Abstract
Background
The brain motor control assessment (BMCA) for the upper limb has been developed to add resolution to the clinical evaluation in patients with spinal cord injury (SCI). BMCA is a surface electromyography (sEMG)-based measure of motor output from the central nervous system during a variety of reflex and voluntary motor tasks performed under strictly controlled conditions.
Method
Nine participants were recruited and assessed four times over a period of 1 year in a prospective cohort study design. The sEMG of 15 muscles (7 muscles from each upper limb and rectus abdominis) were recorded throughout the following stages of the BMCA protocol: (i) relaxation, (ii) reinforcement maneuvers, (iii) voluntary tasks, (iv) tendon-tap reflex responses, (v) vibration responses.
Results
Similarity index (SI) values were significantly lower in the SCI group for unilateral shoulder abduction (P = 0.006) and adduction (P = 0.021), elbow extension (P = 0.038), wrist flexion/extension with palm up (P < 0.001; P < 0.001) and wrist flexion with palm down (P = 0.016). sEMG magnitudes were also significantly lower in the SCI group for wrist flexion/extension with palm up (P < 0.001; P = 0.042). SI changes over time were significant for tasks related to wrist joint (P = 0.002).
Conclusion
Clinicians who are involved in rehabilitation of patients with SCI can use the BMCA to assess their patients’ motor control abilities and monitor their progression throughout their rehabilitation process. The results of this type of neurophysiological assessment might be useful to tailor therapeutic strategies for each patient.
Keywords: Spinal cord injury, Upper limb function
Introduction
The loss of upper limb function is one of the most significant and devastating losses after spinal cord injury (SCI) leading to subsequent dependence on others.1 The severity and extent of upper limb dysfunction are highly individualized after this injury and people with SCI most frequently report that arm and hand function is one of the main functions that they would like to be restored above all others.1–3 Neurorehabilitation after SCI is based on the concept that rehabilitative training recruits neuronal systems that remain intact after the injury to take over the impaired function. Understanding the neural mechanism of recovery will surely contribute to the development of evidence-based rehabilitation therapies.
In daily clinical practice, persons with SCI are evaluated using the International Standards for Neurological and Functional Classification of SCI4 and are classified according to the American Spinal Injury Association Impairment Scale (AIS). This evaluation involves a sensory and motor examination to determine neurological level of the injury and whether the injury is complete or incomplete. Using this scale, the severity of SCI will be categorized as A to E. The AIS grade A classification encompasses those individuals who have complete loss of voluntary muscle control and sensory function in limbs caudal to the injury. During motor examination, five key muscles will be examined bilaterally based on a six-point scale. Using this scale, the pattern of movement, e.g. the activation of synergistic muscles with concurrent inhibition of antagonistic muscles necessary to efficiently perform functional volitional movement, cannot be captured.
Discrepancies between neuropathological and clinical findings in paralysis after SCI led to development of the brain motor control assessment (BMCA), which can add resolution to the clinical evaluation of patients with SCI.5 This protocol is a surface electromyography (sEMG)-based measure of motor output from the central nervous system (CNS) during a variety of reflex and voluntary motor tasks of the upper6 and lower limbs7 performed under strictly controlled conditions. Even though the BMCA can provide valuable information about patients with SCI and has been used in evaluating lower limb function in several studies, there is limited reporting of similar information about upper limb function.
Our modification of the BMCA protocol for upper limb function has been previously published and we tested it on 19 neurologically intact individuals.6 The study presented here was undertaken to use this modified BMCA protocol to evaluate patients with SCI over time. The aim of this study was to investigate whether the pattern of voluntary movements in patients with SCI could be improved over time and whether this improvement could be evaluated with the BMCA protocol.
Method
Nine traumatic spinal cord-injured participants were recruited from the Royal Talbot Rehabilitation Centre to participate in this study (Table 1). No changes were made in clinical management of these patients for this study. All the participants gave their written informed consent before the assessments were carried out. All procedures used conformed with the Declaration of Helsinki, and the protocol was approved by the Human Research Ethics Committees at The University of Melbourne and Austin Health. The participants were assessed up to four times over a period of 1 year. The assessment sessions have been reported based on the number of days post-SCI (Table 2).
Table 1.
Participants' injury level, ASIA impairment scale category, motor and sensory levels
| Participant (sex) | ASIA score | Elbow flexors (C5) | Wrist extensors (C6) | Elbow extensors (C7) | Finger flexors (C8) | Finger abductors (T1) | Neurological level: |
|---|---|---|---|---|---|---|---|
| 1 (F) | D | R: 5 | R: 5 | R: 5 | R: 1 | R: 1 | Sensory: R: C6, L: T3 |
| Incom. | L: 5 | L: 5 | L: 5 | L: 3 | L: 3 | Motor: R: C7, L: C8 | |
| 2 (M) | A | R: 5 | R: 1 | R: 1 | R: 0 | R: 0 | Sensory: R: C4 L: C4 |
| Com. | L: 5 | L: 1 | L: 1 | L: 0 | L: 0 | Motor: R: C5, L: C5 | |
| 3 (M) | C | R: 5 | R: 2 | R: 1 | R: 0 | R: 0 | Sensory: R: C4 L: C4 |
| Incom. | L: 5 | L: 1 | L: 1 | L: 0 | L: 0 | Motor: R: C5, L: C5 | |
| 4 (M) | D | R: 5 | R: 5 | R: 4 | R: 4 | R: 1 | Sensory: R: C4 L: C4 |
| Incom. | L: 5 | L: 4 | L: 2 | L: 3 | L: 2 | Motor: R: C7, L: C6 | |
| 5 (M) | D | R: 5 | R: 3 | R: 1 | R: 4 | R: 2 | Sensory: R: C4 L: C4 |
| Incom. | L: 5 | L: 3 | L: 1 | L: 3 | L: 2 | Motor: R: C6, L: C6 | |
| 6 (M) | A | R: 5 | R: 4 | R: 5 | R: 4 | R: 3 | Sensory: R: C4 L: C4 |
| Com. | L: 5 | L: 5 | L: 5 | L: 4 | L: 4 | Motor: R: C6, L: C8 | |
| 7 (M) | B | R: 5 | R: 5 | R: 5 | R: 0 | R: 0 | Sensory: R: C7 L: C7 |
| Incom. | L: 5 | L: 5 | L: 5 | L: 1 | L: 1 | Motor: R: C7, L: C7 | |
| 8 (M) | D | R: 4 | R: 3 | R: 5 | R: 0 | R: 0 | Sensory: R: C4 L: C5 |
| Incom. | L: 4 | L: 4 | L: 5 | L: 4 | L: 3 | Motor: R: C5, L: C5 | |
| 9 (F) | A | R: 5 | R: 2 | R: 1 | R: 0 | R: 0 | Sensory: R: C4 L: C4 |
| Com. | L: 5 | L: 1 | L: 1 | L: 0 | L: 0 | Motor: R: C5, L: C5 |
ASIA: American Spinal Injury Association; Com: complete; Incom: incomplete; F: female; M: Male; R: right; L: left.
Table 2.
