Abstract
Objectives
To test the analgesic effect of 5-HT-3 receptor antagonist, tropisetron, in a clip compression injury model of spinal cord pain in rats.
Methods
Four weeks post compression of the spinal cord at lumbar level, tropisetron was administered intrathecally at 100 μg and 150 μg dosages. Behavioral tests were assessed before administration. Fifteen minutes after injection, behavioral tests were repeated. Randall-Sellitto and plantar test was used for mechanical and thermal hyperalgesia, respectively. Mechanical and cold allodynia were evaluated by Von Frey filament and acetone droplets, respectively. The analgesic effect of tropisetron was compared with intrathecal administration of salicylate. Locomotor score was evaluated by Basso, Beattie and Bresnahan (BBB) test every week after spinal cord injury.
Results
Intrathecal administration of tropisetron, decreased hyperalgesia and mechanical allodynia, but not cold allodynia were observed after compression of the spinal cord.
Conclusion
Blockade of 5-HT-3 receptors by tropisetron at the spinal level induces an antinociceptive effect on chronic central neuropathic pain and suggests that this compound may have potential clinical utility for the management of central neuropathic pain, particularly in patients with hyperalgesia and tactile allodynia.
Keywords: 5-hydroxytryptamine-3, Spinal cord, Central neuropathic pain, Clips compression injury
Introduction
Pharmacotherapy still represents the main common option for treating central neuropathic pain (NP), but results are not desirable and satisfactory and most of the patients do not obtain sufficient pain relief. So finding a new therapeutic strategy to reduce pain and increase the quality of life of sufferers is necessary.1 Clinical trials published in recent years showed a low efficacy of the current agents for management of NP. These studies depict that effectiveness of approved drugs is variable, dosing can be complicated, analgesic onset is delayed, and side effects are common. Despite the discovery of several pathological conditions which induced neuropathic pain within the last few decades, it was not matched by a similar improvement in the treatment efficacy. Pain relief strategies relying on disease mechanisms cause an increased quality of treatments.1
The 5-hydroxytryptamine-3 (5-HT3) receptor is a non-selective ligand-gated ion channel that is a member of the Cys-loop family of receptors. The 5-HT3 receptors located on central and peripheral nociceptive system, play a substantial role in pain transmission.2 Their functions in this context have not been completely defined yet, and studies have yielded partly contradictory results. For example, both 5-HT3-receptor agonists and antagonists led to reduced pain reactions to noxious stimuli.3,4 Studies have shown that pharmacological block of spinal 5HT3 receptors using a selective antagonist (ondansetron) causes an antinociceptive effect following peripheral formalin injection.5,6 This is paralleled by clinical studies.7 Intrathecal (IT) administration of ondansetron produced a significant reduction in the second phase of formalin test using genetic, pharmacological and electrophysiological approaches. Interestingly, acute pain responses are normal, but persistent pain responses are affected in 5-HT3 mutant mice,8 which indicate the contribution of 5HT receptors on chronic but not acute pain.
It has been suggested that in chronic pain models 5HT3 receptors have a pronociceptive function and blockade of these receptors could be a useful strategy in pain attenuation.6,8 Based on these hypotheses, a 5HT3 receptor antagonist should alter the coding of noxious stimuli in chronic pain states. Tropisetron is a highly selective 5-HT3 receptor antagonist used mainly as an antiemetic to reduce nausea and vomiting. Based on results on experimental animals, tropisetron may have an analgesic effect on different pain models and may have the potential to reduce pain threshold in chronic condition. The results from clinical trial studies are controversial. For example, human clinical trials suggest that a single intravenous dose of tropisetron failed to affect the intensity of low back pain and parameters of central hypersensitivity.9 These results are in contrast with those of a previous observational study, where tropisetron was found to have had a long-lasting analgesic effect in patients with chronic low back pain.10 It has been reported that a single intravenous injection of ondansetron, a selective 5-HT-3 receptor antagonist, produced pain relief in patients with central neuropathic pain condition.7 The results of these studies suggest that the effect of 5-HT-3 receptor antagonists on pain modulation may be different in different pain conditions.
Central neuropathic pain can be a consequence of spinal cord injury (SCI), multiple sclerosis, and stroke. Subsequent to SCI, a majority of individuals experience long-lasting and moderate to severe pain. In spite of improvements in understanding the mechanism of this pain, central neuropathic pain following SCI remains problematic to treat. Therefore, the aim of this study was to investigate the hypothesis that the 5-HT-3 receptor antagonist, tropisetron, may attenuate chronic pain in a SCI model.
Materials and methods
Study design
The protocol of the present experimental study was reviewed and approved by the ethics committee of Iran University of Medical Sciences in Tehran, Iran. Throughout the study, the researchers adhered to guidelines of the “Guide for the Care and Use of Laboratory Animals.” In this study, to obtain the central model of central neuropathic pain, compression of spinal cord was induced, and then to confirm SCI, locomotor function was assessed.
