad.1385806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stein JL, et al. A quantitative framework to evaluate modeling of cortical development by neural stem cells. Neuron. 2014;83:69–86. doi: 10.1016/j.neuron.2014.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Miller JA, et al. Transcriptional landscape of the prenatal human brain. Nature. 2014;508:199–206. doi: 10.1038/nature13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tuoc TC, et al. Chromatin regulation by BAF170 controls cerebral cortical size and thickness. Dev Cell. 2013;25:256–269. doi: 10.1016/j.devcel.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 133.Cotney J, et al. The autism-associated chromatin modifier CHD8 regulates other autism risk genes during human neurodevelopment. Nat Commun. 2015;6:6404. doi: 10.1038/ncomms7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nishiyama M, Skoultchi AI, Nakayama KI. Histone H1 recruitment by CHD8 is essential for suppression of the Wnt–β-catenin signaling pathway. Mol Cell Biol. 2012;32:501–512. doi: 10.1128/MCB.06409-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vaags AK, et al. Rare deletions at the neurexin 3 locus in autism spectrum disorder. Am J Hum Genet. 2012;90:133–141. doi: 10.1016/j.ajhg.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gauthier J, et al. Truncating mutations in NRXN2 and NRXN1 in autism spectrum disorders and schizophrenia. Hum Genet. 2011;130:563–573. doi: 10.1007/s00439-011-0975-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jamain S, et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Berkel S, et al. Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nat Genet. 2010;42:489–491. doi: 10.1038/ng.589. [DOI] [PubMed] [Google Scholar]
- 139.Durand CM, et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet. 2007;39:25–27. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Piton A, et al. Analysis of the effects of rare variants on splicing identifies alterations in GABAA receptor genes in autism spectrum disorder individuals. Eur J Hum Genet. 2013;21:749–756. doi: 10.1038/ejhg.2012.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lionel AC, et al. Rare exonic deletions implicate the synaptic organizer gephyrin (GPHN) in risk for autism, schizophrenia and seizures. Hum Mol Genet. 2013;22:2055–2066. doi: 10.1093/hmg/ddt056. [DOI] [PubMed] [Google Scholar]
- 142.Fassio A, et al. SYN1 loss-of-function mutations in autism and partial epilepsy cause impaired synaptic function. Hum Mol Genet. 2011;20:2297–2307. doi: 10.1093/hmg/ddr122. [DOI] [PubMed] [Google Scholar]
- 143.Corradi A, et al. SYN2 is an autism predisposing gene: loss-of-function mutations alter synaptic vesicle cycling and axon outgrowth. Hum Mol Genet. 2014;23:90–103. doi: 10.1093/hmg/ddt401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tang G, et al. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron. 2014;83:1131–1143. doi: 10.1016/j.neuron.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hutsler JJ, Zhang H. Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res. 2010;1309:83–94. doi: 10.1016/j.brainres.2009.09.120. [DOI] [PubMed] [Google Scholar]
- 146.Blatt GJ, et al. Density and distribution of hippocampal neurotransmitter receptors in autism: an autoradiographic study. J Autism Dev Disord. 2001;31:537–543. doi: 10.1023/a:1013238809666. [DOI] [PubMed] [Google Scholar]
- 147.Oblak A, Gibbs TT, Blatt GJ. Decreased GABAA receptors and benzodiazepine-binding sites in the anterior cingulate cortex in autism. Autism Res. 2009;2:205–219. doi: 10.1002/aur.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Oblak AL, Gibbs TT, Blatt GJ. Decreased GABAB receptors in the cingulate cortex and fusiform gyrus in autism. J Neurochem. 2010;114:1414–1423. doi: 10.1111/j.1471-4159.2010.06858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fatemi SH, et al. mRNA and protein levels for GABAA-α4, -α5, -β1 and GABABR1 receptors are altered in brains from subjects with autism. J Autism Dev Disord. 2010;40:743–750. doi: 10.1007/s10803-009-0924-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yip J, Soghomonian JJ, Blatt GJ. Increased GAD67 mRNA expression in cerebellar interneurons in autism: implications for Purkinje cell dysfunction. J Neurosci Res. 2008;86:525–530. doi: 10.1002/jnr.21520. [DOI] [PubMed] [Google Scholar]