Assessment dates (days post injury)
| Participant | First Ax (days) | Second Ax (days) | Third Ax (days) | Fourth Ax (days) |
|---|---|---|---|---|
| 1 | 130 | 215 | 347 | 542 |
| 2 | 181 | 381 | 479 | 678 |
| 3 | 38 | Not assessed | Not assessed | Not assessed |
| 4 | 67 | 179 | 246 | 478 |
| 5 | 59 | 143 | 234 | 423 |
| 6 | 89 | 208 | 281 | 460 |
| 7 | 50 | 134 | 232 | 421 |
| 8 | 75 | 159 | 299 | Not assessed |
| 9 | 87 | Not assessed | Not assessed | Not assessed |
Upper limb BMCA protocol
The upper limb BMCA protocol was performed with participants lying supine. Participants wore a singlet to allow access to the skin overlying upper limb muscles. At the beginning of the test, participants were transferred on to a plinth to lie in the supine position in a quiet and warm room with minimized distractions (e.g. noise and traffic).
The sEMG of 15 muscles (7 muscles from each upper limb and rectus abdominis (RA)) were recorded throughout the experiment with self-adhesive pre-gelled disposable surface electrodes (Noraxon Dual electrodes, Scottsdale AZ, USA). They were pectoralis major, deltoid (middle fibers), biceps, triceps, wrist flexor muscle group, wrist extensor muscle group, opponens pollicis, and RA. Following skin preparation, pairs of sEMG electrodes, spaced 2 cm apart, were attached to the skin, oriented parallel to the long axis of the selected muscles. The skin under the electrodes was shaved and cleaned with alcohol. In each session the impedance between the two electrodes in the pair was less than 5 Ω. Electromyography (EMG) signals were amplified (×1000) by Zero Wire electrodes (Cometa, Milan, Italy) and then filtered (20–500 Hz) and digitized online (1 kHz sampling rate) using a PowerLab recording system (ADInstruments Ltd, Bella Vista, NSW, Australia). Voltages shown are as recorded by the PowerLab.
The protocol included the following stages: (i) relaxation, (ii) reinforcement maneuvers, (iii) voluntary tasks, (iv) tendon-tap reflex (TTR) responses, (v) vibration responses.
Relaxation
The EMG activity of 15 muscles was recorded for 5 minutes at rest. The instructions to participants were “Please place your arms on the plinth next to your body and try to relax to the best of your ability.” If the participant was unable to relax all muscles, attempts were made to facilitate relaxation through repositioning, coaching the participant and giving verbal feedback.
Reinforcement maneuvers
The maneuvers were “deep breath with forceful exhale” and “neck flexion.” Each reinforcement maneuver was repeated three times cued by a 3 seconds audible tone. For “deep breath with forceful exhale” participants were asked to start inhaling as soon as they heard the tone, hold their breath for the duration of the tone and finally, forcefully exhale at the end of the tone. The assessor monitored the sEMG for complete relaxation before the next trial could begin. For “neck flexion” the participants were requested to lift the head with the start of the tone, pressing against a force manometer placed on the forehead and then to relax again at the end of the tone.
Voluntary tasks
The voluntary tasks included one bilateral task with two phases (shoulder abduction/adduction) and four unilateral tasks with two phases (shoulder abduction/adduction; elbow flexion/extension; wrist flexion/extension with palm up and wrist flexion/extension with palm down). They were performed on both sides. All voluntary tasks in the BMCA protocol were cued by two 5-second tones with a brief pause between them, less than 1 second. Participants were asked to start the first task at the tone and not to start the second task until they heard the second tone. All tasks were repeated three times. After each trial, the participants were given time to relax all the muscles to their best ability before starting a new trial.
TTR response
A tendon hammer was designed for this study. The hammer was balanced about its axis of rotation so that gravity did not affect its motion. A leaf spring imparted energy to the hammer upon release, completed well before the hammer struck the tendon. This meant that the hammer made each strike with a consistent energy, independent of orientation and relative position. A minimum of 10 taps were applied to biceps and triceps tendon (right or left sides) at 5-second intervals. If a response was not observed, the tap position was adjusted, the limb was repositioned and the maneuver repeated until 10 responses were recorded.
Vibration responses
Vibration was applied for 30 seconds to each of the tendons that were tapped to elicit the vibration response. The vibrator used for this part of the BMCA was custom-constructed from a pneumatic hand-grinder fitted with an offset weight and protective barrel (frequency: 115 Hz, and a motion amplitude of 0.8 mm peak to peak). Electrical vibrators were found incapable of developing an adequate stimulus and all those tested caused artifacts in the sEMG channels.
Data reduction
A prototype response vector for each phase of each movement in the protocol was generated from 19 neurologically intact participants (38 limbs).6 These values were used to calculate the similarity index (SI), which compares the relative distribution of sEMG activity across the set of muscles chosen for the voluntary tasks6 and to evaluate the progression of participants with SCI during their rehabilitation. If patients were able to recruit the prime movers for a specific task and decrease unnecessary muscle activity in the other muscles, their SI scores approximated neurologically intact values, indicating better control of their movements. A value of 1.0 for the SI means that the test participant had an identical distribution of sEMG activity across muscles to the neurologically intact group for that task.
Linear model analysis was used to evaluate involuntary muscle activation at rest in neurologically intact participants vs. participants with SCI. Two-tailed unpaired t-test calculations were carried out to compare SI values of neurologically intact participants with the participants with SCI. Linear mixed model analysis was also used to assess the SI changes over time in participants with SCI. A significance level of P < 0.05 was adopted for all comparisons. This analysis was conducted using Statistical Package for the Social Sciences (SPSS) Statistics 22 software (IBM Corp., Armonk, NY, USA).
Results
Relaxation
Participants with SCI showed a significant level of involuntary muscle activity at rest in their first (P = 0.02) and second assessment (P < 0.001) compared to neurologically intact participants. These involuntary activations were reduced over time and became similar to neurologically intact participants (P < 0.05) (Fig. 1A). All the participants showed different degrees of long-lasting, involuntary activity in their muscles at rest. The summary of these observations can be seen in Table 3. The patterns of motor unit firing in different muscles were regular or irregular at rest. RA showed a regular pattern of firing during the relaxation period in all participants and during all assessment sessions except in participant 5. The firing pattern of RA was irregular in this participant in his first assessment; but it remained relaxed in the rest of his assessments. Left pectoralis major showed long-lasting involuntary activity at rest in every single assessment of each participant. Figure 1B shows the involuntary activation of upper limb muscles in participant 2 (Complete A) during relaxation at all his assessment sessions. As can be seen, the muscles with long-lasting involuntary activity were different at each session.
Figure 1.