Animals and drugs
Fifty male Wistar rats (10 in each group) weighing 150–170 g at the initiation of the study were used. Animals were maintained under a 12:12-hour light:dark cycle and had free access to water and food. All tests were carried out between 10:00 a.m. and 02:00 p.m. The experiments were performed after approval of the protocol by the Ethics Committee of the institution and all efforts were made to minimize animal suffering.
All the drugs used in the study were purchased from Sigma Chemical Company, St Louis, MO, USA. The drugs were dissolved in saline immediately before administration and IT administration was performed in a constant volume of 10 µl.
To evaluate the antinociceptive effect of tropisetron in central neuropathic pain model, two different doses of tropisetron (100 & 150 µg) were used. Salicylate was administrated in the dose of 100 µg. Analgesic effect of tropisetron was compared with IT administration of salicylate. Animals in vehicle group received IT injection of normal saline in a volume of 10 µl. Uninjured rats were followed without any intervention. Four weeks after induction of spinal cord injury, animals were evaluated behaviorally. Animals (10 animals in each group) were tested for mechanical and thermal hyperalgesia and tactile and cold allodynia before drug administration. Fifteen minutes after administration of drugs, behavioral tests were administered.
Surgical procedure
For induction of spinal cord injury, rats were anesthetized with a mixture of ketamin (80 mg/kg) and Xylazin (10 mg/kg). After anesthesia the hair from the back was shaved and under aseptic surgical condition, laminectomy was done to expose the spinal cord on the location of T6–T8. A micro-vascular clip (Harvard Apparatus, MA) was placed vertically on the exposed thoracic spinal cord for 60 seconds.11,12 Dura was intact and care was taken not to disturb nearby spinal nerve roots. Following spinal compression, the clip was removed, the muscles sutured shut and the skin closed with 4/0 silk. Three weeks following compression surgery, rats underwent another surgery for placing a PE10 catheter intrathecally. For this reason rats were anaesthetized again with the same dose of the mixture of ketamin and xylasin. According to the method introduced by Xu et al. a PE10 tube was placed in the lumbar part of the spinal cord.13 Briefly, the lumbar part of the skin was shaved and under an aseptic situation a longitudinal skin incision was made over L4-L5 location. Paravertebral muscles were bluntly separated to expose intervertebral space. Intervertebral foramen was exposed and a slice was made on the dura by using the tip of a needle resulting leakage of clear CSF. A 6 cm long PE10 catheter was inserted into the subarachnoid space and passed 1 cm rostrally. The outer part of the catheter was secured by suturing onto the paravertebral muscle. The muscles and skin were sutured by 4/0 silk separately. At the end, 8 microliter of saline was injected and the tip of the catheter was closed by melting the end.12
Behavioral evaluation
For evaluation of pain responses, a battery of sensory tests was performed in sequence. Behavioral testing was performed by a blinded examiner to minimize experimenter bias. Responses to noxious heat (Hargreaves method) were evaluated using paw withdrawal test. Before testing, rats were habituated to the environment for approximately 20 minutes. A radiant heat test source beneath the glass was aimed at the plantar hind paw and activated a timer. Withdrawal latencies were measured automatically with photocell light. Withdrawal latencies were the length of time between the activation of the heat source and the hind paw withdrawal from the glass. A cut-off time was set at 25 seconds to avoid tissue damage. Three trials were given at least 5 minutes apart and the average values used for statistical analysis.
Responses to noxious mechanical pressure (mechanical hyperalgesia) were assessed using a pressure withdrawal test (Randall-Selitto test). The rats were wrapped in a towel and an increasing force (48 geram/second) was applied to the plantar surface of the hind paw until the rat reacted with a withdrawal response. The apparatus automatically terminated at 1000 gram (g) (25 in scale units) in the absence of a response. Two trials were given at least 1 minute apart and the average values were used for statistical analysis.
The thresholds for mechanical allodynia were measured using a series of von Frey filaments. The third metatarsal bone area of the both hindpaw was stimulated with von Frey filaments at using the up-down method.14 The threshold was recorded as 15.0 g if the strongest hair did not elicit a response. Avoidance responses, such as lifting, shaking or licking the paw and running away, were considered as positive responses.
Cold allodynia was measured by foot withdrawal responses after applying a drop of acetone to the plantar surface of the paw. The tests were repeated five time intervals of approximately 3–5 minutes between each test The response frequency to acetone is expressed as the percentage response frequency [(number of paw withdrawals/number of trials) × 100].
To test the locomotor functions, an open field locomotor scale, described by Basso, Beattie and Bresnahan (BBB) from complete paralysis (score 0) to normal locomotion (score 21) was used.15 Rats were allowed to move freely and were scored during 4 minutes by two observers for their ability to use their hindlimbs. Joint movements, paw placement, weight support, and fore/hindlimb coordination were judged according to the 21-point BBB locomotion scale. Locomotor score was evaluated every week after spinal cord injury.