- 151.Yip J, Soghomonian JJ, Blatt GJ. Decreased GAD65 mRNA levels in select subpopulations of neurons in the cerebellar dentate nuclei in autism: an in situ hybridization study. Autism Res. 2009;2:50–59. doi: 10.1002/aur.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yip J, Soghomonian JJ, Blatt GJ. Decreased GAD67 mRNA levels in cerebellar Purkinje cells in autism: pathophysiological implications. Acta Neuropathol. 2007;113:559–568. doi: 10.1007/s00401-006-0176-3. [DOI] [PubMed] [Google Scholar]
- 153.Fatemi SH, et al. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol Psychiatry. 2002;52:805–810. doi: 10.1016/s0006-3223(02)01430-0. [DOI] [PubMed] [Google Scholar]
- 154.Südhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions and mechanisms. Neuropsychopharmacology. 2008;33:18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- 156.Mayford M, Siegelbaum SA, Kandel ER. Synapses and memory storage. Cold Spring Harb Perspect Biol. 2012;4:a005751. doi: 10.1101/cshperspect.a005751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yizhar O, et al. Neocortical excitation-inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Palop JJ, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fritschy JM. Epilepsy, E/I balance and GABAA receptor plasticity. Front Mol Neurosci. 2008;1:5. doi: 10.3389/neuro.02.005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kehrer C, Maziashvili N, Dugladze T, Gloveli T. Altered excitatory-inhibitory balance in the NMDA-hypofunction model of schizophrenia. Front Mol Neurosci. 2008;1:6. doi: 10.3389/neuro.02.006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.van de Lagemaat LN, et al. Age-related decreased inhibitory versus excitatory gene expression in the adult autistic brain. Front Neurosci. 2014;8:394. doi: 10.3389/fnins.2014.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rojas DC, Singel D, Steinmetz S, Hepburn S, Brown MS. Decreased left perisylvian GABA concentration in children with autism and unaffected siblings. Neuroimage. 2014;86:28–34. doi: 10.1016/j.neuroimage.2013.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bejjani A, et al. Elevated glutamatergic compounds in pregenual anterior cingulate in pediatric autism spectrum disorder demonstrated by 1H MRS and 1H MRSI. PLoS One. 2012;7:e38786. doi: 10.1371/journal.pone.0038786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Greer PL, Greenberg ME. From synapse to nucleus: calcium-dependent gene transcription in the control of synapse development and function. Neuron. 2008;59:846–860. doi: 10.1016/j.neuron.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 165.Buffington SA, Huang W, Costa-Mattioli M. Translational control in synaptic plasticity and cognitive dysfunction. Annu Rev Neurosci. 2014;37:17–38. doi: 10.1146/annurev-neuro-071013-014100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Amir RE, et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG–binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 167.Splawski I, et al. CaV1.2 calcium channel dysfunction causes a multisystem disorder, including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 168.Ebert DH, Greenberg ME. Activity-dependent neuronal signaling and autism spectrum disorder. Nature. 2013;493:327–337. doi: 10.1038/nature11860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nicholls RD, Knepper JL. Genome organization, function and imprinting in Prader-Willi and Angelman syndromes. Annu Rev Genomics Hum Genet. 2001;2:153–175. doi: 10.1146/annurev.genom.2.1.153. [DOI] [PubMed] [Google Scholar]
- 170.Huang TN, et al. Tbr1 haploinsufficiency impairs amygdalar axonal projections and results in cognitive abnormality. Nat Neurosci. 2014;17:240–247. doi: 10.1038/nn.3626. [DOI] [PubMed] [Google Scholar]
- 171.Chuang HC, Huang TN, Hsueh YP. Neuronal excitation upregulates Tbr1, a high-confidence risk gene of autism, mediating Grin2b expression in the adult brain. Front Cell Neurosci. 2014;8:280. doi: 10.3389/fncel.2014.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Flavell SW, et al. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science. 2006;311:1008–1012. doi: 10.1126/science.1122511. [DOI] [PubMed] [Google Scholar]