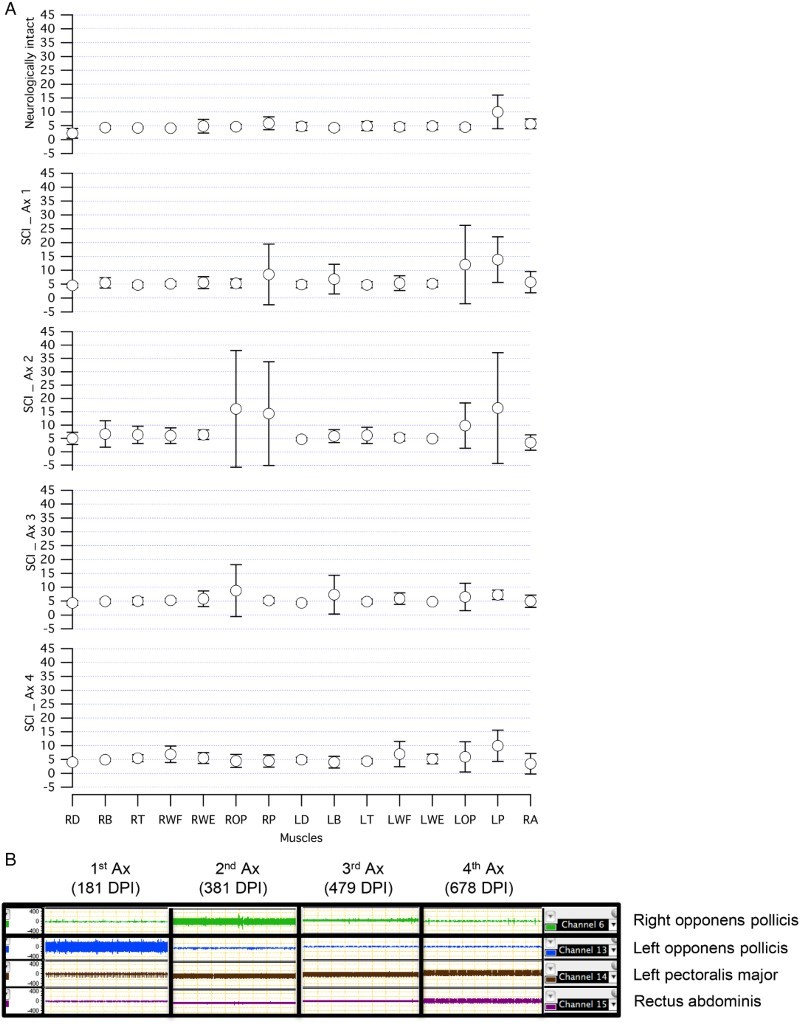
(A) Spontaneous muscle activity in participants with SCI over time compared to neurologically intact participants at rest. (B) Spontaneous muscle activity in participant number 2 during the relaxation period over time. (A) Averaged RMS ± SD for each muscle at rest are presented for participants with SCI and neurologically intact participants. The number of muscles with spontaneous activities, frequency and the amplitude of the motor unit firing were increased in participants with SCI during first and second assessment. These involuntary activities were reduced over time (Ax 3 and Ax 4) in participants with SCI. (B) An example of spontaneous muscle activities at rest in one participant with SCI. The frequency and the amplitude of the motor unit firing were increased over time in LPM. But the high level of spontaneous activity in LOP were cleared completely in the following assessments. Similar activity was seen in ROP in the second Ax which was cleared in the fourth Ax. In addition, the spontaneous muscle activity was only observed in RA in fourth Ax. RMS: root mean square, DPI: days post injury, LOP: left opponens pollicis, LPM: left pectoralis major, Ax: assessment.
Table 3.
Muscles identified as showing long-lasting involuntary activation during the relaxation part of BMCA and their firing pattern
| First Ax |
Second Ax |
Third Ax |
Fourth Ax |
|||||
|---|---|---|---|---|---|---|---|---|
| P | IFP | RFP | IFP | RFP | IFP | RFP | IFP | RFP |
| 1 | LOP | LPM | RT, LOP | LPM, RA | LOP | LPM, RA | LOP | LPM, RA |
| 2 | RWF, LOP | LPM, RA | RWE, ROP | LPM, RA | RT, LB | RPM, LPM, RA | – | RWE, LPM, RA |
| 3 | RB, RWE, LB | RPM, LPM | Not assessed | Not assessed | Not assessed | |||
| 4 | LPM, RA | LPM | LPM | |||||
| 5 | RB, RWF, LB | LPM, RA | LT | LPM, RPM | RWF | RPM, LPM, RA | ROP | RPM, LPM |
| 6 | ROP | LPM, RA | ROP, LOP | LPM | ROP, LOP | LPM, RA | ROP, LOP | LPM |
| 7 | RPM, LPM | – | RB, RT, RPM, LB, LT, LWF, LPM | – | RT, LWF | RPM, LPM, RA | RT, RWF, LWF LPM | – |
| 8 | LT, LWF, LPM | – | RWE | LPM, RA | RWE, ROP | RPM, LPM, RA | Not assessed | |
| 9 | RWF, LWF | RPM, LOP, LPM, RA | Not assessed | Not assessed | Not assessed | |||
P: participant; RB: right biceps; RT: right triceps; RWF: right wrist flexors; RWE: right wrist extensors; ROP: right opponens pollicis; RPM: right pectoralis major; LB: left biceps; LT: left triceps; LWF: left wrist flexors; LWE: left wrist extensors; LOP: left opponens pollicis; LPM: left pectoralis major. RA: rectus abdominis; IFP: irregular firing pattern; RFP: regular firing pattern.
Reinforcement maneuvers
None of the participants showed involuntary activity in their muscles related to these maneuvers.
Voluntary tasks
The response vectors for each task from 19 neurologically intact participants (38 limbs)6 were used as a normal pattern to evaluate the voluntary movements in participants with SCI. All the SI values for those tasks were around 1.0, which means that all the participants showed a similar pattern of muscle activations during those tasks (Fig. 2). SI values were significantly lower in the SCI group compared to the neurologically intact group for unilateral shoulder abduction (P = 0.006), unilateral shoulder adduction (P = 0.021), elbow extension (P = 0.038), wrist flexion with palm up (P < 0.001), wrist extension with palm up (P < 0.001), and wrist flexion with palm down (P = 0.016). sEMG magnitudes were also significantly lower in the SCI group compared to the neurologically intact group for wrist flexion with palm up (P < 0.001) and wrist extension with palm up (P = 0.042). Neurologically intact and SCI group mean SI and magnitude, for each motor task have been presented in Table 4.
Figure 2.
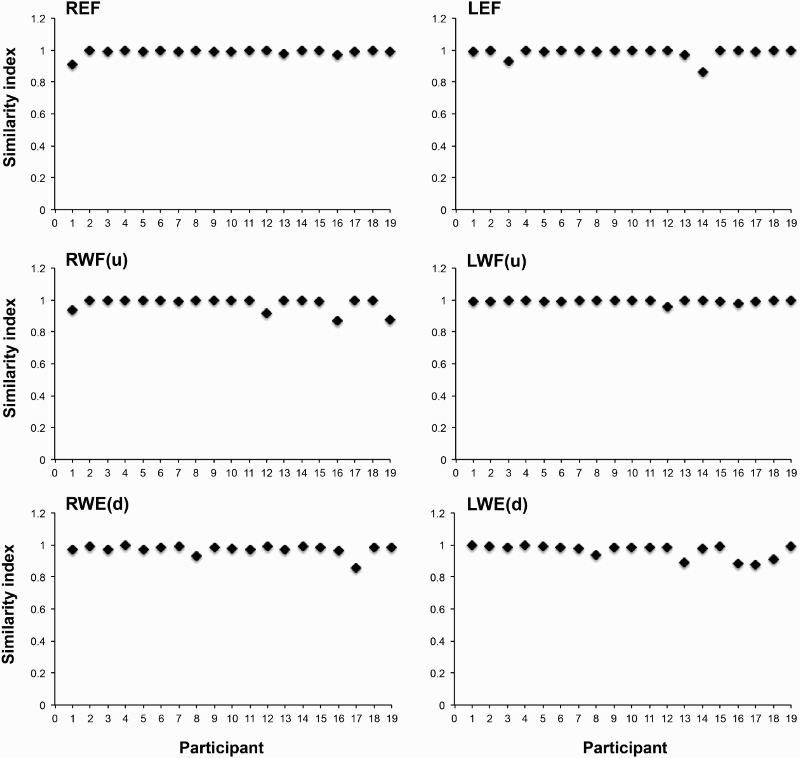
Examples of the SI values of 19 individual neurologically intact participants for three different voluntary tasks on both sides. It can be seen that neurologically intact participant showed a similar pattern of movements during these three voluntary tasks on both sides. R: right; L: left; EF: elbow flexion; WF(u): wrist flexion with palm up; WE(d): wrist extension with palm down.