Statistical analysis
Data are presented as mean ± SEM of paw withdrawal threshold. Data for the time course (comparison of before vs after treatment) and dose effects (100 and 150 µg/10 µl) of the agonists were analyzed using two-way analysis of variance followed by the Bonferroni multiple-comparison test The level of significance was considered P < 0.05.
Results
Mechanical allodynia
The baseline 50% paw withdrawal threshold (PWT) measured with von-Frey filaments was significantly lower in SCI-induced animals compared to uninjured animals [F (5, 45) = 7.92; P < 0.001]. The baseline values in salicylate-treated (100 µg/10 µl) and tropisetron-treated (150 µg/10 µl) rats were 9.4 ± 1.4 g and 9.35 ± 1.2 g which increased after injection to 13.1 ± 0.77 g and 13.4 ± 0.7 g, respectively (Fig. 1). The increase in the 50% PWT in salicylate-treated (100 µg/10 µl) and tropisetron-treated (150 µg/10 µl) rats was significant compared to pre-injection values [F (5, 45) = 4.86; P < 0.001]. After injection, paw withdrawal threshold (PWT) in tropisetron-treated animal and salicylate-treated group reached that of uninjured animals (P > 0.99). In group with IT injection of 100 µg/10 µl of tropisetron, the 50% withdrawal threshold was higher than preinjeciton values but no significant difference was observed between pre-injection and post-injection values. The 50% PWT in animals treated with salysilate and tropicetron (150 µg/10 µl) was significantly higher than the saline-treated animals (9.06 ± 1.4, P < 0.01).
Figure 1.
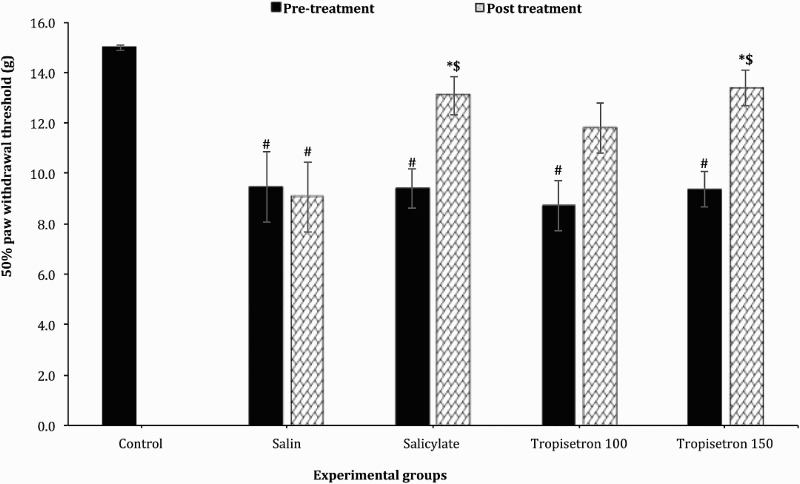
Effect of a single IT injection of salicylate 100 µg/10 µl, Tropisetron 100 µg/10 µl and 150 µg/10 µl on mechanical allodynia. Data are expressed as mean ± SEM of 50% withdrawal threshold. The mean 50% withdrawal threshold is significantly increased following administration of salicylate (100 µg/10 µl), and Tropisetron (150 µg/10 µl) compared to saline-treated animals and before injection. * statistical difference with saline-treated groups (P < 0.01), $ statistical defference with pre-treatment (P < 0.001). #, statistical difference with control groups (p < 0.001).
Cold allodynia
Cold allodynia was evaluated with acetone droplet to the hind paw. The percentage of response to acetone droplet before drug administration in four treated groups was similar. However, the percentage of response in the uninjured was significantly lower than other groups [F (5, 45) = 20.57; P < 0.001]. Cold allodynia of hind paw in the SCI animals was reduced by administration of salicylate (100 µg/10 µl) when compared with pre-injection values or saline treated animals. The mean value in salicylate treated rats was 62.5 ± 11.6 percent which was signinficantly lower compared with before injection (95.0% ± 3.8%) (P = 0.035) and saline-treated group (88.75% ± 5.5%) (P = 0.04). IT adminstration of tropicetron, in both doses, had little effect on percentage of response to acetone droplet compared with pre-injection values or saline treated animals. These differences were not statistically significant (P > 0.99) (Fig. 2).
Figure 2.