- 173.Chen WG, et al. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- 174.Zhou Z, et al. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth and spine maturation. Neuron. 2006;52:255–269. doi: 10.1016/j.neuron.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Castro J, et al. Functional recovery with recombinant human IGF1 treatment in a mouse model of Rett Syndrome. Proc Natl Acad Sci USA. 2014;111:9941–9946. doi: 10.1073/pnas.1311685111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tropea D, et al. Partial reversal of Rett syndrome–like symptoms in MeCP2-mutant mice. Proc Natl Acad Sci USA. 2009;106:2029–2034. doi: 10.1073/pnas.0812394106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bozdagi O, Tavassoli T, Buxbaum JD. Insulin-like growth factor 1 rescues synaptic and motor deficits in a mouse model of autism and developmental delay. Mol Autism. 2013;4:9. doi: 10.1186/2040-2392-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Subramaniam S, et al. Insulin-like growth factor 1 inhibits extracellular signal– regulated kinase to promote neuronal survival via the phosphatidylinositol 3-kinase–protein kinase A–c-Raf pathway. J Neurosci. 2005;25:2838–2852. doi: 10.1523/JNEUROSCI.5060-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ehninger D, Silva AJ. Rapamycin for treating tuberous sclerosis and autism spectrum disorders. Trends Mol Med. 2011;17:78–87. doi: 10.1016/j.molmed.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Napoli I, et al. The fragile X syndrome protein represses activity-dependent translation through CYFIP1, a new 4E-BP. Cell. 2008;134:1042–1054. doi: 10.1016/j.cell.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 182.Nishimura Y, et al. Genome-wide expression profiling of lymphoblastoid cell lines distinguishes different forms of autism and reveals shared pathways. Hum Mol Genet. 2007;16:1682–1698. doi: 10.1093/hmg/ddm116. [DOI] [PubMed] [Google Scholar]
- 183.Tsai PT, et al. Autistic-like behavior and cerebellar dysfunction in Purkinje cell Tsc1-mutant mice. Nature. 2012;488:647–651. doi: 10.1038/nature11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhou J, et al. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular and behavioral abnormalities in neural-specific Pten-knockout mice. J Neurosci. 2009;29:1773–1783. doi: 10.1523/JNEUROSCI.5685-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gkogkas CG, et al. Autism-related deficits via dysregulated eIF4E-dependent translational control. Nature. 2013;493:371–377. doi: 10.1038/nature11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Santini E, et al. Exaggerated translation causes synaptic and behavioral aberrations associated with autism. Nature. 2013;493:411–415. doi: 10.1038/nature11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Berg JM, et al. JAKMIP1, a novel regulator of neuronal translation, modulates synaptic function and autistic-like behaviors in mouse. Neuron. 2015;88:1173–1191. doi: 10.1016/j.neuron.2015.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Darnell JC, et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146:247–261. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci USA. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cohen S, Greenberg ME. Communication between the synapse and the nucleus in neuronal development, plasticity and disease. Annu Rev Cell Dev Biol. 2008;24:183–209. doi: 10.1146/annurev.cellbio.24.110707.175235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hua JY, Smith SJ. Neural activity and the dynamics of central nervous system development. Nat Neurosci. 2004;7:327–332. doi: 10.1038/nn1218. [DOI] [PubMed] [Google Scholar]
- 192.Tsai NP, et al. Multiple autism-linked genes mediate synapse elimination via proteasomal degradation of a synaptic scaffold PSD-95. Cell. 2012;151:1581–1594. doi: 10.1016/j.cell.2012.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wilkerson JR, et al. A role for dendritic mGluR5-mediated local translation of Arc (Arg 3.1) in MEF2-dependent synapse elimination. Cell Rep. 2014;7:1589–1600. doi: 10.1016/j.celrep.2014.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Abrahams BS, Geschwind DH. Connecting genes to brain in the autism spectrum disorders. Arch Neurol. 2010;67:395–399. doi: 10.1001/archneurol.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yerys BE, Herrington JD. Multimodal imaging in autism: an early review of comprehensive neural circuit characterization. Curr Psychiatry Rep. 2014;16:496. doi: 10.1007/s11920-014-0496-2. [DOI] [PubMed] [Google Scholar]