Table 4.
Neurologically intact and patients with SCI mean SI and magnitude, for each motor task
| Voluntary tasks | Subject group | SI | EMG Mag (μV) |
|---|---|---|---|
| BShAb | NI | 0.84 ± 0.08 | 126.15 ± 65.82 |
| SCI first Ax | 0.79 ± 0.10 | 101.59 ± 56.95 | |
| SCI last Ax | 0.82 ± 0.05 | 116.52 ± 59.49 | |
| BShAd | NI | 0.84 ± 0.08 | 86.72 ± 50.20 |
| SCI first Ax | 0.82 ± 0.05 | 74.45 ± 54.43 | |
| SCI last Ax | 0.80 ± 0.13 | 69.83 ± 46.70 | |
| ShAb | NI | 0.87 ± 0.10 | 87.98 ± 52.82 |
| SCI first Ax | 0.72 ± 0.20** | 74.99 ± 45.93 | |
| SCI last Ax | 0.75 ± 0.16 | 82.30 ± 35.58 | |
| ShAd | NI | 0.86 ± 0.11 | 59.87 ± 37.45 |
| SCI first Ax | 0.73 ± 0.22* | 58.79 ± 44.61 | |
| SCI last Ax | 0.71 ± 0.21 | 60.65 ± 45.72 | |
| EF | NI | 0.99 ± 0.03 | 89.72 ± 71.70 |
| SCI first Ax | 0.88 ± 0.27 | 91.73 ± 66.33 | |
| SCI last Ax | 0.91 ± 0.25 | 82.09 ± 68.18 | |
| EE | NI | 0.91 ± 0.08 | 36.91 ± 33.12 |
| SCI first Ax | 0.78 ± 0.22* | 43.23 ± 27.34 | |
| SCI last Ax | 0.79 ± 0.23 | 30.95 ± 24.85 | |
| WFu | NI | 0.98 ± 0.03 | 84.06 ± 50.52 |
| SCI first Ax | 0.67 ± 0.28** | 26.03 ± 13.99** | |
| SCI last Ax | 0.85 ± 0.19 | 64.73 ± 51.02 | |
| WEu | NI | 0.95 ± 0.08 | 18.74 ± 10.80 |
| SCI first Ax | 0.58 ± 0.26** | 12.80 ± 9.46* | |
| SCI last Ax | 0.70 ± 0.21 | 22.39 ± 23.49 | |
| WEd | NI | 0.94 ± 0.10 | 77.76 ± 46.03 |
| SCI first Ax | 0.84 ± 0.26 | 68.09 ± 50.58 | |
| SCI last Ax | 0.98 ± 0.02 | 88.63 ± 76.80 | |
| WFd | NI | 0.90 ± 0.14 | 18.68 ± 13.37 |
| SCI first Ax | 0.69 ± 0.33* | 28.88 ± 19.59 | |
| SCI last Ax | 0.77 ± 0.20 | 23.65 ± 22.80 |
Comparisons were made between SCI first assessment values and neurologically intact group values (*p < 0.05, **p < 0.01). SI: similarity index, Mag: magnitude, BShAb: bilateral shoulder abduction, BShAd: bilateral shoulder adduction, EF: elbow flexion, EE: elbow extension, WFu: wrist flexion with palm up, WEu: wrist extension with palm up, WEd: wrist extension with palm down and WFd: wrist flexion with palm down.
Changes in SI values occurred at different rates for different tasks. Linear mixed model analysis showed that the effect of “Task” as an independent factor was significant (P < 0.001) but, the effect of “Time” did not reach significance (P < 0.05). Further analysis on separate task groups (shoulder tasks, elbow tasks, and wrist tasks) showed that the effect of “Time” was significant for wrist tasks (P = 0.001) and it did not reach a significant level for shoulder or elbow tasks (P < 0.05). The rate of SI changes over time can be seen for three different groups of tasks in Fig. 3.
Figure 3.
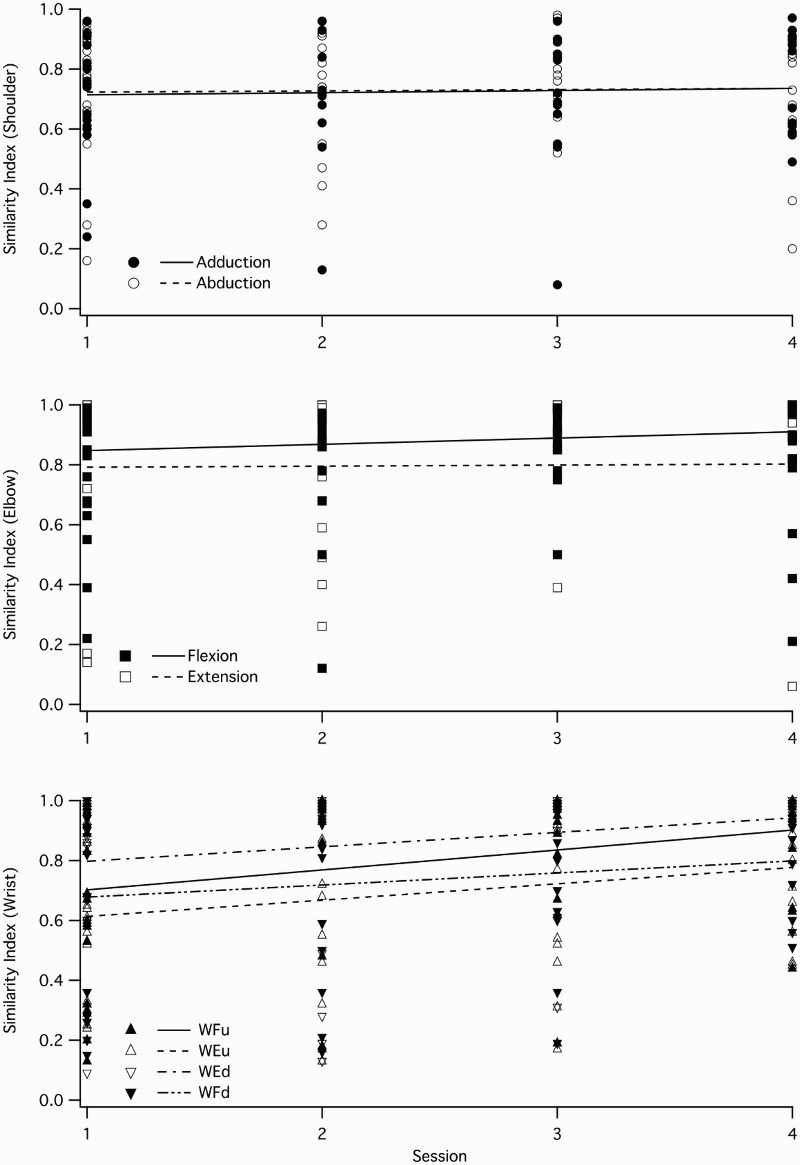
SI changes over time for unilateral tasks at three different joints in patients with SCI. The top and the middle panels show the tasks related to shoulder and elbow joints, respectively. As it can be seen, the two tasks in shoulder complex and elbow joint did not improve over time, but the four wrist tasks are showing some improvements over time (the bottom panel).