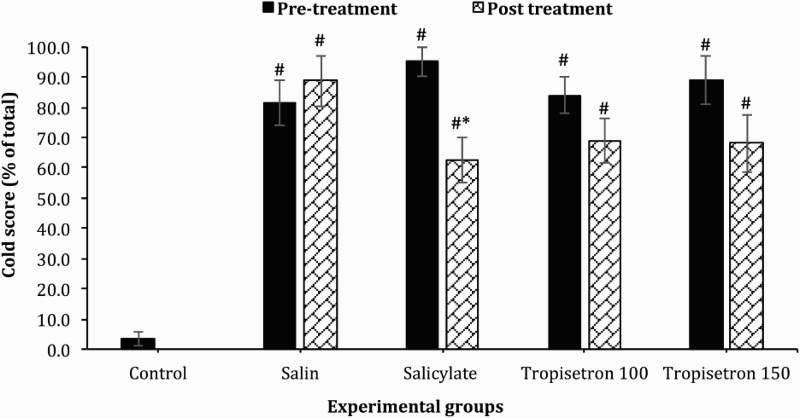
Effect of a single IT injection of salicylate (100 µg/10 µl), and Tropisetron (100 µg/10 µl and 150 µg/10 µl) on cold allodynia. Data are expressed as mean ± SEM cold score. The mean cold score is significantly reduced after administration of salicylate compared to saline-treated animals and stimulation before treatment, * statistical difference with saline-treated groups (P < 0.05), $ statistical defference with pre-treatment (P < 0.05). #, statistical difference with control groups (P < 0.001).
Mechanical hyperalgesia
PWT measured with Randall Selitto test was significantly higher before intervention in the control group compared to SCI-induced animals [F (5, 45) = 55.99; P < 0.001]. The mean ± SEM of PWT was increased to 9.05 ± 0.3 g, 8.6 ± 0.2 g and 8.06 ± 0.4 g in salicylate and tropisetron (100 µg/10 µl and 150 µg/10 µl) treated rats respectively which were significantly higher than the values before injection. The baseline values before injection were 6.9 ± 0.26 g, 7.7 ± 0.21 g and 6.7 ± 0.3 g in salicylate- and tropisetron-treated rats respectively (100 µg/10 µl and 150 µg/10 µl). Salicylate (P < 0.001) and different doses of tropisetron [100 µg/10 µl (P < 0.001) and 150 µg/10 µl (P < 0.003)] strikingly increased paw withdrawal threshold in response to noxious stimulus compared with before injection, but PWT did not reach that of uninjured animals (P < 0.001) (Fig. 3). PWT in animals that received saline was 7.05 ± 0.07, which was significantly lower than the PWT in animals injected with salicylate, tropisetron or uninjured rats (P < 0.001).
Figure 3.
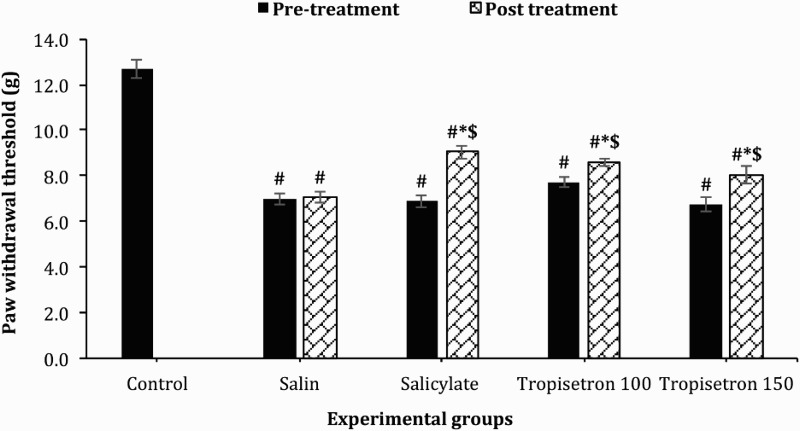
Effect of a single IT injection of salicylate 100 µg/10 µl, Tropisetron 100 µg/10 µl and 150 µg/10 µl on paw-withdrawal threshold using an Ugo-Basile analgesimeter (Randall and Selitto test). Data are expressed as mean ± SEM of withdrawal threshold (g). The mean withdrawal threshold shows a drastic increase after administration of salicylate, Tropisetron 100 µg/10 µl and Tropisetron 150 µg/10 µl compared to saline-treated animals and to pre-treatment values, * statistical difference with saline-treated groups (P < 0.01), $ statistical difference with pre-treatment (P < 0.001). #, statistical difference with control groups (P < 0.001).