- 196.Luckhardt C, Jarczok TA, Bender S. Elucidating the neurophysiological under-pinnings of autism spectrum disorder: new developments. J Neural Transm. 2014;121:1129–1144. doi: 10.1007/s00702-014-1265-4. [DOI] [PubMed] [Google Scholar]
- 197.Ecker C, Bookheimer SY, Murphy DG. Neuroimaging in autism spectrum disorder: brain structure and function across the lifespan. Lancet Neurol. 2015;14:1121–1134. doi: 10.1016/S1474-4422(15)00050-2. [DOI] [PubMed] [Google Scholar]
- 198.Minshew NJ, Williams DL. The new neurobiology of autism: cortex, connectivity and neuronal organization. Arch Neurol. 2007;64:945–950. doi: 10.1001/archneur.64.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zikopoulos B, Barbas H. Changes in prefrontal axons may disrupt the network in autism. J Neurosci. 2010;30:14595–14609. doi: 10.1523/JNEUROSCI.2257-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.McPartland JC, Coffman M, Pelphrey KA. Recent advances in understanding the neural bases of autism spectrum disorder. Curr Opin Pediatr. 2011;23:628–632. doi: 10.1097/MOP.0b013e32834cb9c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rudie JD, et al. Autism-associated promoter variant in MET impacts functional and structural brain networks. Neuron. 2012;75:904–915. doi: 10.1016/j.neuron.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 203.Vissers ME, Cohen MX, Geurts HM. Brain connectivity and high functioning autism: a promising path of research that needs refined models, methodological convergence and stronger behavioral links. Neurosci Biobehav Rev. 2012;36:604–625. doi: 10.1016/j.neubiorev.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 204.Ellegood J, et al. Clustering autism: using neuroanatomical differences in 26 mouse models to gain insight into the heterogeneity. Mol Psychiatry. 2015;20:118–125. doi: 10.1038/mp.2014.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kloth AD, et al. Cerebellar associative sensory learning defects in five mouse autism models. eLife. 2015;4:e06085. doi: 10.7554/eLife.06085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wang SS, Kloth AD, Badura A. The cerebellum, sensitive periods and autism. Neuron. 2014;83:518–532. doi: 10.1016/j.neuron.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Amaral DG. The amygdala, social behavior and danger detection. Ann NY Acad Sci. 2003;1000:337–347. doi: 10.1196/annals.1280.015. [DOI] [PubMed] [Google Scholar]
- 208.Baron-Cohen S, et al. The amygdala theory of autism. Neurosci Biobehav Rev. 2000;24:355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- 209.Langen M, Durston S, Kas MJ, van Engeland H, Staal WG. The neurobiology of repetitive behavior: … and men. Neurosci Biobehav Rev. 2011;35:356–365. doi: 10.1016/j.neubiorev.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 210.Peça J, et al. Shank3-mutant mice display autistic-like behaviors and striatal dysfunction. Nature. 2011;472:437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Rothwell PE, et al. Autism-associated neuroligin–3 mutations commonly impair striatal circuits to boost repetitive behaviors. Cell. 2014;158:198–212. doi: 10.1016/j.cell.2014.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Geschwind DH, Rakic P. Cortical evolution: judge the brain by its cover. Neuron. 2013;80:633–647. doi: 10.1016/j.neuron.2013.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kasthuri N, et al. Saturated reconstruction of a volume of neocortex. Cell. 2015;162:648–661. doi: 10.1016/j.cell.2015.06.054. [DOI] [PubMed] [Google Scholar]