Figure 4 shows the pattern of muscle activation during wrist extension for all participants with SCI and one of the neurologically intact participants. Neurologically intact participants were able to activate the prime movers during each task with no involuntary activity in the other muscles (one example in Fig. 4), but some participants with SCI were not able to do so and they showed activation of muscles irrelevant to the tasks on both sides, even when they had enough strength in their wrist extensor muscles on the active side (3–5/5).
Figure 4.
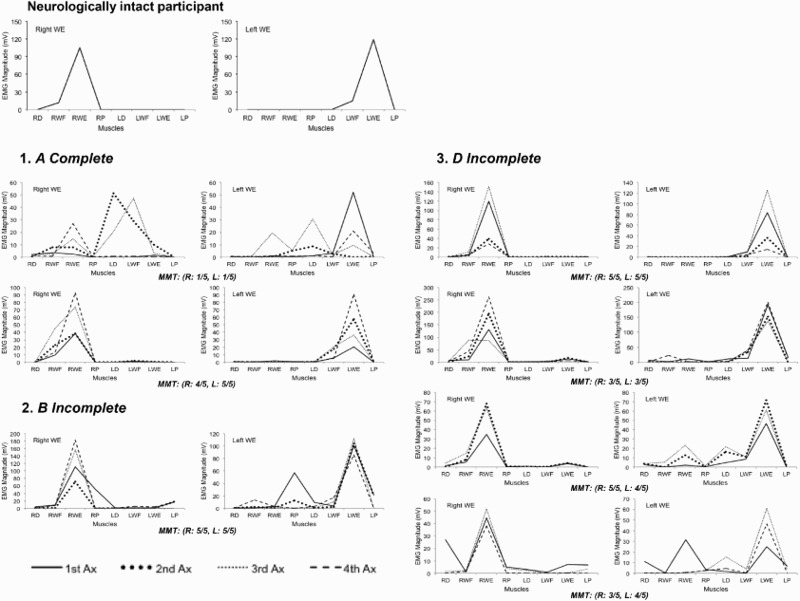
Pattern of muscle activation during wrist extension in participant with SCI and a neurologically intact participant. The neurologically intact participant was able to activate the prime movers during wrist extension and keep the other muscles quiet on both sides, but some participants with SCI were not able to do so and they showed activation of muscles irrelevant to the tasks on both sides even when they had enough strength in their wrist extensor muscles on the active side (3–5/5). WE: wrist extension, MMT: manual muscle testing. R: right, L: left.
Figure 5 shows changes in SI values across participants with SCI for all voluntary tasks. It can be seen that SI values were variable between assessment sessions and did not necessarily show improvement over time. For instance, participant 8 had very good SI scores for left shoulder abduction/adduction in his first assessment (0.8/0.9) and then it gradually dropped to (0.7/0.8) in his second assessment session and to 0.5/0.7 in the following assessments. The same pattern can be seen for his left elbow flexion/extension (Fig. 5). The same participant also showed some gradual improvements in his SI scores for right and left wrist flexion in his second and third assessments, but he did not maintain that level in his fourth assessment.
Figure 5.
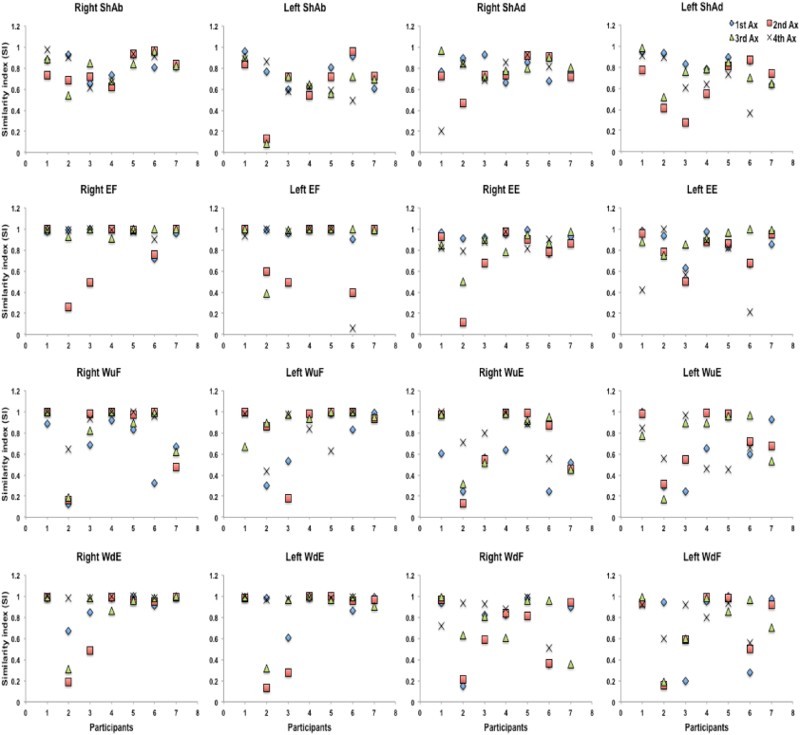
Individual SI scores for each unilateral voluntary task of participants with SCI. The SI scores of nine participants with SCI can be seen for four unilateral tasks with two phases (shoulder abduction/adduction; elbow flexion/extension; wrist flexion/extension with palm up; and wrist flexion/extension with palm down) at different assessment sessions (up to four assessments). Y-axis shows the SI scores and X-axis shows individual participant. ShAb: shoulder abduction; ShAd: shoulder adduction; EF: elbow flexion; EE: elbow extension; WF(u): wrist flexion with palm up; WE(d): wrist extension with palm down.
TTR and tonic vibratory response (TVR): participant 2 showed a clear response to vibration on his right biceps and triceps, 181 days post his injury (Fig. 6). All the other muscles in his upper limbs remained quiet during the vibration period. These responses are expected responses to vibration under supraspinal influences. But in the following assessments, he showed evidence of losing these supraspinal influences over right biceps and triceps muscles. In his third assessment session, the same vibratory stimulus produced responses in other muscles. A similar pattern of responses was seen in these muscles with TTR responses as well (Fig. 7). Five participants showed multiple-level responses for both TTR and TVR in all their assessment sessions. Table 5 shows a summary of TTR and TVR responses from biceps and triceps in all nine participants with SCI during their assessment sessions.
Figure 6.