Heat hyperalgesia
Thermal hyperalgesia was measured by the paw withdrawal latencies (PWL) to noxious heat stimulation. The baseline values were not significant between SCI-induced animals. However, PWL was significantly higher in control group compared to SCI-induced animals [F (5, 45) = 16.44; P < 0.001]. A significant increase in PWL was observed after administration of 100 µg/10 µl salicylate (P = 0.001 vs. pre-treatment) and 100 µg/10 µl tropisetron (P = 0.03 vs. pre-treatment) which indicate the inhibition of thermal hyperalgesia. Injection of tropisetron with the dose of 150 µg/10 µl made an increase in the PWL which was not statistically significant. No significant difference was observed between the baseline PWL and the values after saline injection (Fig. 4). The PWL after administration of salicylate (P = 0.19) and tropisetron 100 µg/10 µl (P > 0.99) were similar to the uninjured group. PWT in animals that received saline injection was 7.05 ± 0.07 which was significantly lower than the PWT in animals injected with salicylate (14.5 ± 0.48; P = 0.02) or tropisetron 100 µg/10 µl (15.1 ± 0.66; P = 0.003) (P < 0.001).
Figure 4.
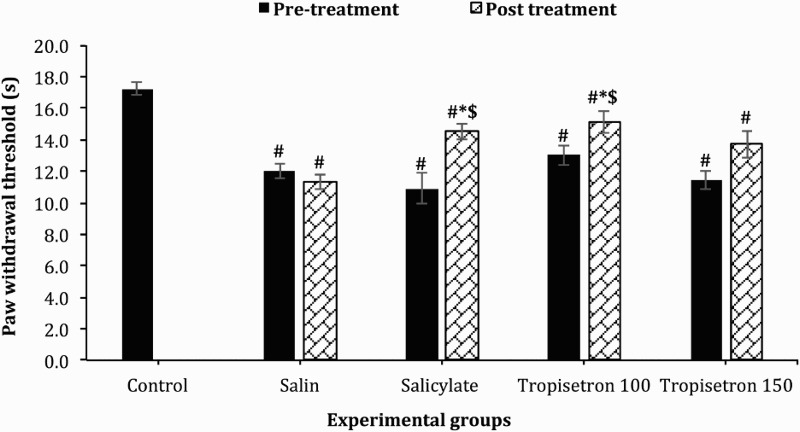
Effect of single IT injection of salicylate 100 µg/10 µl, Tropisetron 100 µg/10 µl and 150 µg/10 µl on paw-withdrawal threshold after themal (heat) stimulus . Data are expressed as means ± SEM withdrawal threshold (second). The mean withdrawal threshold is significantly increased after administration of salicylate and tropisetron 100 µg/10 µl compared to saline-treated animals and to stimulation before treatment, * statistical difference with saline-treated groups (P < 0.05), $ statistical defference with pre-treatment (P < 0.05). #, statistical difference with control groups (P < 0.001).
Figure 5.
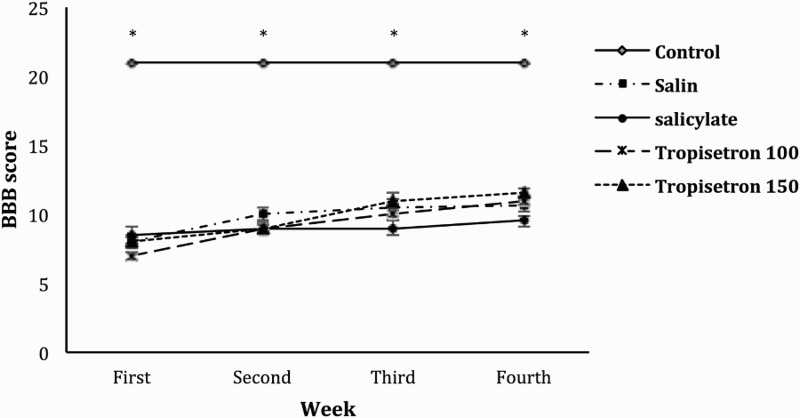
BBB scores of experimental animals that received normal saline, salicylate and different doses of tropicetron (100 & 150 µg). The locomotor function of the animals is compared with control (intact) animals. Data are expressed as mean ± SEM and P < 0.05 is considered significant. * shows statistical difference compared to experimental groups (P < 0.001).
Locomotor function
locomotor function evaluated by BBB score. Parameters as joint movements, paw placement, weight support, and fore/hindlimb coordination are judged according to the 21-point BBB locomotion scale. The score of normal (control) animals were all 21. Rats receiving spinal cord injury showed significantly lower BBB score when compared to intact non-injured animals (P < 0.001). No significant improvement was observed during the one-month period of the experiment. No significant difference was observed between groups injected with normal saline, salicylate or tropicetron.