- 214.Zingg B, et al. Neural networks of the mouse neocortex. Cell. 2014;156:1096–1111. doi: 10.1016/j.cell.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Gunaydin LA, et al. Natural neural projection dynamics underlying social behavior. Cell. 2014;157:1535–1551. doi: 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Marlin BJ, Mitre M, D’amour JA, Chao MV, Froemke RC. Oxytocin enables maternal behavior by balancing cortical inhibition. Nature. 2015;520:499–504. doi: 10.1038/nature14402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Morgan JT, et al. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol Psychiatry. 2010;68:368–376. doi: 10.1016/j.biopsych.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 218.Suzuki K, et al. Microglial activation in young adults with autism spectrum disorder. JAMA Psychiatry. 2013;70:49–58. doi: 10.1001/jamapsychiatry.2013.272. [DOI] [PubMed] [Google Scholar]
- 219.Tetreault NA, et al. Microglia in the cerebral cortex in autism. J Autism Dev Disord. 2012;42:2569–2584. doi: 10.1007/s10803-012-1513-0. [DOI] [PubMed] [Google Scholar]
- 220.Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- 221.Laurence JA, Fatemi SH. Glial fibrillary acidic protein is elevated in superior frontal, parietal and cerebellar cortices of autistic subjects. Cerebellum. 2005;4:206–210. doi: 10.1080/14734220500208846. [DOI] [PubMed] [Google Scholar]
- 222.Lopez-Hurtado E, Prieto J. A microscopic study of language-related cortex in autism. Am J Biochem Biotechnol. 2008;4:130–145. [Google Scholar]
- 223.Gupta S, et al. Transcriptome analysis reveals dysregulation of innate immune response genes and neuronal activity–dependent genes in autism. Nat Commun. 2014;5:5748. doi: 10.1038/ncomms6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Paolicelli RC, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 225.Zhan Y, et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci. 2014;17:400–406. doi: 10.1038/nn.3641. [DOI] [PubMed] [Google Scholar]
- 226.Chung WS, et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504:394–400. doi: 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Clarke LE, Barres BA. Emerging roles of astrocytes in neural circuit development. Nat Rev Neurosci. 2013;14:311–321. doi: 10.1038/nrn3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Schafer DP, et al. Microglia sculpt postnatal neural circuits in an activity- and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.de Vrij FM, et al. Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice. Neurobiol Dis. 2008;31:127–132. doi: 10.1016/j.nbd.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology. 2010;35:976–989. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Tian D, et al. Contribution of mGluR5 to pathophysiology in a mouse model of human chromosome 16p11.2 microdeletion. Nat Neurosci. 2015;18:182–184. doi: 10.1038/nn.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Won H, et al. Autistic-like social behavior in Shank2-mutant mice improved by restoring NMDA receptor function. Nature. 2012;486:261–265. doi: 10.1038/nature11208. [DOI] [PubMed] [Google Scholar]
- 233.Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major fragile X syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49:1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 234.Benson AD, Burket JA, Deutsch SI. Balb/c mice treated with D-cycloserine arouse increased social interest in conspecifics. Brain Res Bull. 2013;99:95–99. doi: 10.1016/j.brainresbull.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 235.Blundell J, et al. Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior. J Neurosci. 2010;30:2115–2129. doi: 10.1523/JNEUROSCI.4517-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Burket JA, Benson AD, Tang AH, Deutsch SI. D-cycloserine improves sociability in the BTBR T+ Itpr3tf/J mouse model of autism spectrum disorders with altered Ras-Raf-ERK1/2 signaling. Brain Res Bull. 2013;96:62–70. doi: 10.1016/j.brainresbull.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Auerbach BD, Osterweil EK, Bear MF. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature. 2011;480:63–68. doi: 10.1038/nature10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Han S, Tai C, Jones CJ, Scheuer T, Catterall WA. Enhancement of inhibitory neurotransmission by GABAA receptors having a2,3-subunits ameliorates behavioral deficits in a mouse model of autism. Neuron. 2014;81:1282–1289. doi: 10.1016/j.neuron.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Han S, et al. Autistic-like behaviour in Scn1a+/− mice and rescue by enhanced GABA-mediated neurotransmission. Nature. 2012;489:385–390. doi: 10.1038/nature11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ehninger D, et al. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat Med. 2008;14:843–848. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Oguro-Ando A, et al. Increased CYFIP1 dosage alters cellular and dendritic morphology and dysregulates mTOR. Mol Psychiatry. 2015;20:1069–1078. doi: 10.1038/mp.2014.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ru W, Peng Y, Zhong L, Tang SJ. A role of the mammalian target of rapamycin (mTOR) in glutamate-induced downregulation of tuberous sclerosis complex proteins 2 (TSC2) J Mol Neurosci. 2012;47:340–345. doi: 10.1007/s12031-012-9753-1. [DOI] [PubMed] [Google Scholar]