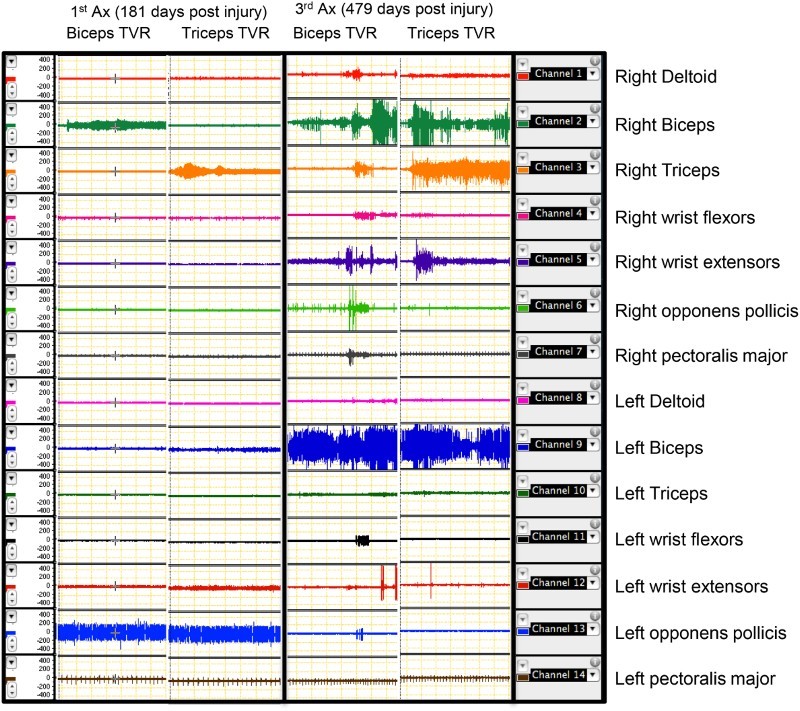
Responses of participant number 2 to vibration on right biceps and triceps in two different assessment sessions. Each panel includes two columns. The right-side columns show the responses to vibration on right biceps in seven muscles on both sides (right and left). The left-side columns show the responses to vibration on right triceps in seven muscles on both sides (right and left). It can be clearly seen that the selective responses to vibration in these two muscles have been lost after the first assessment. Note the multi-level responses on both sides. Ax: assessment. TVR: tonic vibratory response.
Figure 7.
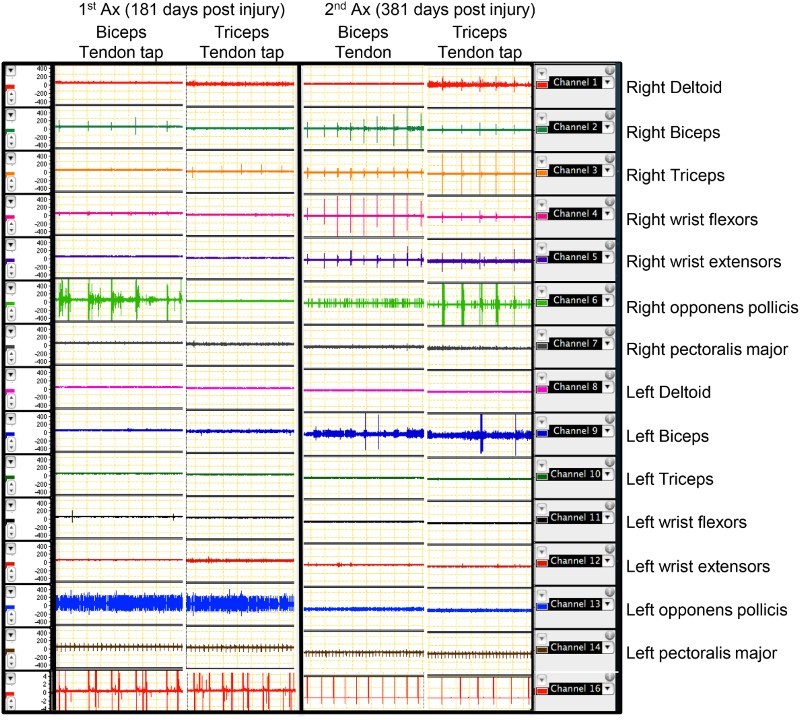
Tendon reflex responses of right biceps and triceps in participant number 2 at two different assessment sessions. Each panel includes two columns. The right-side columns show the responses in seven muscles on both sides during tendon tapping on right biceps. The left-side columns show the responses in seven muscles on both sides during tendon tapping on right triceps. It can be clearly seen that the selective responses to tendon tapping in these two muscles have been lost after the first assessment. Note the multi-level responses on both sides. Ax: assessment.
Table 5.
Tendon tap and TVRs from biceps and triceps during four assessment sessions in nine participants with SCI
| First Ax |
Second Ax |
Third Ax |
Fourth Ax |
|||||
|---|---|---|---|---|---|---|---|---|
| P | Biceps | Triceps | Biceps | Triceps | Biceps | Triceps | Biceps | Triceps |
| 1 | TTR: √ | TTR: √ | TTR: √ | TTR: √ | TTR: √ | TTR: √ | TTR: √ | TTR: √ |
| TVR: √ | TVR: √ | TVR: √ | TVR: √ | TVR: √ | TVR: NR | TVR: √ | TVR: NR | |
| 2 | TTR: √ | TTR: √ | TTR: MLR | TTR: MLR | TTR: MLR | TTR: MLR | TTR: MLR | TTR: MLR |
| TVR: √ | TVR: √ | TVR: MLR | TVR: MLR | TVR: MLR | TVR: MLR | TVR: MLR | TVR: MLR | |
| 3 | TTR: MLR | TTR: MLR | Not assessed | Not assessed | Not assessed | |||
| TVR: MLR | TVR: MLR | |||||||
| 4 | TTR: MLR | TTR: MLR | TTR: MLR | TTR: MLR | TTR: MLR | TTR: MLR | TTR: MLR | TTR: MLR |
| TVR: MLR | TVR: MLR | TVR: MLR | TVR: MLR | TVR: MLR | TVR: MLR | TVR: MLR | TVR: MLR | |
| 5 | TTR: MLR | TTR: MLR | TTR: MLR | TTR: MLR | TTR: MLR | TTR: MLR | TTR: MLR | TTR: MLR |
| TVR: MLR | TVR: MLR | TVR: MLR | TVR: MLR | TVR: MLR | TVR: MLR | TVR: MLR | TVR: MLR | |
| 6 | TTR: NR | TTR: √ | TTR: NR | TTR: √ | TTR: NR TVR: NR | TTR: √ | TTR: few Rs | TTR: √ |
| TVR: NR | TVR: √ | TVR: NR | TVR: √ | TVR: √ | TVR: √ | TVR: √ | ||
| 7 | TTR: NR TVR: NR | TTR: NR TVR: NR | TTR: MLR | TTR: MLR | TTR: MLR | TTR: MLR | TTR: MLR | TTR: MLR |
| TVR: NR | TVR: NR | TVR: MLR | TVR: MLR | TVR: MLR | TVR: NR | |||
| 8 | TTR: MLR | TTR: MLR | TTR: MLR | TTR: MLR | TTR: MLR | TTR: MLR | Not assessed | |
| TVR: NR | TVR: NR | TVR: MLR | TVR: MLR | TVR: MLR | TVR: MLR | |||
| 9 | TTR: MLR | TTR: MLR | Not assessed | Not assessed | Not assessed | |||
| TVR: MLR | TVR: MLR | |||||||
The TTR and TVR responses are two markers that can be used to investigate the existence of supraspinal influences over the motor circuitry of the examined muscle. As it can be seen, participant 2, had normal TTR and TVR in both biceps and triceps, but he lost these responses over time. MLR indicates the loss of inhibitory supraspinal influences on these muscles.