Disscusion
In this study, the effects of selective 5-HT-3 receptor antagonists, tropisetron, on the central neuropathic pain threshold for mechanical and thermal stimulation were analyzed using clips compression injury model of spinal cord. The results showed that tropisetron inhibit the hyperalgesia and mechanical alldynia, with no effect on cold alodynia. The pain alleviating effect of tropisetron on mechanical and thermal hyperalgesia and mechanical allodynina is comparable to salicylate. The results of this study is consistent with the study of Zeitz et al. who showed that the 5-HT3 receptor is not involved in tissue or nerve injury-induced allodynia but it could attenuate formalin-induced hyperallgesia in chronic phase.8 It is necessary to take into consideration that the mechanisms inducing hyperalgesia are quite different and distinct from those mediating nociception or allodynia.16 Interestingly, Nitanda et al. showed that in chronic constriction injury model, tropisetron did not affect the mechanical threshold of the hyperalgesic paw, while the antagonist of 5-HT 2A receptors like sarpogrelate significantly reduce the mechanical pain following peripheral pain injury model.17 They used the Ugo Basil analgesiometer, which is used in our study to measure the mechanical hyperalgesia. The difference between their results and the result of the present study may relate to the route of administration of the antagonist The central and peripheral receptors may be responsible for difference in the results. They administrated tropisetron intrapertoneally while we injected it intrathecally. On the other hand, Suzuki et al. demonstrated that intrathecally administration of ondansetron, a 5HT3-antagonist, produced considerable abatement of the neuronal responses evoked by mechanical allodynia and heat stimuli (hyperalgesia) in neuropathic rats. This inhibitory effect was significantly enhanced after peripheral nerve injury.18
Human studies have demonstrated contradictory results. For example, Neziri et al. in a randomized, double-blind, placebo-controlled crossover study suggest that a single intravenous dose of tropisetron has no effect on modulation of pain and central hypersensitivity in patients with chronic low back pain.9 In another study, Tuveson found that ondansetron does not alter dynamic mechanical allodynia or spontaneous ongoing pain in peripheral neuropathy.19 This is consistent with the results obtained from an animal study with peripheral injection of the tropisetron.15 On the contrary, McCleane et al. showed pain scores were significantly reduced 2 hours after ondansetron injection. Results of their study suggest that ondansetron can have an analgesic effect in central neuropathic pain.7 5HT-receptors antagonists have beneficial effect in pain reduction of other situation. Mueller and Stratz showed that tropisetron is a very successful agent for local treatment of rheumatoid arthritis, tendinopathies, peri-arthropathies, and myofascial pain syndrome and found a long-lasting analgesic and an antiphlogistic effect.20
As mentioned above, it seems that the effects of 5HT receptors in human study are controversial and some experts believe that the 5HT3 receptor antagonists are able to reduce the pain while others have reported the contrary. From the experimental study it seems that 5HT3-receptor antagonists like tropisetron or ondansetron have more attenuating effect on the hyperalgesia than allodynia.
Disparity between human and experimental studies resulted from two reasons. A paramount discrepancy and a potential confounding factor is the administration route, that is, IT in the experimental studies and intravenously in human studies. Also it is necessary to consider that animal studies evaluated pain threshold, but human studies explored pain intensity.
Previous Studies revealed that the excitation of spinal 5-HT-3 receptors in the dorsal horn led to an antinociceptive effect in acute pain models, probably via release of GABA and activation of the descending inhibitory system.4,21 This antinociceptive effect is abrogated by administration of 5-HT3-receptor antagonists.22 But more recent studies in 5-HT3-knockout mice disclosed the involvement of this receptor in acute pain since substantial decrease of the second-phase, but not the first phase, in the formalin test after IT administration of a 5-HT3-receptor antagonist has been demonstrated.8 This means that administration of an antagonist alone did not alter pain thresholds in the absence of ongoing injury. Therefore, it seems that for involvement of 5HT3 receptor, first spinal cord levels of serotonin should be increased under conditions of injury and presumably of pain.
The experimental and clinical findings related to stimulation or inhibition of 5-HT3 receptors suggest that 5-HT-3 receptor antagonism is effective intervention in chronic pain reduction strategies, especially in situations linked to inflammatory stimuli. This intervention can alter pain perception in chronic pain. Some conflicting observations are not hard to understand, because serotonin-mediated reactions are complex. Spread serotoninergic neurotransmitter network in spinal cord and brain should be considered in exploration and interpretation of analgesic effects of this neurotransmitter. The serotoninergic system has a main role in mood, anxiety, sleep and temperature control and so could connect affective and vegetative behaviors with pain. Therefore, alterations in central 5HT neuronal activity may cause changes in this facilitatory system and thereby alter pain transmission at the spinal cord level. This may contribute to the fluctuations in pain reported by patients with neuropathy. Extensive projection of serotoninergic neurons causes different effects. For example, Faris et al. demonstrated ondansetron not only leads to a normalization of the elevated pain threshold but also reduces the vomit episodes.23 Furthermore, the therapeutic effects of tropisetron in fibromyalgia including pain reduction and improvement of various autonomic/functional symptoms were proved.24 Investigating the unexplained mechanisms underlying these effects is a task for the future research.