- 243.Chang Q, Khare G, Dani V, Nelson S, Jaenisch R. The disease progression of Mecp2-mutant mice is affected by the level of BDNF expression. Neuron. 2006;49:341–348. doi: 10.1016/j.neuron.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 244.Kline DD, Ogier M, Kunze DL, Katz DM. Exogenous brain-derived neurotrophic factor rescues synaptic dysfunction in Mecp2-null mice. J Neurosci. 2010;30:5303–5310. doi: 10.1523/JNEUROSCI.5503-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Mellios N, et al. b2-adrenergic receptor agonist ameliorates phenotypes and corrects microRNA-mediated IGF1 deficits in a mouse model of Rett syndrome. Proc Natl Acad Sci USA. 2014;111:9947–9952. doi: 10.1073/pnas.1309426111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Deogracias R, et al. Fingolimod, a sphingosine-1 phosphate receptor modulator, increases BDNF levels and improves symptoms of a mouse model of Rett syndrome. Proc Natl Acad Sci USA. 2012;109:14230–14235. doi: 10.1073/pnas.1206093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin and affiliative behavior. Neuron. 2010;65:768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Peñagarikano O, et al. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci Transl Med. 2015;7:271ra8. doi: 10.1126/scitranslmed.3010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Sala M, et al. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression and seizure susceptibility in oxytocin receptor–null mice: a neurobehavioral model of autism. Biol Psychiatry. 2011;69:875–882. doi: 10.1016/j.biopsych.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 251.Tyzio R, et al. Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science. 2014;343:675–679. doi: 10.1126/science.1247190. [DOI] [PubMed] [Google Scholar]
- 252.Owen SF, et al. Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature. 2013;500:458–462. doi: 10.1038/nature12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Hsiao EY, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Ey E, et al. Absence of deficits in social behaviors and ultrasonic vocalizations in later generations of mice lacking neuroligin 4-like. Genes Brain Behav. 2012;11:928–941. doi: 10.1111/j.1601-183X.2012.00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Bernardet M, Crusio WE. Fmr1 KO mice as a possible model of autistic features. Scientific World Journal. 2006;6:1164–1176. doi: 10.1100/tsw.2006.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Chadman KK, et al. Minimal aberrant behavioral phenotypes of neuroligin-3R451C–knockin mice. Autism Res. 2008;1:147–158. doi: 10.1002/aur.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Jacquemont S, et al. Epigenetic modification of the FMR1 gene in fragile X syndrome is associated with differential response to the mGluR5 antagonist AFQ056. Sci Transl Med. 2011;3:64ra1. doi: 10.1126/scitranslmed.3001708. [DOI] [PubMed] [Google Scholar]
- 258.Tachibana M, et al. Long-term administration of intranasal oxytocin is a safe and promising therapy for early adolescent boys with autism spectrum disorders. J Child Adolesc Psychopharmacol. 2013;23:123–127. doi: 10.1089/cap.2012.0048. [DOI] [PubMed] [Google Scholar]
- 259.Dadds MR, et al. Nasal oxytocin for social deficits in childhood autism: a randomized controlled trial. J Autism Dev Disord. 2014;44:521–531. doi: 10.1007/s10803-013-1899-3. [DOI] [PubMed] [Google Scholar]
- 260.Guastella AJ, et al. The effects of a course of intranasal oxytocin on social behaviors in youth diagnosed with autism spectrum disorders: a randomized controlled trial. J Child Psychol Psychiatry. 2015;56:444–452. doi: 10.1111/jcpp.12305. [DOI] [PubMed] [Google Scholar]