P: participant; TTR: tendon-tap response; TVR: tonic vibratory response; MLR: multi-level response; R: response; NR: no response; √: normal response.
Discussion
Nine participants with different levels of SCI in the cervical region were assessed up to four times with the BMCA protocol throughout their rehabilitation process in this prospective cohort study design.
In the present study, during the relaxation period, long-lasting involuntary activity was seen in muscles that are innervated from near to, within, or caudal to the injury zone similar to previous studies on participants with acute and chronic SCI using the multi-muscle surface EMG recording at rest.7–12 Similar continuous spinal motor output has also been reported in animal models of SCI.13,14
Many of these contractions are weak, occur in response to no obvious stimuli and involve spontaneous firing of motor units for long periods at low frequencies. It has been argued that these prolonged, involuntary activations of muscles are due, in large part, to the uncontrolled activation of sodium and calcium persistent inward currents in motoneurons.14–16
This long-lasting activity became more widespread in most participants over time, similar to previous studies.8 Even though no direct link between the presence of this long-lasting muscle activation and clinically measured function has been established to date, additional studies are needed to establish the clinical relevance and elucidate the precise cellular mechanism or mechanisms that should be targeted to provide effective treatment and avoid the impairment of volitional ability in patients with SCI.
Information regarding the pattern of muscular activation during upper limb tasks in patients with SCI is very limited in the literature. This study showed that the SI values were significantly lower in patients with SCI compared to the neurologically intact group for unilateral shoulder abduction/adduction, elbow extension, wrist extension with palm up, and wrist flexion with palm up/down. Only one study has assessed several upper limb voluntary tasks using the BMCA in 11 patients with SCI.17 Their results regarding elbow extension and wrist extension were similar to our study, but they showed a similar trend for elbow flexion as well, which was not the case for the present study. There are no previous data regarding shoulder abduction/adduction and wrist flexion that can be compared to the results of this study.
Assessing the voluntary movements in participants with SCI showed abnormal patterns of muscle activation during different tasks in patients with different levels of injury and different levels of muscle strength. Bilateral muscle activation can be seen during left wrist extension (Fig. 2) even though the participant had strong wrist extensor muscles (4–5/5). We also showed that the changes in SI values occurred at different rates for different tasks.
Plasticity is the ability of neurons to rearrange their anatomical and functional connectivity in response to environmental input, thereby achieving new or modified outputs. Following injury, neurons can spontaneously increase their plasticity, thereby enabling the creation of new networks as the basis for recovery and compensatory behavior.18 Anatomically, spontaneous injury-induced plasticity includes regenerative sprouting from damaged and intact neurons, synaptogenesis, and synaptic remodeling. Functional plasticity includes changes in neuronal excitability and inhibition, conduction velocity, and synaptic efficacy.
Courtine et al.19 investigated the neural basis of spontaneous recovery after SCI in mice. Interestingly, they showed that propriospional connections are able to mediate spontaneous functional recovery of stepping by bypassing the injury sites without the existence of the descending supraspinal pathways. They concluded that for functional recovery of lumbosacral circuits, the reorganization of interactions between intrinsic spinal cord circuits and descending inputs that relay information past lesion sites is sufficient.19
Not all spontaneous sprouting is useful. Clinically, it has been shown that stimulation of lower limbs following cervical SCI induces involuntary short-latency contractions in the distal upper limbs.20 Such inter-limb reflexes can appear by 6 months and increase thereafter, indicating ongoing synaptic plasticity and strengthening connections.20 But it has been suggested that these connections do not necessarily confer functional benefit and they might block access to synaptic sites by supraspinal axons that might eventually be encouraged to sprout.
Plasticity will also occur at the supraspinal level after SCI. A longitudinal functional magnetic resonance imaging (fMRI) study was performed on six patients with SCI over a 1-year period.21 During fMRI, individuals in the sub-acute stage of their SCI performed a simple self-paced wrist extension motor task. The results showed little task-related activation within the primary motor cortex and extensive activation in sensorimotor areas. The pattern was reversed over time and with improved wrist movement, with greater recruitment of motor areas.21 When the movement was performed with a normal pattern of activity, the overall pattern of cortical activation was similar to that of able-bodied individuals. In a recent study, Freund et al.22 assessed 13 patients after acute traumatic SCI clinically and by magnetic resonance imaging in a prospective longitudinal study over a year. They showed progressive structural changes that were associated with neurological and functional improvements.22 They concluded that patients with greater corticospinal tract integrity recovered more than those patients with low corticospinal tract integrity.22
Participant 2 showed expected responses to tendon tapping and vibration on right biceps (5/5) and triceps (1/5) muscles in his first assessment which was 181 days post his injury. Unfortunately, he lost the selectivity of these responses over time, which is similar to the behavior of neurons that have lost their inhibitory supraspinal influences. This behavior has been reported in previous studies as well.7,23,24
The TTR and TVR responses are two markers that can be used to investigate the existence of supraspinal influences over the motor circuitry of the examined muscle.25,26 Eklund and Hagbarth27 described the TVR response as a reflex muscular contraction in response to a vibratory stimulus. In neurologically intact people, mechanical vibration of low amplitude (3 mm) and a frequency of about 100 cycles per second, efficiently produces a reflex contraction, which increases slowly till a plateau is reached. Such a contraction remains as long as the vibration continues and dies away a few seconds after vibrator removal. There are five mechanisms whereby higher centers influence spinal reflexes: direct input to alpha motor neurons, excitation of segmental inhibitory interneurons, actions on propriospinal neurons that travel to other segmental levels, input to gamma motor neurons and synapses on afferent terminals.
Propriospinal interneurons, whose axons do not leave the spinal cord, account for about 90% of spinal neurons, and have either short or long axons which project over several segments and regulate activity of other local interneurons. It has been shown that loss of brain control often yields a condition where the central state of excitability within internuncial and propriospinal interneuron networks is very high, promoting activation of motor units serving antagonistic, ipsilateral, and contralateral musculature.28
Conclusion
Neuorehabilitation interventions aim to minimize the impact of the injury on spinal cord functions and maximize the restoration of functional capabilities. To achieve this goal, therapists need to be able to assess their patients with more resolution, so they can tailor their treatment plans based on an individual's needs. The quantifiable features of surface EMG may increase the resolution of SCI characterization by adding subclinical details to the clinical picture of lesion severity and distribution.
Disclaimer statements
Contributors MZ was involved in designing the study, obtaining the ethics approval, collecting the data, analyzing the data, interpreting the data and writing the article in whole. MG was involved in designing the study, obtaining the ethics approval, analyzing the data, interpreting the data, and editing the article. DM was involved in analyzing the data, interpreting the data, and editing the article.
Funding This study was supported by a grant from the Victorian Neurotrauma Initiative (Transport Accident Commission).