In clinical situations, 5HT3 receptor antagonists, such as tropisetron, are widely used as anti-emetics and are conventionally used in chemotherapy situations. Protective properties of tropisetron in neurodegenerative disorders hast attracted attention and have been under consideration.25 Only limited evidence exists for the application of this antagonist in pain management. Despite remarkable progress in the comprehension of pathophysiology of central neuropathic pain, alleviation of central neuropathic pain stand still difficult and the main analgesic drugs, such as antidepressants and opioids, have low efficacy in many patients.26 In addition, there is limited evidence for the effect of 5-HT-3 receptor antagonists on central neuropathic pain following injury in the central nervous system.
The present study demonstrated that tropisetron can reduce mechanically and thermally induced hyperalgesia and allodynia following spinal cord injury and suggests that this compound may have potential clinical utility for the management of central neuropathic pain, particularly in patients with hyperalgesia and tactile allodynia. However, the present study does not assess a time course of the analgesic effects of tropisetron. This would allow a better evaluation of the efficacy of tropisetron. Therefore, the result of the present study suggests tropisetron as an ultra-rapid acting agent for reducing neuropathic pain symptoms The long-term effects of tropisetron on alleviating neuropathic pain symptoms need to be investigated.
Conclusion
The results presented here show that IT administration of tropisetron alleviates mechanical and thermal hyperalgesia and mechanical allodynia in clip compression injury model of central neuropathic pain. These results impressed on the potential role of 5-HT3-antagonists in the clinical management of central neuropathic pain.
Disclaimer statements
Contributors MH and ZK participated in data collection. FN, MY, and AJ participated in analysis, interpretation and study design. MH and ZK wrote first draft of the work. FN and AJ revised it critically for important intellectual content. All authors have provided final approval of the version to be published and has agreed to be accountable for all aspects of the work.
Funding None.
Conflicts of interest None.
Ethics approval The protocol of present experimental study were reviewed and approved by the ethic committee of Iran University of medical sciences, Tehran, Iran. Throughout the study, the researchers adhere to guidelines of the “Guide for the Care and Use of Laboratory Animals.”
References
- 1.Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 2010;9(8):807–19. doi: 10.1016/S1474-4422(10)70143-5 [DOI] [PubMed] [Google Scholar]
- 2.Barnes NM, Hales TG, Lummis SC, Peters JA. The 5-HT 3 receptor–the relationship between structure and function. Neuropharmacology 2009;56(1):273–84. doi: 10.1016/j.neuropharm.2008.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gu M, Miyoshi K, Dubner R, Guo W, Zou S, Ren K, et al. Spinal 5-HT3 receptor activation induces behavioral hypersensitivity via a neuronal-glial-neuronal signaling cascade. J Neurosci 2011;31(36):12823–36. doi: 10.1523/JNEUROSCI.1564-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Takagi J, Yonehara N. Serotonin Receptor Subtypes Involved in Modulation of Electrical Acupuncture. Jpn J Pharmacol 1998;78(4):511–4. doi: 10.1254/jjp.78.511 [DOI] [PubMed] [Google Scholar]
- 5.Ali Z, Wu G, Kozlov A, Barasi S. The role of 5HT3 in nociceptive processing in the rat spinal cord: results from behavioural and electrophysiological studies. Neurosci Lett 1996;208(3):203–7. doi: 10.1016/0304-3940(95)12600-7 [DOI] [PubMed] [Google Scholar]
- 6.Green GM, Scarth J, Dickenson A. An excitatory role for 5-HT in spinal inflammatory nociceptive transmission; state-dependent actions via dorsal horn 5-HT 3 receptors in the anaesthetized rat. Pain. 2000;89(1):81–8. doi: 10.1016/S0304-3959(00)00346-8 [DOI] [PubMed] [Google Scholar]
- 7.McCleane GJ, Suzuki R, Dickenson AH. Does a single intravenous injection of the 5HT3 receptor antagonist ondansetron have an analgesic effect in neuropathic pain? A double-blinded, placebo-controlled cross-over study. Anesth Analg 2003;97(5):1474–8. doi: 10.1213/01.ANE.0000085640.69855.51 [DOI] [PubMed] [Google Scholar]