- 261.Jeste SS, Geschwind DH. Clinical trials for neurodevelopmental disorders: at a therapeutic frontier. Sci Transl Med. 2016;8:321fs1. doi: 10.1126/scitranslmed.aad9874. [DOI] [PubMed] [Google Scholar]
- 262.Bailey KR, Rustay NR, Crawley JN. Behavioral phenotyping of transgenic and knockout mice: practical concerns and potential pitfalls. ILAR J. 2006;47:124–131. doi: 10.1093/ilar.47.2.124. [DOI] [PubMed] [Google Scholar]
- 263.Werling DM, Lowe JK, Luo R, Cantor RM, Geschwind DH. Replication of linkage at chromosome 20p13 and identification of suggestive sex-differential risk loci for autism spectrum disorder. Mol Autism. 2014;5:13. doi: 10.1186/2040-2392-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Cloughesy TF, Cavenee WK, Mischel PS. Glioblastoma: from molecular pathology to targeted treatment. Annu Rev Pathol. 2014;9:1–25. doi: 10.1146/annurev-pathol-011110-130324. [DOI] [PubMed] [Google Scholar]
- 265.Werker JF, Hensch TK. Critical periods in speech perception: new directions. Annu Rev Psychol. 2015;66:173–196. doi: 10.1146/annurev-psych-010814-015104. [DOI] [PubMed] [Google Scholar]
- 266.Falconer DS. The inheritance of liability to certain diseases, estimated from the incidence among relatives. Ann Hum Genet. 1965;29:51–76. [Google Scholar]
- 267.Isshiki M, et al. Enhanced synapse remodeling as a common phenotype in mouse models of autism. Nat Commun. 2014;5:4742. doi: 10.1038/ncomms5742. [DOI] [PubMed] [Google Scholar]
- 268.Nakatani J, et al. Abnormal behavior in a chromosome-engineered mouse model for human 15q11–13 duplication seen in autism. Cell. 2009;137:1235–1246. doi: 10.1016/j.cell.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Lee EJ, et al. Trans-synaptic zinc mobilization improves social interaction in two mouse models of autism through NMDAR activation. Nat Commun. 2015;6:7168. doi: 10.1038/ncomms8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Lim CS, et al. Pharmacological rescue of Ras signaling, GluA1-dependent synaptic plasticity and learning deficits in a fragile X model. Genes Dev. 2014;28:273–289. doi: 10.1101/gad.232470.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Gross C, et al. Selective role of the catalytic PI3K subunit p110-β in impaired higher-order cognition in fragile X syndrome. Cell Reports. 2015;11:681–688. doi: 10.1016/j.celrep.2015.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Osterweil EK, Krueger DD, Reinhold K, Bear MF. Hypersensitivity to mGluR5 and ERK1/2 leads to excessive protein synthesis in the hippocampus of a mouse model of fragile X syndrome. J Neurosci. 2010;30:15616–15627. doi: 10.1523/JNEUROSCI.3888-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Etherton MR, Blaiss CA, Powell CM, Südhof TC. Mouse neurexin I alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proc Natl Acad Sci USA. 2009;106:17998–18003. doi: 10.1073/pnas.0910297106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Grayton HM, Missler M, Collier DA, Fernandes C. Altered social behaviors in neurexin-1α–knockout mice resemble core symptoms in neurodevelopmental disorders. PLoS One. 2013;8:e67114. doi: 10.1371/journal.pone.0067114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Tabuchi K, et al. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Etherton M, et al. Autism-linked neuroligin-3R451C mutation differentially alters hippocampal and cortical synaptic function. Proc Natl Acad Sci USA. 2011;108:13764–13769. doi: 10.1073/pnas.1111093108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Baudouin SJ, et al. Shared synaptic pathophysiology in syndromic and nonsyndromic rodent models of autism. Science. 2012;338:128–132. doi: 10.1126/science.1224159. [DOI] [PubMed] [Google Scholar]
- 278.Jamain S, et al. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci USA. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Schmeisser MJ, et al. Autistic-like behaviors and hyperactivity in mice lacking ProSAP1 (Shank2) Nature. 2012;486:256–260. doi: 10.1038/nature11015. [DOI] [PubMed] [Google Scholar]