Conflicts of interest None.
Ethics approval This study was approved by the Human Research Ethics Committees at The University of Melbourne and Austin Health.
References
- 1.Snoek GJ, IJzerman MJ, Hermens HJ, Maxwell D, Biering-Sørensen F. Survey of the needs of patients with spinal cord injury: impact and priority for improvement in hand function in tetraplegics. Spinal Cord 2004;42(9):526–32. doi: 10.1038/sj.sc.3101638 [DOI] [PubMed] [Google Scholar]
- 2.Hanson RW, Franklin MR. Sexual loss in relation to other functional losses for spinal cord injured males. Arch Phys Med Rehab 1976;57(6):291–93. [PubMed] [Google Scholar]
- 3.Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma 2004;21(10):1371–83. doi: 10.1089/neu.2004.21.1371 [DOI] [PubMed] [Google Scholar]
- 4.Kirshblum SC, Burns SP, Biering-Sørensen SP, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med 2011;34(6):535–46. doi: 10.1179/204577211X13207446293695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimitrijevic MR, Dimitrijevic MM, Faganel J, Sherwood AM. Suprasegmentally induced motor unit activity in paralyzed muscles of patients with established spinal cord injury. Ann Neurol 1984;16(2):216–21. doi: 10.1002/ana.410160208 [DOI] [PubMed] [Google Scholar]
- 6.Zoghi M, Galea M, Morgan D. A brain motor control assessment (BMCA) protocol for upper limb function. PLoS One 2013;8(11):e79483. doi: 10.1371/journal.pone.0079483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherwood AM, McKay WB, Dimitrijevic MR. Motor control after spinal cord injury: assessment using surface EMG. Muscle Nerve 1996;19(8):966–79. doi: [DOI] [PubMed] [Google Scholar]
- 8.McKay WB, Ovechkin AV, Vitaz TW, Terson de Paleville DG, Harkema SJ. Long-lasting involuntary motor activity after spinal cord injury. Spinal Cord 2011;49(1):87–93. doi: 10.1038/sc.2010.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKay WB, Lim HK, Priebe MM, Stokic DS, Sherwood AM. Clinical neurophysiological assessment of residual motor control in post-spinal cord injury paralysis. Neurorehab Neural Repair 2004;18(3):144–53. doi: 10.1177/0888439004267674 [DOI] [PubMed] [Google Scholar]
- 10.Stein RB, Brucker BS, Ayyar DR. Motor units in incomplete spinal cord injury: electrical activity, contractile properties and the effects of biofeedback. J Neurol Neurosurg Psychiatry 1990;53(10):880–5. doi: 10.1136/jnnp.53.10.880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas CK, Ross BH. Distinct patterns of motor unit behavior during muscle spasms in spinal cord injured subjects. J Neurophysiol 1997;77(5):2847–50. [DOI] [PubMed] [Google Scholar]
- 12.Zijdewind I, Thomas CK. Spontaneous motor unit behavior in human thenar muscles after spinal cord injury. Muscle Nerve 2001;24(7):952–62. doi: 10.1002/mus.1094 [DOI] [PubMed] [Google Scholar]
- 13.Gelfan S, Tarlov IM. Interneurones and rigidity of spinal origin. J Physiol 1959;146(3):594–617. doi: 10.1113/jphysiol.1959.sp006214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol 2003;90(2):857–69. doi: 10.1152/jn.00236.2003 [DOI] [PubMed] [Google Scholar]
- 15.Eken T, Hultborn H, Kiehn O. Possible functions of transmitter-controlled plateau potentials in alpha motoneurones. Prog Brain Res 1989;80:257–67. doi: 10.1016/S0079-6123(08)62219-0 [DOI] [PubMed] [Google Scholar]
- 16.Kuo JJ, Siddique T, Fu R, Heckman CJ. Increased persistent Na(+) current and its effect on excitability in motoneurones cultured from mutant SOD1 mice. J Physiol 2005;563(Pt 3):843–54. doi: 10.1113/jphysiol.2004.074138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKay WB, Ovechkin AV, Vitaz TW, Terson de Paleville DG, Harkema SJ. Neurophysiological characterization of motor recovery in acute spinal cord injury. Spinal Cord 2011;49(3):421–9. doi: 10.1038/sc.2010.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolpaw JR. Spinal cord plasticity in acquisition and maintenance of motor skills. Acta Physiol (Oxf) 2007;189(2):155–69. doi: 10.1111/j.1748-1716.2006.01656.x [DOI] [PubMed] [Google Scholar]
- 19.Courtine G, Song B, Roy RR, Zhong H, Herrmann J, Ao Y, et al. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med 2008;14(1):69–74. doi: 10.1038/nm1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calancie B, Alexeeva N, Broton JG, Molano MR. Interlimb reflex activity after spinal cord injury in man: strengthening response patterns are consistent with ongoing synaptic plasticity. Clin Neurophysiol 2005;116(1):75–86. doi: 10.1016/j.clinph.2004.07.018 [DOI] [PubMed] [Google Scholar]
- 21.Jurkiewicz MT, Mikulis DJ, McIlroy WE, Fehlings MG, Verrier MC. Sensorimotor cortical plasticity during recovery following spinal cord injury: a longitudinal fMRI study. Neurorehabil Neural Repair 2007;21(6):527–38. doi: 10.1177/1545968307301872 [DOI] [PubMed] [Google Scholar]
- 22.Freund P, Weiskopf N, Ashburner J, Wolf K, Scutter R, Altmann D, et al. MRI investigation of the sensorimotor cortex and the corticospinal tract after acute spinal cord injury: a prospective longitudinal study. Lancet Neurol 2013;12:873–81. doi: 10.1016/S1474-4422(13)70146-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dimitrijevic MR, Nathan PW. Studies of spasticity in man. Some features of spasticity. Brain 1967;9(Part I):1–30. doi: 10.1093/brain/90.1.1 [DOI] [PubMed] [Google Scholar]
- 24.Dimitrijevic MR, Nathan PW. Studies of spasticity in man. 2. Analysis of stretch reflexes in spasticity. Brain 1967;90(Part II):333–58. doi: 10.1093/brain/90.2.333 [DOI] [PubMed] [Google Scholar]
- 25.Sherwood AM, Dimitrijevic MR, Bacia T, McKay WB. Characteristics of the vibratory reflex in humans with reduced suprasegmental influence due to spinal cord injury. Restor Neurol Neurosci 1993;5(2):119–29. [DOI] [PubMed] [Google Scholar]
- 26.Sherwood AM, Dimitrijevic MR, McKay WB. Evidence of subclinical brain influence in clinically complete spinal cord injury: discomplete SCI. J Neurol Sci 1992;110(1–2):90–8. doi: 10.1016/0022-510X(92)90014-C [DOI] [PubMed] [Google Scholar]
- 27.Eklund G, Hagbarth KE. Motor effects of vibratory muscle stimuli in man. Electron Clin Neuro 1965;19:619. [Google Scholar]
- 28.Kern H, McKay WB, Dimitrijevic MM, Dimitrijevic MR. Motor control in the human spinal cord and the repair of cord function. Curr Pharm Des 2005;11(11):1429–39. doi: 10.2174/1381612053507882 [DOI] [PubMed] [Google Scholar]