- 8.Zeitz KP, Guy N, Malmberg AB, Dirajlal S, Martin WJ, Sun L, et al. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci 2002;22(3):1010–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neziri AY, Dickenmann M, Scaramozzino P, Andersen OK, Arendt-Nielsen L, Dickenson AH, et al. Effect of intravenous tropisetron on modulation of pain and central hypersensitivity in chronic low back pain patients. Pain 2012;153(2):311–8. doi: 10.1016/j.pain.2011.10.008 [DOI] [PubMed] [Google Scholar]
- 10.Stratz T, Müller W. Treatment of chronic low back pain with tropisetron. Scand J Rheumatol 2004;33(S119):76–8. doi: 10.1080/03009740410007113 [DOI] [PubMed] [Google Scholar]
- 11.Hama A, Sagen J. Behavioral characterization and effect of clinical drugs in a rat model of pain following spinal cord compression. Brain Res 2007;1185:117–28. doi: 10.1016/j.brainres.2007.09.013 [DOI] [PubMed] [Google Scholar]
- 12.Hosseini M, Karami Z, Janzadenh A, Jameie SB, Mashadi ZH, Yousefifard M, et al. The effect of intrathecal administration of muscimol on modulation of neuropathic pain symptoms resulting from spinal cord injury; an experimental study. Emergency 2014;2(4):151–7. [PMC free article] [PubMed] [Google Scholar]
- 13.Xu F, Li T, Zhang B. An improved method for protecting and fixing the lumbar catheters placed in the spinal subarachnoid space of rats. J Neurosci Methods 2009;183(2):114–8. doi: 10.1016/j.jneumeth.2009.06.020 [DOI] [PubMed] [Google Scholar]
- 14.Chaplan S, Bach F, Pogrel J, Chung J, Yaksh T. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9 [DOI] [PubMed] [Google Scholar]
- 15.Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol 1996;139(2):244–56. doi: 10.1006/exnr.1996.0098 [DOI] [PubMed] [Google Scholar]
- 16.Oliveira M, Pelegrini-da-Silva A, Parada C, Tambeli C. 5-HT Acts on nociceptive primary afferents through an indirect mechanism to induce hyperalgesia in the subcutaneous tissue. Neuroscience 2007;145(2):708–14. doi: 10.1016/j.neuroscience.2006.12.021 [DOI] [PubMed] [Google Scholar]
- 17.Nitanda A, Yasunami N, Tokumo K, Fujii H, Hirai T, Nishio H. Contribution of the peripheral 5-HT 2A receptor to mechanical hyperalgesia in a rat model of neuropathic pain. Neurochem Int 2005;47(6):394–400. doi: 10.1016/j.neuint.2005.06.002 [DOI] [PubMed] [Google Scholar]
- 18.Suzuki R, Rahman W, Hunt SP, Dickenson AH. Descending facilitatory control of mechanically evoked responses is enhanced in deep dorsal horn neurones following peripheral nerve injury. Brain Res 2004;1019(1):68–76. doi: 10.1016/j.brainres.2004.05.108 [DOI] [PubMed] [Google Scholar]
- 19.Tuveson B, Leffler A-S, Hansson P. Ondansetron, a 5HT3-antagonist, does not alter dynamic mechanical allodynia or spontaneous ongoing pain in peripheral neuropathy. Clin J Pain 2011;27(4):323–9. doi: 10.1097/AJP.0b013e31820215c5 [DOI] [PubMed] [Google Scholar]
- 20.Müller W, Stratz T. Local treatment of tendinopathies and myofascial pain syndromes with the 5-HT3 receptor antagonist tropisetron. Scand J Rheumatol 2004;33(S119):44–8. doi: 10.1080/03009740410007032 [DOI] [PubMed] [Google Scholar]
- 21.Alhaider A, Lei S, Wilcox G. Spinal 5-HT3 receptor-mediated antinociception: possible release of GABA. J Neurosci 1991;11(7):1881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelissier T, Alloui A, Paeile C, Eschalier A. Evidence of a central antinociceptive effect of paracetamol involving spinal 5HT3 receptors. Neuroreport 1995;6(11):1546–8. doi: 10.1097/00001756-199507310-00020 [DOI] [PubMed] [Google Scholar]
- 23.Faris PL, Won Kim S, Meller WH, Goodale RL, Hofbauer RD, Oakman SA, et al. Effect of ondansetron, a 5-HT3 receptor antagonist, on the dynamic association between bulimic behaviors and pain thresholds. Pain 1998;77(3):297–303. doi: 10.1016/S0304-3959(98)00108-0 [DOI] [PubMed] [Google Scholar]
- 24.Kohnen R, Färber L, Späth M. The assessment of vegetative and functional symptoms in fibromyalgia patients: the tropisetron experience. Scand J Rheumatol 2004;33(S119):67–71. doi: 10.1080/03009740410007096 [DOI] [PubMed] [Google Scholar]
- 25.Rahimian R, Fakhfouri G, Ejtemaei Mehr S, Ghia JE, Genazzani AA, Payandemehr B, et al. Tropisetron attenuates amyloid-beta-induced inflammatory and apoptotic responses in rats. Eur J Clin Invest. 2013;43(10):1039–51. doi: 10.1111/eci.12141 [DOI] [PubMed] [Google Scholar]
- 26.Sindrup SH, Jensen TS. Efficacy of pharmacological treatments of neuropathic pain: an update and effect related to mechanism of drug action. Pain 1999;83(3):389–400. doi: 10.1016/S0304-3959(99)00154-2 [DOI] [PubMed] [Google Scholar]