- 280.Wang X, et al. Synaptic dysfunction and abnormal behaviors in mice lacking major isoforms of Shank3. Hum Mol Genet. 2011;20:3093–3108. doi: 10.1093/hmg/ddr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Bozdagi O, et al. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction and social communication. Mol Autism. 2010;1:15. doi: 10.1186/2040-2392-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Duffney LJ, et al. Autism-like deficits in Shank3-deficient mice are rescued by targeting actin regulators. Cell Rep. 2015;11:1400–1413. doi: 10.1016/j.celrep.2015.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Gdalyahu A, et al. The autism-related protein contactin-associated protein-like 2 (CNTNAP2) stabilizes new spines: an in vivo mouse study. PLoS One. 2015;10:e0125633. doi: 10.1371/journal.pone.0125633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Derecki NC, et al. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature. 2012;484:105–109. doi: 10.1038/nature10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Lioy DT, et al. A role for glia in the progression of Rett’s syndrome. Nature. 2011;475:497–500. doi: 10.1038/nature10214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Langen M, Kas MJ, Staal WG, van Engeland H, Durston S. The neurobiology of repetitive behavior: of mice…. Neurosci Biobehav Rev. 2011;35:345–355. doi: 10.1016/j.neubiorev.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 287.Lui JH, et al. Radial glia require PDGFD–PDGFR-β signaling in human but not mouse neocortex. Nature. 2014;515:264–268. doi: 10.1038/nature13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Merkle FT, Eggan K. Modeling human disease with pluripotent stem cells: from genome association to function. Cell Stem Cell. 2013;12:656–668. doi: 10.1016/j.stem.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 289.Griesi-Oliveira K, et al. Modeling nonsyndromic autism and the impact of TRPC6 disruption in human neurons. Mol Psychiatry. 2015;20:1350–1365. doi: 10.1038/mp.2014.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Ricciardi S, et al. CDKL5 ensures excitatory synapse stability by reinforcing NGL-1–PSD95 interaction in the postsynaptic compartment and is impaired in patient iPSC-derived neurons. Nat Cell Biol. 2012;14:911–923. doi: 10.1038/ncb2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Kadoshima T, et al. Self-organization of axial polarity, inside-out layer pattern and species-specific progenitor dynamics in human ES cell–derived neocortex. Proc Natl Acad Sci USA. 2013;110:20284–20289. doi: 10.1073/pnas.1315710110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Lepski G, et al. Delayed functional maturation of human neuronal progenitor cells in vitro. Mol Cell Neurosci. 2011;47:36–44. doi: 10.1016/j.mcn.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 293.Mariani J, et al. Modeling human cortical development in vitro using induced pluripotent stem cells. Proc Natl Acad Sci USA. 2012;109:12770–12775. doi: 10.1073/pnas.1202944109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Nicholas CR, et al. Functional maturation of hPSC-derived forebrain interneurons requires an extended timeline and mimics human neural development. Cell Stem Cell. 2013;12:573–586. doi: 10.1016/j.stem.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.van de Leemput J, et al. CORTECON: a temporal transcriptome analysis of in vitro human cerebral cortex development from human embryonic stem cells. Neuron. 2014;83:51–68. doi: 10.1016/j.neuron.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 296.Ben-David E, Shifman S. Combined analysis of exome sequencing points toward a major role for transcription regulation during brain development in autism. Mol Psychiatry. 2013;18:1054–1056. doi: 10.1038/mp.2012.148. [DOI] [PubMed] [Google Scholar]
- 297.Paşca AM, et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods. 2015;12:671–678. doi: 10.1038/nmeth.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Espuny-Camacho I, et al. Pyramidal neurons derived from human pluripotent stem cells integrate efficiently into mouse brain circuits in vivo. Neuron. 2013;77:440–456. doi: 10.1016/j.neuron.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 299.Hansen DV, Lui JH, Parker PR, Kriegstein AR. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010;464:554–561. doi: 10.1038/nature08845. [DOI] [PubMed] [Google Scholar]
- 300.Lancaster MA, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.